Colorectal cancer (CRC) is the third most frequently diagnosed cancer in the US and the second leading cause of cancer death. CRCs arise from a multistep carcinogenic process, beginning with early changes from normal to hyperproliferative epithelium, then to the development of adenomas, which in turn are believed to be precursors of carcinomas. These histological changes are associated with the accumulation of genetic and epigenetic changes involving the activation of oncogenes and the inactivation of tumor suppressor genes.
Approximately two thirds of new cases of CRC appear to be sporadic (i.e., occur without a family history of the disease), and the necessary genetic changes seem to arise de novo, fueled by etiologic agents that can include age, environmental exposures, and lifestyle factors. Nearly one third of cases, however, tend to cluster in families, so that first-degree relatives of patients with newly diagnosed adenomas or invasive CRCs are at increased risk. While many of these familial clusters may be linked to shared behaviors or environmental exposures among family members, some are associated with germline mutations in specific genes.
Although specific genetic mutations have been identified for a wide variety of syndromes that may involve CRC, screening is normally directed at the most common of these: hereditary nonpolyposis CRC (HNPCC), also known as Lynch syndrome, and familial adenomatous polyposis (FAP). Lynch syndrome is the most common hereditary cancer syndrome predisposing to CRC, accounting for approximately 3% of all cases. It is associated with mutations in four DNA-mismatch repair (MMR) genes: MSH2, MLH1, MSH6, and PMS2. Individuals with a germline mutation in any of these genes have a significant lifetime risk of developing CRC (70% for men, 40% for women), as well as a heightened risk for the development of cancers at other sites, including the endometrium, ovary, stomach, small bowel, urinary tract, and pancreas. FAP, about one tenth as common as Lynch syndrome, is associated with mutations in the APC gene, which is classified as a tumor suppressor gene. Individuals with FAP may have hundreds of adenomas, making this syndrome relatively straightforward to detect. Because so many adenomas develop at an early age, classic FAP carries a lifetime risk of nearly 100% for the development of CRC. Additional hereditary CRC syndromes that are not routinely tested for include Peutz-Jeghers syndrome, familial juvenile polyposis, Cowden's disease, Bannayan-Ruvalcaba-Riley syndrome, and Li-Fraumeni syndrome.
For both sporadic and familial CRC, a key early change is the development of genetic and epigenetic instability, which increases the rate at which further changes can be accumulated. At least three patterns of instability can contribute to the development of CRC; typically, one type will predominate in a specific cancer.
One common pattern, prominent in at least 50% of CRCs, is chromosome instability (CIN), which can result in genetic deletions, duplications, and rearrangements. CRCs with CIN are characterized by aneuploid tumor cells.
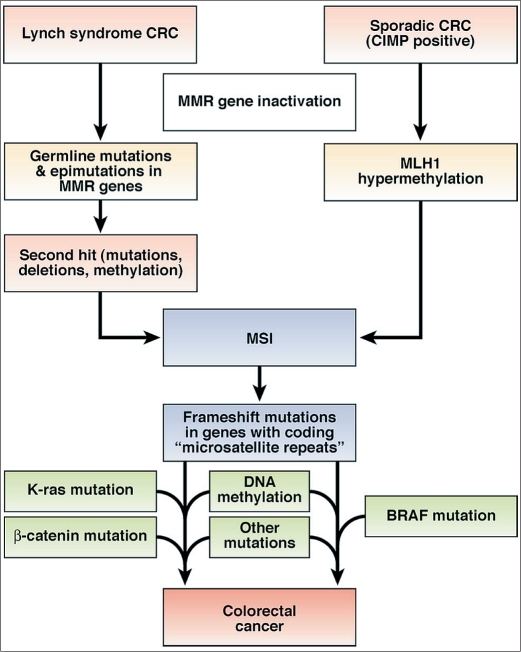
A second pattern is microsatellite instability (MSI), which occurs in about 15% of CRCs (Figure 1). Microsatellites are simple repeat sequences 1 to 6 base pairs in length that occur thousands of times across the genome. MSI occurs through the inactivation of the DNA mismatch repair (MMR) system, resulting in sequences that accumulate errors and become abnormally long or short. In some instances, this creates a frameshift mutation in a gene, such as a tumor suppressor gene. Unlike CIN, most CRCs with MSI are diploid or near diploid. More than 90% of the CRCs attributable to Lynch syndrome are associated with MSI.
Figure 1.
Two molecular pathways to the development of colorectal cancer with microsatellite instability. From Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138(6):2073–2087.e3. Reprinted with permission from Elsevier.
