Abstract
The authors investigated objective response rate to high dose methotrexate (HDMTX)-based combination chemotherapy in primary central nervous system lymphoma (PCNSL), and sought to identify factors that influence response to HDMTX-based combination therapy. Prospective observational analysis was performed on 52 PCNSL patients. All patients received HDMTX (3.5 g/m2) and vincristine (1.4 mg/m2/day) for one day during weeks 1, 3, 5, 7, and 9, and procarbazine (100 mg/m2/day) for one week during weeks 1, 5, and 9. Forty-one patients (78.8%) achieved complete or partial remission. Higher objective response rates were observed for patients with: 1) age < 60 yr; 2) Eastern Cooperative Oncology Group (ECOG) performance score of < 2; 3) low risk status as defined by the International Extranodal Lymphoma Study Group; 4) p53 positivity; 5) XBP-1 negativity; 6) MUM-1 negativity; and 7) homogenous gadolinium enhancement in MR images. Multivariate analysis showed that ECOG performance score of < 2, low risk, negativity for XBP-1, homogenous gadolinium enhancement by MRI, and response to chemotherapy were associated with longer overall survival. In particular, it is interesting to note that patients with a PCNSL that is homogenously enhanced by gadolinium have a higher objective response rate, and a longer progression-free survival and overall survival.
Keywords: Central Nervous System, Lymphoma, Response, Prognosis, Survival, Methotrexate, Chemotherapy
INTRODUCTION
Primary central nervous system lymphomas (PCNSLs) are extranodal malignant lymphomas, which arise within the brain, eyes, leptomeninges, or spinal cord in the absence of systemic lymphoma at the time of diagnosis. PCNSL accounts for fewer than 2% of brain tumors; however, its incidence has increased steadily over the last two decades, particularly in immunocompetent individuals (1). Today, PCNSL represents 1.8% of all primary brain tumors in Korea (1).
PCNSL is a highly aggressive tumor with a poor prognosis in untreated patients (2); however, in contrast to most primary brain tumors, PCNSL is sensitive to corticosteroids, chemotherapy, and radiotherapy, and durable complete responses and long-term survival seem possible with these treatments. However, the outcome of PCNSL is substantially poorer than that of similar stage systemic non-Hodgkin's lymphoma. The combinatorial chemotherapeutic regimen of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) provides the best treatment for comparable systemic non-Hodgkin's lymphoma; however, three large, independent, multicenter trials on CHOP with cranial irradiation in PCNSL have concluded that they offered no survival advantage over radiotherapy alone (3, 4). The apparent inability of these chemotherapeutic regimens to cross the brain-blood barrier (BBB) and eradicate microscopic disease is a major difficulty.
Radiotherapy alone for PCNSL provides a median survival of only 12 to 18 months (5); however, addition of high dose (HD) (> 1 g/m2) methotrexate (MTX)-based chemotherapy to radiotherapy has been reported to result in substantial improvement of median survival time (6-8).
Present therapeutic knowledge of PCNSL is the result of several single-group phase 2 trials, meta-analyses, and large retrospective studies. However, to date, only one randomized trial has been undertaken, and this was halted prematurely due to unsatisfactorily slow accrual (3). The rarity of PCNSL prevents randomized trials, and the different opinions expressed on many of the therapeutic aspects have resulted in a lack of consensus with regard to overall strategy; therefore, the main endpoints should be investigated in a randomized setting. In addition, assessments of new first-line chemotherapy combinations in non-randomized trials, with divergent study designs and entry criteria, do not allow proper comparisons to be made between different regimens, and have resulted in only modest therapeutic progress (9, 10).
Despite limited evidence of the efficacies of specific regimens, HDMTX chemotherapy followed by whole brain radiotherapy (WBRT) is the approach most commonly used for newly diagnosed PCNSL (10, 11), and has been reported to result in a 5-yr survival of 20%-35% (6, 8, 12-15). MTX is a folate antagonist that interrupts DNA synthesis, and is the most widely studied drug in the context of PCNSL.
Following reports of a better outcome for HDMTX-based combination chemotherapy for PCNSL by the Radiation Therapy Oncology Group (RTOG) in the first multicenter trial (8), we started HD (3.5 g/m2/day) MTX-based combination chemotherapy with vincristine and procarbazine. Although many studies have been conducted for identification of the prognostic factors of PCNSL, no comprehensive studies have been undertaken to identify factors that influence response to HDMTX-based combination chemotherapy. In the present prospective observational study, we investigated objective response rates (ORR) to HDMTX-based combination therapy with vincristine and procarbazine chemotherapy and progression-free survival (PFS) and overall survival (OS) rates after a follow-up period of 2 yr. We then sought to identify factors that influence ORR after HDMTX-based combination chemotherapy for PCNSL.
