Abstract
This study was to assess the relation of thyroid dysfunction to metabolic syndrome (MetS) at an earlier stage in Korean population. Metabolic parameters such as body composition, blood pressure (BP), fasting glucose, total cholesterol, triglyceride (TG), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), thyroid-stimulating hormone (TSH) and free thyroxine 4 (fT4) were measured. During a mean follow-up of 3 yr, 5,998 Koreans ages over 18 yr were assessed. There were 694 cases of MetS at follow-up. The mean age of the subjects was 45.6 ± 9.5 yr. Mean level of TSH was 2.02 ± 1.50 mIU/L, mean level of fT4 was 1.23 ± 0.20 ρM/L. At baseline, TSH levels and fT4 levels were associated to waist circumference, BP, glucose and lipids in the subjects. Increase in systolic blood pressure, diastolic blood pressure (DBP), total cholesterol and TG were significantly associated with changes in TSH levels after adjustment. Changes in DBP, TG, HDL-C and fasting glucose were significantly associated with changes in fT4 levels after adjustment. Increase in TSH levels even after further controlling for baseline TSH level predicted the MetS over the study period. In conclusion, there is a relationship between thyroid function and cardiovascular risk factors, such as BP, total cholesterol, TG, HDL-C and fasting glucose. Also, higher levels of TSH may predict the MetS in Korean.
Keywords: Thyroid Function, Cardiovascular Risk Factors, Metabolic Syndrome, Insulin Resistance
INTRODUCTION
Hypothalamic corticotrophin-releasing hormone (CRH) plays an important role in inhibiting gonadotropin-releasing hormone secretion during stress, while via somatostatin it also inhibits growth hormone, thyrotropin-releasing hormone and thyrotropin secretion, suppressing thus reproduction, growth and thyroid function (1). Excessive and sustained cortisol secretion or chronic administration of pharmacologic doses of glucocorticoids have been long associated with depression, hypertension, osteoporosis, immune suppression and the entire spectrum of the metabolic syndrome (MetS), including visceral obesity, insulin resistance, dyslipidemia, dyscoagulation and hypertension, along with their morbid sequelae of atherosclerosis and cardiovascular disease (CVD) (2-4). Each of these manifestations could in theory be produced, despite the presence of normal, nonhyperfunctioning HPA (Hypothalmo-pituitary-adrenal) axis, by tissue-specific hypersensitivity to glucocorticoids of, the amygdala or mesocorticolimbic system, cardiovascular system, bone, immune system or adipose tissue (5).
Thyroid disease is associated with atherosclerotic CVD (6-8). However, there is controversy as to whether this association is also present in subclinical hypothyroidism (7, 9, 10). It is known that over hypothyroidism leads to an increase in plasma cholesterol levels and blood pressure (BP) (9, 10). Most studies in subclinical hypothyroidism show comparable but less pronounced associations (11, 12). Most subjects at risk for CVD are euthyroid. Moreover, little is known about the relationship between insulin resistance and thyroid function. In a cross-sectional study in 47 healthy euthyroid subjects, it was found that insulin resistance modified the relationship between levels of thyroid-stimulating hormone (TSH) and low-density lipoprotein cholesterol (LDL-C) (13). A similar study in 117 diabetic patients revealed a comparable interaction between thyroid function and insulin sensitivity in contributing to diabetic dyslipidemia (14). Also, a recent study showed that low normal free thyroxine 4 (fT4) levels were significantly associated with hyperlipidemia and increased insulin resistance. These findings are consistent with an increased cardiovascular risk in subjects with low normal thyroid function (15). However, a longitudinal study has not yet been performed to assess a possible role for thyroid dysfunction in terms of metabolic abnormality. We aimed to investigate the relationships between thyroid function and MetS components and whether thyroid function predicts the MetS in a large sample of Korean population with normal thyroid function as a prospective cohort study.
MATERIALS AND METHODS
Study population
We enrolled 7,757 Koreans ages over 18 yr who had undergone medical evaluation two times or more at Ajou University Hospital between May 2002 and February 2009.
