Abstract
Near infrared (NIR) light energy has been used in medical applications both for diagnostic and treatment purposes. A priory knowledge of optical tissue properties is necessary in these applications; not only of human but also in animals for testing of devices. However, published data on the optical properties of neural tissue in rodents are rare. The aim of this study was to measure the penetration depth of light into the rat peripheral nerve and brain cortex at NIR wavelengths. Penetration depth was calculated from measurements of transmitted light for various thicknesses of the neural tissue. We found the penetration depth in the rat sciatic nerve to be 0.35 ± 0.023 mm and in the white matter 0.35 ± 0.026 mm. The penetration depth of the gray matter was 0.41 ± 0.029 mm. Compared to the data reported in literature for the human brain, the rat peripheral and the brain cortex attenuate the NIR light much more strongly.
Keywords: near infrared light, penetration depth, reflectance, transmittance
I. INTRODUCTION
Near-infrared light (NIR), ranging from 700 to 900 nm, has the maximum penetration depth into the white and gray matters of the brain. This property has been utilized in numerous applications ranging from spectroscopic imaging [1] to treatment of brain tumors [2]. In these applications, a priory knowledge of optical properties of the human neural tissue is crucial to determine the penetration depth of light for a specific wavelength range [3]. However, it is equally important to know the optical properties in the animal models for development of such applications. The rat nervous system has been frequently used as an animal model in testing of these devices [4]. In our laboratory, we are also interested in studying energy transfer to an implantable microstimulator by optical means in the NIR wavelengths [5]. The light energy collected by the microstimulator depends on the tissue properties as well as implantation depth.
There are numerous reports on optical properties of human neural tissue [3], [6], [7]. However, the literature from the experimental animals is very limited and the reported values vary substantially. In this short paper, we report the penetration depth for NIR light in the rat peripheral nerves and the brain cortex.
As the light travels into the tissue, its intensity decreases due to absorption and scattering. Some of the incident light also reflects back at the surface of entry. The intensity profile by depth is governed by Beer’s law if multiple scattering can be avoided. But the intensity profile may deviate from an exponential in thick tissue slices where multiple scattering may occur. The penetration depth here is defined as the distance at which the total optical power is reduced to 37% of the incident light
| (Equ. 1) |
where It is the transmitted and Io is the incident light power. R is the reflection at the surface of entry and a is the penetration depth.
II. METHODS
A. Methods for Sciatic Nerve Measurements
Six Sprague-Dawley rats (400–500 g) were used for this part. The anesthesia was induced (50 mg/kg) and maintained with sodium pentobarbital with further doses as needed. The body temperature was continuously monitored and maintained between 36–37°C using a temperature regulated heating pad. Tracheotomy was performed to connect the animal to a respirator. Respiration rate and end-tidal CO2 were monitored. The sciatic nerves were dissected out and explanted bilaterally. The epineurium and connective tissue were carefully removed with minimal stretching of the nerve in a Petri dish. The nerve was kept moist using saline solution at room temperature. All experimental procedures were approved by the Animal Care Committee at Rutgers University.
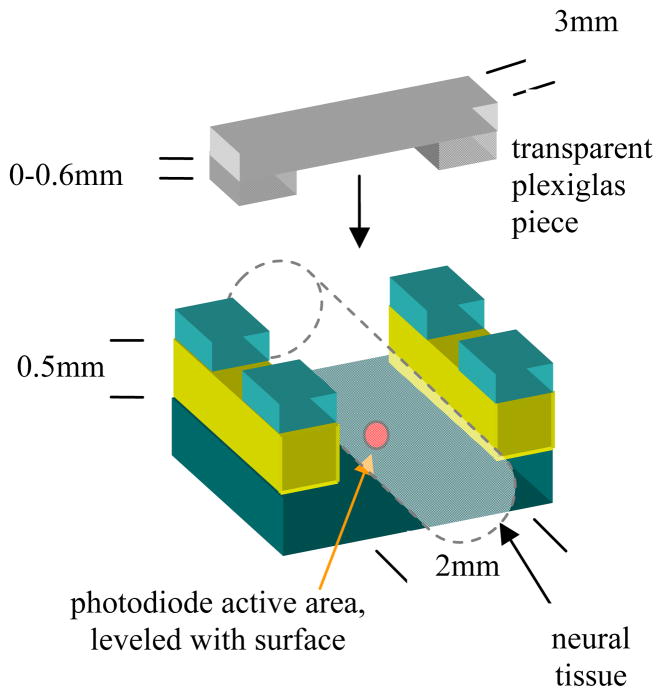
A commercial photodiode (G9842, circular active area, ø0.08 mm, Hamamatsu, GaAs PIN photodiode) was used to measure the transmitted light power through the sciatic nerve. The photodiode was first secured at the bottom of a plastic channel that is 2 mm wide and 0.5 mm deep (Fig. 1). The explanted rat sciatic nerve was then placed inside the channel, which then took the shape of the channel filling it wall-to-wall.
