Abstract
The aim of this study was to determine the effects of transplanted neural differentiated human mesenchymal stem cells (hMSCs) in a guinea pig model of auditory neuropathy. In this study, hMSCs were pretreated with a neural-induction protocol and transplanted into the scala tympani of the guinea pig cochlea 7 days after ouabain injury. A control model was made by injection of Hanks balanced salt solution alone into the scala tympani of the guinea pig cochlea 7 days after ouabain injury. We established the auditory neuropathy guinea pig model using 1 mM ouabain application to the round window niche. After application of ouabain to the round window niche, degeneration of most spiral ganglion neurons (SGNs) without the loss of hair cells within the organ of Corti and increasing the auditory brain responses (ABR) threshold were found. After transplantation of neural differentiated hMSCs, the number of SGNs was increased, and some of the SGNs expressed immunoreactivity with human nuclear antibody under confocal laser scanning microscopy. ABR results showed mild hearing recovery after transplantation. Based on an auditory neuropathy animal model, these findings suggest that it may be possible to replace degenerated SGNs by grafting stem cells into the scala tympani.
Keywords: Mesenchymal Stem Cells, Auditory neuropathy, Hearing Loss, Spiral ganglion neuron
INTRODUCTION
The loss of auditory afferent neurons, the spiral ganglion neurons (SGNs), can lead to sensorineural hearing loss (SNHL). This can occur from primary neuronal loss involving degeneration of neurons in the absence of hair cell degeneration (1) or secondary to the loss of hair cells that normally supply trophic support to SGNs (2). Auditory neuropathy (AN) results when degeneration of SGNs occurs in the presence of functional hair cells (3). A recent study demonstrated that application of ouabain, a well-known cardiac glycoside, to the round window resulted in a loss of SGNs, which serves as a model of AN (4, 5).
Currently, the use of a cochlear implant is the only method to treat the deaf resulting from the loss of hair cells or the loss of SGNs. However, survival of SGNs is a significant issue for obtaining the hearing benefits provided by cochlear implants. Many researchers seek methods for protection of SGNs (6-8) because SGNs do not regenerate to any clinically significant extent after neural degeneration.
Stem cell replacement therapy raises the hope for hearing loss (9, 12). Among the various types of stem cells, bone marrow-derived mesenchymal stem cells (MSCs) are one of the most promising stem cell sources for cell replacement therapy because they can differentiate into neurons (13) and can be used by the patient's own bone marrow (14). Moreover, bone marrow transplantation has been shown to be a safe procedure for decades without any incidents of tumor formation in host tissues after transplantation. Primary MSCs have been proven to survive in the inner ear and to replace the lost SGNs (15, 16). However, it remains unclear whether neural differentiated MSCs can serve as better sources for cell replacement therapy in injured cochleae. Previously, it has been reported that neuronal differentiation of MSCs could be induced by application of basic fibroblast growth factor (bFGF), forskolin, and ciliary neurotrophic factor (14, 17, 18). In this study we have shown that neural differentiated human MSCs have the potential not only to replace the lost SGNs, but also to promote hearing recovery in the AN guinea pig model.
MATERIALS AND METHODS
Preparation of human MSCs and in vitro neural differentiation
Previously, we have reported neural differentiation of human MSCs (19, 20). Briefly, human bone marrow was obtained from the mastoid process during mastoidectomy for ear surgery. Informed consent was obtained from all donors according to the guidelines of the Ethics Committee of the Chonnam National University Hospital. Human MSCs were isolated from the bone marrow and grown as adherent cultures in Dulbecco's Modified Eagle's Medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin-streptomycin (Hyclone). After 4 weeks, MSCs were grown to approximately 80% confluence in a 37℃ humidified incubator with a 5% CO2 and 95% air environment. This stage was termed passage 0 (P0). The human MSCs used in the present study were obtained from passages 3-6.
To induce neural differentiation, MSCs were grown in DMEM containing 1% FBS and supplementary 100 ng/mL of bFGF (Invitrogen Corporation, Carlsbad, CA, USA) for 7 days. The cells were then incubated in the presence of 10 µM forskolin (Sigma Chemical Co., St. Louis, MO, USA). Over the next 7 days, the cells were characterized and used for transplantation into the AN guinea pig model.
