Abstract
Alcoholic liver disease (ALD) features increased hepatic exposure to bacterial lipopolysaccharide (LPS). Toll-like receptor-4 (TLR4) recognizes LPS and activates signaling pathways depending on MyD88 or TRIF adaptors. We previously showed that MyD88 is dispensable in ALD. TLR4 induces Type-I interferons (IFN) in MyD88-independent manner that involves interferon regulatory factor-3 (IRF3). We fed alcohol or control diets to wild-type (WT) and IRF3 knock-out (KO) mice, and to mice with selective IRF3 deficiency in liver parenchymal and bone marrow-derived cells. Whole-body IRF3-KO mice were protected from alcohol-induced liver injury, steatosis and inflammation. In contrast to WT or bone-marrow specific IRF3-KO mice, deficiency of IRF3 only in parenchymal cells aggravated alcohol-induced liver injury, associated with increased pro-inflammatory cytokines, lower anti-inflammatory cytokine IL-10 and lower Type-I IFNs compared to WT mice. Co-culture of WT primary murine hepatocytes with liver mononuclear cells (LMNC) resulted in higher LPS-induced IL-10 and IFN-β, and lower TNF-α levels compared to LMNC alone. Type-I IFN was important since co-cultures of hepatocytes with LMNC from Type-I IFN receptor KO mice showed attenuated IL-10 levels compared to control co-cultures from WT mice. We further identified that Type-I IFNs potentiated LPS-induced IL-10 and inhibited inflammatory cytokine production in both murine macrophages and human leukocytes, indicating preserved cross-species effects. These findings suggest that liver parenchymal cells are the dominant source of Type-I IFN in TLR4/IRF3-dependent manner. Further, parenchymal cell-derived Type-I IFNs increase anti-inflammatory and suppress pro-inflammatory cytokines production by LMNC in paracrine manner. In conclusion, our results indicate that IRF3 activation in parenchymal cells and resulting type I IFNs have protective effects in ALD via modulation of inflammatory functions in macrophages. These results suggest potential therapeutic targets in ALD.
Keywords: alcoholic liver disease; toll-like receptor 4; interferons, type I; tumor necrosis factor alpha; interleukin 10
Alcoholic liver disease (ALD) is the most common drug abuse-induced liver disease and accounts for 40% of deaths from cirrhosis in the United States (1). Gut-derived lipopolysaccharide (LPS), a component of the gram-negative bacterial wall, has been proposed as a key player in the pathogenesis of ALD (2, 3). Exposure to LPS during chronic alcohol consumption results in increased production of inflammatory mediators, leading to progression of liver injury (4). Indeed, mice treated with antibiotics to eliminate gut microflora, or mice deficient in tumor necrosis factor-alpha (TNF) type I receptor were protected from alcohol-induced liver injury (5, 6).
Recognition of pathogen-derived molecules occurs through pattern recognition receptors such as Toll-like receptors (TLR), which are widely expressed on parenchymal and non-parenchymal cell types in the liver (7). TLR4 recognizes LPS and activates two signaling pathways via recruitment of adaptor molecules (8, 9). Recruitment of the common TLR adaptor, myeloid differentiation factor 88 (MyD88), leads to rapid activation of nuclear factor B (NF-κB) and increased TNFα production, while recruitment of TIR domain-containing adaptor inducing interferon-beta (TRIF) activates TANK-binding kinase 1/inhibitor of κB kinase epsilon (TBK1/IKKε) and interferon regulatory factor 3 (IRF3), leading to production of type I interferons (IFNs) and delayed NF-κB activation (10-12).
We have previously reported that MyD88 deficiency failed to prevent alcohol-induced liver damage and inflammation suggesting that TLR4-mediated MyD88-independent pathways are important in induction of ALD (13). The significance of MyD88-independent pathways including activation of IRF3 in ALD is yet to be evaluated.
Considering the importance of LPS-induced inflammatory activation in ALD (3) and the role of MyD88-independent downstream pathways in TLR4 signaling (13), we hypothesized that IRF3 was critical in alcohol-induced liver injury. Given the differential input of parenchymal and nonparenchymal cells in pathophysiology of ALD, we further hypothesized that IRF3 may be critical in alcoholic liver injury in a cell-specific manner. Therefore, we employed a chimeric mouse model to evaluate the effect of chronic alcohol feeding on liver damage, steatosis and inflammation in animals with selective deficiency of IRF3 in liver parenchymal cells.
Here we demonstrate that IRF3 activation and downstream type I IFN induction in parenchymal cells have protective effects in ALD. We report that disruption of IRF3 in liver parenchymal cells decreases type I IFN production and increases liver injury due to dysregulated expression of pro- and anti-inflammatory cytokines.
Materials and methods
Animal studies
All animals received proper care in agreement with animal protocols approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School. Six to eight-week-old, female C57Bl/6 wild-type, IRF3-deficient (IRF3-KO) and Type I interferon α/β receptor 1-deficient (IFNAR-KO) mice (kind gift of Jonathan Sprent, Scripps Research Institute, La Jolla, CA), all on C57Bl/6 background, were employed. Some animals were fed with the Lieber-DeCarli diet (Dyets, Inc., Bethlehem, PA) with 5% (vol/vol) ethanol (36% ethanol-derived calories) for 4 weeks; pair-fed control mice matched the alcohol-derived calories with dextran-maltose (13). Chimeric mice were generated by transplanting wild-type (C57Bl/6) bone marrow into irradiated, IRF3 deficient mice (IRF3-KO/WT-BM). Transplanted IRF3-deficient bone marrow into irradiated, WT mice (WT/IRF3KO-BM), and wild-type mice with transplanted wild-type bone marrow (WT/WT-BM) were used as controls. Serum was stored at -80°C. Livers were snap-frozen in liquid nitrogen for proteins, or stored in RNAlater (Qiagen GmbH, Hilden, Germany) for RNA extraction, or fixed in 10% neutral-buffered formalin for histopathological analysis.