Fig. 1.
The preparation used to measure the penetration depth in the rat neural tissue.
Plexiglas pieces, transparent at NIR wavelengths, with varying heights from the photodiode plane were placed over the nerve and clamped securely. This set-up allowed us to control the length of the pathway precisely that the laser beam traveled inside the neural tissue. The laser was centered above the set-up using a 3-axis micromanipulator and aimed at the photodiode’s active area.
The transmittance values were obtained for a few different thicknesses of the nerve sample. The penetration depth (a) was estimated using an exponential curve fit through the data points.
The incident light power (Io) was measured with no neural tissue present. The horizontal extent of the tissue sample perpendicular to the light beam was assumed to be sufficiently large to neglect the boundary effects. The laser source (DLS-500-830FS-100, Stocker Yale, Canada) was a 74 mW, 830 nm semiconductor source with a circular beam shape that had a Gaussian intensity profile with a measured standard deviation of about 260 μm at the surface of the tissue slab. The laser was placed above 15 cm from the tissue surface.
B. Methods for Brain Cortex Measurements
Three Sprague-Dawley rats (400–500 g) were used for this part. The brain cortex samples were explanted following a similar procedure to that of the sciatic nerve in part A. The samples were placed in 4% formalin and kept in refrigerator for 24 hours for fixation. The white and gray matters were separated and cut into slices. The same setup and measurement techniques described in Fig. 1 were used.
III. RESULTS
A. Results from the Sciatic Nerve Measurements
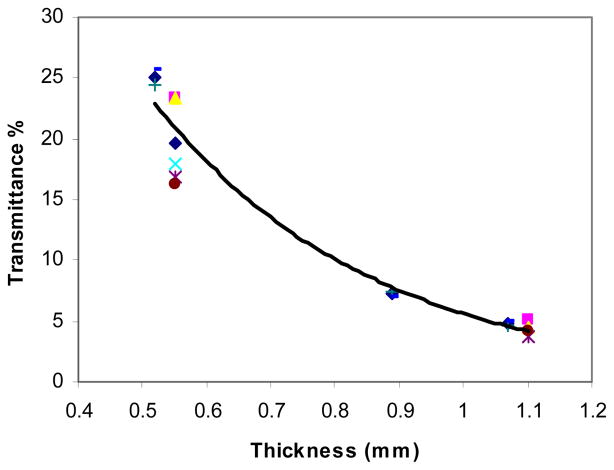
Two point measurements (thickness of 0.55 mm and 1.1 mm) were made on the first five samples and three point measurements (0.52, 0.89, and 1.07 mm) on the remaining two samples. Fig. 2 shows the transmittance values measured from seven samples as a function of nerve sample thickness. The exponential line is a curve fit to the mean values at each thickness (R=0.99). The mean transmittances were 25.06 ± 0.96%, 19.58 ± 3.48%, 7.19 ± 0.26%, 4.79 ± 0.22%, and 4.22 ± 0.61% at thickness values of 0.52, 0.55, 0.89, 1.07, and 1.1 mm respectively. The calculated penetration depth had a mean of 0.35 mm with a standard deviation of 0.023 mm.
Fig. 2.
The transmittance of seven samples as a function of the nerve thickness. The exponential curve is a curve fit to the mean values shown in diamonds (◆) and each symbol type indicates data from a different tissue sample.
B. Results from the Brain Cortex Measurements
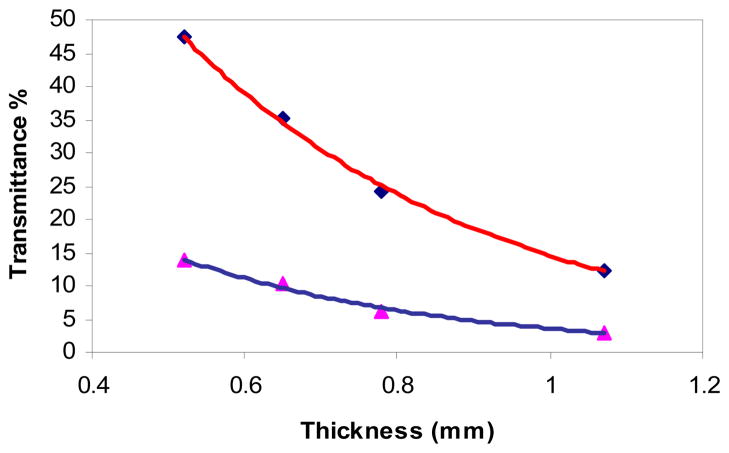
Four point measurements (thicknesses of 0.52, 0.65, 0.78, and 1.07 mm) were made on four samples of the gray and four samples of the white matter. Fig. 3 shows the mean transmittance measurements and the exponential curve fit for both (R >0.99).