MATERIALS AND METHODS
Patients
Newly diagnosed immunocompetent patients with PCNSL who were registered at our institute from March 2003 to August 2008 were evaluated for participation in the study. Sixty-one patients were diagnosed as CNS lymphoma with histological confirmation. Of these, five refused treatment at our institute, and another four did not fulfill the inclusion criteria; 2 had creatinine clearance below 60, 1 had a lymphoma in the chest, and 1 had severe thrombocytopenia, which originated from liver cirrhosis. Therefore, clinical data for 52 patients were available, and these patients constituted the study cohort. All patients had one or more intracranial mass lesions. Histological confirmation of PCNSL was required by the following: brain biopsy or resection, CSF analysis demonstrating lymphoma cells, or vitrectomy documenting concomitant ocular lymphoma. To exclude evidence of systemic lymphoma, a negative staging evaluation by chest, abdominal, and pelvic computed tomography (CT) and by bone marrow biopsy was required. All patients underwent cranial neuroimaging at diagnosis, preferably by MRI. All patients underwent lumbar puncture and a complete ophthalmologic evaluation including a slit-lamp examination.
Patients with newly diagnosed PCNSL were registered within 4 weeks of receiving a histological diagnosis and were considered eligible for enrollment if a pathologic diagnosis had been established and all of the following criteria were met: 1) age over 18 yr; 2) Eastern Cooperative Oncology Group (ECOG) performance status grade ≤ 3; 3) negative HIV serology; 4) creatinine clearance ≥ 60; 5) no evidence of lymphoma elsewhere in the body after CT scans of the chest, abdomen, and pelvis; 6) an absolute neutrophil count of ≥ 2,000 cells/µL and a platelet count of ≥ 100,000 cells/µL; and 7) a bilirubin of ≤ 2.0 mg% and a SGOT of ≤ 2 times the upper normal limit. The ECOG definition of performance status was used throughout (16). Before chemotherapy, laboratory diagnosis of Epstein-Barr virus (EBV) infection was made on a single serum sample using a standard immunofluorescence test for antibodies to EBV-associated antigens simultaneously; immunoglobulin M and G to the viral capsid antigen, the early antigen, and the EBV nuclear antigen.
Treatment protocol
Our protocol was modified by the RTOG 93-10 protocol (8). Treatment consisted of five cycles of chemotherapy administered at fortnight intervals within 5 days intercycle delay, consisting of the following: methotrexate 3.5 g/m2/day for one day during weeks 1, 3, 5, 7, and 9, followed by leucovorin rescue, vincristine (1.4 mg/m2/day with a cap at 2.0 mg/day) for one day during weeks 1, 3, 5, 7, and 9, and procarbazine (100 mg/m2/day) for one week during weeks 1, 5, and 9. Initially, all of the patients started to receive intravenous methotrexate at the dose of 3.5 g/m2/day. During the cycle of chemotherapy, the dose of methotrexate was altered to 3.0 g/m2/day or 2.5 g/m2/day when creatine clearance was between 50 and 60, or between 40 and 50, respectively. And if creatine clearance fell below 40, we halted the schedule and delayed for as long as 2-3 weeks until recovery of creatine clearance. Methotrexate (12 mg) was injected into an Ommaya reservoir for five cycles on one day during the week following each intravenous methotrexate dose. Dexamethasone was initiated at a dose of 16 mg/day from the first cycle of HDMTX and tapered by 4 mg/day each week, providing that the patient's neurological status remained stable.
Follow-up
The mean follow-up period was 32.1 months. Repeat neuroimaging was required at the completion of neoadjuvant chemotherapy and radiotherapy; and then every 2 months for one year, every 4 months for the second year, and every 6 months thereafter. A comprehensive review of chemotherapy records was performed for assessment of adherence to the protocol, and a radiologic review was undertaken for assessment of response after neoadjuvant chemotherapy by two neuroradiologists. MRI was performed using a 1.5T clinical scanner (Signa Horizon; GE Healthcare, Milwaukee, WI, USA). Basal ganglia, the corpus callosum, brain stem, and cerebellum were defined as deep brain structures. Peritumoral edema was categorized as < or ≥ 2 cm from the brain tumor as assessed in T2-weighted MR images. In cases of multiple brain lesions, regardless of the number of lesions, different patterns of enhancement between them in MRI were counted as heterogeneous enhancement.
Evaluation of response to treatment
Response to treatment was assessed by contrast enhanced brain MRI, which was performed at most 7 days before commencement of chemotherapy, after the second and fifth HDMTX chemotherapy cycles, and 4 weeks after termination of chemotherapy. National Cancer Institute standardized response criteria regarding changes in the size of enhanced lesions on T1 weighted MR images were used to define treatment response (17). In brief, complete remission (CR) was defined as the complete disappearance of all evidence of lymphoma; partial response (PR) as ≥ 50% decrease in tumor size; progressive disease (PD) as a ≥ 25% increase in tumor size or the appearance of any new lesion; and stable disease (SD) as situations that did not meet any of these three previous criteria.
Risk group as defined by the International Extranodal Lymphoma Study Group (IELSG)
Using the prognostic scoring system devised by the IELSG, we categorized patients into three risk groups, namely, low, intermediate, and high risk groups (18). Variables found to be independently associated with survival in the present study were used to design a scoring system, as follows. These variables included age (≤ or > 60 yr), ECOG performance status grade (0 to 1 vs 2 to 3), LDH serum level (normal vs elevated), protein concentration in CSF (normal vs elevated), and involvement of deep brain structures (yes vs no). Each variable was assigned a value of 0 if favorable or 1 if unfavorable. Values of these five variables were summed for calculation of final risk scores. Patients with final risk scores of 0 or 1, 2 or 3, or 4 or 5 were assigned to the low, intermediate, or high risk groups, respectively.