Bone marrow transplantation protocol
Bone marrow was collected from the long bones of 8-week old donor male mice by flushing with a 25g needle and passed through a cell strainer to remove clumps. Female mice were irradiated (9 Gy) from a Cesium irradiator (Gammacell 40, Atomic Energy of Canada), and 4 hours later transplanted with 5 million freshly isolated donor bone marrow cells via a single tail vein injection. Transplanted mice were housed in microisolator cages and placed on medicated water (Sulfamethoxazole/Trimethoprim, Hi-Tech Pharmacal Inc.) until engraftment was complete 6 weeks later, as discussed in (14). The engraftment rate for all transplanted mice was >90%, as indicated by PCR analysis of peripheral blood 6 weeks post-transplant.
Biochemical assays
Serum alanine aminotransferase (ALT) was determined using a kinetic method (D-Tek LLC., Bensalem, PA). Liver triglyceride levels were assessed using the L-Type Triglyceride H kit (Wako Chemicals USA Inc., VA).
Cytokine measurement
Mouse IL-1β ELISA kit was purchased from R&D (R&D systems, Inc., Minneapolis, MN), mouse and human TNF-α, IL-1β and IL-10 kits from BD Bioscience (BD Biosciences, San Jose, CA) and mouse IFN-β kit from PBL (PBL interferon source, Piscataway, NJ).
Protein quantification
Whole-cell lysates were extracted from liver, as described (15). Equal amounts of proteins were separated on polyacrylamide gel, and transferred to a nitrocellulose membrane. Target proteins were detected by western blot and immunostaining with specific primary antibody, followed by horseradish peroxidase-labeled secondary antibody. Antibodies specific for IRF3 and IL-10 were from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phosphorylated IRF3 and IFN-β antibodies were from Cell Signaling (Cell Signaling Technology, Inc., Danvers, MA) and from ProSci (ProSci, Inc., Poway, CA), respectively. The specific immunoreactive bands of interest were detected by chemiluminescence (Amersham, Piscataway, NJ), and quantified by densitometric analysis.
RNA Analysis
RNA was purified using the RNeasy kit (Qiagen Sciences, Maryland, USA) and on-column DNA digestion. cDNA was transcribed with the Reverse Transcription System (Promega Corp., Madison, WI). SybrGreen-based real-time quantitative polymerase chain reaction was performed using the iCycler (Bio-Rad Laboratories Inc., Hercules, CA), as described (13); primer sequences are shown in Table 1.
Table 1.
Real-time PCR primers.
| Target gene | Forward primer (5’ → 3’) | Reverse primer (5’ → 3’) |
|---|---|---|
| 18S | gta acc cgt tga acc cca tt | cca tcc aat cgg tag tag cg |
| TNF-A | cac cac cat caa gga ctc aa | agg caa cct gac cac tct cc |
| Pro-IL-1B | tct ttg aag ttg acg gac cc | tga gtg ata ctg cct gcc tg |
| IL-10 | ctg gac aac ata ctg cta acc g | ggg cat cac ttc tac cag gta a |
| IFN-B | agc tcc aag aaa gga cga aca t | gcc ctg tag gtg agg gtt gat ct |
| ISG-56 | ggg cct tgc agg cat cac ctt | tcc tgc ctt ctg ggc tgc ct |
Histopathological analysis
Liver sections were stained with hematoxylin & eosin or oil-red-O and analyzed by microscopy, as we previously described (13).
Isolation of hepatocytes and liver mononuclear cells
Anesthesized animals were perfused via portal vein with saline solution followed by digestion, as we previously described (13). The hepatocytes were separated by centrifugation, liver mononuclear cells (LMNCs) were purified by centrifugation in Percoll gradient.
Phenotype analysis by flow cytometry
Primary hepatocytes were washed in PBS, permeabilitzed using the Cytofix/Cytoperm kit (BD Pharmingen, San Diego, CA), and incubated with anti-albumin (FITC) antibody (Thermo Fisher Scientific Inc., Fremont, CA) for 30 minutes on ice. After incubation, cells were washed with permeabilisation solution as indicated by the manufacturer, fixed in paraformaldehyde and analyzed by flow cytometry.
Isolation of human peripheral blood mononuclear cells
Human peripheral blood mononuclear cells (PBMCs) were separated from blood of healthy volunteers by centrifugation in Ficoll gradient, as we previously described (16).
In vitro experiments
Primary hepatocytes and LMNCs were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% insulin, transferrin, selenium (ITS) solution. Primary hepatocytes were seeded in 6-well collagen-coated plates, LMNCs (106/insert) were plated in cell-culture inserts with pore diameter 0.4 μm (Becton Dickinson Labware, Bedford, MA). Before starting stimulation experiments, hepatocytes were rested for 4 hours. Subsequently culture media was replaced, and stimulation was performed as indicated in the figure legends. LPS (Sigma, St. Louis, MO) was used at 100 ng/mL. IFN-β, IL-10 and TNF-α were measured in supernatants using ELISA.
RAW264.7 macrophages were stimulated with LPS, recombinant mouse IFN-α2a (eBioscience, San Diego, CA), recombinant mouse IL-10 (Peprotech Inc., Rocky Hill, NJ) or with anti-mouse IL-10 receptor antibody (Biolegend, San Diego, CA).
Human PBMCs were stimulated with LPS, recombinant human IFN-α (PBL, Piscataway, NJ), recombinant IL-10 (Ebioscience, San Diego, CA) or IL-10 receptor antibody (R&D systems, Inc., Minneapolis, MN).
Statistical Analysis
Statistical significance was determined using the T-test or the nonparametric Kruskal-Wallis test. Regression plots were constructed using the Graphpad Prism 5.01 (GraphPad software, Inc., La Jolla, CA). Data are shown as mean ± standard error of the mean (SEM) and were considered statistically significant at P < 0.05.