Fig. 3.
The transmittance measured from four samples of gray (◆) and four samples of white (▲) matter with varying thicknesses.
The mean transmittance of the gray matter was 47.42 ± 3.07%, 35.24 ± 3.61%, 24.13 ± 2.69%, and 12.33 ± 1.88% at thickness of 0.52, 0.65, 0.78 and 1.07 mm respectively. The calculated penetration depth of the samples had a mean of 0.41 mm with a standard deviation of 0.029 mm.
The mean transmittance of the white matter was 13.93 ± 2.63%, 10.36 ± 2.18%, 6.02 ± 0.44%, and 3.00 ± 0.53% at the same thicknesses. The calculated penetration depth of the samples had a mean of 0.35 ± 0.026 mm.
IV. DISCUSSION
The penetration depth of rat sciatic nerve and the white matter of the brain were very similar but much smaller than that of the human white matter of the brain, which was reported as 2.3 mm by Roggan et al. [6], 0.9 mm by Yaroslavsky et al. [3], and 0.75 mm by Eggert et al. [7], all at 850 nm. The penetration depth was higher for the rat gray matter than the white matter and the peripheral nerve in our experiments. However, the penetration depth of the rat gray matter is still much smaller than that of the human, which was reported as 1.6 mm at 850 nm by Eggert et al. [7].
Sample preparation techniques play a vital role in measurements of tissue optical properties. Because of the practical limitations of the in vivo preparations we chose to do the measurements in the explanted tissue samples. For instance, the excessive connective tissue and the vascularization around the sciatic nerve induced large variations into the measurements. Reports in literature suggest that in vitro measurement of optical properties can adequately match the in vivo case [3]. In our work, the explanted sciatic nerves were not frozen or subjected to any fixation procedure and the measurements were made immediately after removing the tissue from the host. However, the brain cortex samples were too soft to make slices without pre-processing.
We assumed an exponential decrease for the intensity profile as in Beer’s formulation. The fact that the exponential curve was a good fit to the data taken for multiple sample thicknesses suggests that the intensity profile did not deviate from Beer’s law substantially.
Acknowledgments
This study was supported by a grant from National Institute of Health (1-R21-NS 050757-01A1).
References
- 1.Koehler Frederick W, IV, Lee Eunah, Kidder Linda H, Neil Lewis E. Near Infrared Spectroscopy: the Practical Chemical Imaging Solution. Spectroscopy Europe. 2002;14(3) [Google Scholar]
- 2.Amin Z, Donald JJ, Masters A, Kant R, Steger AC, Bown SG, Lees WR. Hepatic Metastases: Interstitial Laser Photocoagulation with Real-time US Monitoring and Dynamic CT Evaluation of Treatment. Radiology. 1993;187:339–347. doi: 10.1148/radiology.187.2.8475270. [DOI] [PubMed] [Google Scholar]
- 3.Yaroslavsky AN, Schulze PC, Yaroslavsky IV, Schober R, Ulrich F, Schwarzmaier H-J. Optical Properties of Selected Native and Coagulated Human Brain Tissues in Vitro in the Visible and Near Infrared Spectral Range. Phys Med Biol. 2002;47:2059–2073. doi: 10.1088/0031-9155/47/12/305. [DOI] [PubMed] [Google Scholar]
- 4.Berg Rune W, Kleinfeld David. Vibrissa Movement Elicited by Rhythmic Electrical Microstimulation to Motor Cortex in the Aroused Rat Mimics Exploratory Whisking. J Neurophysiol. 2003;90:2950–2963. doi: 10.1152/jn.00511.2003. [DOI] [PubMed] [Google Scholar]
- 5.Gray Kimberlyn, Sahin M. Floating Light Activated Micro-Electrical Simulators. 35th Neural Interface Workshop; Bethesda, MD. Sept 2004. [Google Scholar]
- 6.Roggan A, Minet O, Schroder C, Muller G. The Determination of Optical Tissue Properties with Double Integrating Sphere Technique and Monte Carlo Simulations. SPIE Vol 2100 Cell and Biotissue Optics. 1994 [Google Scholar]
- 7.Eggert Hans R, MD, Blazek V., PhD Optical Properties of Human Brain Tissue, Meninges, and Brain Tumors in the Spectral Range of 200 to 900 nm. Neurosurgery. 21(4):1987. doi: 10.1227/00006123-198710000-00003. [DOI] [PubMed] [Google Scholar]