Immunohistochemistry and immunofluorescence
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissues. In addition, MIB-1 (Ki-67) staining was performed for evaluation of the proliferative activities of large neoplastic lymphocytes. Paraffin section immunohistochemistry was performed using the avidin-biotin-peroxidase technique after antigen epitope enhancement using a pressure cooker. Final detection was performed by treatment of sections with diaminobenzidine.
Anti-BCL-6, anti-p53, and anti-XBP-1 antibodies were purchased from Santa-Cruz Biotechnology (Santa Cruz, CA, USA), and monoclonal anti-IL-4 and anti-MUM-1 antibodies were purchased from Pharmingen (San Diego, CA, USA). Anti-MUM-1 antibody was used directly for immunofluorescence staining of tumor tissue for flow cytometric analysis (20 µL/106 cells).
Statistical methods
Objective response (OR) was defined as complete remission or partial remission after the fifth course of HDMTX-based combination chemotherapy. OS was calculated from the date of histological diagnosis to death or the last date of follow-up, while PFS was calculated from the first day of treatment to relapse or progression, or to the last date of follow-up.
SPSS version 12.0 (SPSS Institute, Inc., Chicago, IL, USA) was used for statistical analyses. Student's t-test or a nonparametric test was used as appropriate for testing of continuous and ordinal variables. Discrete variables were analyzed using Pearson's chi-square test. The cumulative proportions of ORR and their 95% confidence intervals (CIs), adjusted for a competing event, were calculated using the cumulative incidence method devised by Prentice et al. (19). Survival curves were drawn using the Kaplan-Meier method. The Cox proportional hazards method was used for identification of factors associated with ORR. Variables found to be significantly associated with ORR by univariate analyses were subjected to multivariate analyses. The Hazard Ratio (HR) for overall survival and progression free survival were calculated using a Cox proportional hazards model, with ORR considered as a time-dependent covariate. Statistical significance was accepted for P values of < 0.05. All tests were 2-tailed.
Ethics statement
This study was reviewed and approved by the institutional review board of Masan Samsung Hospital (MSH 2003-014). Written informed consent was obtained from all patients who participated in this study.
RESULTS
Clinical characteristics
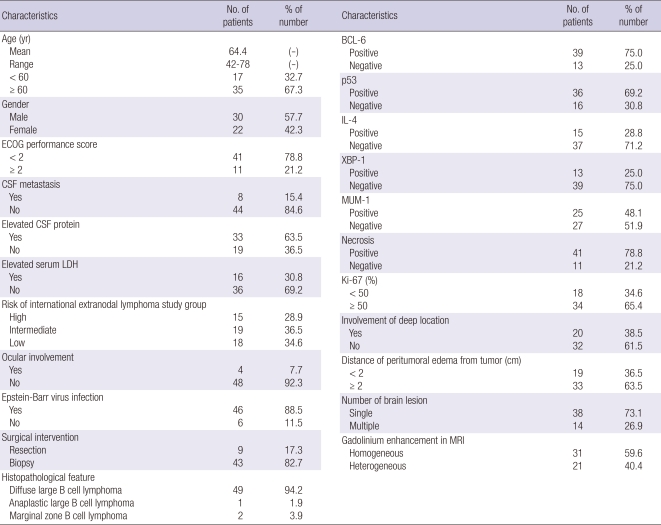
Fifty-two patients underwent HDMTX-based combination chemotherapy for PCNSL. Detailed clinical features in the study are listed in Table 1. Mean age of patients at commencement of chemotherapy was 64.3 yr (range 42-78 yr). Forty-one (78.8%) patients had an ECOG performance score of < 2 and 11 (21.2%) had a score of ≥ 2. Fifteen (28.9%) were categorized as high risk, 19 (36.5%) as intermediate risk, and 18 (34.6%) as low risk.
Table 1.
Clinical characteristics of patients who underwent the high dose methotrexate based-combination chemotherapy for primary central nervous system lymphoma (n = 52)
In histological features, 49 (94.2%) cases were diffuse large B-cell lymphoma, 1 (1.9%) case was anaplastic large B-cell lymphoma, and 2 (3.8%) cases were marginal zone B-cell lymphoma. In terms of MRI findings, homogeneous gadolinium enhancement was observed for 31 (59.6%) tumors and heterogeneous enhancement for 21 (40.4%).
Five cycles of HDMTX-based combination chemotherapy were administered to all patients. No dose-reduction was performed during the first cycle. However, during the second and third cycles, one patient received MTX at 3.0 g/m2/day, during the fourth cycle, 1 patient received MTX at 2.5 g/m2/day, and 3 patients received MTX at 3.0 g/m2/day; and during the fifth cycle, 2 patients received MTX at 2.5 g/m2/day and 5 at 3.0 g/m2/day.
