Abstract
We investigated the patterns of pretreatment expression of the epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and cyclooxygenase-2 (COX-2) by immunohistochemical staining and determined their correlation with treatment response and survival in 44 patients with esophageal squamous cell carcinoma (ESCC) treated with definitive concurrent chemoradiotherapy (CCRT). The definitive CCRT consisted of a median dose of 54 Gy (range: 40.0-68.4 Gy) and two cycles of concurrent administration of mostly 5-fluorouracil + cisplatinum. High expression of EGFR, VEGF, and COX-2 was found in 79.5%, 31.8%, and 38.6%, respectively. The Cox regression analysis for overall survival (OS) showed that both the treatment response and COX-2 expression were significant. The 3-yr OS rates of patients that achieved a complete response and those that did not were 46.7% and 5.3%, respectively (P = 0.006). The logistic regression analysis for treatment response with various parameters showed that only a high expression of VEGF was significantly associated with a complete response. Unlike other well-known studies, higher expression of VEGF was significantly correlated with a complete response to CCRT in this study. However, higher expression of COX-2 was significantly associated with shorter survival. These results suggest that VEGF might be a predictive factor for treatment response and COX-2 a prognostic factor for OS in patients with ESCC after definitive CCRT.
Keywords: Esophageal Neoplasms, Chemoradiotherapy, Epidermal Growth Factor Receptor, Vascular Endothelial Growth Factor, Cyclooxygenase 2
INTRODUCTION
The majority of patients with esophageal squamous cell carcinoma (ESCC) are diagnosed as having advanced disease with regional or distant metastasis at the time of presentation; the 5-yr survival rate for these patients is still below 25% (1). The main treatment modality currently used is concurrent chemoradiotherapy (CCRT) in patients with unresectable disease and the survival outcomes for this combined modality are much better than for radiotherapy alone (2, 3). However, local failure rates after CCRT are still as high as 45%-60% and improved local control is urgently needed (2, 3). Recent phase III European studies of preoperative CCRT, with or without surgery, in patients with ESCC have shown that adding surgery to CCRT resulted in improvement of locoregional control compared to continuation of CCRT; however, the overall survival rates were similar (4, 5). A German study demonstrated that good responders to induction treatment had a better survival rate, regardless of the treatment group, whereas nonresponders generally had a shorter survival (4).
In patients with persistent or residual disease, the survival improved after receiving complete resection. However, the treatment-related mortality was 9%-13% in the surgery group compared to 1%-4% in the CCRT group. Therefore, pretreatment evaluation should be undertaken to predict which patients are likely to have a complete response to CCRT, or in whom surgery should be added due to an incomplete response.
Various factors have been evaluated as possible predictive or prognostic factors of esophageal cancer outcome; however, the data is conflicting and continues to be debated (6). In the present study, three potential factors, namely, the epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and cyclooxygenase-2 (COX-2) were selected for evaluation. The expressions of EGFR has been reported to be associated with better treatment response after CCRT (7) but poor treatment outcomes after primary surgery (8). VEGF is known to play a key role in tumor angiogenesis. It has been known that VEGF expression in esophageal carcinoma was associated with more advanced stage or poor prognosis (9, 10). Elevated COX-2 expression is associated with resistance to treatment and reduced overall survival for patients who received CCRT (11). We performed retrospective analysis of the expression patterns of EGFR, VEGF and COX-2 in pretreatment endoscopic biopsy specimens by immunohistochemical staining. The findings were assessed in an attempt to identify their predictive association with the treatment response and survival.
MATERIALS AND METHODS
Patient characteristics
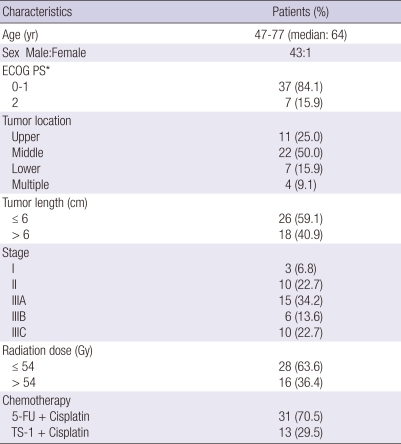
From February 2001 to May 2008, a total of 44 patients with ESCC, that received definitive CCRT, without double primary lesions, were diagnosed as stage I-III according to the American Joint Committee on Cancer staging system with the 7th edition (12), and had endoscopic biopsy specimens pre- and post-treatment available, were enrolled in the present study. To determine the stage, all patients underwent a thorough medical history, physical examination, complete blood cell count, serum biochemistry, esophagography, upper gastrointestinal endoscopy with or without ultrasound, a computerized tomography (CT) of the chest and abdomen, and if necessary 18F-fluorodeoxyglucose-positron emission tomography. The clinical and tumor characteristics of the 44 patients are presented in Table 1. Most patients were male and all patients had squamous cell carcinoma. For 26 cases (59.1%), the primary tumors were located in the middle esophagus. Thirty-three (75.0%) primary lesions were staged as T3, and 31 (70.5%) as stage III.
