Abstract
The formation of a provisional scaffold is essential in wound healing. However, for tissues inside of joints, this process is impeded by the synovial fluid environment and wound healing is significantly impaired as a result. Therefore, development of substitute provisional scaffolds which are effective in the intra-articular environment is of great interest. Collagen-platelet hydrogels have recently been found useful as substitute provisional scaffolding materials. In this study, our hypothesis was that increasing the collagen density in the hydrogel would result in physiologic changes that would be likely to affect their function as provisional scaffold substitutes. The primary functional outcome measures were modulus of the hydrogel, platelet activation, fibroblast proliferation, and scaffold retraction. Increased collagen density resulted in collagen-platelet hydrogels with a higher storage modulus. Platelet activation was not found to be dependent on the collagen density within the range tested. Increasing the collagen density had a suppressive effect on both fibroblast proliferation and scaffold retraction. These studies suggest that the collagen density may be able to significantly influence the function of collagen-platelet hydrogels used as substitute provisional scaffolds.
Keywords: collagen, hydrogel, ligament, ACL, scaffold, provisional scaffold, fibroblast, wound healing, PDGF, tissue engineering, platelet
INTRODUCTION
The formation of an effective provisional fibrin scaffold is essential in wound healing. The provisional scaffold serves multiple functions, it physically fills the wound site and provides structure for the migration of surrounding cells, it entraps platelets and activates them to start the complex growth factor release pattern typical of wound healing, and it provides a supportive environment for the fibroblasts that invade and remodel it into functional tissue.
However, injured tissues inside of joints do not form this provisional scaffold.1–5 One possible explanation is the postinjury up-regulation of urokinase plasminogen activator in the synovial environment,6 which results in increased circulating plasmin levels and, consequently, in the premature dissolution of the fibrin provisional scaffold. For example, recent work has shown that for intra-articular ligaments, such as the anterior cruciate ligament (ACL), no provisional scaffold forms between the ruptured ligament ends, and consequently, there is no formation of functional scar.1–4 Similar findings have also been reported in meniscus as well.5 Therefore, part of a novel strategy to enhance healing of tissues within joints is the placement of a substitute provisional scaffold that can resist early degradation and provide an environment supportive of the wound healing process.2–4
Collagen-based scaffolds combined with platelet concentrates have recently been found to stimulate repair in the joint environment in two large animal models.2–4 This is likely due in part to the collagen attenuation of the fibrin degradation by plasmin7 and the low levels of normal catabolic activity of matrix metalloproteinases in the synovial environment,8 which prevents the premature dissolution of this substitute provisional scaffold, and allows it to fill the wound site and bridge the ACL ruptured ends. In addition, collagen stimulates the platelet release of growth factors, including platelet-derived growth factor (PDGF), which subsequently promote cellular proliferation within and adjacent to the wound site.4,9 This provisional scaffold would be supported by the neovascularization that is part of the natural response of the ruptured ligament.1,9
For optimal function in the intra-articular environment, the scaffold must meet mechanical requirements including (1) sufficient stiffness to remain in the wound site and (2) minimal scaffold retraction (to facilitate scaffold retention in the wound and maintain tissue continuity over the early healing period). In addition to the mechanical requirements, the scaffold must also provide several biologic functions. One of the key biologic functions that must also be supported by the engineered scaffold is platelet activation, a function that occurs when platelets come into contact with collagen10,11 and therefore is likely to be dependent on both platelet density and collagen density. Finally, the scaffold must support fibroblast growth as the functioning fibroblasts are needed to remodel the provisional scaffold into more ligamentous-like tissue over time.12,13
Collagen-based hydrogels have been used as 3-D scaffolds to study cell properties14–16 and to develop tissue analogs, such as vasculature,17,18 skin,19 spinal cord,20 bone,21 and tendon.22 In all cases, the interaction of cells and scaffold is of foremost importance. Despite the wide use of collagen-based hydrogels, relatively few studies have assessed the effect of the collagen density on scaffold performance.22–25 Furthermore, these studies have looked into only one scaffold characteristic at a time, for example, retraction,23 migration,24 rheology,25 or lower collagen concentrations.22 The effect of collagen density on the overall mechanical and physiologic performance of the hydrogel has yet to be fully defined.
In this study, we hypothesized that higher collagen density within the hydrogel would minimize provisional scaffold retraction and improve its rheologic properties, at the same time increasing the degree of platelet activation and stimulation of fibroblast proliferation. To test this hypothesis, provisional scaffolds containing three different densities of collagen were evaluated in vitro using rheologic behavior, platelet activation, and fibroblast proliferation as the primary outcome measures. A secondary outcome of scaffold retraction was also assessed.
