Abstract
Decompression sickness (DCS) with alterations in coagulation system and formation of platelet thrombi occurs when a subject is subjected to a reduction in environmental pressure. Blood platelet consumption after decompression is clearly linked to bubble formation in humans and offers an index for evaluating DCS severity in animal models. Previous studies highlighted a predominant involvement of platelet activation and thrombin generation in bubble-induced platelet aggregation (BIPA). To study the mechanism of the BIPA in DCS, we examined the effect of acetylsalicylic acid (ASA), heparin (Hep), and clopidogrel (Clo), with anti-thrombotic dose pretreatment in a rat model of DCS. Male Sprague-Dawley rats (n = 208) were randomly assigned to one experimental group treated before the hyperbaric exposure and decompression protocol either with ASA (3×100 mg·kg−1·day−1, n = 30), Clo (50 mg·kg−1·day−1, n = 60), Hep (500 IU/kg, n = 30), or to untreated group (n = 49). Rats were first compressed to 1,000 kPa (90 msw) for 45 min and then decompressed to surface in 38 min. In a control experiment, rats were treated with ASA (n = 13), Clo (n = 13), or Hep (n = 13) and maintained at atmospheric pressure for an equivalent period of time. Onset of DCS symptoms and death were recorded during a 60-min observation period after surfacing. DCS evaluation included pulmonary and neurological signs. Blood samples for platelet count (PC) were taken 30 min before hyperbaric exposure and 30 min after surfacing. Clo reduces the DCS mortality risk (mortality rate: 3/60 with Clo, 15/30 with ASA, 21/30 with Hep, and 35/49 in the untreated group) and DCS severity (neurological DCS incidence: 9/60 with Clo, 6/30 with ASA, 5/30 with Hep, and 12/49 in the untreated group). Clo reduced fall in platelet count and BIPA (−4,5% with Clo, −19.5% with ASA, −19,9% with Hep, and −29,6% in the untreated group). ASA, which inhibits the thromboxane A2 pathway, and Hep, which inhibits thrombin generation, have no protective effect on DCS incidence. Clo, a specific ADP-receptor antagonist, reduces post-decompression platelet consumption. These results point to the predominant involvement of the ADP release in BIPA but cannot differentiate definitively between bubble-induced vessel wall injury and bubble-blood component interactions in DCS.
Keywords: platelet activation, bubble formation
decompression after a hyperbaric exposure occurs when ambient pressure decreases at the end of a dive and it can produce tissue and vascular bubbles of inert gas (usually nitrogen). Generally, bubbles are removed from the tissues by the venous blood stream and then carried to the pulmonary microvasculature where venous gas emboli are filtered and eliminated from the body through exhalation. The occurrence of an increased amount of bubbles is clearly linked to a high risk of decompression sickness (DCS) (20). The presence of bubbles leads to the development of DCS through venous/arterial embolization, vessel occlusion, and pulmonary vessel obstruction resulting in hemodynamic and biochemical changes. Venous gas emboli can cause pulmonary injury, impairing gas exchange, and it can also disturb microvascular circulation in the central nervous system. In animal models, symptoms of pulmonary DCS range from respiratory manifestations, vascular hypertension, pulmonary edema, and bronchial constriction to cardiorespiratory arrest and death (5). The nervous system is also frequently involved in dive-related complications, and neurological DCS may range from weakness to permanent disability with paralysis, mainly through a spinal cord or cerebral vascular stroke (3).
If venous gas embol is the pivotal pathologic event in DCS, many clinical symptoms cannot be explained only by the hypothesis of bubble-induced mechanical obstruction of the microvasculature (22). Bubble-induced platelet aggregation is a key factor in the thrombotic event that contributes to the pathogenesis of the disease (19). For more than a decade, the post-dive decrease in platelet count (PC) is assumed to be induced by a platelet aggregation likely to be linked to the bubble-platelet interactions in the bloodstream (6, 8, 14). In a rat model of DCS, Philp et al. (27) observed platelet microthrombi in the pulmonary microcirculation and a correlation between the extent of lung pathology and DCS severity. Later, authors showed that blood platelets recognized circulating bubbles in the bloodstream as a foreign surface that comes into contact with the formed elements of the blood, leading to platelet adhesion and aggregation around the bubbles (9, 10, 26, 34). In previous studies, we showed in humans that percent fall in PC after a scuba dive without DCS symptoms could be a predictor of bubble formation (29). In a rat model of DCS, we previously highlighted a relationship between the post-dive decrease in PC and the DCS severity (28).