To avoid confounding of the association between thyroid function and the risk of preexisting MetS, 1,271 subjects with MetS and 35 patients with hypothyroidism or hyperthyroidism during the enrollment period were excluded. In addition, 453 subjects with history of thyroid disease, cancer or antithyroid treatment were excluded. The final sample included 5,998 subjects.
Data collection
The sociodemographic characteristics of subjects were assessed by questionnaire. Life style factors such as smoking status, alcohol intake, and exercise were assessed as well. Height and weight were measured using bioelectrical impedance analysis (Inbody 3.0, Biospace, Seoul, Korea) following overnight fast. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2) (16). Waist circumference (WC) was measured at middle part between the lower rib and iliac crest by a trained nurse. Blood pressure was measured electrically (TM-2650A, PMS Instruments, Tokyo, Japan) after a rest of at least 15 min.
Venous blood was drawn following an 8-hr overnight fast and 24-hr abstinence from vigorous activity. Standard enzymatic measurements of total cholesterol, triglycerides, high-density lipoprotein cholesterol, and fasting glucose were made on fresh serum samples by oxidase-peroxidase enzymatic assay (TBA-200FR, Toshiba, Tokyo, Japan). TSH and fT4 were measured using a radioimmunoassay. The reference ranges were 0.25-5.0 mIU/L for TSH and 0.7-1.8 ρM/L for fT4.
Outcome definition and follow-up procedures
We defined thyroid function status of the subjects by level of thyroid hormones so we divided the cohort into hypothyroidism (fT4 < 0.7 ρM/L), subclinical hypothyroidism (TSH > 5.0 mIU/L and normal fT4 level), subclinical hyperthyroidism (TSH < 0.25 mIU/L and normal fT4 level), hyperthyroidism (fT4 > 1.8 ρM/L), and euthyroid state. We followed the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) guideline component to define MetS, but the WC cutoff point for central obesity in Koreans, which was 90 cm for men and 85 cm for women (17). The last follow-up was conducted on 28 February 2009, and the mean follow-up period was 2.96 yr.
Statistical analysis
We used multiple linear regression analyses to assess the relationship between cardiovascular risk factors and thyroid function at baseline, adjusting for age, gender, BMI, smoking status, alcohol intake and exercise habits. Linear regression analyses were also used to assess effects of cardiovascular risk factors as predictors of changes in thyroid function, controlling for age, gender, BMI, smoking status, alcohol intake, exercise habits, baseline level of thyroid function and the baseline level of each metabolic factors. Logistic regression analyses were used to evaluate associations of changes in thyroid function status to MetS at follow up, while controlling for age, gender, BMI, smoking status, alcohol intake and exercise habits. In all analyses, a two-sided α level of 0.05 was considered statistically significant. All analyses were conducted with the use of SPSS software (SPSS Inc., Chicago, IL, USA), in version 12.0.
Ethics statement
The institutional review boards of Ajou University approved the study (AJIRB-MED-MDB-10-022). In the IRB approval, informed consent from the survey participants was waived.
RESULTS
Demographic characteristics
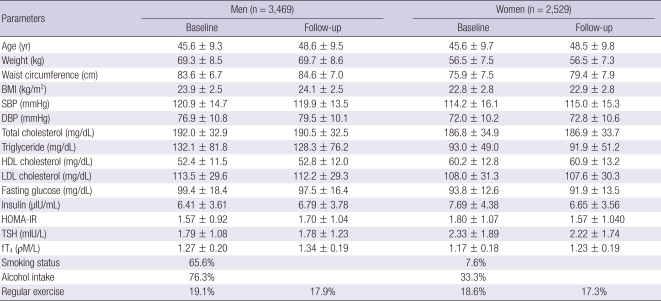
Demographic characteristics are presented in Table 1. The total of 5,998 individuals included 57.8% men (n = 3,469) and 42.2% women (n = 2,529). The mean age of the subjects was 45.6 ± 9.5 yr. The mean level of TSH was 2.02 ± 1.50 mIU/L, the mean level of fT4 was 1.23 ± 0.20 ρM/L. Men, in comparison to women, had higher smoking and alcohol drinking rates. Over 3 yr of follow-up, there were 694 incident cases (11.6%) of MetS (507 in men, 187 in women).