The third pattern seen in CRC is an epigenetic change that involves the methylation of promoters of human genes. This can lead to the silencing of certain tumor suppressor genes in CRC. About half of the promoters of human genes are embedded in CpG islands, so this phenotype in tumor DNA is called the CpG island methylator phenotype (CIMP). Overlap between MSI and CIMP occurs in about 12% of CRCs when the promoter of the MMR gene MLH1 is methylated (and inactivated), leading to MSI.
COLON CANCER SCREENING AT BAYLOR DALLAS
C. Richard Boland, MD, a gastroenterologist on the medical staff and chief of the Division of Gastroenterology at Baylor University Medical Center at Dallas, oversees the testing of patients with suspected hereditary CRC syndromes. These patients are referred by other gastroenterologists, oncologists, and surgeons. A sample of patients' DNA is taken and used to determine if there is a familial aspect to their cancer or some other feature that would allow their oncologists to personalize their care. After testing, most patients opt to be put into a registry so that they can be alerted to new treatments or invited to join clinical studies. These patients are also reassessed in light of new research that may provide information about their cancer. “In some cases,” commented Dr. Boland, “a new genetic variation is reported somewhere that fits a patient we looked at 2 or 3 years earlier, and we are able to contact the individuals and their physicians to provide them with useful information. We see patients one time for genetic screening; then they become research subjects.”
RESEARCH IN COLON CANCER GENETICS AT BAYLOR DALLAS
Dr. Boland has been studying the genetics of CRC for over 25 years. His work studying the relationship of MSI to CRC led to the unexpected discovery that, in addition to genetic mutations in MMR genes, epigenetic changes involving DNA methylation in promoter genes could lead to MMR gene inactivation, resulting in MSI in cases of sporadic CRC. Currently, Dr. Boland and his colleagues Ajay Goel, PhD, and Minoru Koi, PhD (Figure 2), are engaged in exciting research aimed at developing new genetic-based screening tests for CRC, identifying genes associated with the metastatic phenotype, and investigating a ubiquitous virus that may be involved in the etiology of CRC. Dr. Goel is an investigator and Dr. Koi is a senior research associate at Baylor Research Institute on the Baylor Dallas campus.
Figure 2.
Colorectal cancer researchers at Baylor University Medical Center at Dallas: (a) C. Richard Boland, MD, (b) Ajay Goel, PhD, and (c) Minoru Koi, PhD.
New screening tests for CRC
One of the most effective ways to prevent CRC is to find and remove polyps and other areas of abnormal cell growth before they develop into malignancies. The current gold standard for screening is colonoscopy, which can provide detection and treatment in one procedure. However, as few as 25% of people older than 50 get colonoscopies. A combination of cost, an unpleasant colon-cleansing preparation required prior to the screening, and fears about potential mishaps during the procedure discourage most eligible adults from having this screening test.
To overcome this difficulty, researchers have worked for many years on developing a noninvasive screening test using stool samples that could be collected at home. The earliest tests looked for blood in the stool as an indication of cancerous tissue or polyps. Subsequent tests looked at gene mutations in cells shed from the colonic lumen. The lumen continually sheds cells to renew the colonic epithelium. If a cancer is present, normal cell-to-cell contact breaks down, and more cells are shed.
These early assays based on the detection of occult blood or a small number of gene mutations proved to be highly inaccurate. About 5 years ago, Drs. Goel and Boland postulated that the tests based on gene mutations did not work because CRCs are heterogeneous genetically, and not all cancers carried the mutations that were used in the screening panel. Instead, they opted to look at DNA methylation; it is much more common, with a measurable frequency even in healthy individuals. They selected tumor suppressor genes (e.g., those that regulate and restrain cell growth) that are frequent targets for methylation in CRC and demonstrated that a screening test based on these markers could detect abnormal cell growth in the colon with a high degree of sensitivity. The test could distinguish polyps from carcinomas, and small polyps from larger polyps, based on the degree of methylation. By adding additional markers, a single stool sample could be used to look for gastric and pancreatic cancers, in addition to CRC. Because a screening test based on methylation is both noninvasive and less expensive than colonoscopy, it could be performed more frequently in high-risk individuals. Methylation is a reversible phenomenon, so the test may also be of use to monitor treatment effects over time.
Now, Dr. Goel is looking at another type of genetic marker for use in CRC screening tests. MicroRNAs (miRNAs) are short RNA molecules that bind to messenger RNA transcripts (mRNAs), resulting in gene silencing. Each miRNA may regulate up to several hundred genes. MiRNAs are involved in multiple types of cancers, and it appears that there are specific patterns of miRNA expression associated with different cancers. In comparison to mRNA, miRNAs, perhaps because of their small size, are resistant to endogenous degradation and thus remain stable during the process necessary to isolate them from stool samples. Preliminary work recently published by Dr. Goel and colleagues has demonstrated that miRNAs can be successfully isolated from stool samples and may be useful as biomarkers for the early detection of CRC.