Circulating bubbles in the bloodstream could produce damage to the vascular wall that activates the endothelial cells and coagulation system. Circulating bubbles could produce mechanical damage to endothelial cells going as far as complete abrasion of the endothelium, revealing the collagen and the subendothelial basal cell layer (21). The pulmonary capillary network is the first to be locally affected by the formation of bubbles (5). Physiologically, platelet activation and aggregation leads to immediate filling to the vascular lesion by forming a clot of platelets. Damage to the vascular wall causes the vessel to contract and triggers changes in local haemodynamic and rheological conditions. Although the resting endothelial cells present a thromboresistant surface, the collagen-rich subendothelium can cause thrombosis. The damaged endothelial cells release tissue factor, which, in conjunction with factor VII, initiates the coagulation process leading to rapid thrombin formation. Thrombin, a powerful platelet agonist, plays a major role in thrombus formation activating blood platelets and converting fibrinogen to fibrin. By activating factor V, VIII, and XI, thrombin initiates the appearance of platelet aggregates in the vascular system, which in turn leads to a thrombotic state. For decades, this pathological condition has been described in DCS in animals, as well as in humans (11). Finally in DCS, the blood platelets could be activated by the subendothelium they adhere to, through the local production of thrombin, which is a powerful platelet agonist, and by bubble-platelet interactions in the bloodstream. The activated platelets would then secrete the contents of their granules and initiate the arachidonic acid pathway (7). Both the adenosine diphosphate (ADP) and the thromboxane A2 released from the activated cells induce platelet aggregation via the GPIIb/IIIa-receptors, if fibrinogen is present. In previous studies, we showed that thrombin generation and platelet activation are increased in a rat model of DCS and contribute to a thrombotic event in the pathogenesis of the disease (30).
Since recognizing DCS as a possible thrombotic response, rather than a phenomenon exclusive to gas supersaturation and bubble load, we therefore hypothesize that platelet activation in DCS is partially dependent on increased bubble formation. While recompression followed by controlled decompression is the standard treatment, an effective adjunctive therapy could prevent or minimize secondary pathophysiological pathways. Antiplatelet treatment reduces the incidence of thombotic events in several pathologies. In the past, few studies have pursued drug therapies targeting the thrombotic event with platelet activation and coagulation cascade involving ADP stimulation (2).
To study the mechanism of bubble-induced platelet aggregation in DCS, we examined the effect that several agents affecting the coagulation system and platelet aggregation have on DCS outcome and platelet consumption severity in a rat model. Acetylsalicylic acid (ASA), the most widely administered antiplatelet treatment used to reduce the incidence of thrombotic events including inhibition of platelet release and aggregation; heparin (Hep), a powerful inhibitor of thrombus formation blocking thrombin generation; and clopidogrel (Clo), a selective and a specific ADP-receptor antagonist.
MATERIALS AND METHODS
Study population.
Male Sprague-Dawley rats (n = 209; Charles River, France) weighing 379 ± 53 g (mean ± SD) were used for this study. They were kept at 22 ± 1°C on a 12:12-h light/dark cycle (lights on at 7:00 AM) with standard laboratory food and water available ad libitum. Before the experiments, rats were housed in an accredited animal care facility under veterinary supervision. Procedures were in accordance with the European Communities Council rules (Bruxelles, Belgium) directive of November 24, 1986 (86/609/EEC), as enshrined in French law (decree 87/848).
Drug protocol.
According to previous results in our animal model of DCS, rats were randomly assigned to one of three groups; the first group with administered treatment before the compression and decompression protocol (C/DC group, n = 120), the second group with treatment before an atmospheric exposure (no compression and decompression protocol, non-C/DC, n = 39), and a third control group with a placebo pretreatment before the same hyperbaric exposure and the same decompression protocol as the first group (control group, n = 49). The first two groups (C/DC and non-C/DC) were split into three subgroups according to the treatment they received: ASA (n = 30 and n = 13 in C/DC and non-C/DC groups, respectively), Clo (n = 60 and n = 13), or Hep (n = 30 and n = 13). ASA and Clo were dissolved in water and administered by gavage using a rodent feeding needle. ASA (acetyl salicylate lysine salt, Amilly, France) was delivered once a day (100 mg/kg) for 2 days before the hyperbaric exposure. Clo (Sanofi Recherche, Toulouse, France) was delivered once a day (50 mg/kg) for 3 days before the experiment. Hep (Calciparine, Sanofi-Choay, Gentilly, France) was injected subcutaneously 3 h before the experiment at a dose of 500 IU/kg using a 1-ml syringe and a 26-gauge needle (33). The control group with placebo pretreatment received both an oral gavage administration with sterile water and a subcutaneous injection with normal saline, without any other treatment.
