Abstract
We present a case of Kounis-Zavras syndrome in the setting of aspirin-induced asthma, or the Samter-Beer triad of asthma, nasal polyps, and aspirin allergy. The Kounis-Zavras syndrome, also known as Kounis syndrome, leads to angina pectoris or acute coronary syndrome secondary to coronary vasospasm in response to an allergic stimulus, leading to mast-cell degranulation of vasospastic mediators. The vasospasm can result in myocardial infarction, as it did in our patient.
CASE REPORT
A 25-year-old woman with previous asthma and nasal polyps presented to the emergency department with severe chest pain. Over the past 2 years, she had sporadic chest pain episodes; an earlier exercise stress echocardiogram and esophagogastroduodenoscopy showed no significant findings. During a repeat exercise stress echocardiogram, a small pericardial effusion was noted, and she was started on colchicine and naproxen. Thirty minutes after taking naproxen, the patient developed severe chest pain and came to the emergency department. She had previously experienced past episodes of chest pain after taking aspirin or nonsteroidal antiinflammatory drugs (NSAIDs).
Her pain was squeezing and substernal and was worse than at any of her prior episodes. On arrival, her heart rate was approximately 110 beats/minute, and her systolic blood pressure was 75 mm Hg. The jugular veins were not distended. There were no precordial murmurs, gallops, or rubs. She had bilateral lung crackles. Examination of the abdomen and legs showed no abnormalities. A chest radiograph revealed bilateral pulmonary edema; an echocardiogram showed a pericardial effusion without evidence of tamponade; and an electrocardiogram displayed ST-segment elevations in V2 to V5 as well as I and aVL, with ST depressions in leads II, III, and aVF. Her initial laboratory values are presented in Table 1.
Table 1.
Laboratory values
| Test | Day 1 | Day 2 |
| Creatine kinase (U/L) | 1708 | 1999, 3503 |
| Creatinine kinase-MB (ng/mL) | 1.4, 268 | 382, 592 |
| Troponin I (ng/mL) | <0.05, 25 | 81, 91 |
| Thyroid-stimulating hormone (μIU/mL) | 1.99 | |
| Hemoglobin (g/dL) | 14.7 | |
| Blood urea nitrogen (mg/dL) | 12 | |
| Creatinine (mg/dL) | 0.9 | |
| Total cholesterol (mg/dL) | 161 | |
| High-density lipoprotein cholesterol (mg/dL) | 62 | |
| Low-density lipoprotein cholesterol (mg/dL) | 88 | |
| Triglycerides (mg/dL) | 53 |
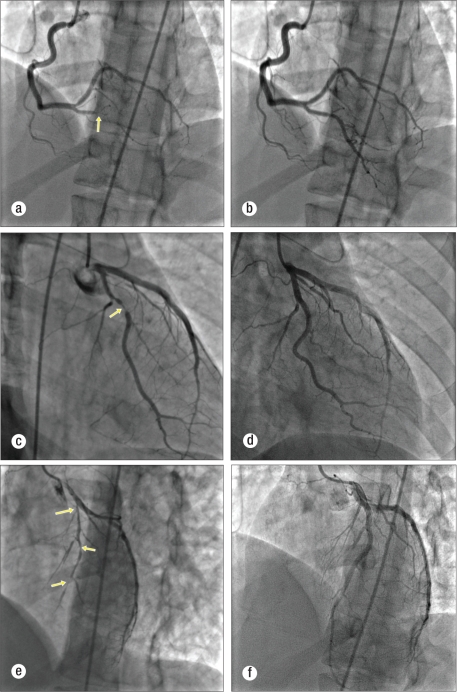
Cardiac catheterization disclosed multivessel coronary vaso- spasms. Intracoronary nitroglycerin alleviated the vasospasms (Figure). A left ventriculogram showed severe hypokinesis of the anteroseptal and posterior walls, with an ejection fraction of 15%. The patient was in the hospital for 2 weeks for the acute decompensated heart failure with cardiogenic shock. Prior to discharge, a repeat echocardiogram showed the ejection fraction to be 40%. She had been treated with carvedilol, lisinopril, zileuton, and inhaled corticosteroids. During the 7 months since discharge, she has not had angina pectoris.
Figure.
Coronary angiograms both before and after intracoronary nitroglycerin: (a) right coronary artery spasm (arrow), (b) right coronary artery after intracoronary nitroglycerin, (c) left circumflex and obtuse marginal artery spasm (arrow), (d) left circumflex and obtuse marginal artery after intracoronary nitroglycerin, (e) left anterior descending artery spasm (arrows), (f) left anterior descending artery after intracoronary nitroglycerin.