AN animal model and human MSC transplantation
Twenty-four female guinea pigs, weighing 250-350 g, were used in the present study. Animal care complied with institutional guidelines. All surgical procedures were performed under aseptic conditions. The guinea pigs were anesthetized with pentobarbital sodium (40 mg/kg) by intraperitoneal injection. The microsurgical techniques were performed under an operating microscope (Zeiss OPMI® pico; Carl Zeiss, Goettingen, Germany).
To make an AN model, the right post-auricular region of the guinea pigs was shaved and sterilized with potadine and 70% ethanol following anesthesia (see above). The animal was then placed on a heating pad (37℃), and the right bulla was opened widely and the round window niche was exposed. A piece of gelatin sponge soaked with 1 mM ouabain (Sigma Chemical Co.) was placed on the round window membrane. Then, the incision was closed with sutures. The animals were randomly divided into 2 groups (n = 8 per group), as follows: control group, Hanks balanced salt solution (HBSS; Hyclone) without neuralinduced human mesenchymal stem cell (nhMSC) transplantation into the cochlea; and stem cell group, HBSS with nhMSC transplantation into the cochlea.
Neural-induced human MSC transplantation
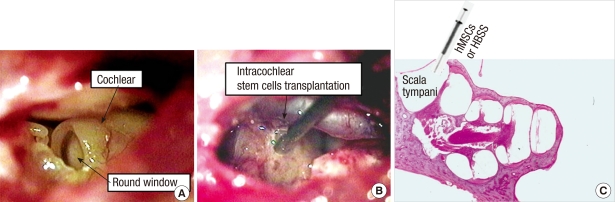
Transplantation of nhMSCs was performed 7 days after ouabain application. Procedures for animal preparation and anesthesia were similar to those described above. The right bulla was surgically exposed and opened to visualize the basal cochlea using a post-auricular incision. A small hole was made into the scala tympani at the basal cochlear turn using a 0.8-mm diamond drilling burr. HBSS with nhMSCs (1 × 105 cells in 5 µL) or HBSS alone was then injected into the right scala tympani through the small hole using a Hamilton syringe and microinfusion pump at a speed of 2 µL/min (Fig. 1). After injection, the hole was plugged with small connective tissue and a fibrin-based adhesive agents (Greenplast®; Green-Cross, Seoul, Korea), and the incision was approximated with sutures.
Fig. 1.
Photomicrographs of stem cell transplantation method. (A) Visualization of the round window after opening the bulla. (B, C) Note the tip of a needle of a Hamilton syringe is inserted into the most basal turn of the scala tympani, where neural-induced human mesenchymal stem cells (nhMSCs) or Hanks balanced salt solution (HBSS) were injected (C, H&E staining, × 40).
Outcome measurement by histologic analysis
Histologic evaluation was performed 6 weeks after the transplantation. The animals were sacrificed by administration of a high dose of pentobarbital sodium. The cochleae were carefully removed, trimmed, fixed in 5% paraformaldehyde, then decalcified for 2 weeks in 10% EDTA. The fixed, decalcified cochleae were embedded in paraffin wax for paraffin section. The paraffin-embedded cochlea was sliced info 5-µm-thick serial paramodiolar sections. The sections were mounted on glass slides and the morphology was checked via hematoxylin and eosin staining. We obtained spiral ganglion images with a magnification of × 400 using a Nikon camera (Tokyo, Japan). These images were then printed on 10 × 7.5 cm photographic paper for neuron counting. Quantitative histology values were used to calculate the neuron density (neurons per field divided by the partial spiral ganglion cross-sectional area in each turn).
After blocking in 1% normal goat serum, the sections were incubated overnight at 4℃ with a primary antibody diluted in phosphate-buffered saline (pH 7.4). The primary antibodies used in this study were anti-human nuclei (1:200), Math1 (1:200), and NF-H (1:300). Then, the sections were incubated with FITC- and rhodamine-conjugated secondary antibodies to visualize primary antibodies. 4'6-diamidino-2-phenylindole (DAPI) was used to label nuclei. All primary and secondary antibodies were purchased from Chemicon, except for Math1 (Ambion, Austin, TX, USA). Fluorescent images were obtained using a Zeiss LSM510 confocal microscope (Carl Zeiss Inc., Jena, Germany). The captured images were processed using Zeiss LSM Image Browser version 4, 2, 0, 121 (Carl Zeiss Inc.) and Adobe photoshop CS.