Decompression protocol.
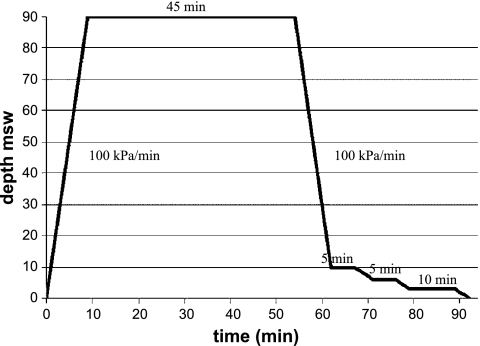
In the C/DC and control groups, animals were identified and then weighed 30 min before and immediately after diving. They were placed in a cage containing six animals, and the whole group was compressed together in a 200-liter hyperbaric chamber (DCS, Toulon, France). The animals were unrestrained and could move freely inside the cage. They were observed throughout the dive via the portholes of the chamber for any signs of distress during compression and decompression. Rats underwent the compression procedure at a rate of 100 kPa/min to a pressure of 1,000 kPa (90 msw) and maintained at pressure for 45 min while breathing air (30). At the end of the exposure period, rats were decompressed down to 200 kPa at a rate of 100 kPa/min with a 5-min stop at 200 kPa, a 5-min stop at 160 kPa, and a 10-min stop at 130 kPa. Decompression between 200 kPa and surface was performed at a rate of 10 kPa/min (Fig. 1). In a previous study using this hyperbaric exposure and decompression profile, we found that 50% of the rats died within 5 min after surfacing with pulmonary DCS signs, 32% of rats presented neurological DCS signs before dying within 24 min, and 18% of rats survived with no DCS signs (28). Carbon dioxide levels were kept below 300 ppm by continuous circulation of gases in the chamber through a soda-lime canister. Humidity (40–60%) was controlled with silicagel, and the ambient temperature was adjusted to 27°C to prevent hypothermia and ensure that the rodents remained comfortable. A light was on throughout the protocol.
Fig. 1.
Experimental hyperbaric exposure profile. Rate of compression 100 kPa/min, bottom time 45 min. Initial rate of decompression 100 kPa/min to 10 msw and 10 kPa/min from 10 msw to surface. Total decompression time: 38 min.
In the non-C/DC group, rats were placed in the same decompression chamber but exposed at sea level.
After decompression, rats were weighed. The animals were observed during a 60-min period, and the time of death was recorded when all respiratory movements ceased. Onset of DCS was recorded to the nearest 30 s by a dedicated observer. DCS signs were categorized according to severity using a 1–4 scale with 1 for no signs, 2 for neurological DCS signs, 3 for pulmonary DCS signs when the animals exhibited pronounced respiratory maneuvers, and 4 when animals died before surfacing. Neurological DCS signs included walking difficulties and forelimb and/or hindlimb paralysis.
Following the 60-min post-decompression observation period, the animals were anesthetized by intraperitoneal injection of a mixture of 16 mg/kg xylazine (Rompum 2%, Bayer Pharma) and 100 mg/kg ketamine (Imalgène 1000, Laboratoire Rhône-Merieux) for blood sample collection and killed by exsanguination.
Blood sampling techniques and platelet count.
For platelet count analysis, blood samples were taken sequentially by means of a small incision at the end of the rat tails. Stroking the tail gently results in blood droplets at the incision site. Up to 20 μl of blood were collected within 90 s by specially trained staff. The samples were collected into an Eppendorf tube 30 min before the experimental protocol and 30 min after the exposure in all groups. The samples were fixed by EDTA (12 mM) to avoid coagulation and analyzed with an animal blood counter (Scil vet ABC, Scil, France).
Statistical analysis.
For statistic processing, we used the Sigmastat 3.0 software program (SPSS, Chicago, IL). The data were distributed normally and presented as means ± standard deviation. However, we used non-parametric statistics because of the small sample size. Wilcoxon signed rank test was used for paired data, whereas a Mann Whitney test was used for comparisons in different groups. A value of P < 0.05 was considered statistically significant. Data are presented as means ± SD throughout the article.