Table 1.
General characteristics of the study population at baseline
Values are mean ± SD unless otherwise indicated. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HG, hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance; TSH, thyroid stimulating hormone; fT4, free thyroxine 4.
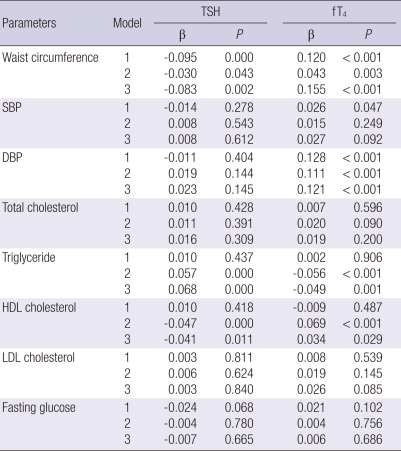
Correlations between TSH levels, fT4 levels and cardiovascular risk factors at baseline
TSH levels were negatively associated to WC, the association that remained significant after adjustment for age, gender, BMI, smoking status, alcohol intake, and exercise, while TSH levels were positively associated to TG after controlling for age, gender, and even after further adjustment. There was a significant correlation between HDL-C and TSH levels after adjustment.
fT4 levels were positively associated to WC and diastolic blood pressure (DBP), the association that remained significant after adjustment for age, gender, BMI, smoking status, alcohol intake, and exercise. fT4 levels were also positively associated to systolic blood pressure (SBP), but this association was reduced to non-significance after adjustment. fT4 levels were negatively associated to TG after adjustment, while HDL-C was positively associated with fT4 levels after adjustment. There were no associations between thyroid function and total cholesterol, LDL-cholesterol and fasting glucose with or without adjustment (Table 2).
Table 2.
Associations of thyroid function with serum lipid concentrations and the components of the metabolic syndrome at baseline
Values of β are standardized regression coefficients: model 1, crude; model 2, after adjustment for age and gender; model 3, further adjustment for BMI, smoking status, alcohol intake and exercise; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein.
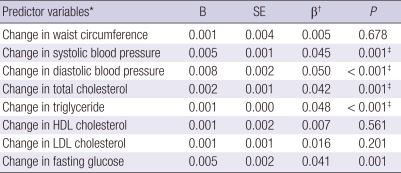
Changes in cardiovascular risk factors in the prediction of changes in thyroid function
To assess the associations between cardiovascular risk factors and thyroid function, linear regression models were conducted, controlling for age, gender, the baseline level of thyroid function and the baseline level of each metabolic factors. As Table 3 shows, higher SBP, DBP, total cholesterol, TG, and fasting glucose were significantly associated with changes in TSH levels. Further adjustments for BMI, smoking status, alcohol intake and exercise habits showed that changes in SBP, DBP, total cholesterol and triglyceride were still positively associated with changes in TSH levels.
Table 3.
Summary of results from 8 standardized multiple linear regression coefficients predicting change in TSH levels
*Computed as difference between baseline and follow-up levels. Results are based on 5,998 individuals with complete data; †Adjusted for age, gender, baseline TSH level, and the baseline level of each metabolic factors; ‡P < 0.01 controlling for age, gender, BMI, baseline TSH level, the baseline level of each metabolic factors, smoking status, alcohol intake and exercise. B, unstandardized regression coefficients; SE, standard error.
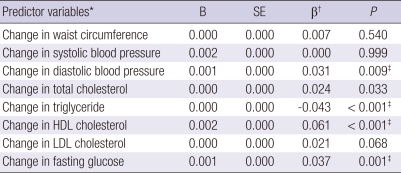
As seen Table 4, higher DBP, total cholesterol, HDL-C, LDL-C and fasting glucose, and lower triglyceride were significantly associated with changes in fT4 levels. Further adjustments for BMI, smoking status, alcohol intake and exercise habits showed that changes in DBP, triglyceride, HDL-C and fasting glucose were still associated with changes in fT4 levels.