Results
IRF3-deficiency protects against alcohol-induced liver damage
TLR4 recognizes LPS and activates two signaling pathways by utilizing the adaptor molecules MyD88 or TRIF, respectively. We showed that MyD88 is dispensable in ALD (13). In addition to induction of inflammatory cytokines via NF-κB, MyD88-independent activation of TLR4 triggers production of Type I IFNs, which is largely dependent on activation of intracellular pathways involving interferon regulatory factor-3 (IRF3) (12). To define the importance of the MyD88-independent, IRF3-dependent signaling cascade and Type I IFNs in alcohol-induced liver injury, we fed ethanol or isocaloric control (pair feeding) diet to wild-type (WT) and IRF3-KO mice.
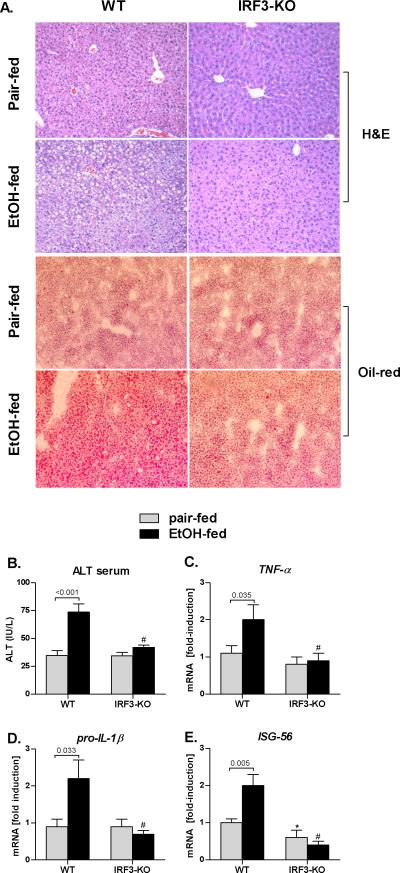
Histopathological analysis revealed that chronic alcohol feeding induced micro- and macrovesicular steatosis and inflammatory cell recruitment in ethanol-fed wt mice, suggestive of ALD (Fig 1A). In contrast, none of the histopathological features of ALD were observed in IRF3-KO mice (Fig. 1A). Consistent with the histopathology, serum ALT levels were significantly higher in alcohol-fed WT mice, but not in the IRF3-KO mice, compared to the pair-fed controls (Fig. 1B). We also found that the expression of inflammatory cytokines TNF-α and IL-1β in the liver was significantly higher in alcohol-fed WT mice compared to pair-fed controls; this alcohol-induced pro-inflammatory state was absent in IRF3-KO mice (Fig. 1C,D). Alcohol feeding to WT mice triggered expression of Type I IFN stimulated gene (ISG) 56, suggesting activation of Type I IFN signaling in alcohol-induced liver injury. In contrast, alcohol feeding of IRF3KO mice failed to upregulate ISG56 (Fig. 1E). These data suggested a role of IRF3 and/or Type I IFNs in alcohol-induced liver injury.
Fig. 1. IRF3-deficiency protects against alcohol-induced liver damage.
Wild-type (WT) and IRF3-deficient (IRF3-KO) were fed Lieber DeCarli ethanol or control (pair-fed) diet and sacrificed after 4 weeks. Livers were fixed in formalin and stained with H&E or with Oil-red-O; magnification 200x (A). Serum ALT levels (B) were analyzed. Messenger RNA levels of liver (C) tumor necrosis factor α (TNFA), (D) pro-interleukin 1-beta (IL-1β) and (E) interferon stimulated gene ISG56 were analyzed by real-time PCR and normalized to 18s.Values are shown as mean ± SEM fold increase over WT pair-fed control group (9-12 mice per group). Numbers in graphs denote p values; *) p < 0.05 vs. pair-fed WT mice; #) p < 0.05 vs. ethanol-fed WT mice.
IRF3 deficiency in parenchymal cells aggravates alcohol-induced liver damage
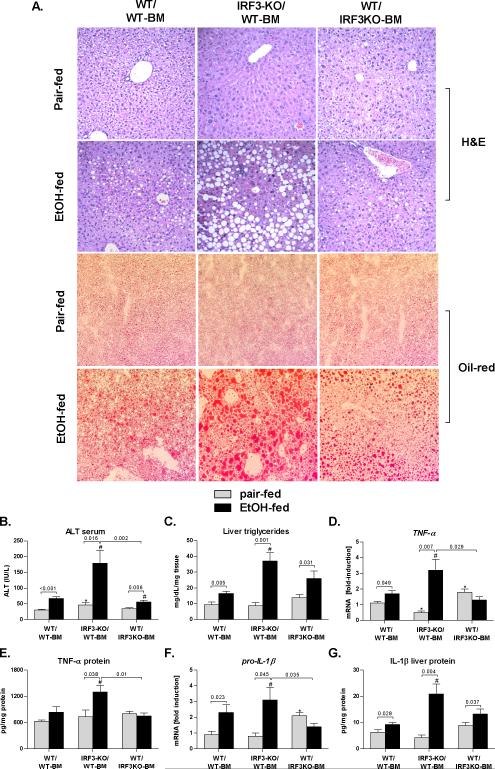
The liver functions with a complex co-existence of parenchymal and non-parenchymal cells. To explore whether the protective effect of IRF3 in alcoholic liver injury is mediated by parenchymal cells or (BM)-derived immune cells, we generated IRF3-chimeric mice by transplanting wild-type BM into irradiated, IRF3-deficient mice (IRF3-KO/WT-BM mice), or by transplanting IRF3-deficient BM into irradiated WT mice (WT/IRF3KO-BM). Wild-type mice with wild-type bone marrow transplant served as controls (WT/WT-BM). As expected, WT/WTBM mice developed ALD after 4 weeks of Lieber-DiCarli diet, as indicated by liver steatosis, inflammatory infiltrate and liver injury, compared to pair-fed controls (Fig. 2A,B,C).
Fig. 2. Parenchymal cell-specific IRF3 deficiency aggravates alcohol-induced liver damage.