RESULTS
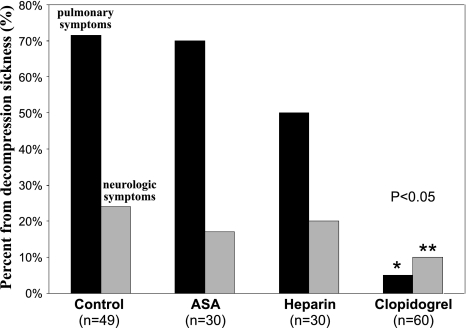
For DCS incidence and severity, there was no difference among the control group and both ASA and Hep groups (Table 1). The presence of bubbles leads to the development of DCS through venous/arterial embolization vessel occlusion and pulmonary vessel obstruction, resulting in hemodynamic and biochemical changes. Venous gas emboli can cause pulmonary injury, impairing gas exchange and causing symptoms of pulmonary DCS ranging from respiratory manifestations, vascular hypertension, pulmonary edema, and bronchial constriction to cardiorespiratory arrest and death in an animal model (5). Lethal DCS with pulmonary symptoms including abnormal breathing, respiratory arrest, and ultimately death, occurred during the 60-min observation period in 35 of 49 (72%) rats in the control group, 21 of 30 (70%) rats in the Hep group, 15 of 30 (50%) rats in the ASA group, and in 3 of 60 (5%) animals pretreated with Clo, with a significant difference between this last group and the three others (P < 0.01; Fig. 2). Neurological DCS signs without death, including limb paralysis and walking difficulties, occurred in 12 of 49 (24%) rats in the control group, 5 of 30 (17%) rats in the Hep group, 6 of 30 (20%) rats in the ASA group, and 9 of 60 (15%) animals pretreated with Clo (Fig. 2). Finally, 48 of 60 (85%) rats pretreated with Clo survived following surfacing with no apparent DCS symptoms compared with 2 of 49 (4%) in the control group, 4 of 30 (13%) in the Hep group, and 9 of 30 (30%) in the ASA group, with the Clo group being statistically significantly different than the three others (P < 0.001).
Table 1.
| Nb (%) | Control Group | Heparine Group | ASA Group | Clopidogrel Group |
|---|---|---|---|---|
| Lethal DCS | 35 (72 %) | 21 (70 %) | 15 (50 %) | 3 (5 %) |
| DCS without death | 12 (24 %) | 5 (17 %) | 6 (20 %) | 9 (15 %) |
| No DCS | 2 (4 %) | 4 (13 %) | 9 (30 %) | 48 (80 %) |
| Total | 49 (100 %) | 30 (100 %) | 30 (100 %) | 60 (100 %) |
In non C/DC groups, there was no significant change in platelet count after the experiment regardless of the pretreatment drug used, heparin, ASA or Clopidogrel (433 ± 64 versus 417 ± 61 × 103 mm−3, 356 ± 49 versus 346 ± 58 × 103 mm−3, 401 ± 41 versus 391 ± 96 × 103 mm−3 respectively, P = 0.72.
Fig. 2.
Percent forms (%) of lethal decompression sickness (DCS) preceded by pulmonary symptoms (abnormal breathing and respiratory arrest) during the 60-min observation period (in black) and neurologic DCS with symptoms including limb paralysis and walking difficulties (in gray) in heparin (Hep), acetylsalicylic acid (ASA), and clopidogrel group (Clo). *Significant difference (P < 0.01) for the pulmonary DCS form between the Clo group and the groups with different antithrombotic pretreatment. **Significant difference (P < 0.01) for neurological DCS form between Clo group and the other groups with different antithrombotic pretreatment.
In non-C/DC groups, there was no significant change in platelet count after the experiment regardless of the pretreatment drug used: Hep, ASA, or Clo (433 ± 64 vs. 417 ± 61×103 mm−3, 356 ± 49 vs. 346 ± 58×103 mm−3, 401 ± 41 vs. 391 ± 96×103 mm−3, respectively, P = 0.72).