Table 4.
Summary of results from 8 standardized multiple linear regression coefficients predicting change in fT4 levels
*Computed as difference between baseline and follow-up levels. Results are based on 5,998 individuals with complete data; †Adjusted for age, gender, baseline fT4 level, and the baseline level of each metabolic factors; ‡P < 0.01 controlling for age, gender, BMI, baseline fT4 level, the baseline level of each metabolic factors, smoking status, alcohol intake and exercise. B, unstandardized regression coefficients; SE, standard error.
Correlations between thyroid function, insulin and HOMA
Insulin and homeostatis model assessment of insulin resistance (HOMA-IR) measures were only available in a subpopulation of the study participants (n = 1,173). The mean insulin level of the subjects was 6.4 ± 3.6 µIU/mL. At baseline, the associations of TSH and fT4 to insulin and HOMA were not significant, adjusting for age, gender and BMI.
Over a 3 yr follow-up, higher levels of TSH was significantly associated with changes in insulin levels and HOMA-IR after controlling for age, gender, BMI, the baseline TSH level and the each baseline variable factors, smoking status, alcohol intake and exercise (β = 0.166, P = 0.008, β = 0.164, P = 0.008, respectively). Lower levels of fT4 was significantly associated with changes in HOMA-IR after controlling for age, gender, BMI, the baseline fT4 level and the baseline HOMA-IR (β = -0.143, P = 0.016). Further adjustments for smoking status, alcohol intake and exercise reduced the association of fT4 to HOMA-IR non-significant. There was no relation of change in fT4 levels to change in insulin levels with or without adjustment (n = 326).
Associations of changes in thyroid function to the metabolic syndrome
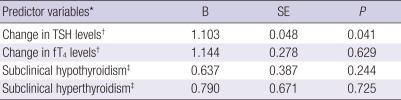
The associations of changes in thyroid function to the MetS were examined in separate logistic regression models that controlled for age, gender, BMI, smoking status, alcohol intake, exercise, and baseline level of each thyroid function (Table 5). The MetS was significantly associated with increase in TSH levels over the study period after adjustment. fT4 levels, and individuals with subclinical hypothyroidism (n = 158), subclinical hyperthyroidism (n = 49), or overt thyroid dysfunction (n = 38) did not predict the MetS after adjustment.
Table 5.
Summary of results for independent logistic regression analyzes of different metabolic markers predicting metabolic syndrome
*controlled for age, gender, BMI, smoking status, alcohol intake and exercise; †further adjustment for baseline level of each thyroid function; ‡compared to euthyroid state.
DISCUSSION
In the current study, we found a significant relationship between levels of thyroid hormones and cardiovascular risk factors as repoted in previous cross-sectional studies (9, 15), as well as over the follow-up period. Our results also indicate that higher levels of TSH may predict the MetS in this study subjects, suggesting that the influence of thyroid function on metabolic abnormality extends into subjects without MetS. These findings might be implicated that subjects with low or high normal thyroid function are already at increased cardiovascular risk. Bakker et al. (13) found that TSH seemed to affect this important cardiovascular risk factor especially in subjects who are already at risk of developing cardiovascular disease because of their insulin-resistant state. They showed that a regression model consistent with a virtual absence of an association between TSH with LDL-C in very insulin-sensitive subjects, whereas in insulin-resistance subjects a steep association exits. A recent cross-sectional study also reported that a close relationship between TSH and MetS in euthyroid postmenopausal women (18).
Thyroid hormones play an essential role in a variety of metabolic and developmental processes in human body. Most effects are mediated via mechanisms that stimulate resting metabolic rate, increase ATP expenditure, and modulate adrenergic receptor number and thus responsiveness to catecholamines. Thyroid hormones also influence carbohydrate metabolism in skeletal muscle and adipose tissue via the positive transcriptional regulation of the muscle/fat-specific GLUT4, and stimulate lipolysis. All these steps interact with insulin action (19). Otherwise, the pathophysiological process behind the influence of thyroid function on lipid metabolism is known from subjects with overt thyroid dysfunction. The elevation of TGs in hypothyroidism is caused by a reduced removal rate of TG from plasma due to a decrease in the activity of hepatic TG lipase (20, 21). In addition, studies investigating thyroid hormone receptors in obese individuals demonstrated a decrease in thyroid hormone receptor density (22, 23). Over 3 yr of follow-up, our results showed the associations of thyroid hormones to cardiovascular factors. Similarly, several important cardiovascular risk factors have been identified in patients with mild and subclinical hypothyroidism: diastolic dysfunction (24), increased arterial stiffness (25), endothelial dysfunction (26), and increase in systemic vascular resistance (27).