Wild-type mice with transplanted WT bone marrow (WT/WT-BM), IRF3-deficient mice with transplanted wild-type bone marrow (IRF3-KO/WT-BM) and WT mice with transplanted IRF3-deficient bone marrow (WT/IRF3-KO) were fed Lieber DeCarli ethanol or control (pair-fed) diet and sacrificed after 4 weeks. Livers were fixed in formalin and stained with H&E or with Oilred-O; magnification 200x (A). Serum ALT levels (B) and liver triglycerides (C) were analyzed. Messenger RNA levels of liver (D) tumor necrosis factor α (TNFA) and (F) pro-interleukin 1-beta (IL-1β) were analyzed by real-time PCR and normalized to 18s. Liver TNF-α and IL-1β levels were analyzed using ELISA (E,G). Values are shown as mean ± SEM fold increase over wild-type pair-fed control group (11-17 mice per group). Numbers in graphs denote p values; *) p < 0.05 vs. pair-fed wild-type mice; #) p < 0.05 vs. ethanol-fed wild-type mice.
In sharp contrast to WT/WT-BM mice, IRF3-KO/WT-BM mice showed increased alcohol-induced liver injury, as indicated by exaggerated steatosis and inflammatory infiltrate on histology (Fig. 2A). This finding was accompanied by a significant elevation in serum ALT and in liver triglycerides, compared to WT/WT-BM ethanol-fed mice (Fig. 2B,C). Further, IRF3-KO/WT-BM mice showed significantly increased expression of inflammatory cytokines TNF-α and IL-1β, compared to WT/WT-BM ethanol-fed mice (Fig. 2D-G). These data suggested a protective role of IRF3 in parenchymal cells in ALD by limiting liver inflammation and injury.
Furthermore, WT/IRF3KO-BM mice showed no protection against alcohol-induced liver damage and steatosis (Fig. 2A, B, C), compared to WT/WT-BM mice, in spite of deficient induction of pro-inflammatory cytokine TNF-α (Fig. 2D,E) and IL-1β (Fig. 2G,F). These findings contrasted with the complete protection against alcohol-induced liver injury in global IRF3-KO mice (Fig. 1), and suggested that both parenchymal cell-specific and myeloid-specific IRF3 is required for the pathogenesis of alcohol-induced liver damage. More importantly, they indicated a protective role of parenchymal cell-specific IRF3 in alcohol-induced liver damage.
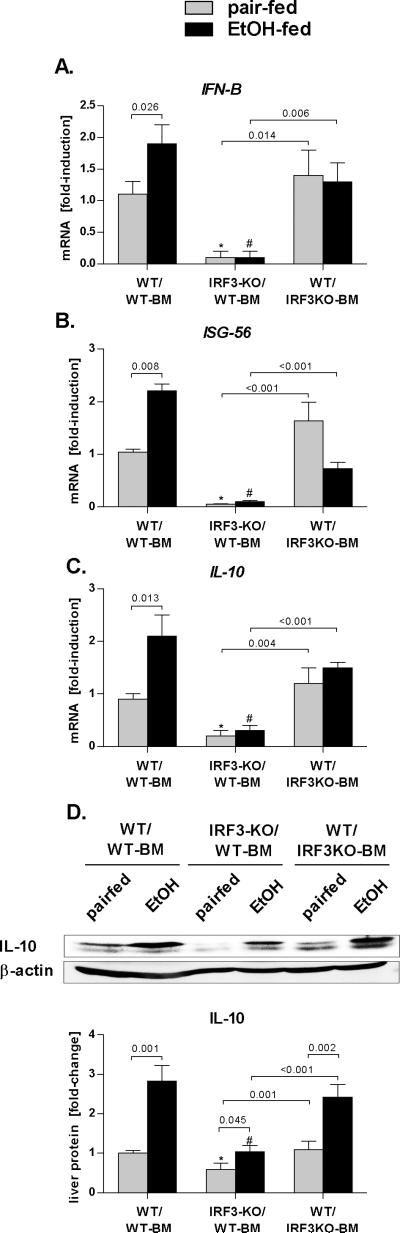
Deficiency of IRF3 in parenchymal cells is associated with decreased Type I IFN and IL-10 induction
Activation of IRF-3 leads to preferential induction of IFN-β (17). We identified that, in contrast to WT and to WT/IRF3KO-BM mice, IRF3-KO/WT-BM mice showed a significantly decreased expression of IFN-β (Fig. 3A) and of interferon-inducible gene ISG-56 (Fig. 3B). This finding indicated that aggravated liver injury in IRF3-KO/WT-BM mice is associated with deficiency in IRF3-dependent type I IFNs induction and signaling and suggested possible involvement of IRF3- and Type I IFN-dependent anti-inflammatory factors in alcohol-induced liver injury. We thus analyzed the expression of the Type I IFN-dependent gene, IL-10, which is a major anti-inflammatory cytokine (18). The extent of IL-10 increase was significantly higher in the liver of WT/WT-BM and WT/IRF3KO-BM mice, compared to IRF3-KO/WT-BM mice after alcohol feeding (Fig. 3C,D); the latter showed a significantly lower baseline IL-10 expression, compared to controls (Fig. 3C,D). Collectively, these findings suggested that parenchymal cell-specific IRF3 is required for expression of IFN-β and IL-10 in alcohol-induced liver injury.
Fig. 3. Parenchymal cell-specific IRF3 deficiency is associated with decreased Type I IFN and IL-10 induction.
Wild-type mice with transplanted WT bone marrow (WT/WT-BM), IRF3-deficient mice with transplanted wild-type bone marrow (IRF3-KO/WT-BM) and WT mice with transplanted IRF3-deficient bone marrow (WT/IRF3-KO) were fed Lieber DeCarli ethanol or control (pair-fed) diet and sacrificed after 4 weeks. Messenger RNA levels of (A) liver interferon β (IFN-β), interferon sstimulated gene 56 (ISG-56) and (C) interleukin 10 (IL-10) were analyzed by real-time PCR and normalized to 18s. Liver IL-10 protein levels were analyzed using immunoblot analysis (D). Values are shown as mean ± SEM fold increase over wild-type pair-fed control group (11-17 mice per group). Numbers in graphs denote p values. *) p < 0.05 vs. pair-fed wild-type mice; #) p < 0.05 vs. ethanol-fed wild-type mice.
