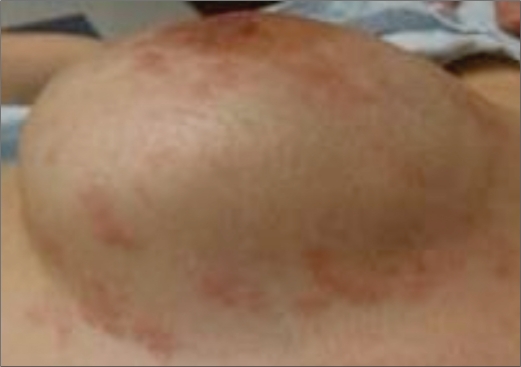
It is estimated that 207,090 women will be diagnosed with and 39,840 women will die of breast cancer in 2010 in the United States. Inflammatory breast cancer (IBC) accounts for approximately 1% to 6% of all breast cancer cases in the US (1, 2). This rare and aggressive form of breast cancer is diagnosed clinically by the rapid onset of diffuse erythema and edema (peau d'orange) of at least a third of the skin overlying the breast (3) (Figure 1). Tumor emboli blocking dermal lymphatic channels lead to the characteristic “inflammatory” skin changes; however, this is not necessary to make the diagnosis (4). The primary tumor of IBC is classified as T4d by definition, even if no underlying palpable mass is present in the breast.
Figure 1.
Typical clinical appearance of inflammatory breast cancer.
Women diagnosed with IBC have inferior survival outcomes compared with women with other forms of breast cancer. IBC patients tend to be younger, and IBC tumors are more likely to overexpress HER2 than non-IBC tumors (5). Hormone receptor negativity also occurs at a higher frequency in IBC tumors (6). At presentation, most women with IBC have lymph node involvement, and approximately one third have distant sites of disease (7, 8). Historically, attempts to treat IBC with surgery alone or surgery combined with radiation therapy resulted in median overall survival times of less than 15 months and local recurrence rates as high as 50% (9).
The treatment of IBC has dramatically improved with the advent of multimodality therapy. Results from a large retrospective study of patients with IBC performed over a 20-year period demonstrated that initial treatment with an anthracycline-based regimen followed by local therapy resulted in 5 - and 10-year survival rates of 40% and 33%, respectively (10). The incorporation of taxanes has also been associated with higher pathologic complete response rates and better survival outcomes. According to data from the National Cancer Institute's Surveillance, Epidemiology, and End Results database, for women who were diagnosed with IBC between 1988 and 2001, the 5-year survival rate was approximately 40%. This compares with about 87% for all breast cancers combined.
The National Comprehensive Cancer Network (NCCN) panel recommends preoperative chemotherapy with an anthracycline-based regimen with or without taxanes for the initial treatment of patients with IBC. Inclusion of trastuzumab in the chemotherapy regimen is recommended for patients with HER2-positive disease. Patients responding to preoperative treatment should then undergo mastectomy with axillary lymph node dissection. Any remaining planned chemotherapy should be completed after the mastectomy, followed sequentially by endocrine therapy in patients with hormone receptor–positive disease. Finally, postmastectomy chest wall and regional node irradiation is recommended after the completion of any planned chemotherapy. As a historical reference, the Baylor Sammons Cancer Center medical committee and breast site committee constructed IBC treatment guidelines in 1989 that are in all practical purposes identical to the current NCCN guidelines.
In 2010, the joint medical committee at Baylor Sammons Cancer Center requested a review of the IBC cases seen at our institution from 2003 to 2009 to evaluate compliance with the aforementioned guidelines as well as measure Baylor Dallas' outcomes data in accordance with the National Cancer Data Base (NCDB). This report presents those findings.
METHODS
Using the NCDB data from Baylor University Medical Center at Dallas, we retrospectively analyzed 51 cases entered into the database from 2003 to 2009 with the diagnosis of cancer of the breast (ICD-O-2/3 codes C50.0 through C50.9), with a recorded T stage of 4, who were managed at Baylor Sammons Cancer Center. Our data were evaluated for compliance with the NCCN practice guidelines for IBC, with outcomes measured against survival data from the NCDB. To evaluate our outcomes data internally, we extracted data on age, race/ethnicity, type of insurance, and chemotherapy regimens.