Response to high dose methotrexate-based combination chemotherapy
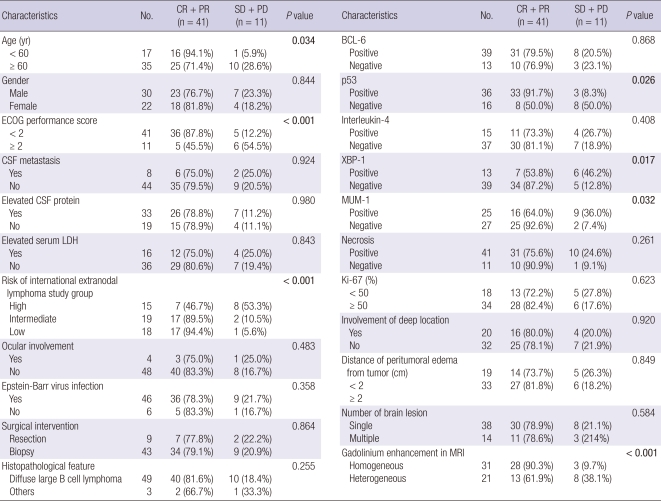
The overall crude incidence of objective response to HDMTX chemotherapy was 78.8% (CR of 63.5% and PR of 15.3%), and the overall incidence after adjusting for competing events was 82.4% (95% CI, 75.3%-89.5%). The ORR was 94.1% for patients aged < 60 yr and 71.4% for those aged ≥ 60 yr, which was a significant difference (P = 0.034). In patients with an ECOG performance score of < 2, the ORR was 87.8%, and in those with an ECOG performance score of ≥ 2, the ORR was 45.5% (P < 0.001). ORRs of patients in the high, intermediate, and low risk groups were 46.7%, 89.5%, and 94.4%, respectively (P < 0.001). However, no significant ORR differences were found for the other clinical variables studied. Table 2 details the clinical variables subjected to univariate analysis. A younger age (< 60 yr), a good performance status (ECOG performance score < 2), and a low risk were found to be associated with a significantly higher ORR.
Table 2.
Response to the high dose methotrexate based combination chemotherapy in patients with primary central nervous system lymphoma (n = 52)
In terms of the features of brain tumors, ORR was 91.7% for patients positive for p53 expression, and 50.0% for those negative for p53 (P = 0.026). Those positive for XBP-1 had an ORR of 53.8%, and those negative for XBP-1 an ORR of 87.2% (P = 0.017), and those positive for MUM-1 had an ORR of 64.0%, and those negative for MUM-1 an ORR of 92.6% (P = 0.032). Patients with heterogeneous gadolinium enhancement in MR images had an objective response of 61.9%; however, those with homogeneous enhancement had an objective response of 90.3% (P < 0.001). We found no other variable with a significant effect on ORR. The crude ORRs of tumors that were heterogeneously or homogeneously enhanced were 61.9% and 90.3%, and ORRs after adjusting for competing events were 63.4% (95% CI, 56.6%-70.2%) and 88.8% (95% CI, 81.9%-95.7%), respectively. This finding confirms that an enhancing pattern independently predicts objective response to HDMTX chemotherapy.
Progression free survival and overall survival
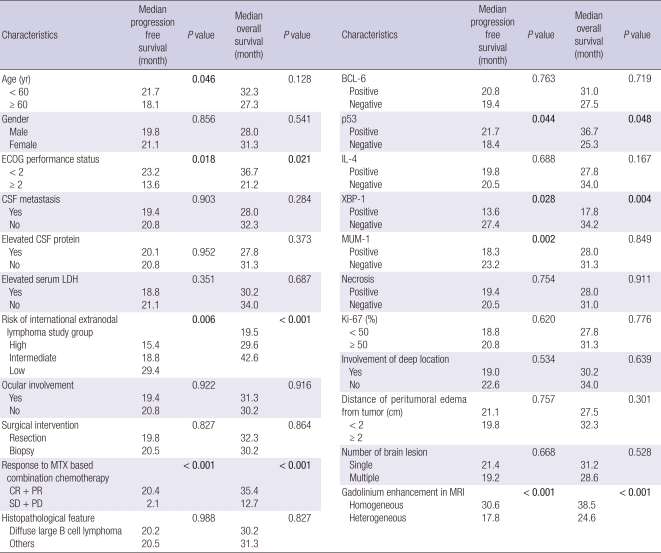
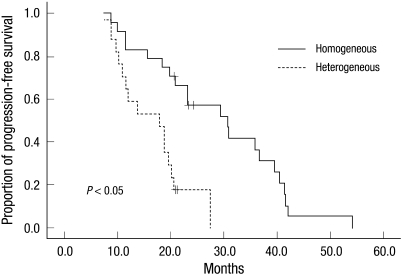
Table 3 illustrates median PFSs and median OSs of all 52 study subjects. Median PFS was 20.8 months (95% CI, 16.2-24.8 months). Median PFS was 21.7 months for those < 60 yr and 18.1 months for those ≥ 60 yr (P = 0.046). Patients with an ECOG performance score of < 2 had a median PFS of 23.2 months, and those with an ECOG performance score of ≥ 2 had a median PFS of 13.6 months (P = 0.018). Median PFSs in the high, intermediate, and low risk groups were 15.4, 18.8, and 29.4 months, respectively (P = 0.006). In terms of features of brain tumors, median PFS was 21.7 months for patients positive for p53 expression, and 18.4 months for patients negative for p53 (P = 0.044). Patients negative for XBP-1 had a greater median PFS than those positive for XBP-1 (27.4 months vs 13.6 months, respectively; P = 0.028). Patients positive for MUM-1 had a median PFS of 18.3 months, and those negative for MUM-1 had a median PFS of 23.2 months (P = 0.002). Of particular interest, median PFS was 17.8 months for patients with a heterogeneously gadolinium enhanced PCNSL, and 30.6 months for those with a homogeneous enhanced PCNSL (P < 0.001). Fig. 1 shows the progression-free survival curve for homogeneously vs heterogeneously enhanced PCNSL.