MATERIALS AND METHODS
Manufacture of the provisional scaffolds
Tail tendons were obtained from breeder rats undergoing euthanasia for other Institutional Animal Care and Use Committee approved studies. The tendons were sterilely harvested, minced, and acid-solubilized into a slurry. Collagen content within the slurry was indirectly determined by quantifying hydroxyproline and adjusted to 12 mg/mL, which correspond to a collagen density in the provisional scaffold of 4 mg/mL. Aliquots of the slurry were further diluted in order to have provisional scaffolds of 2 and 3 mg/mL. This range of collagen density (2–4 mg/mL) is appropriate for the in vivo delivery of the gel. Lower collagen density gels do not achieve sufficient viscosity for delivery, and gelation is too slow. Higher collagen density gels would be extremely viscous, which would be problematic in terms of delivery of the gel.
The collagen slurry was mixed with HEPES Buffer (Cellgro, Mediatech, Herndon, VA), Ham's F-10 medium (MP Biomedicals LCC, Aurora, OH), Antibiotic-Antimycotic solution (Cellgro, Mediatech, Herndon, VA), and sterile water. The solution was neutralized to a pH of 7.4 using 7.5% sodium bicarbonate (Cambrex BioScience Walkersville, Walkersville, MD) and kept on ice until adding the fibrin-platelet concentrate (FPC) component of the scaffold.
Platelet preparation
A platelet solution was produced using the Harvest Smart PreP2 System (Harvest Technologies, Plymouth, MA). Briefly, 54 mL of human whole blood was drawn into a 60 mL syringe containing 6 mL acid-citrate dextrose (Harvest Technologies, Plymouth, MA). The blood was centrifuged to allow for the decantation of plasma, platelets, and white blood cells from most of the red blood cells. A second centrifugation step was used to form a pellet of platelet concentrate at the bottom of the decantation chamber. Excess plasma was removed and the platelet pellet resuspended in the remaining plasma to obtain a FPC. This method resulted in platelet enrichment of at least 7×.
Low passage, human ACL fibroblastic cells were obtained by culturing explants of ruptured human ACL tissue, retrieved at ACL reconstruction, in Dulbecco's Minimum Essential Medium (DMEM) (Cellgro, Mediatech, Herndon, VA) supplemented with 10% defined fetal bovine serum (FBS) (Hyclone, Logan, UT) and 1% antibiotic-antimycotic solution (Cellgro, Mediatech, Herndon, VA). Media was changed twice a week and third passage fibroblasts were used in this experiment. The fibroblasts were suspended in the platelet solution at 1.5 × 106 cells/mL just before incorporation in the hydrogels.
Culture of fibroblast-seeded provisional scaffolds
Collagen-platelet hydrogels were prepared at three different collagen densities (2, 3, and 4 mg/mL). For each collagen density, 12 hydrogels were seeded with human ACL fibroblasts and cultured for one (n = 6) or 10 days (n = 6); six hydrogels without cells were also cultured for one and 10 days. The cell-seeded hydrogels had a final fibroblast density of 0.5 × 106 cells/mL. All hydrogels had a platelet concentration of 4 × 108 platelets/mL. Scaffolds without platelets were not included in this study because previously no PDGF-AB was detected in the conditioned medium of similar collagen gels cultured without platelets.26 To make the scaffolds, 0.5-mL aliquots were cast in 3 cm long silastic semitubular molds between two sections of polyester mesh to produce tethered hydrogel constructs.
The collagen-platelet hydrogels were maintained at 37°C for 1 h before adding enough complete medium (DMEM + 10% FBS + 1% antibiotic/antimycotic solution) to cover the constructs. Fibroblasts were allowed to equilibrate in the scaffolds overnight before baseline fibroblast number (at day 1) was determined for the three collagen density groups. Media was collected at 12 and 36 h and on days 3, 5, and 7. On days 1, 7, and 10, the cultures were photographed for further image analysis to evaluate provisional scaffold retraction. At the 10 day time point, fibroblast number was determined again for the three collagen density groups.
Rheological properties
For rheological properties, the collagen slurry aliquots (n = 5 for 2, 3, and 4 mg/mL groups) and the other components used to manufacture the provisional scaffolds (except the sodium bicarbonate and the platelet solution) were kept on ice until testing. Immediately after being mixed, 1 mL of the collagen-platelet slurry was placed onto the center of the plate of a AR 1000 Rheometer (TA Instruments, New Castle, DE) at 25°C, fitted with a 60 mm, 1° angle acrylic cone, to measure the rheological properties of gelation. As they gelled, the collagen-platelet slurries were cyclically loaded to 1% strain with angular frequency equal to 6.3 rad/s. The shear viscoelastic parameters storage and loss moduli (G′, G″, respectively) were recorded as a function of time. The storage modulus represents the elastic component of the provisional scaffold, whereas the loss modulus represents its viscous component.
