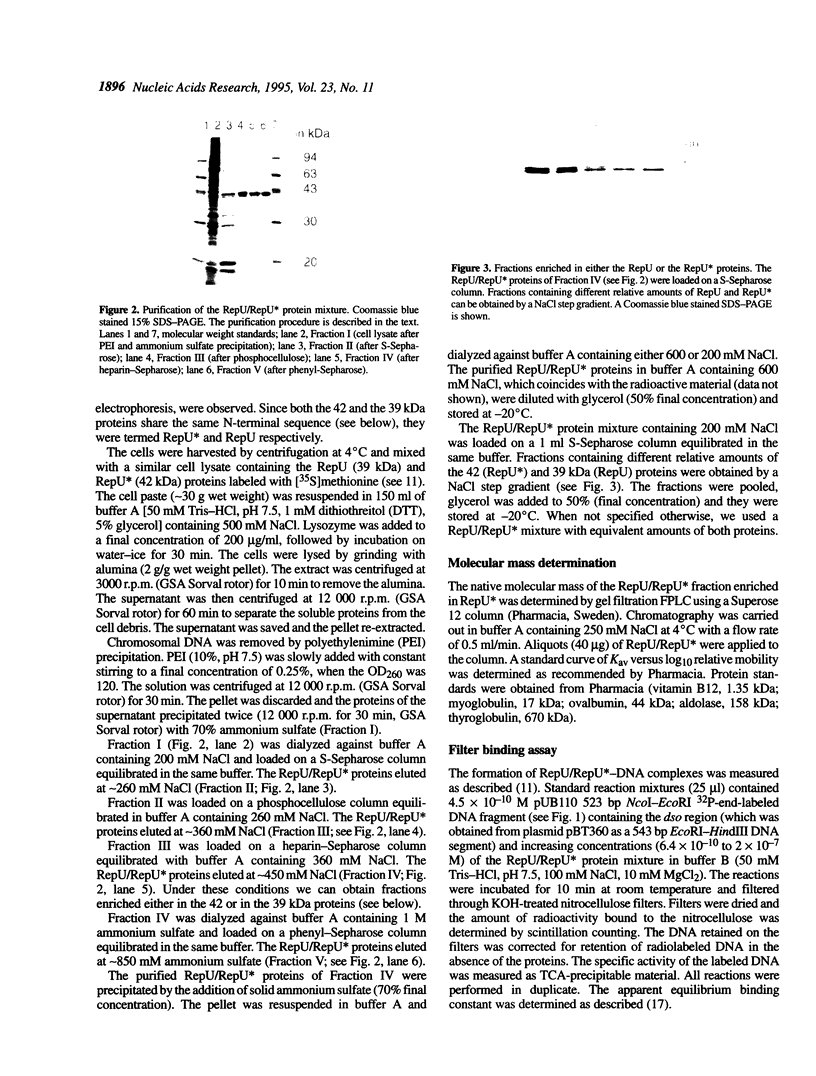
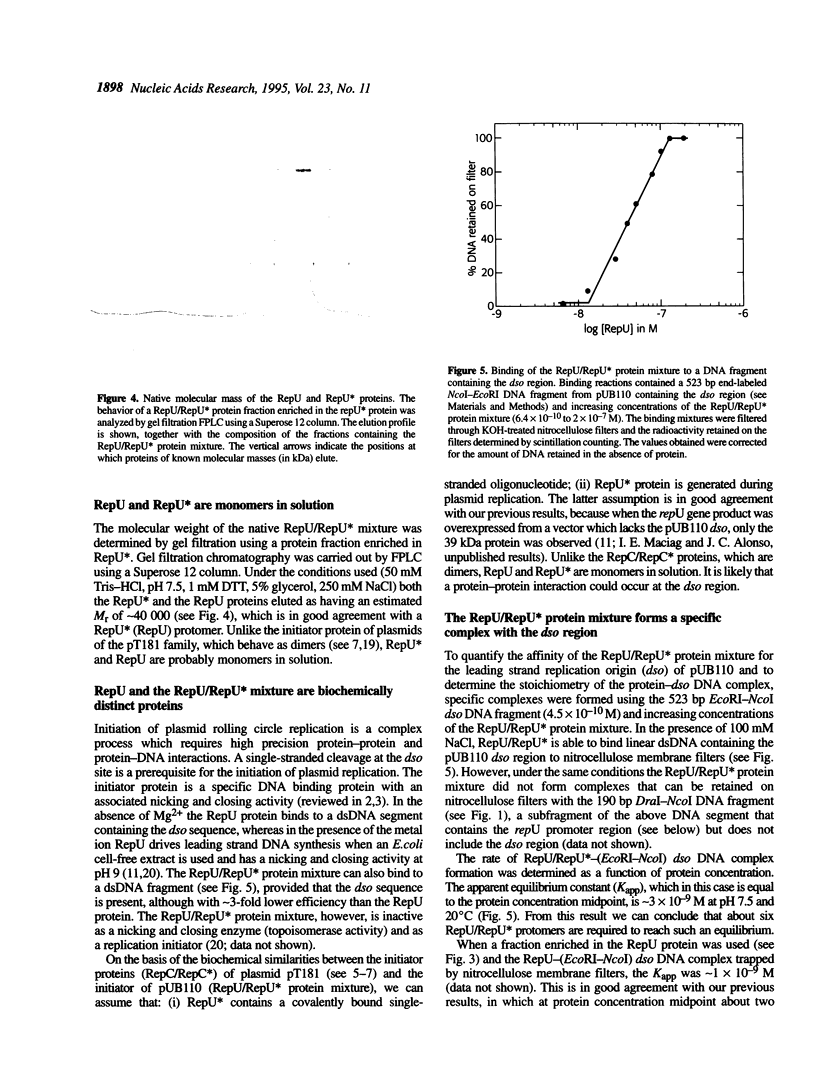
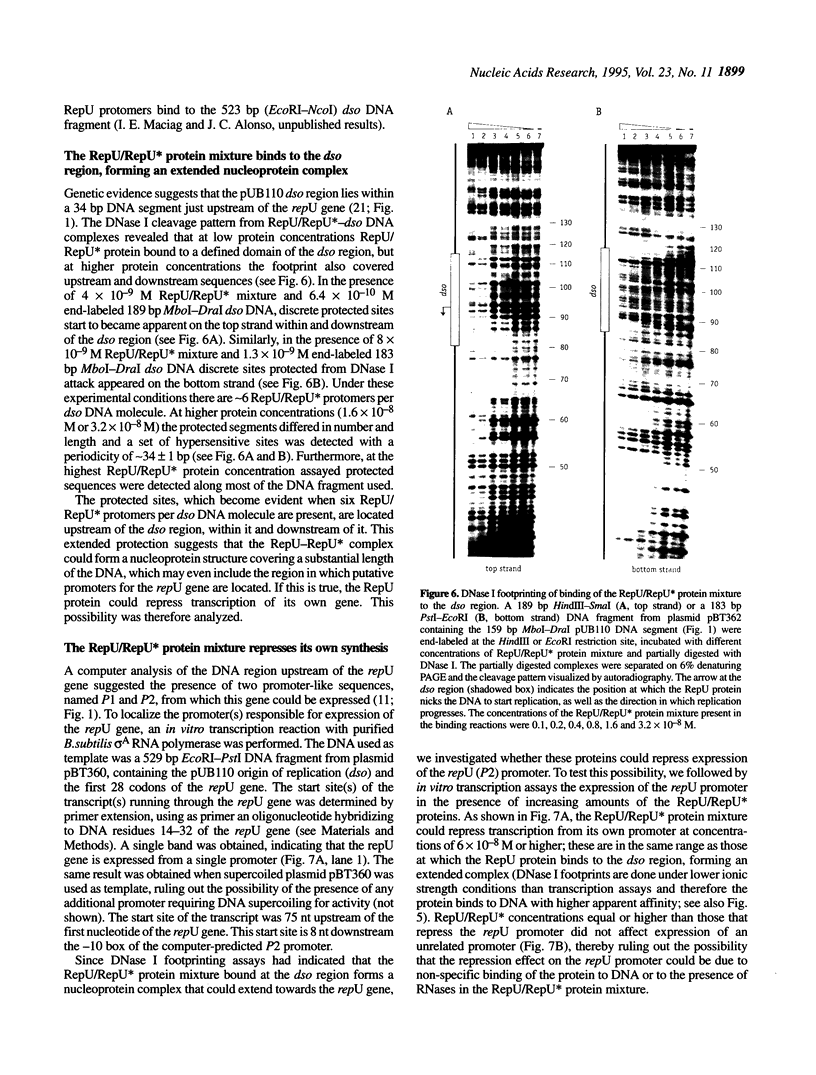
Abstract
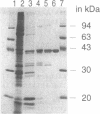
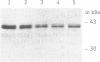
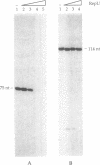
Plasmids control their copy number by limiting the amount of the initiator for DNA replication. The plasmid pUB110 initiator protein is termed RepU. Expression of the pUB110 repU gene is controlled by two antisense RNAs that interfere with repU mRNA translation. Genetic evidence suggests that Rep protein levels may be regulated by additional uncharacterized mechanisms. The repU gene product was radiolabeled and purified by monitoring the radioactive label. RepU overproduction was performed in cells containing the plasmid leading strand replication origin (dso), to allow for a putative inactivation of RepU. Polypeptides with apparent molecular masses of 42 (RepU*) and 39 (RepU) kDa were purified, both having the N-terminal sequence expected for the repU gene. The RepU/RepU* protein mixture bound specifically to dso. At low protein concentrations, about six RepU/RepU* protomers bound to the dso region. At higher concentrations, an extended nucleoprotein complex was formed. The promoter for the repU gene was localized downstream of the dso region. The results suggest that the extended RepU/RepU*-dso DNA complex interferes with repU promoter utilization. This provides an additional copy number control by limiting RepU concentration. Our results suggest that during replication the RepU protein might