Auditory Brain Responses (ABR) measurement and analysis
ABR recordings were carried out using commercial auditory-evoked potential equipment (Navigator SE evoked potential system; Bio-logic Systems Corp., Mundelein, IL, USA) at various intervals (1, 3, and 5 weeks) after the transplantation. The animals were placed in a prone position after anesthesia (see above). ABRs were elicited using 11.1/sec alternating polarity clicks (150-3,000 Hz, stimulation rate 11/sec [reflecting lower frequency responses]) and 8 kHz (reflecting middle frequencies) alternating polarity tone bursts, a rise and fall time of 5 msec, and a plateau of 5 msec shaped with a Blackman window. The stimuli were delivered to the ear using an insert earphone. Response signals were recorded by three silver needle electrodes. The electrodes were placed in the vertex for an active electrode and in the bilateral retroauricular region for ground and reference electrodes. The responses were digitally band-passed filtered (100-3,000 Hz), amplified, and time-averaged (n = 512 to 1,024) for a post-stimulus time window of 9.98 msec. Stimulus intensity from a 90-decibel (dB) sound pressure level (SPL) was used, down to threshold in 10 dB, then 5 dB steps around the threshold level. The thresholds of ABRs were measured by detecting the presence of wave V. At the threshold intensity, at least two sequences of recordings were made to confirm response reproducibility.
Statistical analysis
All results were reported as the mean ± standard error of the mean. Statistical analysis was performed with an independent sample t-test for comparison of two experimental groups using SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA). A value of P < 0.05 was considered to be significant.
Ethics statement
This study protocol and procedures were reviewed and approved by the institutional review board (I-2009-03-016) and the institutional animal care and use committee of the Chonnam National University Hospital.
RESULTS
Effect of ouabain on SGNs
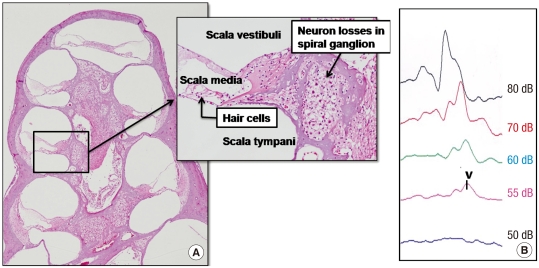
All experimental animals exhibited normal hearing with click-evoked ABR thresholds of -10 ± 10 dB prior to application of ouabain. Fig. 2 shows the effect of ouabain on SGNs. After 7 days of ouabain application, a severe decrease in SGNs was generally representative for all turns in the right ears. Despite the apparent loss of afferent innervation, hair cells were preserved in all turns. Also, the hearing threshold was increased in ABR measurements.
Fig. 2.
Ouabain-induced spiral ganglion neuropathy. (A) There is a severe loss of spiral ganglion neurons (SGNs) without a loss of hair cells after 7 days of ouabain application (H&E staining, original magnification × 40 and × 200). (B) Also, an increase of the hearing threshold is noted (dB, decibel). V, wave v.
Transplanted neural differentiated hMSCs survive in the spiral ganglion
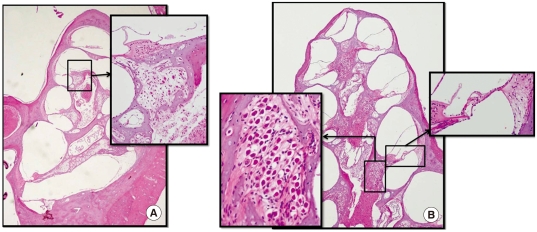
We explored the localization of nhMSCs after transplantation into the AN guinea pig cochlea 6 weeks after nhMSC transplantation. In the control group (Fig. 3A), severe loss of SGNs and sensory hair cells from the basal to the apical turns of the cochlea was noted. In contrast, in the stem cell group (Fig. 3B) the number of SGNs and sensory hair cells was increased in all turns of the cochlea 6 weeks after stem cell grafting.
Fig. 3.
Histologic findings of cochlea after stem cell transplantation. (A) Severe loss of SGNs and sensory hair cells from the basal to the apical turn of the cochlea is noted 6 weeks after HBSS injection (control group). (B) Numerous SGNs and sensory hair cells are observed in all turns of the cochlea 6 weeks after stem cell grafting (stem cell group). (H&E staining, × 40 and × 200).
Even without the use of immunosuppressants, there was no evidence of acute rejection, which usually occurs within the first 6 months following transplantation. No significant inflammation response was observed in the stem cell group.