Table 1.
Patient characteristics
ECOG PS, Performance score by Eastern Cooperative Oncology Group; 5-FU, 5-fluorouracil; TS-1, Tegafur + Gimeracil + Oteracil.
Radiotherapy
Radiotherapy was performed using a LINAC 6- or 10-MV X-ray unit, in daily 1.8 or 2 Gy with five fractions per week. The radiation dose ranged from 40 to 68.4 Gy with a median of 54 Gy. Initially anteroposterior and posteroanterior fields were used with a craniocaudal margin of 5 cm and a transverse margin of 2 cm adjacent to the gross tumor mass as determined by chest CT or esophagography. At doses between 36 and 40 Gy, a chest CT was performed for 3D-conformal radiotherapy planning and a boost dose was delivered to clinical target volumes with a craniocaudal margin of 3 cm and a transverse margin of 2 cm adjacent to the gross tumor volumes. Further radiotherapy boosts were administered to cervical and supraclavicular lymph nodes depending on nodal size.
Chemotherapy
To patients with adequate bone marrow function, as well as hepatic, and renal function, concurrent chemotherapy was delivered with 5-fluorouracil based regimen. Cisplatinum (75 mg/m2) was administered intravenously on the first day of weeks 1, 5, 9, and 13, and 5-fluorouracil (1,000 mg/m2) was administered as a continuous infusion for the first 4 days of weeks 1, 5, 9, and 13; the first two cycles were delivered concurrently with radiotherapy. Thirty-one patients (70.5%) received cisplatinum + 5-fluorouracil with a median of three cycles (range 1-10) and 13 patients received cispatinum plus TS-1 of oral fluorouracil agent.
Immunohistochemical staining
The biopsy specimens were all obtained via pretreatment endoscopy with one sample for each case. Specimens were routinely fixed in 10% buffered formalin and embedded in paraffin. Five serial paraffin-embedded sections (4 µm-thick) were prepared for immunostaining. The sections were dewaxed using xylene, transferred to alcohol, then placed in citric acid buffer (10 mM/L) and heated in a microwave oven (700 W) for 12 min to expose the antigens. Endogenous peroxidase activity was blocked by incubating the sections with 3% hydrogen peroxide in methanol for 30 min; the slides were then washed three times in phosphate-buffered saline (PBS), incubated in 10% normal goat serum for 20 min to reduce nonspecific antibody binding, and washed with PBS. The slides were incubated overnight at 4℃ with the monoclonal antibodies, i.e., with the monoclonal antibody against EGFR (clone H11, diluted 1:200, Dako, Glostrup, Denmark), VEGF (clone VG1, diluted 1:50, Dako), and COX-2 (clone CX-294, diluted 1:100, Dako). The samples were visualized by the EnVision method (Dako) according to the manufacturer's instructions. Lung squamous cell carcinoma strongly expressing EGFR, VEGF, and COX-2 was used as a positive control. For the negative control, sections were similarly treated but without the primary antibody.
Evaluation of immunohistochemical staining
Each slide was examined under the same magnification (× 200, Vanox-S, Olympus, Tokyo, Japan) by one independent pathologist who moved the microscope field randomly across the specimens. For the assessment of EGFR, the immunoreactivity of EGFR was graded into four groups according to the intensity of cell membrane EGFR staining: high (markedly stronger staining than normal esophageal epithelium), medium (moderately stronger staining), low (the same staining level as normal epithelium), and negative (fainter staining). Strong and moderate staining groups were defined as positive for EGFR expression (7). For the evaluation of VEGF expression, when intensive positive staining of VEGF was observed in more than 10% of the tumor cells, the case was considered VEGF-positive (10). For COX-2, the intensity of staining was determined as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). A combined score based on the staining intensity and the percentage of stained cells was used as the final score. Low expression was defined as an intensity of 2 or 3 and < 10% stained cells or an intensity of 0 or 1 and < 50% stained cells. High expression was defined as an intensity of 2 or 3 and > 10% stained cells or intensity 1 and > 50% stained cells (13).