be converted into an inactive RepU-RepU* hetero-oligomer, further limiting the amount of RepU protein available for replication initiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. C. DNA replication of plasmids from gram-positive bacteria in Bacillus subtilis. Plasmid pUB110 as a model system. Microbiologia. 1989 Jun;5(1):5–12. [PubMed] [Google Scholar]
- Alonso J. C., Leonhardt H., Stiege C. A. Functional analysis of the leading strand replication origin of plasmid pUB110 in Bacillus subtilis. Nucleic Acids Res. 1988 Oct 11;16(19):9127–9145. doi: 10.1093/nar/16.19.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J. C., Tailor R. H. Initiation of plasmid pC194 replication and its control in Bacillus subtilis. Mol Gen Genet. 1987 Dec;210(3):476–484. doi: 10.1007/BF00327200. [DOI] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Genetic studies of a multi-resistant strain of Staphylococcus aureus. J Med Microbiol. 1974 May;7(2):285–297. doi: 10.1099/00222615-7-2-285. [DOI] [PubMed] [Google Scholar]
- Maciag I. E., Viret J. F., Alonso J. C. Replication and incompatibility properties of plasmid pUB110 in Bacillus subtilis. Mol Gen Genet. 1988 May;212(2):232–240. doi: 10.1007/BF00334690. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Iordanescu S., Projan S. J., Kornblum J., Edelman I. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell. 1989 Oct 20;59(2):395–404. doi: 10.1016/0092-8674(89)90300-0. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- Rasooly A., Novick R. P. Replication-specific inactivation of the pT181 plasmid initiator protein. Science. 1993 Nov 12;262(5136):1048–1050. doi: 10.1126/science.8235621. [DOI] [PubMed] [Google Scholar]