Platelet activation—PDGF-AB
At 12, 36, 72 h, conditioned media was aspirated from around each of the collagen-platelet hydrogels. Platelet activation was determined by measuring levels of the heterodimer PDGF-AB (which comprises 70% of all PDGF isoforms purified from human platelets11) at each time point. Concentrations of human PDGF-AB were determined using the commercially available Quantikine colorimetric sandwich ELISA kit (R&D Systems, Minneapolis, MN). Assays were performed in duplicate on media samples (n = 5, per group and time point) according to the instructions of the manufacturer. A standard curve was produced by a 2-fold serial dilution of a known concentration of PDGF-AB provided in the kit to make final concentrations of 0, 31.2, 62.5, 125, 250, 500, 1000, and 2000 pg/mL. The color changes of the final reactions were measured at 450 nm and the standard curve was used to calculate the PDGF-AB concentrations in the conditioned media.
Fibroblast proliferation
Fibroblast number in the collagen-platelet hydrogels was measured using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The MTT assay evaluates the ability of the fibroblasts’ mitochondrial dehydrogenase enzymes to convert yellow, soluble MTT salt into purple formazan salt. The MTT solution was prepared fresh at a concentration of 1 mg/mL in serum-free DMEM from a sterile 5 mg/mL MTT (Sigma-Aldrich, St. Louis, MO) stock solution in sterile 1× Dulbecco's Phosphate Buffered Saline (DPBS) (Cellgro, Mediatech, Herndon, VA). For each collagen density, at each time point, there were six experimental, fibroblast-seeded, scaffolds and three control, unseeded, scaffolds. A sterile spatula was used to transfer each provisional scaffold from the culture tubes to individual wells of a fresh 12-well plate. MTT solution (1 mL) was added to each well and the plates incubated for 3 h (at 37°C, 5% CO2). Subsequently, the excess MTT solution was removed and 1 mL of sterile 1× DPBS added to each well. The plates were placed on a horizontal shaker (Fisher Scientific Clinical Rotator, 100 rpm) at room temperature for 30 min. Rinses were repeated until the absorbance values of the wash were less than 0.100. Then each provisional scaffold was transferred with a sterile spatula into sterile 3 mL centrifuge tubes with 1 mL of a detergent reagent obtained by mixing (1:1 volume ratio) 20% Sodium Dodecyl Sulfate (SDS) (Teknova, Hollister, CA) and Formamide (VWR, West Chester, PA), and incubated overnight at 37°C. Finally, the tubes were centrifuged for 5 min at 1500 rpm, and aliquots of the supernatant from each tube (200 μL) were transferred onto a sterile 96-well plate. Absorbance was measured at 562 nm. Control groups had only background values. Fibroblast proliferation was calculated based on the increase in absorbance between days 1 and 10, after subtraction from the control background.
Provisional scaffold retraction
Digital pictures of the cultures (n = 6 for cell-seeded groups and n = 3 for unseeded groups) were taken on days 1, 7, and 10, and construct “area” measured using a public domain image processing software (ImageJ 1.37V, NIH). Retraction, for each construct, was evaluated as the percent decrease of a particular construct's area at days 7 and 10 with respect to that construct's area at day 1.
Statistical analysis
The statistical analysis was performed using SPSS version 15.0 (SPSS Inc, Chicago, IL). The rheological properties of the provisional scaffolds were compared using one-factor ANOVA model. The MTT absorbance at days 1 and 10 (fibroblast proliferation) were compared using two-factor ANOVA mixed model. PDGF-AB levels in conditioned media at 12, 36, and 72 h (platelet activation) were compared using a repeated measures two-factor ANOVA mixed model. Scaffold areas at days 1, 7, and 10 (retraction) were compared using a repeated measures three-factor ANOVA mixed model. Post-hoc multiple comparisons, when necessary, were adjusted using the Bonferroni correction. All values of p < 0.05 were considered statistically significant.
RESULTS
Rheological properties
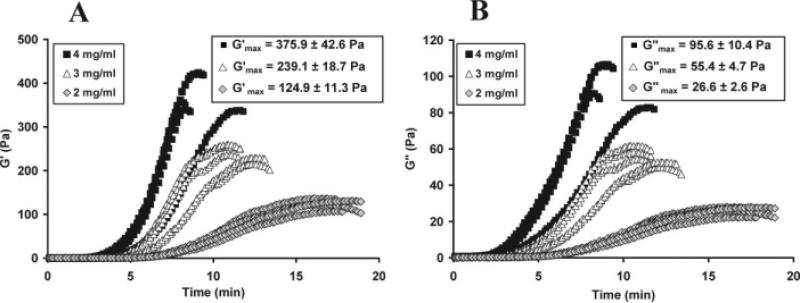
Both the storage (G′) and loss (G″) moduli of the provisional scaffolds were directly proportional to collagen density, as shown in Figure 1(A,B), all comparisons significant, p < 0.001. The time needed to reach the maximum storage modulus () decreased with increasing collagen density (17.2 ± 0.9, 11.6 ± 1.0, and 9.2 ± 1.4 min, mean ± standard deviation, for the 2, 3, and 4 mg/mL groups, respectively (n = 5 per group), all comparisons significant, p ≤ 0.017).