Evaluation of tumor response and statistical analysis
To evaluate tumor responses, an endoscopic biopsy and a chest CT scan were performed within one month of completing radiotherapy. Follow-up CT and esophagography were performed every three months for the first two years and every six months thereafter. A complete disappearance of the primary tumor and regional lymph nodes on radiologic study was defined as complete response (CR) and otherwise as non-complete response (non-CR). Endoscopy and CT were repeated when new clinical symptoms were present. The chi-square test or Fisher's exact test were used to compare frequencies. The survival rates were calculated using the Kaplan-Meier method and the log-rank test was used to compare the survival curves. Logistic regression analysis was performed to identify variables significantly associated with the tumor response. The Cox proportional hazards model was used to identify variables related to overall survival (OS) and cause-specific survival (CSS). When calculating CSS, death of esophageal cancer was regarded as an event but that of unknown cause or other cause as a censored case. All statistical analyses were performed using SPSS 17.0 and P values less than 0.05 was considered statistically significant.
Ethics statement
The study was approved by the institutional review board of Chonnam National University Hwasun Hospital (2010-75). Informed consent was exempted by the board because the study was retrospective.
RESULTS
Treatment response and survival outcomes
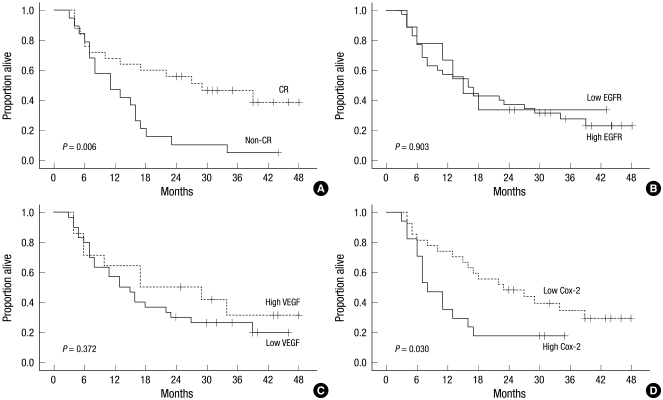
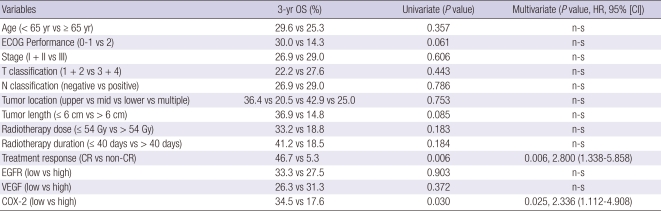
The median follow-up for all patients was 16 months with a range of 3-48 months. At the end of this study, 32 out of the 44 patients had died. Among the 32 dead patients, 17 patients died of progressive or recurrent disease of esophageal carcinoma. The remaining patients died of unknown causes were considered as a censored case on the cause-specific survival analysis. The median overall survival time for all 44 patients was 16 months, with a 3-yr overall survival rate (OSR) of 27.7% and a 3-yr cause-specific survival rate (CSSR) of 45.1%. The 3-yr OSRs for stages I, II, IIIA, IIIB, and IIIC were 66.7%, 20.0%, 33.3%, 50.0%, and 10.0%, respectively (P = 0.37), which lack of significance might be caused by small number of patients groups. The 3-yr CSSRs for stages I, II, IIIA, IIIB, and IIIC were 100%, 40.0%, 55.5%, 62.5%, and 17.5%, respectively (P = 0.04). Twenty-five patients (56.8%) achieved a CR and the 3-yr OSRs of patients that achieved a CR and those that did not were 46.7% and 5.3%, respectively (P < 0.01) (Fig. 1A). The 3-yr CSSRs of patients with a CR and those without were 66.4% and 13.7%, respectively (P < 0.01). For the OS analysis with other clinical and therapeutic parameters, the Eastern Cooperative Oncology Group (ECOG) performance score (P = 0.06) and tumor length (P = 0.08) were marginally significant.
Fig. 1.