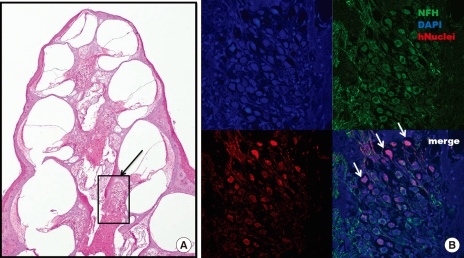
We used human-specific anti-nuclei antibodies to specifically identify human cells in the transplanted guinea pig inner ear cochlea. Transplanted human nuclei-positive cells located in the spiral ganglion expressed NF-H, a neural cell marker, suggesting differentiation into neuronal cells (Fig. 4).
Fig. 4.
Confocal microscope image after immu- nostaining for anti-human nuclei and NF-H. (A) Numerous neurons are observed in the spiral ganglion (black arrow) after 6 weeks of stem cell transplantation (H&E staining, × 40). (B) the expression of anti-human nuclei (red) and NF-H (green) co-localizes with that of DAPI (blue)-positive cells in the spiral ganglion. Grafted stem cells (white arrow) are located in the spiral ganglion (× 200).
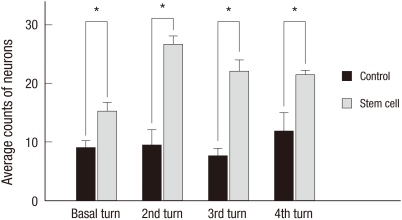
Based on quantitative histologic analysis, the average number of neurons in each turn of the spiral ganglion was significantly greater in the stem cell group than in the control group (Fig. 5). In the control group, the average number of neurons in the basal, 2nd, 3rd, and 4th turns was 9.00 ± 1.27/1,000 µm2, 9.50 ± 2.63/1,000 µm2, 7.63 ± 1.36/1,000 µm2, and 11.88 ± 3.10/1,000 µm2, respectively. In the stem cell group, the average number of neurons in the basal, 2nd, 3rd, and 4th turns was 15.30 ± 1.51/1,000 µm2, 26.64 ± 1.46/1,000 µm2, 22.00 ± 1.99/1,000 µm2, and 21.44 ± 0.77/1,000 µm2, respectively.
Fig. 5.
Average neuron counts in each turn of the spiral ganglion 6 weeks after transplantation. Average number of neurons in each turn of the spiral ganglion is significantly greater in the stem cell group than in the control group (*P < 0.05).
Hearing recovery results after nhMSC transplantation
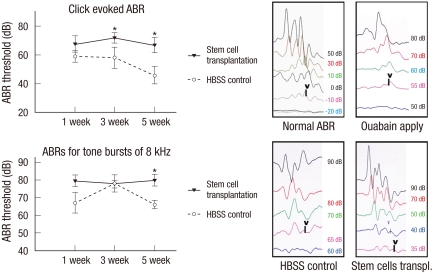
Although evidence exists that transplanted nhMSCs differentiate to NF-H-positive neuronal cells in the spiral ganglion to replace the lost cells in the AN guinea pig model, it is not clear if transplanted stem cells can enhance the hearing threshold. To evaluate the effect of nhMSC transplantation on hearing recovery in an AN guinea pig, we measured the ABR thresholds before, and 1, 3, and 5 weeks after the transplantation. The ABR was evoked by two types of stimuli (alternative click sound and an 8 kHz pure tone burst sound). All experimental animals exhibited normal hearing with click-evoked ABR thresholds of 0 ± 10 dB prior to AN modeling. Seven days after the application of 1 mM ouabain on the round window, an increase of hearing threshold (63.00 ± 4.90 dB at click-evoked stimuli and 70.00 ± 5.70 dB at 8 kHz tone burst stimuli) was noted. After receiving a transplantation of nhMSCs, the guinea pigs showed a significant improvement in hearing threshold compared to the control group. Five weeks after the transplantation, average ABR thresholds for the click and 8 kHz tone burst stimuli were decreased to 46.00 ± 6.00 dB and 66.00 ± 2.45 dB, respectively (Fig. 6). The considerable improvement in hearing function observed in the ABR test is consistent with the restoration of SGNs in the stem cell group.
Fig. 6.
Auditory brainstem response (ABR) results after nhMSC transplantation. Click-evoked ABR waves are recorded up to -10 ± 10 dB in normal guinea pigs. Seven days after application of 1 mM ouabain to the round window, an increase of ABR threshold is noted. After receiving a nhMSC transplantation, the auditory neuropathy guinea pigs show a significant improvement in hearing threshold compared to the control group (*P < 0.05).