Dr. Goel believes that, in the end, the best fecal screening assay for CRC will be a cocktail of multiple assays—gene mutation, methylation, miRNA—each with its unique strength. This combined assay will be noninvasive and could be inexpensive, removing the major roadblocks to consumer acceptance of regular colon screening.
Genes associated with metastasis in CRC
Dr. Koi's research is focused on the identification of metastasis-specific gene markers associated with CRC. Mutations in the four MMR genes, MSH2, MLH1, MSH6, and PMS2, commonly result in MSI. This leads to mistakes in DNA replication, which in turn lead to mutations in many different genes, enhancing cancer development.
There are many types of microsatellites, based on the number of bases involved in the repeated sequence. Most Lynch syndrome CRC tumor specimens with MSH2 or MLH1 germline mutations as well as sporadic CRCs with the MLH1 gene silenced by promoter hypermethylation show a high level of MSI at mono-, di-, tri-, and tetranucleotide repeats.
Dr. Koi has directed his attention to larger microsatellites and is looking at instability in tetranucleotide repeats. He has found that 50% to 60% of sporadic primary CRCs show instability in tetranucleotide repeats and that this is associated with loss of the MSH3 gene. Cells that lose the protein product of this gene show a unique type of MSI profile: the affected repeats are larger (mostly tetranucleotides), and mononucleotides are seldom involved.
Based on published papers indicating a possible association of tetranucleotide repeats and down-regulation of MSH3 with poor outcome, Dr. Koi is examining primary and liver metastatic tissue from patients with sporadic CRC to more carefully define this relationship. His goal is to determine if specific loci targeted by the MSH3 deficiency might be good candidates for metastasis-specific gene markers.
The JC virus and CRC
In the early 1970s, there was keen interest among oncologists in the idea that cancer might be the result of viral infection. Although this idea did not turn out to have the global applicability that many hoped for, viruses are in fact associated with some cancers. As of 2002, Max Park, MD, senior epidemiologist at the Wolffson Institute of Preventive Medicine in London, estimated that about 12% of cancers are related to viral infection, with the most noteworthy examples being cervical cancer (human papillomavirus), hepatocellular carcinoma (hepatitis B/C virus), lymphoma (Epstein-Barr virus), and Kaposi sarcoma (human immunodeficiency virus).
Dr. Boland and colleagues are interested in the role of viruses in the etiology of CRC, especially the JC virus (JCV). JCV is a human polyomavirus that is extremely common, affecting up to 90% of the general population. Most people acquire the virus in their childhood or early adolescence. In most cases, the virus appears to be relatively benign, although it can become activated in patients with immunodeficiency or immunosuppression, resulting in fatal encephalopathies.
Dr. Boland's interest in JCV lies in the fact that, like the closely related SV40 virus, it has a transforming gene that encodes T antigen, which will induce chromosome instability in vitro and make normal cells behave malignantly. However, evidence is missing about whether JCV is sufficient in itself to result in malignancy or whether it is a component of a larger process—that is, whether it is a driver or a passenger. Dr. Boland believes that it is a driver, but one that requires activation. He hypothesizes that JCV is present in almost everyone in a latent state. At some point, another factor (dietary exposure, cell proliferation, etc.) allows the virus to become oncogenic, inducing chromosome instability and making CRC likely to occur.
The idea of a latent virus incubating harmlessly for years before being activated by an environmental factor is consistent with a growing amount of epidemiologic evidence about the relationship between environment and CRC. There is a clear indication of geographic variation in CRC, with incidence rates 20 times higher in north central Europe than in Africa, India, and Southeast Asia. This variation is presumed to be associated with dietary factors. For example, in Japan in the 1970s, the incidence of CRC was very low; the rate has since increased with the growing incursion of Western cultural influence. In Japanese nationals who migrated to Hawaii, CRC incidence quickly became equivalent to that seen in the Caucasian population. Unlike genetically driven changes, the incidence rates shift over time and when the population moves; they don't appear to be tied to smoking or to alcohol consumption but may be related to meat and fat consumption.
A working model for the genetic basis of CRC tumorigenesis was first proposed nearly 25 years ago by Vogelstein and colleagues in a landmark paper published in the New England Journal of Medicine. They visualized CRC as stemming from the sequential activation of four or five genes, include APC, ras, and p53. While subsequent research over the years has supported the involvement of these genes, it has turned out that few CRCs actually evolve along the precise pathway envisioned by Vogelstein. As we are learning, the genetic mechanisms involved in colon cancer (and, indeed, in most cancers) are not straightforward. “Probably there are no two colon cancers that are just alike,” said Dr. Boland. “Most are enormously different from one another.” Drs. Boland, Goel, and Koi continue to piece together a better understanding of this enormously complex field, gathering the data that will lead to the improved personalized care of tomorrow.