Overall survival according to the treatment response and the patterns of protein expression: (A) The Kaplan-Meier survival curve shows a significant difference between the patients with a complete response (CR) and those without a complete response. (B, C) the survival analyses of EGFR (B) and VEGF (C) did not show significant differences. (D) The patients with a higher expression of COX-2 show a significantly lower overall survival.
Relationship between treatment response and expression of EGFR, VEGF, and COX-2
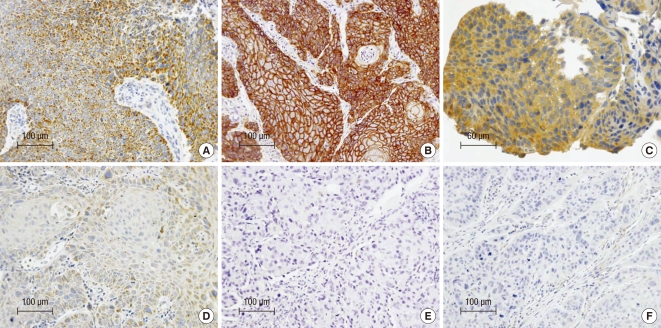
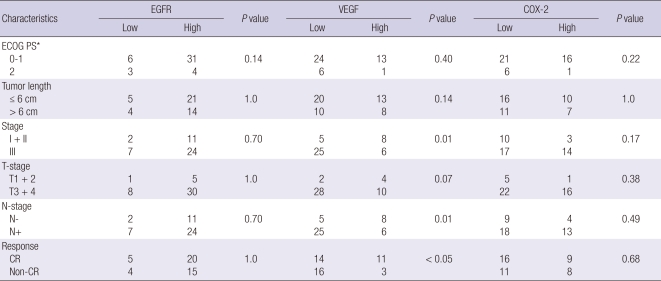
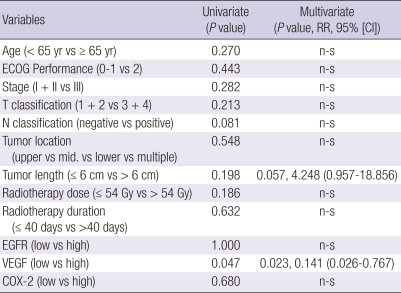
COX-2 immunoreactivity was characteristically cytoplasmic and granular (Fig. 2A). Weak expression of COX-2 was often observed in normal-appearing squamous epithelium adjacent to carcinomas. EGFR expression was mainly seen in the cell membrane of tumor cells (Fig. 2B). Normal appearing epithelium adjacent to the carcinoma showed a few strongly positive cells. VEGF expression was primarily detected in the cytoplasm or cell membranes of the tumor cells (Fig. 2C). The vascular endothelial cells within the carcinomas were weakly stained. According to our scoring criteria, high COX-2, EGFR, and VEGF expression was found in 38.6%, 79.5%, and 31.8%, respectively (Fig. 2). The correlation between the patterns of expression and clinical/pathological factors is listed in Table 2. The patients with a higher stage (III vs I + II), presence of regional lymph node metastases (N+ vs N-), and incomplete response had significantly lower expression of VEGF. However, the expression of EGFR and COX-2 did not have any significant correlation with the clinical/pathological parameters. The multiple logistic regression analysis for determining predictive factors associated with the treatment response with 12 covariates of age (< 65 yr old vs ≥ 65 yr), ECOG score (0-1 vs 2), stage (I + II vs III), tumor location (upper vs mid vs lower vs multiple), tumor length (≤ 6 cm vs > 6 cm), T- stage (T1 + 2 vs T3 + 4), N-stage (N- vs N+), radiation dose (≤ 54 Gy vs > 54 Gy), radiotherapy duration (≤ 40 days vs > 40 days), and the expression of EGFR, VEGF, and COX-2 showed that only a high expression of VEGF was significantly correlated with the primary CR for CCRT in patients with ESCC (Table 3).
Fig. 2.
Immunohistochemical staining of high and low expression of COX-2 (A, D), EGFR (B, E), and VEGF (C, F).
Table 2.
Correlations between clinical/pathological characteristics and EGFR, VEGF, and COX-2 expression (chi-square test or Fisher's exact test)
*Performance score by Eastern Cooperative Oncology Group. CR, complete response.
Table 3.
Logistic regression analysis with potential variables predicting compete response to definitive chemoradiotherapy
RR, relative risk; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; n-s, statistically non-significant.