Table 3.
Progression-free survivals and overall survival (n = 52)
Fig. 1.
Progression-free survival curves for the patients with homogeneously versus heterogeneously enhanced primary central nervous system lymphoma.
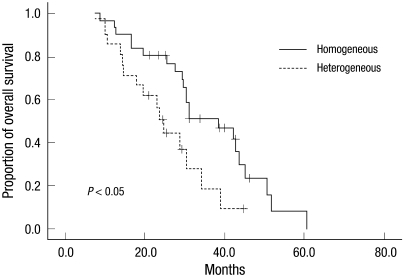
Median OS and the 2-yr survival rate were 30.5 months (95% CI, 21.7-39.3 months) and 62.3%. Furthermore, median OS was 36.7 months in patients with an ECOG performance score of < 2 and 21.2 months in those with a performance score of ≥ 2 (P = 0.021). Patients in the high, intermediate, and low risk groups had OSs of 19.5, 29.6, and 42.6 months, respectively, and these were significantly different (P < 0.001). As expected, patients who achieved an objective response to HDMTX based combination chemotherapy survived longer. The median OS of patients who experienced an objective response was 35.4 months, whereas the median OS of patients who did not experience an objective response was 12.7 months (P < 0.001). In terms of features of brain tumor, the median OS was 36.7 months in patients positive for p53, and 25.3 months in those negative for p53 (P = 0.048). Positivity and negativity for XBP-1 expression were found to be associated with median OSs of 17.8 and 34.2 months, respectively (P = 0.004). Of particular interest, homogeneous gadolinium enhancement was found to be associated with longer median OS (38.5 months vs 24.6 months, P < 0.001). Fig. 2 shows the Kaplan-Meier survival curves of patients for homogeneously vs heterogeneously enhanced PCNSL.
Fig. 2.
Kaplan-Meier survival curves for the patients with homogeneously versus heterogeneously enhanced primary central nervous system lymphoma.
Multivariate analysis of factors that influence progression free and overall survival
In terms of PFS, seven factors, namely, age, ECOG performance score, risk of IELSG, p53, XBP-1, and MUM-1 expression, and gadolinium enhancement were found to be positively associated with PFS by univariate analysis (Table 2). After multifactor adjustment, the HR of an ECOG performance of < 2 over a performance of ≥ 2 for longer PFS was 2.68 (95% CI, 1.50-3.86; P = 0.037). The HR of high risk over low risk, according to the IELSG, for longer PFS was 3.03 (95% CI, 1.54-4.37; P = 0.019). The HR of negativity over positivity for XBP-1 for longer PFS was 2.64 (95% CI, 1.46-3.52; P = 0.042), and the HR of negativity over positivity for MUM-1 for longer PFS was 2.93 (95% CI, 1.68-3.72; P = 0.035). Finally, the HR of homogeneous over heterogeneous gadolinium enhancement for longer PFS was 3.32 (95% CI, 1.90-4.54; P = 0.002). However, multivariate analysis showed that a younger age and p53 expression were not associated with longer PFS.
In terms of OS, 6 factors, namely, ECOG performance score, risk of IELSG, response to HDMTX based chemotherapy, expression of p53 and XBP-1, and gadolinium enhancement pattern were found to be significantly associated with longer OS by univariate analysis (Table 2). After multifactor adjustment, the HR of an ECOG performance score of < 2 over a score of ≥ 2 for longer OS was 2.53 (95% CI, 1.33-3.62; P = 0.040), the HRs of a high risk over intermediate risk, a high risk over a low risk, and an intermediate risk over a low risk were 2.44 (95% CI, 1.71-3.25; P = 0.048), 4.35 (95% CI, 2.70-6.23; P < 0.001), and 2.52 (95% CI, 1.58-3.41; P = 0.045), respectively. The HR of negativity over positivity for XBP-1 expression for longer OS was 3.01 (95% CI, 1.59-4.44; P = 0.022), the HR of homogenous over heterogeneous enhanced PCNSL for longer OS was 4.18 (95% CI, 2.26-4.90; P < 0.001), and the HR of the presence over the absence of an objective response to HDMTX chemotherapy for longer OS was 5.22 (95% CI, 2.84-7.60; P < 0.001). Although p53 expression was found to be associated with longer OS by univariate analysis, multivariate analysis revealed no significant association.