DISCUSSION
Recent advance in studies on stem cells have suggested the possibility of clinical use of stem cell transplantation for ischemic brain disease and neurodegenerative disorders (21, 22). Also, restoration of SGNs is one of the targets for stem cell therapy in the treatment of hearing loss (23). The present study provides the scientific basis for a requirement of neural differentiation of MSCs for better treatment and presents the novel demonstration of a therapeutic approach leading to substantial recovery of hearing in AN patients' ears. Some researchers have reported that transplantation of undifferentiated MSCs into the inner ear repairs injured cochlear fibrocytes in an animal SNHL model and increases survival in the spiral ganglion in an AN model (15, 16).
Various ototoxic treatments (5, 24) and the aging process lead to loss of SGNs (25). The application of ouabain to the round window membrane is a well-known method which induces the death of most SGNs, and thus provides an excellent model for AN (4, 5, 16). In this study, we placed a piece of gelatin sponge soaked with 1 mM ouabain on the round window membrane. One week after ouabain application, there was a selective and permanent destruction of SGNs without loss of hair cells and an increase of the ABR threshold.
In the present study, the number of SGNs was increased at all turns of the cochlea after nhMSC transplantation, and the transplanted nhMSCs were positively stained for NF-H, a mature neuronal marker, at the damaged spiral ganglion. This result suggests that the nhMSCs differentiate into neurons and replace the lost or damaged neural cells in the spiral ganglion. This finding suggests that the migration and differentiation of grafted stem cells are influenced by the cues or signals in the microenvironment of the damaged tissue. It is known that damaged cells and tissues of the host brain are capable of releasing molecules that stimulate the production of neurotrophic factors in transplanted MSCs (26).
The ultimate goal of stem cell therapy in AN patients is not only restoration of missing SGNs, but also to increase hearing function. In this study, the decreased average ABR thresholds for click and 8 kHz tone burst stimuli indicated that nhMSC transplantation is an effective treatment method for AN. Because the presence of some SGNs is known to enhance the function of cochlear implants, the regeneration or protection of SGNs via nhMSC transplantation would also improve the therapeutic effect of cochlear implantation (27, 28). Thus, our results suggest that the transplantation of nhMSCs at the time of cochlear implant surgery could improve hearing recovery.
In this study, it is possible that immunologic rejection of transplanted cells occurred in this experiment because we used the xenotransplantation procedure. Xenotransplantation was performed in this study because we sought the effectiveness of human MSCs on cochlear repair. Although immunologic rejection was expected in this study, there was no evidence of acute rejection in any of the animals used in our study. Escape from immune surveillance is partly due to the fact that MSCs lack MHC class II or inhibit T-cell proliferation (29, 30). Escape from immune surveillance was also caused by some degree of immunoprotection of the cochlea provided by the blood-labyrinth barrier (16). However, further studies will be required to elucidate the long-term immunologic reactions.
In conclusion, neural differentiated MSCs are able to restore damaged SGNs and decrease hearing thresholds in an AN model. Furthermore, transplantation into the scala tympani is a suitable method because numerous grafted nhMSCs were found in the spiral ganglion. These findings suggest that it may be possible to replace degenerated SGNs by grafting stem cells into the scala tympani.
Footnotes
This study was supported by the Korea Research Foundation Grant funded by the Korean Government (Basic Promotion Fund: KRF-2008-E00148) and a grant (CRI09062-1) of Chonnam National University Hospital Research Institute of Clinical Medicine.
AUTHOR SUMMARY
Transplantation of Neural Differentiated Human Mesenchymal Stem Cells into the Cochlea of an Auditory-neuropathy Guinea Pig Model
Yong-Bum Cho, Hyong-Ho Cho, Sujeong Jang, Han-Seong Jeong, and Jong-Seong Park
We investigated the effects of human mesenchymal stem cells (hMSCs) differentiated into neural cells on auditory neuropathy. The auditory neuropathy was induced in guinea pigs using 1mM ouabain applied to the round window niche. The differentiated hMSCs were transplanted into the scala tympani of the guinea pig cochlea 7 days after the ouabain-induced injury. The application of ouabain induced attenuated auditory brainstem response (ABR) and degeneration of spiral ganglion neurons (SGNs) without loss of hair cells in the organ of Corti. After transplantation of neural differentiated hMSCs, the number of SGNs was increased and the ABR results showed mild hearing recovery. It might be possible to replace degenerated SGNs by grafting stem cells into the scala tympani.