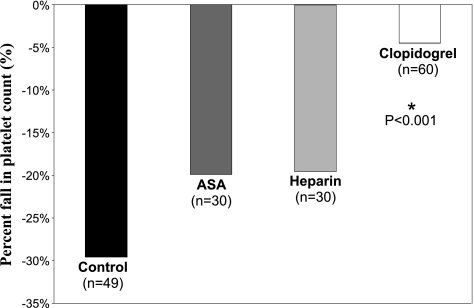
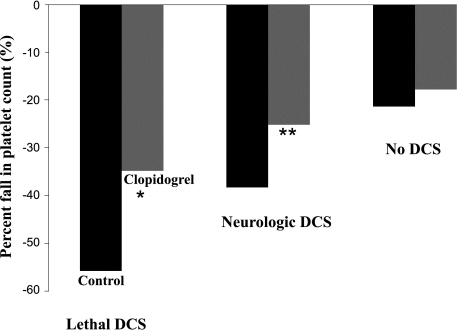
In C/DC groups the decrease in platelet count was significantly lower in animals pretreated with Clo compared with those pretreated with Hep and ASA, as well as the Control groups (−4.5%, −19.9%, −19.5%, and −29.6%, respectively, P < 0.05; Fig. 3). Looking at the DCS severity in C/DC, the mean percent fall in platelet counts was significantly lower in animals pretreated with Clo compared with those not treated in the control group for the lethal DCS form (34.8 ± 14.4% in Clo group vs. 55.8 ± 22.4% in the control group, P < 0.05) and for the neurologic DCS form (25.2 ± 8.1% in the Clo group vs. 38.2 ± 26.5% in the control group, P < 0.05). There was no significant change in percent fall in platelet count in animals without DCS signs (17.8 ± 23.1% in the Clo group vs. 21.4 ± 30.1% in the control group; P < 0.01, Mann-Whitney, P = 0.08; Fig. 4).
Fig. 3.
Mean percent fall in blood platelet count (%) after hyperbaric exposure and decompression protocol in control, ASA, Hep, and Clo. *Significant difference (P < 0.001) between the Clo group and the different antithrombotic pretreatment groups.
Fig. 4.
Mean percent fall in blood platelet count after hyperbaric exposure and decompression protocol in Control (black) and Clo (gray) groups for lethal DCS, neurologic DCS, and no DCS forms. *Significant difference (P < 0.05) between the Control and Clo groups for lethal DCS form. **Significant difference (P < 0.05) between the Control and Clo groups for neurologic DCS form.
DISCUSSION
In the control group, the incidence of lethal DCS occurring during the observation period and the number of rats presenting either the neurologic or the pulmonary form of DCS confirm other results obtained in dry conditions (16). We found that blood platelet count measured after the hyperbaric exposure and decompression protocol was significantly decreased compared with the predive values. This result is in accordance with previous studies in different rat models of DCS (9, 13, 24, 28, 34). The results have shown that Clo reduced the DCS risk and severity with no significant protective effect in the ASA group and Hep group. In our Study, Clo reduced the decrease in platelet count and bubble-induced platelet aggregation (−4.5% with Clo, −19.5% with ASA, −19.9% with Hep, and −29.6% in untreated group). The results were statistically significant compared with Hep, ASA, and controls.
ASA inhibits the cyclooxygenase (COX) pathways, which is one among several mechanisms regulating platelet function. In humans and at low concentration (30–300 mg/day), ASA blocks the platelet-COX (COX-1) and thromboxane A2 synthesis and its subsequent release from the platelets (17). At a higher concentration, ASA inhibits endothelial-COX (COX-2) and prostacyclin generation, which reduces the anti-thrombotic effect of blood platelets but involves the anti-inflammatory effect (37). ASA reduced the production of reactive oxygen species via its uncoupling of oxidative phosphorylation, and changes in redox balance are known to alter platelet activation and aggregation in decompression sickness. In rat models, ASA has long been used to alleviate the pain associated with DCS, but its effects at preventing DCS symptoms have been limited in their scope (1). In our study, there is no difference in DCS outcome and DCS severity between ASA pretreatment and control groups. This result corroborates previous studies in a rat model of DCS subjected to the same dose of 100 mg/kg three times daily for 2 days before hyperbaric exposure (18). However, only one study in a rat model of DCS has shown a significant decrease in DCS incidence (22% in ASA group vs. 40% in control group) and DCS severity (12% incidence of death in ASA group vs. 31% in control group) with ASA pretreatment before a hyperbaric exposure in a dry chamber and a fast decompression. Nevertheless in this study, the protective effect against DCS was obtained for ASA pretreatment at a dose of 100 mg·kg−1·day−1 during 1 mo before the simulated dive (31). In our study, ASA did not significantly reduce bubble-induced platelet aggregation after the hyperbaric exposure and decompression protocol. Our results corroborate those of previous studies in a rat model of DCS (4). Only one study in humans has shown a protective effect of ASA pretreatment on the post-dive decrease in platelet count at the dose of 325 mg·kg−1·day−1 but after a saturation dive (25). On the basis of these results we conclude that the COX pathway does not seem to be the predominant mechanism in bubble-induced platelet aggregation in our rat model of DCS.