Mortality and recurrence
During the follow-up period, thirty-seven (71.2%) patients died. Of these, four patients (7.7%) died during treatments: 2 patients succumbed to disease progression; 1 to acute renal failure; and 1 to sepsis. In patients of diseases progression, acute cerebral edema and hydrocephalic crisis were the cause of death. Acute renal failure and sepsis were attributed to toxicity of HDMTX-based combination chemotherapy. And, three patients were above 60 yr old. There were no deaths from other systemic disease, such as acute myocardiac infarction, pulmonary embolism, complication of diabetes, etc.
Forty patients (76.9%) experienced disease recurrence. Median time to recurrence was 8.3 months (range, 2.4-45.3 months). After recurrence, 27 patients (67.5%) were treated with HDMTX-based combination chemotherapy again, and 13 patients (32.5%) were treated with WBRT.
DISCUSSION
Although many reports have shown that several factors influence the prognosis of PCNSL, no study to date has assessed the factors that influence objective response to HDMTX based combination chemotherapy for PCNSL. In particular, there has been no study to report on the relationship between gadolinium enhancement in MRI and outcome of HDMTX for PCNSL. Ferreri et al. suggested that age, performance status, LDH serum level, HDMTX, and the use of HD cytarabine are significantly associated with overall response rate (18). Their therapeutic modalities included radiotherapy alone, radiotherapy followed by chemotherapy, chemotherapy alone, and chemotherapy followed by radiotherapy. However, their chemotherapeutic regimen was highly heterogeneous, that is, it involved MTX followed by alkylating agents, HD cytarabine, and anthracycline. Other regimens included alkylating agent alone, the CHOP regimen, an HD cytarabine-based regimen, HDMTX alone, HDMTX plus alkylating agents, HDMTX plus CHOP-like regimens, and others. Furthermore, it has not been previously reported that the molecular features of PCNSL can influence objective response to HDMTX-based combination chemotherapy.
Of particular interest, the present study shows that patterns of gadolinium enhancement were predictive of response to HDMTX. According to a report by Algazi et al. (20), contrast enhancement was uniform in 55% to 60% of PCNSL cases. They suggested that the apparent diffusion coefficient (ADC) is correlated with cellular density in PCNSL, and that pre-therapeutic ADC (a possible surrogate marker of cellular density) is predictive of clinical outcome. Low cellularity, which reduces ADC within contrast-enhancing regions, may permit a high influx of contrast into the tumor; therefore, PCNSLs showing homogeneous gadolinium enhancement have lower cellularity and a higher objective response due to the smaller tumor cell burden. Unfortunately, because ADC mapping was not performed on many of the MR images, we are unable to present the association between ADC and response to HDMTX-based combination chemotherapy.
Although many other characteristics were not found to affect objective response to HDMTX-based combination chemotherapy, we found that age, performance status, and low risk status as defined by the IELSG, expression of p53, XBP-1, and MUM-1, and patterns of gadolinium enhancement in MR images significantly affected objective response. Findings by Ferreri et al. (21) who also studied predictors of treatment outcome in PCNSL, concur with those of the present study, namely, that age, performance status, and a lower prognostic score (as defined by the IELSG) influence overall response rate to HDMTX-based combination chemotherapy. However, in the present study, unlike the previous study, multivariate analysis showed no relationship between age and longer PFS or OS; the cause of this discrepancy is not known.
Rubenstein et al. (22) suggested that several clinical features of PCNSL exhibit a distinct biologic phenotype; that is, 1) a short response duration to a combined modality therapy; 2) a unique proclivity to grow in the CNS; and 3) a heightened responsiveness to MTX. In terms of prognostic significance in correlation with biologic phenotype, diffuse large B-cell lymphoma (DLBCL) cases with germinal center B-cell patterns expressing BCL-6 have been known to show a much better prognosis than DLBCL cases with activated B-cell expressing MUM-1 (22, 23). However, other groups observed no difference in survival between DLBCL patients based on BCL-6 expression (24, 25). One reverse report showed that BCL-6 expression indicates poor prognosis (26). In our study, BCL-6 expression was not associated with PFS and OS, and negative expression of MUM-1 was associated with longer PFS in multivariate analysis. And, this multivariate analysis showed that negative expression of XBP-1 was associated with longer PFS and OS. Rubenstein et al. (22) also suggested that XBP-1 was among the genes showing the most significant differential expression in PCNSL, and that the strongest XBP-1 protein expression among dense tumor cell populations was associated with the vasculature. Further studies are needed to determine the role of these biomarkers.
Although the present study shows that several factors, including age < 60 yr, good performance status, low risk status of IELSG, expression of p53, XBP-1, and MUM-1, and homogeneous MRI enhancement patterns, are associated with objective response to HDMTX-based combination chemotherapy for PCNSL, several study shortcomings limit our conclusions.