Relationship between survival outcomes and expression of EGFR, VEGF, and COX-2
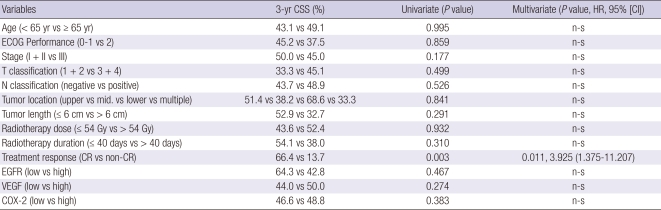
The 3-yr OS analyses according to EGFR expression did not show significant differences (high vs low = 27.5% vs 33.3%. P = 0.90, Fig. 1B). The 3-yr OS of patients according to VEGF expression showed no significant difference (high vs low = 31.3% vs 26.3%, P = 0.37, Fig. 1C). However the patients with high COX-2 expression survived significantly shorter than those of low expression (17.6% vs 34.5%, P = 0.03, Fig. 1D). The Cox regression analysis for OS with the above 12 covariates adding treatment response showed that both treatment response (P < 0.01) and COX-2 expression (P = 0.03) were significantly correlated with OS (Table 4). However, the 3-yr CSS analyses revealed that EGFR (high vs low = 42.8% vs 64.3%, P = 0.47), VEGF (high vs low = 50.0% vs 44.0%, P = 0.27), and COX-2 (high vs low = 48.8% vs 46.6%, P = 0.38) did not show significant survival differences. The Cox regression analysis for the CSS of the above thirteen covariates showed that only the treatment response was significant (Table 5).
Table 4.
Cox proportional hazards model with potential variables predicting overall survival (OS)
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; n-s, statistically non-significant; CR, complete response.
Table 5.
Cox proportional hazards model with potential variables predicting cause-specific survival (CSS)
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; n-s, statistically non-significant; CR, complete response.
DISCUSSION
VEGF is a potential biomarker for the prediction of treatment outcome in patients with esophageal cancer undergoing CCRT (14) or surgery (9, 15). Shih et al. (15) examined the relationship between VEGF expression, microvessel density (MVD), and clinical/pathological factors in 117 patients with ESCC undergoing surgery. They demonstrated that survival of patients with VEGF-positive tumors was significantly decreased compared to the patients with VEGF-negative tumors. A similar tendency for survival was observed for high-MVD versus low-MVD cases (32% vs 60%, P = 0.009). The MVD of the VEGF (+) tumors was higher than the VEGF (-) tumors; however, the difference was not significant (P = 0.08). In a recent study reported by Shimada et al. (14), high pretreatment VEGF expression was associated with a poor response to CCRT and poor survival in patients with ESSC. The response rate of VEGF (+) tumors was significantly lower than VEGF (-) tumors (43% vs 90%, P < 0.001) and the multivariate analysis identified VEGF as a significant independent prognostic factor. However, other studies could not show an association between VEGF expression and survival in patients with esophageal cancer undergoing surgery (16) or CCRT (17, 18).
The results of this study showed that high VEGF expression was significantly correlated with a complete response to CCRT in ESCC. However, no prior study has demonstrated that higher VEGF expression is correlated with better responsiveness to CCRT in patients with esophageal cancer so far. The biological mechanism by which a high VEGF expression could result in improved response to CCRT remains unknown. Hypoxia in tumors is an important factor associated radioresistance; it can also decrease the efficacy of chemotherapeutic agents due to alterations in blood flow and drug diffusion distance. Oxygenation and drug delivery to cancer cells depends mostly on microvessels and blood flow in the tumor microcirculation. Most tumors have hypoxic areas that could drive VEGF production. VEGF has been shown to be the most important pro-angiogenic factor involved in tumor growth (19, 20). Oxygenation and drug delivery might be more effective in tumors with high VEGF expression if VEGF was responsible for the hypervascularization of tumors. The destruction of tumor vessels, by anti-angiogenic agents, has been shown to increase tumor hypoxia, resulting in radioresistance. Indeed, some preclinical studies have shown that prolonged use of anti-VEGF agents has resulted in decreased MVD, and induced hypoxia, which would make radiation less effective (21, 22). Hironaka et al. (17) analyzed the prognostic factors including the clinical/pathological factors and biological markers in pretreatment biopsy specimens from 73 patients with ESCC treated with definitive CCRT. They reported that the patients with a high MVD had significantly better survival than those with low MVD tumors. They suggested that the MVD was a favorable prognostic factor in patients undergoing CCRT because oxygenation and drug delivery could be enhanced in richly vascularized tumors. The association between VEGF expression and MVD is currently an active focus of research. For esophageal cancer, some studies have found a significant correlation between VEGF expression and MVD (10, 23), whereas others have not (15, 17). This discrepancy might be explained by interobserver variation, the markers used, and the techniques; although the MVD has been widely used to quantify intratumor angiogenesis in human tumors (24). Hironaka et al. (17) assessed the MVD by counting intratumor and stromal vessels with the actual lumens around the tumor nests for consideration of tumor oxidation, instead of single endothelial cells and vessels far from the tumor nests. Although MVD was not evaluated in this study, the high VEGF expression might be correlated with high MVD of the tumor, which could enhance the supply of oxygen and radiosensitizing agents to cancer cells. Based on this postulation, we suggest that high VEGF expression could be associated with more improved response to CCRT. Additional study is needed to determine whether the tumor with higher VEGF expression and denser microvessel distribution could show better response to CCRT.