Hep prevents thrombus formation under arterial or venous flow conditions inhibiting thrombin generation in the presence of antithrombin III, a necessary cofactor. In our study, there is no difference in DCS outcome and DCS severity between Hep pretreatment and control groups. Previous studies confirm this result in a rat model (12), in a dog model (32), and in a rabbit model (15) with no significant change in DCS incidence and severity. One study has shown a significant decrease in altitude DCS incidence and severity in rats when Hep was administered before a hypobaric exposure (23). In the bloodstream, circulating bubbles damage the vascular wall and activate endothelial cells. Mechanical damage to the vascular wall can result in a complete abrasion of the endothelium, revealing the collagen and the sub-endothelial basal cell layer (21). Most tissues can be affected by these lesions; however, the pulmonary capillary network is the first to be locally affected by the formation of bubbles (5). Physiologically, the purpose of platelet activation and aggregation is to immediately fill the vascular lesion by forming a clot of platelets. The interaction of platelets with the vascular wall plays a key role in normal hemostasis, ensuring vascular function and preventing hemorrhages in vessels of the microcirculation. Damage to the vascular wall causes the vessel to contract, triggers changes in local hemodynamic and rheological conditions, and in particular, exposes the connective tissue extracellular matrix. Although the resting endothelial cell presents a thromboresistant surface, the collagen-rich subendothelium can cause thrombosis. The effects of hemodynamic forces and the Von Willebrand factor cause the platelets to adhere to the subendothelium. This is the first stage of hemostasis. Alongside this platelet activation, the coagulation system is also activated. The damaged endothelial cells release tissue factor, which in conjunction with factor VII initiates the coagulation process and rapid thrombin formation. Thrombin, which is a powerful platelet agonist, initiates platelet activation and thus the appearance of aggregates in the vascular system, which in turn lead to a thrombotic state, as is the case in disseminated intravascular coagulation. Although this pathological condition has been described in DCS (11, 26, 27), the results of our rat model of DCS cannot support this mechanism for vessel damage-induced platelet activation and aggregation. In our study, there was no difference between the postdecompression decrease in platelet count for rats pretreated with heparin and those without pretreatment. Heparin pretreatment did not significantly reduce bubble-induced platelet aggregation after decompression. Our results using Hep suggest no preferential or benefic effect against bubble-endothelial cell interaction that affects the vessel integrity and enhances coagulation system in DCS. In our animal model of DCS, the results cannot definitively prejudge between bubble-induced vessel wall injury and bubble-blood platelet interaction in activation and aggregation.
Pretreatment with Clo selectively and irreversibly inhibits the low affinity P2Y12-receptor on platelets resulting in noncompetitive inhibition of ADP-induced platelet fibrinogen binding and finally platelet aggregation and reduced stability of platelet aggregates (7). Clo pretreatment may also inhibit platelet aggregation induced by low concentrations of collagen or thrombin, two powerful platelet agonists, presumably by inhibiting the effects of platelet-released ADP (33). In our rat model of DCS, Clo pretreatment has markedly improved the DCS outcome, has reduced DCS severity and percent decrease of platelet count after the decompression protocol. Clo has decreased bubble-induced platelet aggregation severity reducing ADP pathway-induced platelet aggregation. In vitro, platelet aggregation triggered by nitrogen bubbles causes blood platelet consumption. The mechanisms of this aggregation are similar to those caused by platelet agonists such as ADP (35) and the authors suggested that the bubbles were able to activate the platelets in vitro by releasing ADP. This other powerful platelet agonist is stored in the platelets and released on platelet activation reacting with receptors found on the platelets (P2y12) leading to further platelet activation (36). Observations carried out in vitro using electron microscopy have shown that the induction of platelet aggregation by bubbles was linked to the contact and adhesion of plasma proteins and lipids to the interface between the liquid blood phase and the gaseous bubble phase (35). This is an active metabolic process that can be altered by pharmacological agents, in particular those that increase intracellular cAMP concentrations in the platelets (36). If DCS is partly the consequence of the bubble-induced mechanical obstruction of vessels, thrombotic events with platelet activation through ADP release could play a key role in the pathogenesis of the disease. Regarding the results focusing on the protective effect of Clo pretreatment, ADP-platelet agonists appear to be an important cofactor in bubble-induced platelet aggregation.