Unfortunately, insulin resistance was only available in a subsample of the participants. In this group, we found higher levels of TSH to be associated with increased insulin resistance, an association that remained after adjustment for the confounding factors. On contrary, lower levels of fT4 tended to be associated with insulin resistance after adjustment. According to a experimental study, a mutation in the α-isoform of the thyroid hormone receptor has been recently described to lead to thyroid hormone resistance, low heart rate, and insulin resistance in an animal model (28).
Our results indicate that the MetS was significantly associated with increase in TSH levels after adjustment over time, even though the study individuals at baseline were relatively healthy without thyroid disease and MetS. However, fT4 levels, and individuals with subclinical hypothyroidism or subclinical hyperthyroidism did not predict the MetS after adjustment in the study. Previous epidemiological studies in which cardiovascular morbidity and mortality have been evaluated in patients with mild thyroid hormone deficiency have yielded conflicting data (7, 29, 30). These results may be due to differences in the study populations in terms of age, sex, race, definition of mild hypothyroidism, and differences in the duration of follow-up. The weak correlations between thyroid function and metabolic markers in the present study probably reflect the extensive metabolic screening of our subjects to exclude individuals with MetS or thyroid disease. Although we did not evaluate status of insulin resistance intended for in whole subjects, our results suggest that impairment of thyroid function is metabolically fluctuant if insulin resistance in body might not be progressed but still associated with cardiovascular risk factors.
The limitation of this study was that the study population consisted of patients who visited a University Hospital. Therefore, the prevalence of MetS in our data does not reflect that in general population. However, we performed the current study with a relatively large number of subjects and also followed up the individuals over the study period. Another point of consideration is that we did not examine insulin resistance of the subjects using standard method like hyperinsulinemic euglycemic clamp even in subgroup analysis. But we excluded subjects with MetS as well as thyroid disease during the enrollment period. Further larger randomized trials are necessary to evaluate the potential benefit for the management of thyroid dysfunction at an ealier stage.
In conclusion, the results of the present study show that there is a relationship between thyroid function and cardiovascular risk factors, such as BP, total cholesterol, TG, HDL-C and fasting glucose. Also, higher levels of TSH may predict the MetS in Korean.
ACKNOWLEDGMENTS
We would like to thank Miss Ann Wilson for administrative assistance and Mrs. Ju Hee Song for helping with the data collection.
AUTHOR SUMMARY
The Relation of Thyroid Function to Components of the Metabolic Syndrome in Korean Men and Women
Sat Byul Park, Ho Chun Choi, and Nam Seok Joo
This study was to assess the relation of thyroid dysfunction to metabolic syndrome at an earlier stage in Korean population (mean follow-up of 3 yr, 5,998 Koreans, age > 18). There were 694 cases of metabolic syndrome at follow-up. Increase in blood pressure and cholesterol were associated with TSH levels. Also, some parameters of blood pressure and cholesterol were associated with thyroid hormone (fT4) levels. As a whole, there is a relationship between thyroid function and cardiovascular risk factors. Higher levels of TSH might predict the metabolic syndrome in Korean.