References
- 1.Suzuka Y, Schuknecht HF. Retrograde cochlear neuronal degeneration in human subjects. Acta Otolaryngol Suppl. 1988;450:1–20. doi: 10.3109/00016488809098973. [DOI] [PubMed] [Google Scholar]
- 2.Keithley EM, Croskrey KL. Spiral ganglion cell endings in the cochlear nucleus of young and old rats. Hear Res. 1990;49:169–177. doi: 10.1016/0378-5955(90)90103-v. [DOI] [PubMed] [Google Scholar]
- 3.Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol. 2000;11:215–230. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- 4.Lang H, Schulte BA, Goddard JC, Hedrick M, Schulte JB, Wei L, Schmiedt RA. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9:225–240. doi: 10.1007/s10162-008-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmiedt RA, Okamura HO, Lang H, Schulte BA. Ouabain application to the round window of the gerbil cochlea: a model of auditory neuropathy and apoptosis. J Assoc Res Otolaryngol. 2002;3:223–233. doi: 10.1007/s1016200220017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iguchi F, Nakagawa T, Tateya I, Kim TS, Endo T, Taniguchi Z, Naito Y, Ito J. Trophic support of mouse inner ear by neural stem cell transplantation. Neuroreport. 2003;14:77–80. doi: 10.1097/00001756-200301200-00015. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara T, Bredberg G, Ulfendahl M, Pyykkö I, Olivius NP, Kaksonen R, Lindström B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Corrales CE, Edge A, Heller S. Stem cells as therapy for hearing loss. Trends Mol Med. 2004;10:309–315. doi: 10.1016/j.molmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker MA, Cotanche DA. The potential use of stem cells for cochlear repair. Audiol Neurootol. 2004;9:72–80. doi: 10.1159/000075998. [DOI] [PubMed] [Google Scholar]
- 13.Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 14.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, Kusano R, Nakagawa S, Satoh H, Fujii M, Matsunaga T. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214–226. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007;117:1629–1635. doi: 10.1097/MLG.0b013e31806bf282. [DOI] [PubMed] [Google Scholar]
- 17.Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. Neural induction with neurogenin 1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26:2217–2228. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- 18.Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, Park IH, Kim SU. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2:e1272. doi: 10.1371/journal.pone.0001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho HH, Jeong HS, Jang SJ, Park JS, Cho HC, Jang CH, Cho YB. Neural differentiation of mesenchymal stem cells from bone marrow of human mastoid process. Korean J Otorhinolaryngol-Head Neck Surg. 2008;51:422–428. [Google Scholar]
- 20.Cho HH, Jang S, Lee SC, Jeong HS, Park JS, Han JY, Lee KH, Cho YB. Effect of neural-induced mesenchymal stem cells and platelet-rich plasma on facial nerve regeneration in an acute nerve injury model. Laryngoscope. 2010;120:907–913. doi: 10.1002/lary.20860. [DOI] [PubMed] [Google Scholar]
- 21.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama D, Matsuyama T, Ishibashi-Ueda H, Nakagomi T, Kasahara Y, Hirose H, Kikuchi-Taura A, Stern DM, Mori H, Taguchi A. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur J Neurosci. 2010;31:90–98. doi: 10.1111/j.1460-9568.2009.07043.x. [DOI] [PubMed] [Google Scholar]
- 23.Tamura T, Nakagawa T, Iguchi F, Tateya I, Endo T, Kim TS, Dong Y, Kita T, Kojima K, Naito Y, Omori K, Ito J. Transplantation of neural stem cells into the modiolus of mouse cochleae injured by cisplatin. Acta Otolaryngol Suppl. 2004;551:65–68. doi: 10.1080/03655230310016780. [DOI] [PubMed] [Google Scholar]
- 24.Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Dong Y, Yuki K, Naito Y, Lee SH, Ito J. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003;113:994–999. doi: 10.1097/00005537-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Shiga A, Nakagawa T, Nakayama M, Endo T, Iguchi F, Kim TS, Naito Y, Ito J. Aging effects on vestibulo-ocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiol Neurootol. 2005;10:97–104. doi: 10.1159/000083365. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 27.Coleman B, de Silva MG, Shepherd RK. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells. 2007;25:2685–2694. doi: 10.1634/stemcells.2007-0393. [DOI] [PubMed] [Google Scholar]
- 28.Nadol JB, Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Jr, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- 29.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 30.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]