Figure 1.
(A) Storage modulus (G′) and (B) loss modulus (G″) vs. time as a function of collagen concentration (n = 5 per group) demonstrating the direct correlation between maximum moduli and collagen concentration for both storage and loss moduli. All between-group comparisons for and were significant (p < 0.001).
Platelet activation—PDGF-AB
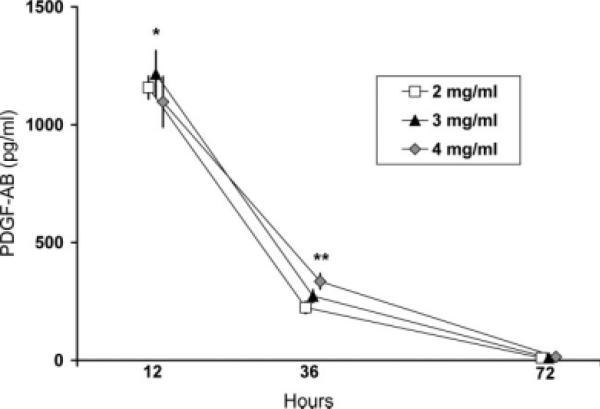
For all collagen concentrations, the majority of the PDGF-AB released into the medium occurred in the first twelve hours after platelet contact with collagen (1157 ± 48, 1215 ± 100, and 1097 ± 107 pg/mL, mean ± standard deviation, for the 2, 3, and 4 mg/mL collagen groups, n = 5 per group, respectively). The release of PDGF-AB declined by more than 75% by 36 h (224 ± 26, 272 ± 27, and 335 ± 33 pg/mL for the 2, 3, and 4 mg/mL collagen groups, respectively), and even further by 72 h (10.2 ± 2.4, 10.1 ± 3.7, and 14.4 ± 3.8 pg/mL for the 2, 3, and 4 mg/mL collagen groups, respectively). ANOVA mixed model analysis showed a significant interaction of the factors “collagen concentration” and “time” (p = 0.014), meaning that the effect of collagen concentration on the PDGF-AB release varied according to the time point evaluated. Post-hoc tests revealed that at 12 h the 3 mg/mL scaffolds had the highest release of PDGF-AB, significant difference with respect to the 4 mg/mL scaffolds, p = 0.005; while the 4 mg/mL scaffolds released most PDGF-AB at 36 h, significant difference with respect the 2 mg/mL scaffolds, p = 0.008 (Fig. 2). No other comparison was statistically significant (p > 0.2).
Figure 2.
PDGF-AB eluted into surrounding medium from collagen provisional scaffolds at 12, 36, and 72 h, n = 5 per cell-seeded group. Post-hoc comparisons showed significant differences as noted by * (3 mg/mL higher than 4 mg/mL, p = 0.005) and ** (4 mg/mL higher than 2 mg/mL, p = 0.008). No other comparison was statistically significant (p > 0.2). Symbols and error bars represent mean and standard deviation, respectively.
Fibroblast proliferation
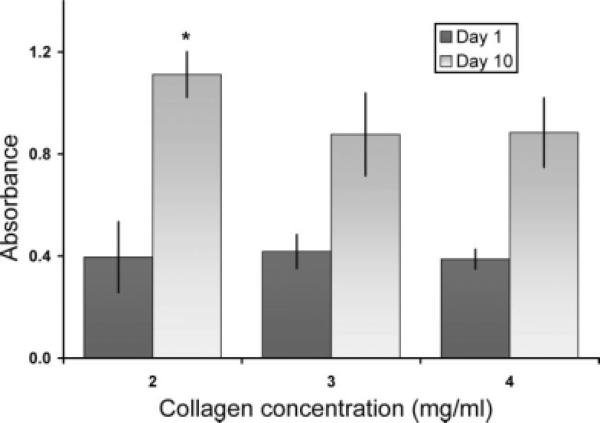
Fibroblast number after 10 days of culture was significantly higher in collagen-platelet hydrogels with collagen density of 2 mg/mL than in hydrogels with higher collagen densities. An 181 ± 42% increase in fibroblast number was seen in the 2 mg/mL scaffolds (mean ± standard deviation) with respect to initial seeding, whereas the 3 mg/mL group had only an increase of 110 ± 41% and the 4 mg/mL group had an increase of 128 ± 30% over the same time point (p < 0.005 for comparisons between 2 mg/mL and the other two groups, Fig. 3). Fibroblast number at day one was similar for all fibroblast seeded hydrogel groups (p = 0.84), suggesting satisfactory uniformity in the fibroblast seeding process. The unseeded control hydrogels had absorbance values comparable to blank readings.