Considering activation of platelet function in DCS, Clo pretreatment could inhibit bubble-induced platelet aggregation acting on the ADP pathway. The main effect of Clo in preventing DCS is unlikely to be due to the inhibition of platelet aggregation induced by low concentration of thrombin but to a benefic effect against bubble-blood platelet interactions. These findings should lead to a better understanding of the use of antiplatelet drugs in the medical treatment of DCS and further studies are needed to validate the effects of antithrombotic therapy.
In conclusion, the results of this study suggest that Clo pretreatment, a powerful ADP inhibitor, has a protective effect on decompression risk, thus significantly improving DCS outcome and reducing DCS severity and bubble-induced platelet aggregation. The results point to the predominant involvement of ADP in bubble-induced platelet aggregation. However, the results cannot differentiate between bubble-induced vessel wall injury and bubble-blood component interactions in platelet activation in our animal model of DCS. Actually, a study in divers should be aimed at demonstrating that Clo can minimize secondary pathophysiological pathways and can offer a benefit as an adjuvant in DCS treatment.
GRANTS
This study was supported by a Grant DGA (COSSA 2008).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Authors are indebted to Dr. Savi for very helpful comments about the study and the manuscript and to B. Zouani and S. Ciret for valuable technical help. Laboratory blood tests were carried out by M. Nicolas and B. Zouani from the Rresearch Department headed by Jean-Jacques Risso, Military Biomedical Research Institute.
REFERENCES
- 1. Bennet PB, Brock AJ. Action of selected drugs on decompression sickness in rats. Aerospace Med 40: 607–610, 1969 [PubMed] [Google Scholar]
- 2. Bove AA. The basis for drug therapy in decompression sickness. Undersea Biomed Res 9: 91–111, 1982 [PubMed] [Google Scholar]
- 3. Broome JR. Association of CNS hemorrhage with failure to respond to recompression treatment-implications for management of refractory cases of decompression illness. In: Undersea and Hyperbaric Medical Society, edited by Moon RE, Sheffield PJ, 1996, p. 364–373 [Google Scholar]
- 4. Broussolle B, Hyacinthe R, Mainart G, Stoltz JF. Use of anti platelet aggregation substances in the treatment of decompression accidents in the rats. J Physiol 67: 334–344, 1973 [PubMed] [Google Scholar]
- 5. Butler BD, Hills BA. Transpulmonary passage of venous air emboli. J Appl Physiol 59: 543–547, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Clay JR. Histopathologiy of experimental decompression sickness. Aerospace Med 34: 1107–1110, 1963 [PubMed] [Google Scholar]
- 7. Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost 86: 222–232, 2001 [PubMed] [Google Scholar]
- 8. Geller F. Uber die Blutgerinung unter dem Einfluss von Gasen (Coagulation and circulating gas bubble). Arch Gesamte Physiol Menschen Tiere 244: 687–695, 1941 [Google Scholar]
- 9. Giry P, Porlier G, Eastman D, Radomski MW. Dive-induced modifications in platelet kinetics in rats. Undersea Biomed Res 4: 147–157, 1977 [PubMed] [Google Scholar]
- 10. Hallenbeck JM, Bove AA, Moquin RB. Accelerated coagulation of whole blood and cell-free plasma by bubbling in vitro. Aerospace Med 44: 712–714, 1973 [PubMed] [Google Scholar]
- 11. Holland HA. Discussion of disseminated intravascular coagulation in decompression sickness. Report 585 Groton, CT: US Naval Submarine Medical Center, 1969 [PubMed] [Google Scholar]
- 12. Inwood BM. Experimental evidence in support of the hypothesis that intravascular bubbles activate the hemostatic process. In: Symposium of Blood-Bubble Interactions in Decompression Sickness DCIEM Rep N° 73-CP-960: 171–200, 1973 [Google Scholar]
- 13. Jacey MJ, Heyder E, Williamson RA. Biochemistry and haematology of decompression sickness: a case report. Aviat Space Environ Med 47: 657–661, 1976 [PubMed] [Google Scholar]
- 14. Jacobs MH, Stewart DR. Observations on the blood of albino rats following rapid decompression. US National Research Council Comm. Aviat Med 1–5, 1942 [Google Scholar]