References
- 1.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 2.Tsigos C, Chrousos GP. Differential diagnosis and management of Cushing's syndrome. Annu Rev Med. 1996;47:443–461. doi: 10.1146/annurev.med.47.1.443. [DOI] [PubMed] [Google Scholar]
- 3.Friedman TC, Mastorakos G, Newman TD, Mullen NM, Horton EG, Costello R, Papadopoulos NM, Chrousos GP. Carbohydrate and lipid metabolism in endogenous hypercortisolism: shared features with metabolic syndrome X and NIDDM. Endocr J. 1996;43:645–655. doi: 10.1507/endocrj.43.645. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP. The hypothalamic - pituitary - adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 5.Chrousos GP, Detera-Wadleigh S, Karl M. Syndromes of glucocorticoid resistance. Ann Intern Med. 1993;119:1113–1124. doi: 10.7326/0003-4819-119-11-199312010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 7.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132:270–278. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 9.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 10.Fommei E, Iervasi G. The role of thyroid hormone in blood pressure homeostasis: evidence from short-term hypothyroidism in humans. J Clin Endocrinol Metab. 2002;87:1996–2000. doi: 10.1210/jcem.87.5.8464. [DOI] [PubMed] [Google Scholar]
- 11.Biondi B, Klein I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine. 2004;24:1–13. doi: 10.1385/ENDO:24:1:001. [DOI] [PubMed] [Google Scholar]
- 12.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 13.Bakker SJ, ter Maaten JC, Popp-Snijders C, Slaets JP, Heine RJ, Gans RO. The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J Clin Endocrinol Metab. 2001;86:1206–1211. doi: 10.1210/jcem.86.3.7324. [DOI] [PubMed] [Google Scholar]
- 14.Chubb SA, Davis WA, Davis TM. Interactions among thyroid function, insulin sensitivity, and serum lipid concentrations: the Fremantle diabetes study. J Clin Endocrinol Metab. 2005;90:5317–5320. doi: 10.1210/jc.2005-0298. [DOI] [PubMed] [Google Scholar]
- 15.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health. Clinical Guidelines on the Identification, evaluation, and treatment of overweight and obesity in adults - the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 17.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Park HT, Cho GJ, Ahn KH, Shin JH, Hong SC, Kim T, Hur JY, Kim YT, Lee KW, Kim SH. Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women. Maturitas. 2009;62:301–305. doi: 10.1016/j.maturitas.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Jameson JL. Mechanisms of thyroid hormone action. In: DeGroot LJ, Jameson JL, editors. Endocrinology. 4th ed. Vol 2. Philadelphia: WB Saunders; 2001. pp. 1327–1344. [Google Scholar]
- 20.Tulloch BR, Lewis B, Fraser TR. Triglyceride metabolism in thyroid disease. Lancet. 1973;1:391–394. doi: 10.1016/s0140-6736(73)90250-x. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Takamatsu J, Matsuo T, Kameoka K, Kubota S, Fukata S, Tamai H, Miyauchi A, Kuma K, Hanafusa T. Serum concentrations of remnant-like particles in hypothyroid patients before and after thyroxine replacement. Clin Endocrinol (Oxf) 2003;58:621–626. doi: 10.1046/j.1365-2265.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore R, Mehrishi JN, Howard AN, Mills IH. Lymphocyte thyroid hormone receptors in obesity. Int J Obes. 1982;6:541–548. [PubMed] [Google Scholar]
- 23.Burman KD, Latham KR, Djuh YY, Smallridge RC, Tseng YC, Lukes YG, Maunder R, Wartofsky L. Solubilized nuclear thyroid hormone receptors in circulating human mononuclear cells. J Clin Endocrinol Metab. 1980;51:106–116. doi: 10.1210/jcem-51-1-106. [DOI] [PubMed] [Google Scholar]
- 24.Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid. 2007;17:625–630. doi: 10.1089/thy.2007.0158. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71:1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 26.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–1508. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 27.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 28.Liu YY, Schultz JJ, Brent GA. A thyroid hormone receptor alpha gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem. 2003;278:38913–38920. doi: 10.1074/jbc.M306120200. [DOI] [PubMed] [Google Scholar]
- 29.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Rodgers H, Tunbridge F, Young ET. The development of ischemic heart disease in relation to autoimmune thyroid disease in a 20-year follow-up study of an English community. Thyroid. 1996;6:155–160. doi: 10.1089/thy.1996.6.155. [DOI] [PubMed] [Google Scholar]
- 30.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]