RESULTS
Patient characteristics
Fifty-one patients with stage 3 (T4) breast cancer were identified from the Baylor cancer registry between 2003 and 2009. Two of the patients were <40 years, 9 were in their 40s, 17 in their 50s, 14 in their 60s, 6 in their 70s, and 3 ≥80 years. In terms of race/ethnicity, 35 were white, 13 were black, 2 were Hispanic, and 1 was Filipino.
Treatment
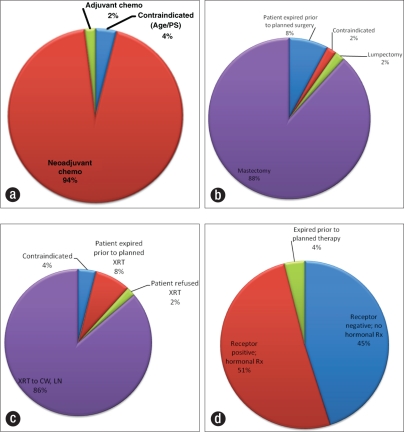
Of the 51 patients, 48 (94%) were administered neoadjuvant chemotherapy; 45 (88%) underwent a mastectomy; 44 (86%) received adjuvant radiation therapy after completion of chemotherapy and surgery; and of the 28 patients who had positive estrogen and/or progesterone receptors, 26 received hormonal therapy (51% of total patient group) (Figure 2). Chemotherapy regimens included doxorubicin and cyclophosphamide (28 patients); fluorouracil, epirubicin, and cyclophosphamide (9 patients); cyclophosphamide, doxorubicin, and fluorouracil (4 patients) followed by a taxane (with or without trastuzumab); and a variety of other combination regimens incorporating a taxane, doxorubicin, cyclophosphamide, trastuzumab, or capecitabine.
Figure 2.
Therapy for the 51 patients: (a) multiagent chemotherapy, (b) surgery, (c) radiation therapy (XRT), and (d) hormonal therapy. PS indicates performance status; CW, chest wall; LN, lymph nodes.
Standard of care
Of the 28 patients with hormone receptor–positive breast cancer, 23 (82%) received the standard-of-care treatment for IBC as outlined in the NCCN practice guidelines (Table). Of those who did not receive standard multimodality therapy, 1 refused radiation, 2 died before receiving additional therapy, 1 had contraindications for surgery, chemotherapy, and radiation, and 1 was referred to Baylor after mastectomy. Nineteen of the 23 patients (83%) with hormone receptor–negative breast cancer received standard-of-care treatment. Two of those who did not expired before additional therapy, and two had a contraindication to either chemotherapy or radiation therapy.
Table.
Treatment for 51 patients with inflammatory breast cancer treated at Baylor University Medical Center at Dallas
| Treatment | Patients (%) | Comments |
| Hormone receptor–positive patients | ||
| Chemotherapy, surgery, radiation therapy, hormonal therapy | 23 (82%) | Standard of care |
| Chemotherapy, surgery, hormonal therapy | 1 (4%) | Refused radiation therapy |
| Chemotherapy only | 2 (7%) | Patients died before additional treatment |
| Hormonal therapy only | 1 (4%) | Additional therapies contraindicated (95-year-old) |
| Surgery, chemotherapy, radiation therapy, hormonal therapy | 1 (4%) | Patient referred after mastectomy |
|
Hormone receptor–negative patients | ||
| Chemotherapy, surgery, radiation therapy | 19 (83%) | Standard of care |
| Chemotherapy, surgery | 1 (4%) | Radiation therapy was contraindicated |
| Chemotherapy only | 2 (9%) | Patients died before additional treatment |
| Surgery, radiation therapy | 1 (4%) | Chemotherapy was contraindicated (83-year-old) |
Survival
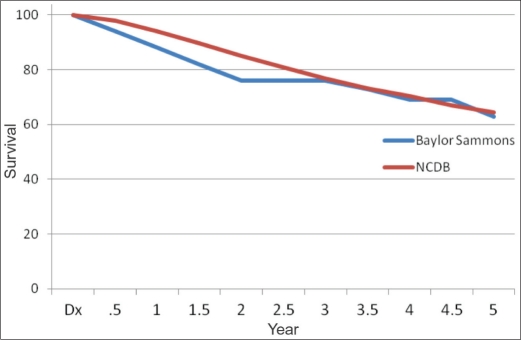
As of December 2010, the median survival for patients entered into the database between 2003 and 2009 was 45 months. Of the 32 patients (63%) who were alive, 27 had no evidence of disease, and 5 were alive with metastatic breast cancer. Of the 19 patients who died, 16 deaths were attributed to breast cancer (5 were never disease free, and 11 had recurrence identified 1 to 3 years after treatment). Three patients had no evidence of breast cancer at the time of death but died secondary to other cancers (lung cancer, cervical cancer, and acute lymphocytic leukemia). When comparing Baylor's data to NCDB data, there was no difference in survival based on chemotherapy agents, race/ethnicity, payer type, or age. The Kaplan-Meier survival curves (Figure 3) appear to be superimposable starting at year 3. The sample size from Baylor Sammons Cancer Center was not large enough to detect any statistically significant differences in survival.