- Rasooly A., Projan S. J., Novick R. P. Plasmids of the pT181 family show replication-specific initiator protein modification. J Bacteriol. 1994 Apr;176(8):2450–2453. doi: 10.1128/jb.176.8.2450-2453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasooly A., Wang P. Z., Novick R. P. Replication-specific conversion of the Staphylococcus aureus pT181 initiator protein from an active homodimer to an inactive heterodimer. EMBO J. 1994 Nov 1;13(21):5245–5251. doi: 10.1002/j.1460-2075.1994.tb06856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Rojo F., Alonso J. C. A novel site-specific recombinase encoded by the Streptococcus pyogenes plasmid pSM19035. J Mol Biol. 1994 Apr 29;238(2):159–172. doi: 10.1006/jmbi.1994.1278. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991 May 5;219(1):37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- Thomas C. D., Balson D. F., Shaw W. V. In vitro studies of the initiation of staphylococcal plasmid replication. Specificity of RepD for its origin (oriD) and characterization of the Rep-ori tyrosyl ester intermediate. J Biol Chem. 1990 Apr 5;265(10):5519–5530. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- del Solar G., Espinosa M. The copy number of plasmid pLS1 is regulated by two trans-acting plasmid products: the antisense RNA II and the repressor protein, RepA. Mol Microbiol. 1992 Jan;6(1):83–94. doi: 10.1111/j.1365-2958.1992.tb00840.x. [DOI] [PubMed] [Google Scholar]