Figure 3.
Fibroblast proliferation over 10 days as a function of collagen concentration, n = 6 per group and per time point (* denotes statistically significant difference with respect to other day-10 groups, p < 0.005). Day 1 absorbancies around 0.4 reflect initial cell density of 5 × 105 cells/mL (i.e., 2.5 × 105 cells per construct). Columns and error bars represent mean and standard deviation, respectively.
Provisional scaffold retraction
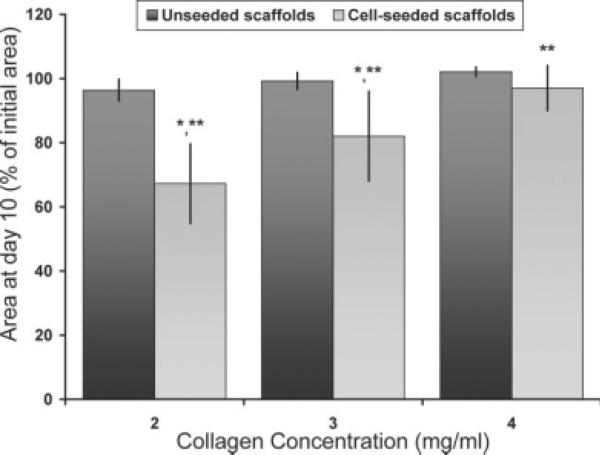
There were no changes in scaffold size between days 1 and 7 for all groups (p > 0.99). At day 10, scaffold retraction was significantly affected by collagen density and fibroblast-seeding condition, as shown in Figure 4, with fibroblast-seeded scaffold retraction inversely proportional to collagen concentration (33.1, 17.6, and 3.3% for the 2, 3, and 4 mg/mL collagen concentration scaffolds, respectively, p < 0.001). There was no significant retraction of unseeded scaffolds, independent of collagen concentration (p ≥ 0.78), or of the 4 mg/mL collagen concentration scaffolds, regardless of time (p ≥ 0.98) or fibroblast-seeding condition (p = 0.25).
Figure 4.
Retraction of cultured FPC-collagen hydrogels shown as percent decrease with respect to initial area (day 1), n = 6 for cell-seeded groups and n = 3 for unseeded groups. Initial areas of all groups were similar, p = 0.14. * denotes statistically significant difference when comparing cell seeding conditions at day 10 and time (i.e., day 10 vs. days 1 and 7), in both cases, p < 0.001. ** denotes statistically significant difference when comparing cell-seeded groups at day 10, p < 0.001. Columns and error bars represent mean and standard deviation, respectively.
DISCUSSION
The main objective of this study was to test the hypothesis that increasing the collagen density within a provisional scaffold would alter the physiologic and biomechanical functions of that scaffold. The results demonstrate that by moderately increasing collagen density we were able to triple the scaffolds’ strength, shorten the time needed for the gelation, and minimize scaffold retraction, without major adverse effect on the scaffolds’ biological functionality.
Three collagen densities were investigated: 2, 3, and 4 mg/mL. These values are higher than usual collagen densities found in the literature for provisional scaffolds used in similar applications,15,16,24,27 but prior in vivo studies showed this range to be desirable in terms of gelation time, viscosity, and retention in the wound site.2 Briefly, in vivo, after combining the neutralized collagen and the fibrin-platelet concentrate, the mixture is injected around the sutured ACL, filling the gaps between the ligament stumps, and attaching to the torn ends of the ligament. Relatively rapid gelation and sufficient mechanical stability to withstand the fluid flow within the joint during motion are desirable properties.2,4 In this study, we used a constrained (anchored) gel model because it has been shown to be advantageous when compared with free-floating cultures23,28–31 and it mimics the attachment of the scaffold to the torn ends of the ruptured ligament.
Collagen density had a significant effect on the rheological properties of the collagen-platelet hydro-gels. Increasing the collagen density of the hydrogels not only increased their maximum storage and loss moduli ( and , respectively) by a factor of 3× to 3.5× but also decreased the time necessary for the hydrogels to reach their maximum storage modulus (i.e., ). Interestingly, the lowest collagen density group transitioned most quickly from liquid to gel. However in practice, it is more important to know when the provisional scaffold reaches its maximum , as that is when the maximum stiffness of the gel (or “full set”) is reached. The average obtained in this study for 4 mg/mL collagen-platelet hydrogel (376 Pa) is higher than that found by Wu et al. for collagen hydrogels made and tested under similar experimental conditions (152 Pa).32 The same authors reported that stiffer scaffolds would favor fibroblast growth,32 whereas in our case the stiffer scaffolds (which had a higher collagen density) had suppressed fibroblast proliferation in comparison with lower density hydrogels. These differences could be due to the presence or absence of other extracellular matrix (ECM) proteins in the acid-solubilized slurries as a consequence of differences in collagen extraction protocols, or possibly due to the presence of the platelets and fibrin in our collagen-platelet hydrogels.