Figure 3.
Survival rates at Baylor Sammons Cancer Center and the National Cancer Data Base (NCDB). At Baylor, survival was 88% at year 1, 76% at year 3, and 63% at year 5. In the NCDB, survival was 94% at year 1, 76.9% at year 3, and 64.5% at year 5.
DISCUSSION
This retrospective analysis of patients treated for IBC at Baylor Sammons Cancer Center from 2003 to 2009 provides insight into practice patterns as well as outcomes data. The 51 patients analyzed during this time frame is likely an underrepresentation of the true number of cases seen at our cancer center, considering that 600 to 650 new patients with breast cancer are seen at our institution each year. Most likely, inconsistencies in coding prevented us from identifying all cases. For instance, cases may have been coded as locally advanced breast cancer, which carries a different ICD code.
Overall, the vast majority of patients at our cancer center were treated with multimodality therapy, which represents the standard of care as outlined in the NCCN practice guidelines. The few patients who did not receive standard treatment had extenuating circumstances. For instance, a few patients died before receiving planned therapy, some had contraindications such as advanced age and poor performance status, and one patient refused. Only two patients in the analysis did not receive the standard of care: one patient underwent a mastectomy without neoadjuvant chemotherapy at an outside institution before seeking care at Baylor, and the other patient underwent a lumpectomy by choice. The median survival of patients in this retrospective analysis of IBC was 45 months at the time of this publication.
In conclusion, IBC remains a rare but aggressive form of breast cancer. The application of multimodality therapy as directed by a multidisciplinary team has improved survival for patients with IBC. Patients treated at Baylor Sammons Cancer Center from 2003 to 2009 received treatment in accordance with the NCCN guidelines, with survival outcomes similar to those published in the NCDB.
References
- 1.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97(13):966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawood S, Cristofanilli M. What progress have we made in managing inflammatory breast cancer? Oncology. 2007;21(6):673–679. [PubMed] [Google Scholar]
- 3.Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, Dirix LY, Levine PH, Lucci A, Krishnamurthy S, Robertson FM, Woodward WA, Yang WT, Ueno NT, Cristofanilli M. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol 2010 Aug 9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Bonnier P, Charpin C, Lejeune C, Romain S, Tubiana N, Beedassy B, Martin PM, Serment H, Piana L. Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? Int J Cancer. 1995;62(4):382–385. doi: 10.1002/ijc.2910620404. [DOI] [PubMed] [Google Scholar]
- 5.Parton M, Dowsett M, Ashley S, Hills M, Lowe F, Smith IE. High incidence of HER-2 positivity in inflammatory breast cancer. Breast. 2004;13(2):97–103. doi: 10.1016/j.breast.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Paradiso A, Tommasi S, Brandi M, Marzullo F, Simone G, Lorusso V, Mangia A, De Lena M. Cell kinetics and hormonal receptor status in inflammatory breast carcinoma. Comparison with locally advanced disease. Cancer. 1989;64(9):1922–1927. doi: 10.1002/1097-0142(19891101)64:9<1922::aid-cncr2820640927>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: a review. J Clin Oncol. 1992;10(6):1014–1024. doi: 10.1200/JCO.1992.10.6.1014. [DOI] [PubMed] [Google Scholar]
- 8.Walshe JM, Swain SM. Clinical aspects of inflammatory breast cancer. Breast Dis. 2005–2006;22:35–44. doi: 10.3233/bd-2006-22105. [DOI] [PubMed] [Google Scholar]
- 9.Zucali R, Uslenghi C, Kenda R, Bonadonna G. Natural history and survival of inoperable breast cancer treated with radiotherapy and radiotherapy followed by radical mastectomy. Cancer. 1976;37(3):1422–1431. doi: 10.1002/1097-0142(197603)37:3<1422::aid-cncr2820370325>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, Frye D, Hortobagyi GN. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40(4):321–329. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]