First, HDMTX is known to be the primary treatment for PCNSL and has been successfully incorporated into multi-drug chemotherapy regimens, which has in turn been associated with notable improvements in response and survival. The authors commenced this 6-yr long study in 2003; thus, the study is somewhat dated in terms of current practice. In fact, results of combination chemotherapies with other drugs, such as, high-dose cytarabine (21), temozolomide (27), and cyclophosphamide (28) have also been reported. In addition, the recently introduced extremely high dose MTX mono-chemotherapy (> 8 g/m2/day) (29), and development of targeted drugs, such as, rituximab (15), may have improved the prognosis for PCNSL. Second, samples were examined as paraffin-embedded tissues and we did not investigate genetic alterations in proteins, which have previously been found to be associated with response to treatment. Therefore, the present study does not shed light on pathophysiologic mechanisms. Third, we did not perform ADC mapping using MR images during the early period of this study in most patients, which would have possibly confirmed the existence of a positive association between homogeneous gadolinium enhancement and high signal intensity of the ADC map. Therefore, we could not examine the association between homogeneous enhancement and low cellularity of tumor. Accordingly, we did not present any confirmative association between responsiveness to HDMTX chemotherapy and low cellularity of PCNCL. Fourth, the number of patients in this study was relatively small. Therefore, we need to perform a randomized and prospective study of more patients in order to obtain stronger evidence to support the results of this study.
In conclusion, through prospective analysis, we suggest that an age of < 60 yr, an ECOG performance score of < 2, a low or intermediate risk status of IELSG, positivity for p53, negativity for XBP-1 or MUM-1, and homogenous gadolinium enhancement in MRI should be associated with a good response of PCNSL to HDMTX-based combination chemotherapy. In particular, it was interesting to note that patients with a PCNSL that is homogeneously enhanced by gadolinium have a higher ORR to HDMTX-based chemotherapy, and a longer PFS and OS, as well as better response to HDMTX-based chemotherapy.
ACKNOWLEDGMENTS
The authors would like to thank Yun Gyu Song, and Ha Young Lee, M.D. for their review of neuroradiological images for this study.
AUTHOR SUMMARY
Factors Influencing the Response to High Dose Methotrexate-based Vincristine and Procarbazine Combination Chemotherapy for Primary Central Nervous System Lymphoma
Kang Hyun Sung, Eun Hee Lee, and Young Zoon Kim
Prospective observational analysis was performed on 52 primary central nervous system lymphoma (PCNSL) patients. All patients received HDMTX, vincristine, and procarbazine. Forty-one patients (78.8%) achieved complete or partial remission after the combination chemotherapy. Higher OR rates were observed in the patients with: 1) age < 60 yr; 2) ECOG performance score of < 2; 3) low risk status as defined by the International Extranodal Lymphoma Study Group (IELSG); 4) p53 positivity; 5) XBP-1 negativity; 6) MUM-1 negativity; and 7) homogenous gadolinium enhancement in MR images. Multivariate analysis showed that ECOG performance score of < 2, low risk, negativity for XBP-1, homogenous gadolinium enhancement by MRI, and response to chemotherapy were associated with longer OS.
References
- 1.Lee CH, Jung KW, Yoo H, Park S, Lee SH. Epidemiology of primary brain and central nervous system tumors in Korea. J Korean Neurosurg Soc. 2010;48:145–152. doi: 10.3340/jkns.2010.48.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher EA, Fine HA. Primary CNS lymphoma. Semin Oncol. 1999;26:346–356. [PubMed] [Google Scholar]
- 3.Mead GM, Bleehen NM, Gregor A, Bullimore J, Shirley D, Rampling RP, Trevor J, Glaser MG, Lantos P, Ironside JW, Moss TH, Brada M, Whaley JB, Stenning SP. A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer. 2000;89:1359–1370. [PubMed] [Google Scholar]
- 4.Schultz C, Scott C, Sherman W, Donahue B, Fields J, Murray K, Fisher B, Abrams R, Meis-Kindblom J. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of Radiation Therapy Oncology Group protocol 88-06. J Clin Oncol. 1996;14:556–564. doi: 10.1200/JCO.1996.14.2.556. [DOI] [PubMed] [Google Scholar]
- 5.Nelson DF. Radiotherapy in the treatment of primary central nervous system lymphoma (PCNSL) J Neurooncol. 1999;43:241–247. doi: 10.1023/a:1006206602918. [DOI] [PubMed] [Google Scholar]
- 6.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 7.Ferreri AJ, Reni M, Dell'Oro S, Ciceri F, Bernardi M, Camba L, Ponzoni M, Terreni MR, Tomirotti M, Spina M, Villa E. Combined treatment with high-dose methotrexate, vincristine and procarbazine, without intrathecal chemotherapy, followed by consolidation radiotherapy for primary central nervous system lymphoma in immunocompetent patients. Oncology. 2001;60:134–140. doi: 10.1159/000055310. [DOI] [PubMed] [Google Scholar]
- 8.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma: lessons from prospective trials. Ann Oncol. 2000;11:927–937. doi: 10.1023/a:1008376412784. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri AJ, Abrey LE, Blay JY, Borisch B, Hochman J, Neuwelt EA, Yahalom J, Zucca E, Cavalli F, Armitage J, Batchelor T. Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol. 2003;21:2407–2414. doi: 10.1200/JCO.2003.01.135. [DOI] [PubMed] [Google Scholar]