DISCUSSION
In 1991, Kounis and Zavras described “the coincidental occurrence of chest pain and allergic reactions accompanied by clinical and laboratory findings of classical angina pectoris caused by inflammatory mediators released during the allergic insult” (1). Kounis-Zavras syndrome is now the term used for allergic angina pectoris or allergic myocardial infarction. Two variants have been described. Type I is an allergic insult resulting in coronary vasospasm leading to angina pectoris with or without myocardial infarction in the setting of normal coronary arteries. The type II variant occurs in a patient with underlying quiescent coronary atherosclerotic disease; the allergic insult is proposed to induce plaque rupture, causing an acute coronary syndrome (2). Many etiologies of allergic angina pectoris have been reported (Table 2).
Table 2.
Causes of the Kounis-Zavras syndrome
| Condition | Drug | Environmental exposure |
| Angioedema | Antibiotic | Ant sting |
| Bronchial asthma | Analgesic | Bee sting |
| Exercise-induced anaphylaxis | Anticoagulant | Grass cutting |
| Food allergy | Antineoplastic | Jellyfish sting |
| Idiopathic anaphylaxis | Bupropion | Latex contact |
| Mastocytosis | Contrast media | Poison ivy |
| Serum sickness | Corticosteroid | Shellfish eating |
| Urticaria | Heparin | Viper venom |
| Intravenous anesthetic | Wasp sting | |
| Lepirudin | ||
| Nonsteroidal antiinflammatory | ||
| drug | ||
| Protamine | ||
| Skin disinfectant | ||
| Thrombolytic |
While the exact pathophysiology is not known, inflammatory mediators released in the setting of anaphylactic or anaphylactoid reactions appear to be the primary mechanism leading to allergic angina syndromes. When immunoglobulin E (IgE) is exposed to an antigen, it activates the mast cell bound through the high-affinity IgE receptor (3). Histamine-releasing factors from macrophages (4) or T lymphocytes (5) and anaphylatoxins from complement system activation also lead to activation and subsequent degranulation of mast cells (6). The mediators released include tryptase, chymase, histamine, platelet-activating factor, cytokines, and others, as well as prostaglandin and leukotriene synthesis. Many of these compounds in susceptible patients induce coronary vasospasm as well as platelet activation.
Studies in patients developing these allergic anginal syndromes have shown that histamine released during the allergic reaction either systemically or by mast cells located at plaque sites leads to paradoxical coronary vasoconstriction causing vasospasm, chest pain, and ultimately an acute coronary syndrome (7, 8). Some studies have shown that even in the nonallergic setting, mast cells are found in considerably higher amounts at acute plaque rupture sites than in stable organized thrombus or nonatherosclerotic coronary walls (9, 10). One hypothesis is that mast cell activation precedes all acute coronary syndromes and, thus, is a common pathway between allergic and nonallergic coronary syndromes (11). If this hypothesis is proven true, novel agents that stabilize mast cell membranes could be developed to prevent acute coronary syndromes.
The treatment of these allergic angina pectoris syndromes involves managing both the acute coronary syndrome and the allergic syndrome simultaneously. Cevik et al suggested treating the anaphylactic or anaphylactoid reaction with epinephrine, corticosteroids, antihistamines (both H1- and H2-blockers), fluid resuscitation, and oxygen (12). During the allergic reaction, if the patient develops chest pain and allergic angina pectoris or if myocardial infarction is suspected, emergent catheterization should be considered for both diagnostic and therapeutic reasons. Without angiographic evidence or a prior history of an allergic angina-type syndrome, it is difficult to start treating someone for this disease in the emergency department. Once vasospasm is diagnosed through cardiac catheterization, vasodilator drugs such as intracoronary nitrates and calcium channel blockers are recommended. Revascularization or antithrombotics may be appropriate in type II variants. If the culprit allergen is known, the implicated causal factor should be strictly avoided. Along with epinephrine, long-term use of mast cell stabilizers, such as cromolyn sodium or nedocromil sodium, and antihistamines may be considered.
Aspirin-induced asthma (AIA) was first described by Widal et al (13) in 1922 and later by Samter and Beers (14) in 1967. The term Samter's triad (asthma, aspirin sensitivity, and nasal polyps) became popular. Now, chronic eosinophilic rhinosinusitis is also considered to be part of the syndrome. AIA is an inflammatory condition of unclear etiology that affects 5% to 10% of adults with asthma (15). Generated hypotheses include viral exposure or hereditary factors (16). The Samter-Beer triad generally starts as chronic rhinitis with development of nasal polyposis. Salicylate intolerance and asthma develop over 1 to 5 years (17).