Contrary to our hypothesis, increasing the collagen density of the collagen-platelet scaffolds did not result in a consistent increase in PDGF-AB release at any time point, with the release over 72 h being very similar for all three concentrations. The decreased level of PDGF-AB released after 12 h is consistent with previous reports demonstrating a peak release of PDGF-AB in the first 12 h of platelet activation in vitro,26,33 likely due to the high percentage of platelet activation on contact with even 2 mg/mL of collagen. Despite the relatively high release of PDGF-AB into the media, when these scaffolds are used in vivo, PDGF-AB has been retained within the collagen-platelet hydrogels up to 42 days after actvation,9 suggesting that while some PDGF-AB is released from the gels, enough is retained to sustain biologic activity within the provisional scaffold substitute. Overall, our results suggest that there in no additional benefit in terms of platelet activation when the collagen density of the scaffolds is increased from 2 to 4 mg/mL.
Other researchers have investigated how fibroblast proliferation is affected by differences in experimental conditions and scaffold composition.14,23,24,27,28,34–36 However, this is the first study to examine the effect of collagen density on fibroblast proliferation. In this study, increasing the collagen density of fibroblast-seeded scaffolds to 3 and 4 mg/mL led to a decrease in cell proliferation of 39.2% and 29.3 %, respectively. The physiologic significance of the higher fibroblast proliferation seen in the 2 mg/mL collagen density group requires further study.
Differences in hydrogel retraction were evident only for fibroblast-seeded scaffolds at 10 days of culture, with significant differences in retraction (33.1, 17.6, and 3.3% for the 2, 3, and 4 mg/mL collagen concentration scaffolds respectively). Notwithstanding, a moderate increase in collagen density from 2 to 4 mg/mL resulted in a substantial decrease in retraction from 33.1% to 3.3%. Because fibroblasts have a role in collagen gel retraction,37,38 the higher fibroblast proliferation in the 2 mg/mL FPC-collagen scaffolds possibly contributed to their higher retraction. In general, comparisons between ours and other studies were not possible because of the diversity in experimental conditions.23,27,36,39 However, our scaffold retraction results (33.1%) are comparable to those reported by Gentleman et al.40 (32%) for similar experimental conditions (i.e., collagen density of approximately 2 mg/mL, initial fibroblast seeding around 2 × 105 cells/scaffold and evaluation at day 10 of culture). The fact that minimal retraction was seen in the fibroblast-free scaffolds suggests that, under our experimental conditions, scaffold retraction was mostly a fibroblast-mediated phenomenon. Interestingly, although platelets have also been shown to stimulate fibroblast-mediated retraction of collagen gels,39 there was hardly any provisional scaffold retraction by day 7 in all experimental groups. Previous studies31,39,41 have reported higher degrees of retraction before 7 days for provisional scaffolds with lower collagen densities (<1 mg/mL) and/or cultured as floating hydrogels. Whether decreasing the collagen density below 1 mg/mL or culturing floating scaffolds without changing other experimental conditions of this work would result in the same findings requires further investigation.
The major limitation of this study is its in vitro nature. Although 3-D ECM models may more closely approximate a wound site than 2-D models, the in vivo biomechanical and biological cues are clearly absent. In addition, the static nature of the cultures does not recapitulate the dynamic mechanical environment of an ACL wound site. Further studies in vivo will be required to determine whether the differences found in vitro also occur in vivo. Another limitation is that higher platelet activity at the first 12 h of culture could have stimulated cell metabolism and affected the MTT assay. However, because platelet activity was similar to all groups at each time point, it is less likely to have affected the increase in absorbance of any particular group between days 1 and 10.
CONCLUSIONS
We have shown that increasing the collagen density of collagen-platelet hydrogels from 2 to 4 mg/mL resulted in stiffer scaffolds which suppress fibroblast proliferation and are more resistant to retraction when seeded with fibroblasts. However, higher collagen densities did not result in higher levels of PDGF-AB in the surrounding medium, suggesting that platelet activation is not significantly affected in this range of collagen density. Future directions of this work include the evaluation of how changes in collagen density may affect wound healing in vivo. These studies suggest that the collagen density may be able to significantly influence the function of collagen-platelet hydrogels used as substitute provisional scaffolds.
Acknowledgments
The authors would like to acknowledge the assistance of Dr. David Zurakowski in the statistical analysis, Drs. May Jacobson and Sherwin Kevy in the preparation of the platelets, and Marie Torres in the collagen density quantification.