- 11.Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, Aondio GM, Ferrarese F, Gomez H, Ponzoni M, Borisch B, Berger F, Chassagne C, Iuzzolino P, Carbone A, Weis J, Pedrinis E, Motta T, Jouvet A, Barbui T, Cavalli F, Blay JY. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58:1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 12.Herrlinger U, Küker W, Uhl M, Blaicher HP, Karnath HO, Kanz L, Bamberg M, Weller M Neuro-Oncology Working Group of the German Society. NOA-03 trial of high-dose methotrexate in primary central nervous system lymphoma: final report. Ann Neurol. 2005;57:843–847. doi: 10.1002/ana.20495. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien PC, Roos DE, Pratt G, Liew KH, Barton MB, Poulsen MG, Olver IN, Trotter GE Trans-Tasman Radiation Oncology Group. Combined-modality therapy for primary central nervous system lymphoma: long-term data from a Phase II multicenter study (Trans-Tasman Radiation Oncology Group) Int J Radiat Oncol Biol Phys. 2006;64:408–413. doi: 10.1016/j.ijrobp.2005.07.958. [DOI] [PubMed] [Google Scholar]
- 14.Ferreri AJ, Dell'Oro S, Foppoli M, Bernardi M, Brandes AA, Tosoni A, Montanari M, Balzarotti M, Spina M, Ilariucci F, Zaja F, Stelitano C, Bobbio F, Corazzelli G, Baldini L, Ponzoni M, Picozzi P, Caligaris Cappio F, Reni M. MATILDE regimen followed by radiotherapy is an active strategy against primary CNS lymphomas. Neurology. 2006;66:1435–1438. doi: 10.1212/01.wnl.0000210464.94122.e1. [DOI] [PubMed] [Google Scholar]
- 15.Shah GD, Yahalom J, Correa DD, Lai RK, Raizer JJ, Schiff D, LaRocca R, Grant B, DeAngelis LM, Abrey LE. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 17.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 18.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borisch B, Tinguely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell'Oro S, Zucca E, Cavalli F. Prognostic scoring system for primary CNS lymphomas: the International Exranodal Lymphoma Study Group Experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 19.Chen BE, Kramer JL, Greene MH, Rosenberg PS. Competing risks analysis of correlated failure time data. Biometrics. 2008;64:172–179. doi: 10.1111/j.1541-0420.2007.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Algazi AP, Kadoch C, Rubenstein JL. Biology and treatment of primary central nervous system lymphoma. Neurotherapeutics. 2009;6:587–597. doi: 10.1016/j.nurt.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Zoppegno L, Aondio GM, Avvisati G, Balzarotti M, Brandes AA, Fajardo J, Gomez H, Guarini A, Pinotti G, Rigacci L, Uhlmann C, Picozzi P, Vezzulli P, Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F International Extranodal Lymphoma Study Group (IELSG) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomized phase 2 trial. Lancet. 2009;374:1512–1520. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 22.Rubenstein JL, Fridlyand J, Shen A, Aldape K, Ginzinger D, Batchelor T, Treseler P, Berger M, McDermott M, Prados M, Karch J, Okada C, Hyun W, Parikh S, Haqq C, Shuman M. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CC, McClintock S, Cleveland RP, Trzpuc T, Vesole DH, Logan B, Kajdacsy-Balla A, Perkins SL. Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol. 2004;28:464–470. doi: 10.1097/00000478-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC, Hsu HC, Tien HF, Cheng AL. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12:1152–1156. doi: 10.1158/1078-0432.CCR-05-1699. [DOI] [PubMed] [Google Scholar]
- 25.Sugita Y, Tokunaga O, Nakashima A, Shigemori M. SHP-1 expression in primary central nervous system B-cell lymphomas in immunocompetent patients reflects maturation stage of normal B cell counterparts. Pathol Int. 2004;54:659–666. doi: 10.1111/j.1440-1827.2004.01677.x. [DOI] [PubMed] [Google Scholar]
- 26.Momota H, Narita Y, Maeshima AM, Miyakita Y, Shinomiya A, Maruyama T, Muragaki Y, Shibui S. Prognostic value of immunohistochemical profile and response to high-dose methotrexate therapy in primary CNS lymphoma. J Neurooncol. 2010;98:341–348. doi: 10.1007/s11060-009-0078-z. [DOI] [PubMed] [Google Scholar]
- 27.Omuro AM, Taillandier L, Chinot O, Carnin C, Barrie M, Hoang-Xuan K. Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly. J Neurooncol. 2007;85:207–211. doi: 10.1007/s11060-007-9397-0. [DOI] [PubMed] [Google Scholar]
- 28.Silvani A, Salmaggi A, Eoli M, Lamperti E, Broggi G, Marras CE, Fariselli L, Milanesi I, Fiumani A, Gaviani P, Erbetta A, Giovagnoli AR, Pollo B, Botturi A, Boiardi A. Methotrexate based chemotherapy and deferred radiotherapy for primary central nervous system lymphoma (PCNSL): single institution experience. J Neurooncol. 2007;82:273–279. doi: 10.1007/s11060-006-9276-0. [DOI] [PubMed] [Google Scholar]
- 29.Ferreri AJ, Crocchiolo R, Assaanelli A, Govi S, Reni M. High-dose chemotherapy supported by autologous stem cell transplantation in patients with primary central nervous system lymphoma: facts and opinions. Leuk Lymphoma. 2008;49:2042–2047. doi: 10.1080/10428190802381238. [DOI] [PubMed] [Google Scholar]