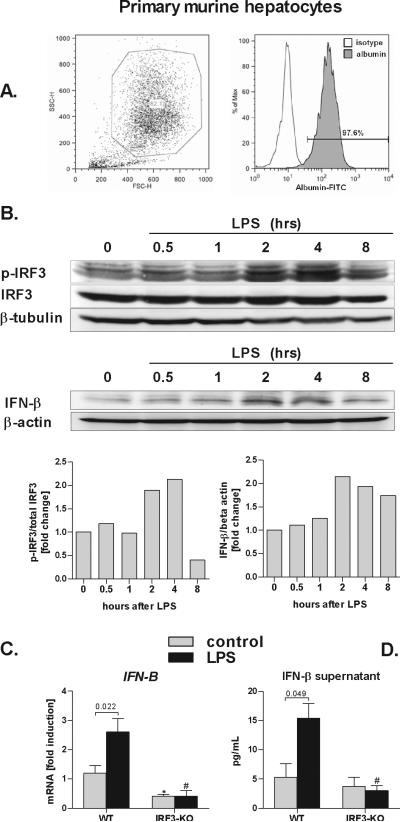
Hepatocytes produce Type I IFN in an IRF3-dependent manner
Our findings suggested that expression of liver IL-10 is linked to activation of of IRF3 in parenchymal cells. To confirm that the parenchymal cell-specific role of IRF3 is attributable to hepatocytes, we isolated primary hepatocytes from WT mice and observed > 97% purity of hepatocyte isolates (Fig. 4A). Next, we stimulated primary WT hepatocytes with LPS ex vivo and observed induction of IRF3 phorphorylation, which was matched by induction of IFN-β (Fig. 4B). While statistically significant induction of IFN-β mRNA and protein was observed in WT hepatocytes, no IFN-β induction occurred in hepatocytes deficient in IRF3 at the mRNA or protein levels (Fig. 4C,D). These in vitro observations suggested that hepatocytes are a major source of IFN-β in ethanol/LPS-induced liver injury.
Fig. 4. Parenchymal cells induce Type I IFN in IRF3-dependent manner.
Primary murine hepatocytes were permabilized, stained with anti-albumin antibody, and analyzed by flow cytometry (A). Wild-type primary hepatocytes were stimulated with 100 ng/mL lipopolysaccharide (LPS) for indicated time points and levels of phosphorylated IRF3 (p-IRF3), total IRF3, IFN-β and, beta tubulin and beta actin were analysed using immunoblotting (B). WT and IRF3-deficient (IRF3-KO) primary murine hepatocytes were stimulated LPS, and messenger RNA levels of interferon β (IFN-β) were analyzed by real-time PCR and normalized to 18s (C). IFN-β protein levels in supernatant were analyzed using ELISA (D). Values are shown as mean ± SEM (5 mice per group). Numbers in graphs denote p values; *) p < 0.05 vs. nonstimulated WT hepatocytes; #) p < 0.05 vs. LPS-stimulated WT hepatocytes.
Hepatocyte-specific Type I IFNs modulate cytokine production in liver mononuclear cells
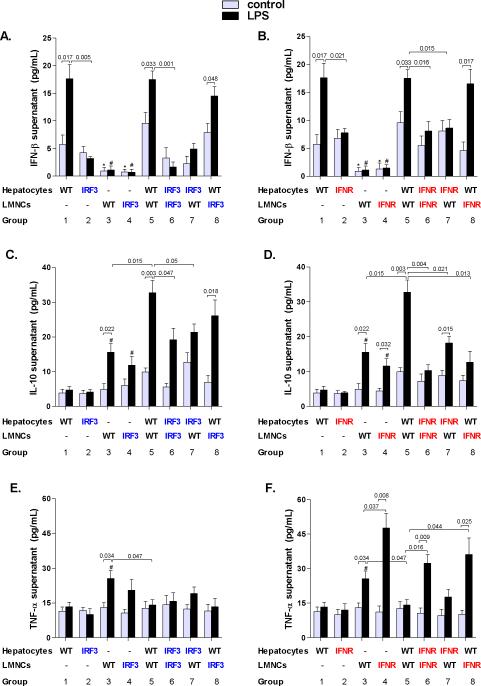
We further employed co-cultures of hepatocytes and liver mononuclear cells (LMNC) to dissect the regulatory loops involved in Type I IFN/IL-10 production. Control unstimulated and LPS-stimulated WT hepatocytes produced significantly more IFN-β than LMNCs (Fig. 5A,B groups 1 and 3). On the contrary, IL-10 was produced mainly by LMNCs (Fig. 5C,D group 3), which supports the data that Kupffer cells stimulated with LPS produce IL-10 (19, 20). Importantly, LMNCs co-culture with primary hepatocytes resulted in increased IL-10 production, compared to either cell types alone, which was further significantly increased upon stimulation with LPS (Fig. 5C,D, groups 1, 3, 5). The induction of IL-10 in hepatocyte/LMNC co-culture exceeded a merely additive contribution of both cell types to the secretion of IL-10, suggesting that hepatocyte-derived IFN-β facilitates the production of IL-10 in LPS-challenged immune cells in the liver. In contrast, we observed significantly lower induction of IL-10 in LPS-stimulated co-cultures of hepatocytes and LMNCs from IRF3-KO or IFNAR-KO mice (Fig. 5C,D, groups 6, 7), or in co-cultures of WT hepatocytes with IFNAR-deficient LMNCs (Fig. 5D, groups 8), compared with co-cultures of WT hepatocytes and WT LMNCs (Fig. 5C,D, group 5). These findings supported our hypothesis that enhancement of LPS-induced IL-10 expression in LMNCs is dependent on production of Type I IFN in parenchymal cells.
Fig. 5. Parenchymal cell-derived Type I IFNs upregulate IL-10 and downregulate TNF-α in liver mononuclear cells stimulated with lipopolysaccharide.