The NSAID intolerance is believed to be non–IgE-mediated and occurs due to shunting of the arachidonic pathway by cyclooxygenase-1 inhibition with overproduction of cysteinyl leukotrienes and release of inflammatory mediators, including histamine and tryptase, from mast cells and eosinophils. Cysteinyl leukotrienes and their receptors are elevated at baseline in these patients, and after NSAID ingestion there is a precipitous rise. Ingestion of NSAIDs simply exacerbates the asthma but does not cause the asthma, as the chronic airway inflammation persists even with strict avoidance of aspirin and other NSAIDs. Treatment includes aspirin desensitization as well as treatments targeted at the asthma and rhinitis.
Typically, when given an NSAID, patients with AIA develop bronchopulmonary reactions (bronchospasm, laryngeal edema), although extrapulmonary manifestations (rhinorrhea, conjunctival injection, urticarial-type lesions) similar to an IgE-mediated or immediate hypersensitivity reaction can occur. The sharp rise in cysteinyl leukotrienes that occurs with NSAID ingestion in AIA has been shown to cause myocardial ischemia in patients with anatomically normal coronary arteries (18), presumably due to coronary vasospasm. Both AIA and allergic anginal syndromes are largely underdiagnosed.
References
- 1.Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract. 1991;45(2):121–128. [PubMed] [Google Scholar]
- 2.Nikolaidis LA, Kounis NG, Gradman AH. Allergic angina and allergic myocardial infarction: a new twist on an old syndrome. Can J Cardiol. 2002;18(5):508–511. [PubMed] [Google Scholar]
- 3.Ishizaka T, Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- 4.Liu MC, Proud D, Lichtenstein LM, MacGlashan DW, Jr, Schleimer RP, Adkinson NF, Jr, Kagey-Sobotka A, Schulman ES, Plaut M. Human lung macrophage-derived histamine-releasing activity is due to IgE-dependent factors. J Immunol. 1986;136(7):2588–2595. [PubMed] [Google Scholar]
- 5.Sedgwick JD, Holt PG, Turner KJ. Production of a histamine-releasing lymphokine by antigen- or mitogen-stimulated human peripheral T cells. Clin Exp Immunol. 1981;45(2):409–418. [PMC free article] [PubMed] [Google Scholar]
- 6.Metzler B, Xu Q. The role of mast cells in atherosclerosis. Int Arch Allergy Immunol. 1997;114(1):10–14. doi: 10.1159/000237636. [DOI] [PubMed] [Google Scholar]
- 7.Vigorito C, Poto S, Picotti GB, Triggiani M, Marone G. Effect of activation of the H1 receptor on coronary hemodynamics in man. Circulation. 1986;73(6):1175–1182. doi: 10.1161/01.cir.73.6.1175. [DOI] [PubMed] [Google Scholar]
- 8.Kern MJ. Histaminergic modulation of coronary vascular resistance: are we missing a therapeutic adjunct for the treatment of myocardial ischemia? J Am Coll Cardiol. 1991;17(2):346–347. doi: 10.1016/s0735-1097(10)80097-x. [DOI] [PubMed] [Google Scholar]
- 9.Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92(5):1084–1088. doi: 10.1161/01.cir.92.5.1084. [DOI] [PubMed] [Google Scholar]
- 10.Constantinides P. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92(5):1083. doi: 10.1161/01.cir.92.5.1083. [DOI] [PubMed] [Google Scholar]
- 11.Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol. 2006;110(1):7–14. doi: 10.1016/j.ijcard.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Cevik C, Nugent K, Shome GP, Kounis NG. Treatment of Kounis syndrome. Int J Cardiol. 2010;143(3):223–226. doi: 10.1016/j.ijcard.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 13.Widal MF, Abrami P, Lermeyez J. Anaphylaxie et idiosyncrasie. Presse Med. 1922;30:189–192. [Google Scholar]
- 14.Samter M, Beers RF., Jr Concerning the nature of intolerance to aspirin. J Allergy. 1967;40(5):281–293. doi: 10.1016/0021-8707(67)90076-7. [DOI] [PubMed] [Google Scholar]
- 15.Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111(5):913–921. doi: 10.1067/mai.2003.1487. [DOI] [PubMed] [Google Scholar]
- 16.Zeitz HJ. Bronchial asthma, nasal polyps, and aspirin sensitivity: Samter's syndrome. Clin Chest Med. 1988;9(4):567–576. [PubMed] [Google Scholar]
- 17.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. 2000;16(3):432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 18.Szczeklik A, Nizankowska E, Mastalerz L, Bochenek G. Myocardial is- chemia possibly mediated by cysteinyl leukotrienes. J Allergy Clin Immunol. 2002;109(3):572–573. doi: 10.1067/mai.2002.121700. [DOI] [PubMed] [Google Scholar]