Contract grant sponsor: CIMIT; contract grant number: DAMD17-02-2-0006
Contract grant sponsor: NIH; contract grant numbers: R01AR054099, K02 AR049346
References
- 1.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 3.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 4.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, Ballard P, Nanney LB, Zurakowski D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 5.Mesiha M, Zurakowski D, Soriano J, Nielson JH, Zarins B, Murray MM. Pathologic characteristics of the torn human meniscus. Am J Sports Med. 2007;35:103–112. doi: 10.1177/0363546506293700. [DOI] [PubMed] [Google Scholar]
- 6.Rosc D, Powierza W, Zastawna E, Drewniak W, Michalski A, Kotschy M. Post-traumatic plasminogenesis in intraarticular exudate in the knee joint. Med Sci Monit. 2002;8:CR371–CR378. [PubMed] [Google Scholar]
- 7.Kroon ME, van Schie ML, van der Vecht B, van Hinsbergh VW, Koolwijk P. Collagen type 1 retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis. 2002;5:257–265. doi: 10.1023/a:1024540701634. [DOI] [PubMed] [Google Scholar]
- 8.Maiotti M, Monteleone G, Tarantino U, Fasciglione GF, Marini S, Coletta M. Correlation between osteoarthritic cartilage damage and levels of proteinases and proteinase inhibitors in synovial fluid from the knee joint. Arthroscopy. 2000;16:522–526. doi: 10.1053/jars.2000.4632. [DOI] [PubMed] [Google Scholar]
- 9.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 10.Vissinger H, Husted SE, Kristensen SD, Nielsen HK. Platelet-derived growth factor release and antiplatelet treatment with low-dose acetylsalicylic acid. Angiology. 1993;44:633–638. doi: 10.1177/000331979304400807. [DOI] [PubMed] [Google Scholar]
- 11.Vassbotn FS, Havnen OK, Heldin CH, Holmsen H. Negative feedback regulation of human platelets via autocrine activation of the platelet-derived growth factor alpha-receptor. J Biol Chem. 1994;269:13874–13879. [PubMed] [Google Scholar]
- 12.Frank C, Woo SL, Amiel, Harwood F, Gomez M, Akeson W. Medial collateral ligament healing. A multidisciplinary assessment in rabbits. Am J Sports Med. 1983;11:379–389. doi: 10.1177/036354658301100602. [DOI] [PubMed] [Google Scholar]
- 13.Frank C, Amiel D, Akeson WH. Healing of the medial collateral ligament of the knee. A morphological and biochemical assessment in rabbits. Acta Orthop Scand. 1983;54:917–923. doi: 10.3109/17453678308992934. [DOI] [PubMed] [Google Scholar]
- 14.Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996;32:427–433. doi: 10.1007/BF02723005. [DOI] [PubMed] [Google Scholar]
- 15.Lin PW, Wu CC, Chen CH, Ho HO, Chen YC, Sheu MT. Characterization of cortical neuron outgrowth in two- and three-dimensional culture systems. J Biomed Mater Res B Appl Biomater. 2005;75:146–157. doi: 10.1002/jbm.b.30276. [DOI] [PubMed] [Google Scholar]
- 16.Yang B, Cao DJ, Sainz I, Colman RW, Guo YL. Different roles of ERK and p38 MAP kinases during tube formation from endothelial cells cultured in 3-dimensional collagen matrices. J Cell Physiol. 2004;200:360–369. doi: 10.1002/jcp.20025. [DOI] [PubMed] [Google Scholar]
- 17.Boccafoschi F, Habermehl J, Vesentini S, Mantovani D. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials. 2005;26:7410–7417. doi: 10.1016/j.biomaterials.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 18.Brinkman WT, Nagapudi K, Thomas BS, Chaikof EL. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules. 2003;4:890–895. doi: 10.1021/bm0257412. [DOI] [PubMed] [Google Scholar]
- 19.van den Bogaerdt AJ, van Zuijlen PP, van Galen M, Lamme EN, Middelkoop E. The suitability of cells from different tissues for use in tissue-engineered skin substitutes. Arch Dermatol Res. 2002;294:135–142. doi: 10.1007/s00403-002-0305-3. [DOI] [PubMed] [Google Scholar]
- 20.Joosten EA, Bar PR, Gispen WH. Collagen implants and cortico-spinal axonal growth after mid-thoracic spinal cord lesion in the adult rat. J Neurosci Res. 1995;41:481–490. doi: 10.1002/jnr.490410407. [DOI] [PubMed] [Google Scholar]
- 21.Wiesmann HP, Nazer N, Klatt C, Szuwart T, Meyer U. Bone tissue engineering by primary osteoblast-like cells in a mono-layer system and 3-dimensional collagen gel. J Oral Maxillofac Surg. 2003;61:1455–1462. doi: 10.1016/j.joms.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Nirmalanandhan VS, Levy MS, Huth AJ, Butler DL. Effects of cell seeding density and collagen concentration on contraction kinetics of mesenchymal stem cell-seeded collagen constructs. Tissue Eng. 2006;12:1865–1872. doi: 10.1089/ten.2006.12.1865. [DOI] [PubMed] [Google Scholar]