Primary hepatocytes and liver mononuclear cells (LMNC) were isolated from WT, IRF3-deficient mice (A,C,E) or Type I interferon α/β receptor 1-deficient (IFNAR-KO) mice (B,D,F) and stimulated with 100 ng/mL lipopolysaccharide (LPS) in transwell cell-culture systems as indicated. Protein levels of (A,B) interferon β (IFN-β), (C,D) interleukin 10 (IL-10) and (E,F) tumor necrosis factor α (TNF-α) in cell-free supernatants were analyzed using specific ELISA. Values are shown as mean ± SEM (5 mice per group). Numbers in graphs denote p values. *) p < 0.05 vs. nonstimulated hepatocytes of the respective genotype; #) p < 0.05 vs. LPS-stimulated hepatocytes of the respective genotype.
Given the tight control of the pro-and anti-inflammatory balance in the liver we further asked whether Type I IFN-dependent IL-10 production may affect the level of TNF-α in liver immune cells. We identified that TNF-α production by WT LMNCs was significantly downregulated upon their co-culture with WT hepatocytes (Fig. 5E,F, groups 3 and 5); such effect was absent in LMNCs of IFNAR-KO mice (Fig. 5F, groups 4, 6, 8). We observed that stimulation with LPS induced TNF-α also in IRF3-deficient LMNCs (Fig. 5E, group 4), whereas no induction was observed in IRF3-deficient livers of alcohol-fed mice (Fig. 1C and Fig. 2D,E). This finding suggests a context-specific role for IRF3: whereas IRF3 is crucial for the synergism of ethanol and LPS in induction of TNF-α from monocytes/macrophages, other transcription factors likely induce TNF-α in monocytes/macrophages which are stimulated only with LPS (21).
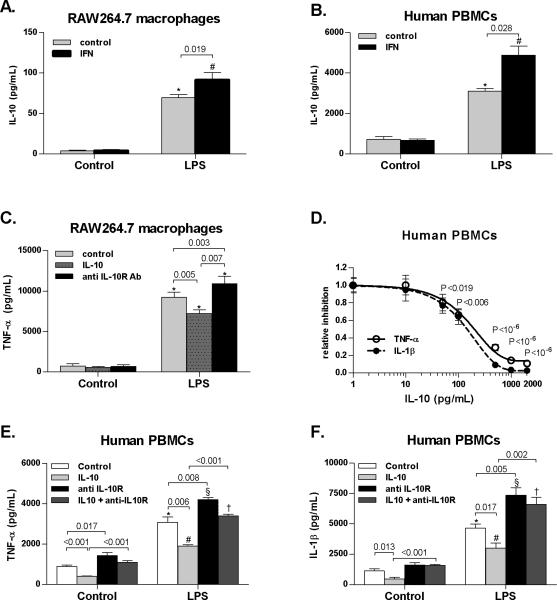
To further evaluate if regulation of IL-10 by Type I IFNs is preserved across species, we stimulated RAW264.7 murine macrophages or human peripheral blood mononuclear cells (PBMC) with LPS and Type I IFN, and identified a significant increase of IL-10 in the presence of Type I IFN compared to stimulation with LPS alone in both species (Fig. 6A,B). We found that expression of TNF-α was significantly decreased in RAW264.7 murine macrophages stimulated with LPS in the presence of recombinant IL-10, whereas neutralization of the IL-10 receptor (IL-10R) with anti-IL10R antibody significantly upregulated TNF-α (Fig. 6C). We also observed a dose-dependent inhibition of TNF-α and IL-1β by IL-10 in human PBMCs (Fig. 6D). Further, a 50% inhibitory concentration (IC50) of IL-10 caused a significant inhibition of LPS-triggered TNF-α and IL-1β (Fig. 6D,E,F), while inhibition of IL-10 receptors using IL-10R Ab significantly upregulated secretion of inflammatory cytokines in human PBMCs (Fig. 6E,F). These data confirmed that Type I IFN potentiates the LPS-induced IL-10 both in human and mouse systems.
Fig. 6. Type I IFN-dependent induction of IL-10 modulates inflammatory cytokines in mononuclear cells.
Murine RAW264.7 macrophages were stimulated with 100 ng/mL LPS, 1000 IU/mL murine recombinant IFNα2a, 10 ng/mL recombinant murine IL-10 and 1 μg/mL anti-mouse IL10 receptor antibody (anti IL-10R Ab). Human peripheral blood mononuclear cells (PBMCs, N=4) were stimulated with 10 ng/mL LPS, 1000 IU/mL human recombinant IFNα2, 10-2000 pg/mL recombinant human IL-10 and 1 μg/mL anti-human IL10 receptor antibody (anti IL-10R Ab). Protein levels of (A,B) interleukin 10 (IL-10), (C,D,E) tumor necrosis factor α (TNF-α) and (D,F) interleukin 1-β (IL-1β) were analyzed using ELISA. Values are shown as mean ± SEM. Numbers in graphs denote p values; *,#,§,†) p < 0.05 vs. respective control group
Taken together, these data demonstrate that parenchymal cell-derived Type I IFNs upregulate IL-10 and downregulate inflammatory cytokines in non-parenchymal cells in the liver. More importantly, they outline the paradigm of intercellular cooperation and regulation in the liver, where hepatocytes control the inflammatory potential of immune cells.