VEGF increases vessel permeability, which subsequently leads to the migration and organization of endothelial and tumor cells and promotes cancer cell invasion into the circulation (20). Therefore, most studies have shown that VEGF is associated with the aggressive characteristics of ESCC. VEGF expression has been significantly correlated with the presence of lymph node metastasis in patients with ESCC; VEGF expression was observed in 30%-49% of LN negative patients and 74%-87% of LN positive patients (9, 25, 26). Sato et al. showed that VEGF expression correlated with LN metastasis in patients with esophageal cancer; 49% in the negative node group and 74% in the positive node group (25). Shih et al. (15) also showed that the number of metastatic LNs was closely related to the expression of VEGF; the average number of metastatic LNs at surgery was 5.6 in the VEGF-positive cases and 3.0 in the VEGF-negative cases (P = 0.04). Some investigators have found that a high VEGF level was significantly associated with advanced stage disease and a high frequency of distant metastases (9, 23), as well as a poorer survival (14, 15). The results of this study showed that the OSR and CSSR of patients with higher VEGF expression were not significantly different from patients with lower VEGF expression. Although higher VEGF expression was related with better treatment response in this study, this phenomenon might be a temporal event which occurred at the time of or shortly after CCRT. It can be assumed that in the long run, the well-known nature of biological aggressiveness of VEGF expression would eventually undermine the prognosis of patient, despite initial good treatment response. Nevertheless, further prospective studies are needed with a larger population of patients to determine accurate survival outcomes. Some studies have reported no significant association between VEGF expression and clinical/pathological factors in patients with esophageal cancer (16, 17, 27). This study showed that a lower VEGF expression was significantly associated with the presence of lymph node metastasis, not with the primary lesion, resulting in a higher overall stage. Sato et al. classified the LN metastasis of esophageal cancer into two groups of patients that had LN dissection, early (cancer cells < 50% of lymph node) and late (≥ 50%) stage, according to the space occupying rates of the cancer cells (25). The VEGF-positive staining decreased from 60.7% in the early stage to 33.1% in the late stage, suggesting that the expression of VEGF changes during the temporal process of LN metastasis. Thus, the patients with high VEGF expression in this study might have actually had earlier stage of lymph node metastases or clinically undetectable nodal disease. Meanwhile it could not be excluded that there might be also a potential limitation in view of staging accuracy in this study. Around only one third of patients received endoscopic ultrasonography or 18F-fluorodeoxyglucose-positron emission tomography at the time of initial workup.
The multivariate analysis of 13 variables including the clinical/ pathological and EGFR, VEGF, and COX-2 factors showed that both the treatment response and COX-2 expression were significant prognostic factors for OS and only treatment response for CSS. Huang et al. (11) reported that the overexpression of COX-2 was significantly associated with a poor response to CCRT and a poor OS as in our study. The exact mechanism behind COX-2 influencing tumor sensitivity to CCRT remains unclear. However, apoptosis suppression might be one explanation. When there is a high level of COX-2 expression in ESCC, tumor cells may not be sensitive to the apoptosis signal, and abnormal proliferation would be the result (11). Gotoh et al. (7) reported that EGFR (+) was significantly correlated with a CR of the primary esophageal lesion to CCRT. However, EGFR did not show a significant correlation with the treatment response nor survival in this study.