- 23.Zhu YK, Umino T, Liu XD, Wang HJ, Romberger DJ, Spurzem JR, Rennard SI. Contraction of fibroblast-containing collagen gels: Initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell Dev Biol Anim. 2001;37:10–16. doi: 10.1290/1071-2690(2001)037<0010:COFCCG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Sun S, Wise J, Cho M. Human fibroblast migration in three-dimensional collagen gel in response to noninvasive electrical stimulus. I. Characterization of induced three-dimensional cell movement. Tissue Eng. 2004;10:1548–1557. doi: 10.1089/ten.2004.10.1548. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Iatridis JC, Hlibczuk V, Ratcliffe A, Mow VC. Determination of collagen-proteoglycan interactions in vitro. J Biomech. 1996;29:773–783. doi: 10.1016/0021-9290(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson M, Fufa D, Abreu EL, Kevy S, Murray MM. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008;16:370–378. doi: 10.1111/j.1524-475X.2008.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31:391–402. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa S, Pawelek P, Grinnell F. Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res. 1989;182:572–582. doi: 10.1016/0014-4827(89)90260-7. [DOI] [PubMed] [Google Scholar]
- 29.Von den Hoff JW. Effects of mechanical tension on matrix degradation by human periodontal ligament cells cultured in collagen gels. J Periodontal Res. 2003;38:449–457. doi: 10.1034/j.1600-0765.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa S, Pawelek P, Grinnell F. Long-term culture of fibroblasts in contracted collagen gels: Effects on cell growth and biosynthetic activity. J Invest Dermatol. 1989;93:792–798. doi: 10.1111/1523-1747.ep12284425. [DOI] [PubMed] [Google Scholar]
- 31.Harada I, Kim SG, Cho CS, Kurosawa H, Akaike T. A simple combined floating and anchored collagen gel for enhancing mechanical strength of culture system. J Biomed Mater Res A. 2007;80:123–130. doi: 10.1002/jbm.a.30835. [DOI] [PubMed] [Google Scholar]
- 32.Wu CC, Ding SJ, Wang YH, Tang MJ, Chang HC. Mechanical properties of collagen gels derived from rats of different ages. J Biomater Sci Polym Ed. 2005;16:1261–1275. doi: 10.1163/156856205774269494. [DOI] [PubMed] [Google Scholar]
- 33.Fufa D, Shealy B, Jacobson M, Kevy S, Murray MM. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meaney Murray M, Rice K, Wright RJ, Spector M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238–244. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 35.Sakai D, Mochida J, Iwashina T, Watanabe T, Suyama K, Ando K, Hotta T. Atelocollagen for culture of human nucleus pulposus cells forming nucleus pulposus-like tissue in vitro: Influence on the proliferation and proteoglycan production of HNPSV-1 cells. Biomaterials. 2006;27:346–353. doi: 10.1016/j.biomaterials.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Murray MM, Forsythe B, Chen F, Lee SJ, Yoo JJ, Atala A, Steinert A. The effect of thrombin on ACL fibroblast interactions with collagen hydrogels. J Orthop Res. 2006;24:508–515. doi: 10.1002/jor.20054. [DOI] [PubMed] [Google Scholar]
- 37.Guidry C, Grinnell F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci. 1985;79:67–81. doi: 10.1242/jcs.79.1.67. [DOI] [PubMed] [Google Scholar]
- 38.Lewus KE, Nauman EA. In vitro characterization of a bone marrow stem cell-seeded collagen gel composite for soft tissue grafts: Effects of fiber number and serum concentration. Tissue Eng. 2005;11:1015–1022. doi: 10.1089/ten.2005.11.1015. [DOI] [PubMed] [Google Scholar]
- 39.Zagai U, Fredriksson K, Rennard SI, Lundahl J, Skold CM. Platelets stimulate fibroblast-mediated contraction of collagen gels. Respir Res. 2003;4:13. doi: 10.1186/1465-9921-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gentleman E, Nauman EA, Dee KC, Livesay GA. Short collagen fibers provide control of contraction and permeability in fibroblast-seeded collagen gels. Tissue Eng. 2004;10:421–427. doi: 10.1089/107632704323061780. [DOI] [PubMed] [Google Scholar]
- 41.Pascher A, Steinert AF, Palmer GD, Betz O, Gouze JN, Gouze E, Pilapil C, Ghivizzani SC, Evans CH, Murray MM. Enhanced repair of the anterior cruciate ligament by in situ gene transfer: Evaluation in an in vitro model. Mol Ther. 2004;10:327–336. doi: 10.1016/j.ymthe.2004.03.012. [DOI] [PubMed] [Google Scholar]