Discussion
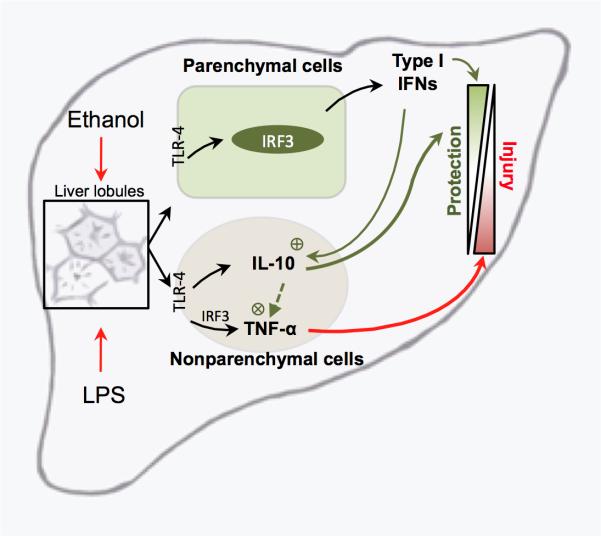
Chronic consumption of ethanol is tightly linked to liver inflammation and steatosis in human disease as well as in experimental models. Whereas activation of TLR4-dependent pathways by gut-derived LPS and induction of inflammatory cytokines has been traditionally attributed to bone marrow-derived Kupffer cells (22), the role of crosstalk between parenchymal and nonparenchymal (bone marrow-derived immune cells) in ALD remains elusive. Here we demonstrate that liver response to insults is a multistep process: IRF3 in parenchymal cells drives Type I IFN induction in the liver and parenchymal cell-derived Type I IFN leads to a modulation of inflammatory cytokines in non-parenchymal BM-derived cells (Fig. 7). Our novel findings outline a link between parenchymal and liver immune cells in modulation of innate immune signaling in ALD.
Fig. 7. Proposed mechanism of parenchymal cell-mediated control of inflammatory responses in alcohol-induced liver injury.
Chronic alcohol consumption results in increased exposure of the liver to the gut-derived lipopolysaccharide (LPS). LPS is recognized via the Toll-like receptor 4 (TLR-4) on nonparenchymal and parenchymal cells. In non-parenchymal cells, LPS increases production of inflammatory cytokines via multiple transcription factors, including IRF3. In parenchymal cells, LPS induces Type I interferons (IFN) in IRF3-dependent manner. In turn, hepatocyte-derived Type I IFNs enhance IL-10 and downregulate TNF-α production in non-parenchymal cells, thus regulating the balance between inflammatory and anti-inflammatory cytokines in the liver.
BM-derived cells are considered to be the main targets of pathogen-derived products in the liver due to their strategical position to encounter pathogens in the portal system and broad-range expression of TLRs. Our study provides novel lines of evidence that parenchymal cells are the main producers of Type I IFNs in response to alcohol/LPS exposure, and that IRF3 is a dominant signaling molecule inducing Type I IFN in alcoholic liver disease.
First, chimeric mice containing IRF3-deficient liver parenchymal cells and wild-type bone marrow-derived cells show a similar reduction in baseline and ethanol-induced expression of Type I IFNs as mice with global IRF3 deficiency. Second, no decrease in liver expression of Type I IFNs was observed in mice with selective deficiency of IRF3 in bone marrow-derived cells. Third, ex vivo stimulation of wild-type primary mouse hepatocyte isolates with LPS resulted in phosphorylation of IRF3 and in a significant upregulation of Type I IFNs, in contrast to hepatocyte isolates from IRF3KO mice that failed to induce Type I IFNs. In addition, phenotypic analysis of hepatocyte isolates employed in our study indicated that the IRF3-dependent Type I IFN induction indeed originates from hepatocytes, while the role of other cell types remains negligible.
Our study defines induction of Type I IFNs via IRF3 in hepatocytes and downregulation of inflammatory cytokines in BM–derived cells as two complementary, yet independent mechanisms by which TLR4 controls the extent of alcohol-induced liver inflammation and injury. Kupffer cells stimulated via TLR4 are a main source of inflammatory cytokines in the liver and promote tissue inflammation, injury and fibrosis (22). Thus, TLR4 seems to activate IRF3 in both parenchymal and non-parenchymal liver cells: here we demonstrate that while the signaling pathways are shared, we observed a cell-specific response to LPS, with a distinct outcome.
Studies by Zhao et al. (21) suggested that IRF3, activated by TLR4/TRIF and ethanol, induces inflammatory cytokines in macrophages, thereby playing a proinflammatory role. We observed no induction of inflammatory cytokines in mice with bone marrow-specific deficiency of IRF3; however, our novel data show that this effect was not sufficient to prevent alcohol-induced liver injury. These findings suggest that both myeloid- and parenchymal-cell specific IRF3 contribute to ALD, i.e. that the solo contribution IRF3 in bone marrow-derived cells is not sufficient for the development of ALD. The type of signal in IRF3 deficient bone-marrow derived cells that improves ALD in the global IRF3 knockouts, and the reason why this signal requires the absence of IRF3 in parenchymal cells remains to be further investigated.
Recently, Klein et al. (23) and Kennedy et al. (24) reported that in chimeric mice, 2 populations of liver macrophages co-exist: radioresistant macrophages which show tolerogenic properties, and radiosensitive macrophages with are immunogenic; the latter macrophages are rapidly replaced by BM transplantation and expected to be the dominant subtype that participates in the immunoinflammatory reactions in the liver post-transplant. Thus, the lack of protection from ALD in mice with bone-marrow specific deficiency of IRF3 could be attributable, at least in part, to an incomplete substitution of the host with BM-derived cells and/or differential host/donor macrophage ratio (24), either of which could influence the nature of the response to LPS and/or alcohol.
The differential role of IRF3 in ALD seems to be dominated by its parenchymal cell-specific protective effect. Our data demonstrate that IRF3 in parenchymal cells dampens TLR4-induced inflammatory response by indirect (paracrine) mechanism mediated by Type I IFNs. The importance of this cell-specific activation of IRF3 and Type I IFNs is emphasized by our finding that aggravated liver inflammation and injury was observed in mice chimeras lacking IRF3 in parenchymal cells, and was further associated with a significantly decreased expression of IL-10, a major anti-inflammatory cytokine, in the liver.