In conclusion, unlike other well known studies, higher expression of VEGF was significantly correlated with the complete response to CCRT in this study, whereas higher COX-2 expression was correlated with poorer survival. Although further prospective studies are needed to determine the mechanism by which the VEGF expression status could influence the response to CCRT with a larger patients population, these results suggest that VEGF might be a predictive factor for treatment response and COX-2 a prognostic factor for OS in patients with ESCC after definitive CCRT.
Footnotes
This study was supported by the CRI 08029-1 grant from Chonnam National University Hospital Research Institute of Clinical Medicine.
AUTHOR SUMMARY
VEGF as a Predictor for Response to Definitive Chemoradiotherapy and COX-2 as a Prognosticator for Survival in Esophageal Squamous Cell Carcinoma
Mee Sun Yoon, Taek-Keun Nam, Ji-Shin Lee, Sang-Hee Cho, Ju-Young Song, Sung-Ja Ahn, Ik-Joo Chung, Jae-Uk Jeong, Woong-Ki Chung, and Byung-Sik Nah
The patients with esophageal squamous cell carcinoma can achieve good prognosis even without surgery if they have complete response after concurrent chemoradiotherapy. This study showed that higher expression of VEGF was significantly correlated with complete response after chemoradiotherapy. We postulated that a higher VEGF expression induces more vascularization of tumor tissues, and thereby improve the response to chemoradiotherapy. However, this result is different from the previous studies by other groups, which requires further prospective study. On the other hand, higher COX-2 expression is correlated with poorer prognosis, which is consistent with previous studies. As a whole, in the patients with esophageal squamous cell carcinoma after definitive chemoradiotherapy, the expression levels of VEGF and COX-2 might be used as a predictive factor for the treatment response and the prognostic factor for survival, respectively.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Araújo CM, Souhami L, Gil RA, Carvalho R, Garcia JA, Froimtchuk MJ, Pinto LH, Canary PC. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer. 1991;67:2258–2261. doi: 10.1002/1097-0142(19910501)67:9<2258::aid-cncr2820670908>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, Cooper J, Byhardt R, Davis L, Emami B. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277–284. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 4.Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, Klump B, Budach W, Teichmann R, Schmitt M, Schmitt G, Franke C, Wilke H. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 5.Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 6.Nam TK, Lee JH, Cho SH, Chung IJ, Ahn SJ, Song JY, Yoon MS, Chung WK, Nah BS. Low hMLH1 expression prior to definitive chemoradiotherapy predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Lett. 2008;260:109–117. doi: 10.1016/j.canlet.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh M, Takiuchi H, Kawabe S, Ohta S, Kii T, Kuwakado S, Katsu K. Epidermal growth factor receptor is a possible predictor of sensitivity to chemoradiotherapy in the primary lesion of esophageal squamous cell carcinoma. Jpn J Clin Oncol. 2007;37:652–657. doi: 10.1093/jjco/hym089. [DOI] [PubMed] [Google Scholar]
- 8.Gibault L, Metges JP, Conan-Charlet V, Lozac'h P, Robaszkiewicz M, Bessaguet C, Lagarde N, Volant A. Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer. 2005;93:107–115. doi: 10.1038/sj.bjc.6602625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida S, Shimada Y, Watanabe G, Tanaka H, Shibagaki I, Miyahara T, Ishigami S, Imamura M. In oesophageal squamous cell carcinoma vascular endothelial growth factor is associated with p53 mutation, advanced stage and poor prognosis. Br J Cancer. 1998;77:1704–1709. doi: 10.1038/bjc.1998.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogata Y, Fujita H, Yamana H, Sueyoshi S, Shirouzu K. Expression of vascular endothelial growth factor as a prognostic factor in node-positive squamous cell carcinoma in the thoracic esophagus: long-term follow-up study. World J Surg. 2003;27:584–589. doi: 10.1007/s00268-003-6866-2. [DOI] [PubMed] [Google Scholar]