- 15. Laborit H, Barthelemy L, Perrimond-Trouchet R. Action of heparin in the treatment of complications of decompression. Agressologie 2: 229–236, 1961 [PubMed] [Google Scholar]
- 16. Lillo RS, Parker EC. Mixed-gas model for predicting decompression sickness in rats. J Appl Physiol 89: 2107–2116, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Michibayashi T. Platelet aggregation and vasoconstriction related to platelet cyclooxygenase and 12-lipoxygenase pathways. J Atheroscler Thromb 12: 154–162, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Montcalm-Smith EA, Fahlman A, Kayar SR. Pharmacological interventions to decompression sickness in rats: comparison of five agents. Aviat Space Environ Med 79: 7–13, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Moon RE, Fawcett TA, Exposito AJ. Platelet count in deep saturation diving. Undersea Biomed Res 19: 279–286, 1992 [PubMed] [Google Scholar]
- 20. Nishi RY. Doppler evaluation of decompression tables. In: Man in the Sea, edited by Lin YC, Shida KK. Honolulu: University of Hawaii Press, 1990, p. 297–316 [Google Scholar]
- 21. Nossum V, Koten S, Brubakk AO. Endothelial damage by bubbles in the pulmonary artery of the pig. Undersea Hyperb Med 26: 1–8, 1999 [PubMed] [Google Scholar]
- 22. Olszanski R, Sicko Z, Baj Z. Effect of nitrox saturation diving upon platelet hemostasis. Bull Inst Marit Trop Med Gdynia 41: 59–62, 1990 [PubMed] [Google Scholar]
- 23. Philp RB. The ameliorative effects of heparine and depolymerized hyaluronate on decompression sickness in rats. Can J Physiol Pharmacol 42: 819–829, 1964 [DOI] [PubMed] [Google Scholar]
- 24. Philp RB. A review of blood changes associated with compression-decompression: relationship to decompression sickness. Undersea Biomed Res 1: 117–150, 1974 [PubMed] [Google Scholar]
- 25. Philp RB, Bennet PB, Andersen JC, Fields GN. Effects of aspirin and dipyridamole on platelet function, hematology and blood chemistry of saturation divers. Undersea Biomed Res 6: 127–146, 1979 [PubMed] [Google Scholar]
- 26. Philp RB, Inwood MJ, Warren BA. Interactions between gas bubbles and components of the blood: implications in decompression sickness. Aerospace Med 43: 946–953, 1972 [PubMed] [Google Scholar]
- 27. Philp RB, Schacham P, Gowdey CW. Involvement of platelets and microthrombi in experimental decompression sickness: similarities with disseminated intravascular coagulation. Aerospace Med 41: 1358–1361, 1971 [PubMed] [Google Scholar]
- 28. Pontier JM, Blatteau JE, Vallée N. Blood platelet count and severity of decompression sickness in rats after a provocative dive. Aviat Space Environ Med 79: 761–764, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Pontier JM, Jimenez C, Blatteau JE. Blood platelet count and bubble formation after a dive to 30 msw for 30 min. Aviat Space Environ Med 79: 1–4, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Pontier JM, Vallée N, Bourdon L. Bubble-induced platelet aggregation in a rat model of decompression sickness. J Appl Physiol 107: 1825–1829, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Popovic P, Popovic V, Honeycutt C. Levodopa and aspirin pretreatment beneficial in experimental decompression sickness. Proc Soc Exp Biol Med 169: 140–143, 1982 [DOI] [PubMed] [Google Scholar]
- 32. Reeves E, Workman RD. Use of heparin for the therapeutic-prophylactic treatment of decompression sickness. Aerospace Med 42: 20–23, 1971 [PubMed] [Google Scholar]
- 33. Savi P, Nurden P, Nurden AT, Levy-Toledano S, Herbert JM. Clopidogrel: a review of its mechanism of action. Platelets 9: 251–255, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Tanoue K, Mano Y, Kuroiwa K. Consumption of platelets in decompression sickness of rabbits. J Appl Physiol 62: 1772–1779, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Thorsen T, Dalen H, Bjerkvig R, Holmsen H. Transmission and scanning electron microscopy of N2 microbubble-activated human platelets in vitro. Undersea Biomed Res 14: 45–58, 1987 [PubMed] [Google Scholar]
- 36. Thorsen T, Ovstedal T, Vereide A, Holmsen H. Effects of platelet antagonist on the reduction in platelet density caused by microbubbles in vitro. Undersea Biomed Res 13: 289–303, 1986 [PubMed] [Google Scholar]
- 37. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 110: 255–258, 2003 [DOI] [PubMed] [Google Scholar]