Our finding of Type I IFN-dependent induction of the anti-inflammatory state in the liver is supported by the fact that the IL-10 promoter contains a Type I IFN-dependent responsive element (25) which makes this cytokine a Type I IFN-dependent anti-inflammatory mediator. We found that, ex vivo, LPS-stimulated liver mononuclear cells synthesized significantly more IL-10 when co-cultured with primary hepatocytes that produced significant amounts of Type I IFNs. This synergism was completely absent in co-cultures of WT hepatocytes with IFNAR1-deficient LMNCs, but only partially abolished in co-cultures containing LMNCs that lacked IRF3, suggesting that it is the parenchymal-cell derived Type I IFN that acts synergistically with LPS on LMNCs to produce IL-10, rather than IRF3 in LMNCs per se. The existence of a hepatocyte/immune cell regulation loop is further supported by our finding that the facilitation of LPS-induced production of IL-10 by hepatocyte-specific Type I IFNs in liver mononuclear cells was abrogated in cells lacking Type I IFN receptor. Furthermore, our data show that administration of IL-10 to mouse macrophages or human PBMCs stimulated with LPS significantly suppresses inflammatory cytokines, and therefore support the critical role of IL-10 in determining the pro- and anti-inflammatory balance in the pathogenesis of ALD (19, 26). Taken together, these findings demonstrate that full expression of anti-inflamatory factors in BM-derived cells is dependent on Type I IFN signaling from parenchymal cells, which is regulated by IRF3.
TLRs fulfill a variety of functions in the liver, and inhibition of TLR4 signaling may alter biological processes related to liver inflammation, injury and fibrosis (27-30). TLR4 also promotes disease progression in alcoholic and nonalcoholic steatohepatitis (13, 31), primary sclerosing cholangitis (32) and ischemia-reperfusion injury (33), and therefore represents a potential therapeutic target. Indeed, use of probiotics, antifibrotics or anti-inflammatory agents are proposed as potential therapeutic options for these diseases (34). However, excessive TLR signaling triggers not only harmful responses, but also beneficial responses, such as clearance of microorganisms (35), tissue regeneration (36), and, as we demonstrate in this study, indirect induction of anti-inflammatory loop via induction of protective Type I IFNs in hepatocytes and anti-inflammatory IL-10 in macrophages in an IRF3-dependent manner. Therefore, it seems plausible that fine tuning, in contrast to approaches that would completely abrogate TLR signaling, may have a future in efforts to translate TLR pathophysiology into clinical practice in human liver diseases.
Financial support
This work was supported by NIAAA grant 1R01AA017729-01A1 (to G.S.) Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 from the National Institute of Diabetes and Digestive and Kidney Diseases were used. Dr. Gyongyi Szabo is a member of the UMass DERC (DK32520).
List of abbreviations
- ALD
alcoholic liver disease
- LPS
lipopolysaccharide
- TNF-α
tumor necrosis factor-alpha
- TLR
toll-like receptor
- IFN
interferon
- MyD88
myeloid-differentiation factor 88
- NF-κB
nuclear factor κB
- TRIF
TIR domain-containing adaptor inducing interferon-beta
- TBK1/IKKε
TANK-binding kinase 1/inhibitor of κB kinase epsilon
- IRF3
interferon regulatory factor-3
- KO
knock-out
- IFNAR
Type I interferon α/β receptor
- BM
bone-marrow
- IL-1β
interleukin-1 beta
- IL-10
interleukin 10
- LMNC
liver mononuclear cells
- PBMC
peripheral blood mononuclear cells
- WT
wild-type
- ISG
interferon stimulated gene
- IL-10R
interleukin 10 receptor
References
- 1.Kim WR, Brown RS, Jr., Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 2.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 3.Nanji AA. Role of Kupffer cells in alcoholic hepatitis. Alcohol. 2002;27:13–15. doi: 10.1016/s0741-8329(02)00207-0. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler MD. Endotoxin and Kupffer cell activation in alcoholic liver disease. Alcohol Res Health. 2003;27:300–306. [PMC free article] [PubMed] [Google Scholar]
- 5.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 6.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 7.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006;44:287–298. doi: 10.1002/hep.21308. [DOI] [PubMed] [Google Scholar]
- 8.Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, et al. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932–936. doi: 10.1053/he.2000.5634. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hritz I, Velayudham A, Dolganiuc A, Kodys K, Mandrekar P, Kurt-Jones E, et al. Bone marrow-derived immune cells mediate sensitization to liver injury in a myeloid differentiation factor 88-dependent fashion. Hepatology. 2008;48:1342–1347. doi: 10.1002/hep.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- 16.Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–1524. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 17.Schafer SL, Lin R, Moore PA, Hiscott J, Pitha PM. Regulation of type I interferon gene expression by interferon regulatory factor-3. J Biol Chem. 1998;273:2714–2720. doi: 10.1074/jbc.273.5.2714. [DOI] [PubMed] [Google Scholar]
- 18.Chang EY, Guo B, Doyle SE, Cheng G. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol. 2007;178:6705–6709. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
- 19.Sermon F, Le Moine O, Gustot T, Quertinmont E, Louis H, Nagy N, et al. Chronic alcohol exposure sensitizes mice to galactosamine-induced liver injury through enhanced keratinocyte chemoattractant and defective IL-10 production. J Hepatol. 2003;39:68–76. doi: 10.1016/s0168-8278(03)00186-7. [DOI] [PubMed] [Google Scholar]
- 20.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 23.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 25.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23(Suppl 1):S50–53. doi: 10.1111/j.1440-1746.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 27.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gabele E, et al. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963–2969. doi: 10.4049/jimmunol.168.6.2963. [DOI] [PubMed] [Google Scholar]
- 28.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 29.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 30.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 31.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karrar A, Broome U, Sodergren T, Jaksch M, Bergquist A, Bjornstedt M, et al. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132:1504–1514. doi: 10.1053/j.gastro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 34.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–1583. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- 36.Su GL, Wang SC, Aminlari A, Tipoe GL, Steinstraesser L, Nanji A. Impaired hepatocyte regeneration in toll-like receptor 4 mutant mice. Dig Dis Sci. 2004;49:843–849. doi: 10.1023/b:ddas.0000030097.52476.aa. [DOI] [PubMed] [Google Scholar]