- 11.Huang WZ, Fu JH, Wang DK, Hu Y, Liu MZ, Yang H, Feng YF, Zheng B, Wang G, Luo KJ, Wen J, Rong TH. Overexpression of cyclooxygenase-2 is associated with chemoradiotherapy resistance and prognosis in esophageal squamous cell carcinoma patients. Dis Esophagus. 2008;21:679–684. doi: 10.1111/j.1442-2050.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 12.American Joint Committee on Cancer. Manual for staging of cancer. 7th ed. Philadelphia: Lippincott; 2010. [Google Scholar]
- 13.Ali-Fehmi R, Che M, Khalifeh I, Malone JM, Morris R, Lawrence WD, Munkarah AR. The effect of cyclooxygenase-2 expression on tumor vascularity in advanced stage ovarian serous carcinoma. Cancer. 2003;98:1423–1429. doi: 10.1002/cncr.11650. [DOI] [PubMed] [Google Scholar]
- 14.Shimada H, Hoshino T, Okazumi S, Matsubara H, Funami Y, Nabeya Y, Hayashi H, Takeda A, Shiratori T, Uno T, Ito H, Ochiai T. Expression of angiogenic factors predicts response to chemoradiotherapy and prognosis of oesophageal squamous cell carcinoma. Br J Cancer. 2002;86:552–557. doi: 10.1038/sj.bjc.6600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih CH, Ozawa S, Ando N, Ueda M, Kitajima M. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2000;6:1161–1168. [PubMed] [Google Scholar]
- 16.Rosa AR, Schirmer CC, Gurski RR, Meurer L, Edelweiss MI, Kruel CD. Prognostic value of p53 protein expression and vascular endothelial growth factor expression in resected squamous cell carcinoma of the esophagus. Dis Esophagus. 2003;16:112–118. doi: 10.1046/j.1442-2050.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 17.Hironaka S, Hasebe T, Kamijo T, Ohtsu A, Boku N, Yoshida S, Saitoh H, Ochiai A. Biopsy specimen microvessel density is a useful prognostic marker in patients with T(2-4)M(0) esophageal cancer treated with chemoradiotherapy. Clin Cancer Res. 2002;8:124–130. [PubMed] [Google Scholar]
- 18.Kii T, Takiuchi H, Kawabe S, Gotoh M, Ohta S, Tanaka T, Kuwakado S, Nishitani H, Katsu K. Evaluation of prognostic factors of esophageal squamous cell carcinoma (stage II-III) after concurrent chemoradiotherapy using biopsy specimens. Jpn J Clin Oncol. 2007;37:583–589. doi: 10.1093/jjco/hym077. [DOI] [PubMed] [Google Scholar]
- 19.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 20.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 21.Murata R, Nishimura Y, Hiraoka M. An antiangiogenic agent (TNP-470) inhibited reoxygenation during fractionated radiotherapy of murine mammary carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:1107–1113. doi: 10.1016/s0360-3016(96)00628-1. [DOI] [PubMed] [Google Scholar]
- 22.Ou G, Itasaka S, Zeng L, Shibuya K, Yi J, Harada H, Hiraoka M. Usefulness of HIF-1 imaging for determining optimal timing of combining bevacizumab and radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:463–467. doi: 10.1016/j.ijrobp.2009.02.083. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997;79:206–213. doi: 10.1002/(sici)1097-0142(19970115)79:2<206::aid-cncr2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Nico B, Benagiano V, Mangieri D, Maruotti N, Vacca A, Ribatti D. Evaluation of microvascular density in tumors: pro and contra. Histol Histopathol. 2008;23:601–607. doi: 10.14670/HH-23.601. [DOI] [PubMed] [Google Scholar]
- 25.Sato F, Shimada Y, Watanabe G, Uchida S, Makino T, Imamura M. Expression of vascular endothelial growth factor, matrix metalloproteinase-9 and E-cadherin in the process of lymph node metastasis in oesophageal cancer. Br J Cancer. 1999;80:1366–1372. doi: 10.1038/sj.bjc.6690530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee T, Kumar A, Mathur M, Chattopadhyay TK, Ralhan R. Ets-1 and VEGF expression correlates with tumor angiogenesis, lymph node metastasis, and patient survival in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2003;129:430–436. doi: 10.1007/s00432-003-0457-3. [DOI] [PubMed] [Google Scholar]
- 27.Ahn MJ, Jang SJ, Park YW, Choi JH, Oh HS, Lee CB, Paik HK, Park CK. Clinical prognostic values of vascular endothelial growth factor, microvessel density, and p53 expression in esophageal carcinomas. J Korean Med Sci. 2002;17:201–207. doi: 10.3346/jkms.2002.17.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]