Abstract
Proteins of the transmembrane immunoglobulin and mucin domain (TIM) family are expressed by multiple cell types within the immune systems of rodents and humans. Studies over the last several years have suggested that these proteins may be promising targets for therapeutic manipulation of immune responses. This review discusses the progress that has been made in understanding TIM protein function in the immune system, as well as some of the unresolved issues that remain on the road to eventually targeting TIM proteins for enhancing or inhibiting immunity.
Introduction
The recent appreciation of the functions carried out by T cell (or transmembrane) immunoglobulin and mucin domain (TIM) family proteins in immunity has opened up the possibility of a new area for immune modulation. This review will cover the most promising members of the TIM family in this regard – TIM-1 and TIM-3. It will also touch on the function of TIM-4, mainly in its context as a ligand for TIM-1. There have been a number of studies of the other Tim protein present in mouse, i.e. Tim-2. However, it appears that this member of the family is not present in the human genome, which has decreased overall interest in this protein. It remains to be seen if the negative regulatory function ascribed to Tim-2 (1–3) has been assumed by another member of the human TIM family or even by an entirely different protein.
Domain structure and classification of TIM family proteins
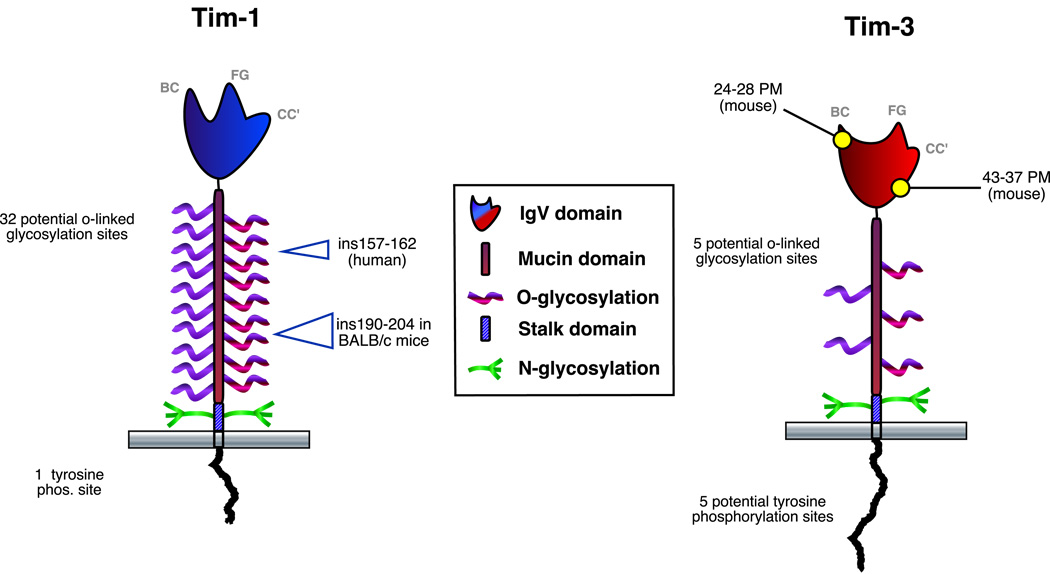
The members of the transmembrane (or T cell) immunoglobulin and mucin domain (TIM) family are type I transmembrane proteins that possess an N-terminal Ig domain of the V type, followed by a mucin domain of variable length and containing from a few to dozens of potential sites of O-linked glycosylation (Fig. 1). There are also predicted sites of N-linked glycosylation in the Ig domain and the stalk domain that lies between the mucin and transmembrane domains (Fig. 1). Following the transmembrane domain is a cytoplasmic tail that ranges in length from approximately 38–65 residues. There are eight predicted tim genes in the murine genome, four of which (tim1-tim4) encode functional proteins, while the human genome only contains three Tim genes (Tim1, Tim3 and Tim4). Based on their domain structure, TIM family proteins appear to be most closely related to mucosal addressin cellular adhesion molecule (MAdCAM) (4), which can bind to distinct ligands through its Ig domain (alpha4beta7 integrin) and carbohydrate side chains (selectin). As discussed below specific ligands have been described for the IgV domains of Tim-1 and Tim-3, although no ligands have yet been described for the respective mucin-like domains.
Figure 1. Domain structures of TIM-1 and TIM-3.
The major structural domains of Tim-1 (left) and Tim-3 (right) are shown. The lettering on the IgV domains refers to the structural loops identified in the published crystal structures of this domain from the two proteins (41, 42). Major insertional polymorphisms for both mouse and human Tim-1 are indicated, as are the best-characterized point mutations (PM) of mouse Tim-3. Polymorphisms of human and mouse TIM’s have previously been reviewed in greater detail (12). Likely sites of O- and N-linked glycosylation in the mucin-like and stalk domains, respectively, are also shown. Possible sites of glycosylation within the IgV domains (41, 42) have been omitted for clarity.
Discovery of the TIM family
The founding member of the TIM family was initially described as kidney injury molecule 1 (Kim-1), a putative adhesion molecule upregulated in a rat model of kidney ischemia (4). Production of a soluble form of Kim-1, generated by proteolytic cleavage, is now employed clinically as a diagnostic markers for acute kidney injury (5). This protein is equivalent to Tim-1, as cloned in the mouse (see below). Later, it was also shown in primates that Kim1/TIM1 is a receptor for Hepatitis A virus (6, 7) and thus the human and monkey forms are sometimes referred to as hepatitis A virus cellular receptor 1 (HAVCR1).
The TIM family was largely ignored by immunologists until a landmark paper by DeKruyff and colleagues (8), who were studying the development of allergic asthma in a commonly used murine model. It is well known that BALB/c mice are much more susceptible to the development of type II immune responses, when compared with other strains, such as C57 BL/6 and DBA/2 (9), and this includes the OVA-induced asthma model (10). This difference is thought to be due, at least in part, to polymorphisms in one or more genes contained in the so-called Th2 cytokine locus, which includes the genes for IL-4, IL-5 and IL-13 (11). DeKruyff and colleagues generated a series of congenic mouse strains to map loci responsible for the differential atopic sensitivity of BALB/c and DBA/2. One congenic strain (“HBA”) contained a segment of mouse chromosome 11, on an otherwise BALB/c background (8). In this strain, the Th2-prone phenotype was “suppressed,” such that production of Th2 cytokines and airway hyper-responsiveness (AHR) were virtually identical to those seen with a pure DBA/2 mouse. When the locus in question was narrowed further, it was found to segregate independently of the Th2 cytokine locus, but included the tim family genes, among others, and was also largely syntenic with human chromosome 5q33, which lies within a region previously linked to human atopic disease (8). Sequence analysis revealed the existence of multiple polymorphisms in the genes encoding Tim-1 and Tim-3 (known polymorphisms in human and mouse Tim-1 and Tim-3 are discussed in more detail elsewhere (12)). While the authors provided evidence to link T cells to the phenotypic differences between the mouse strains, they did not definitively prove that polymorphisms in Tim-1 or Tim-3 (or both) were responsible. However, the case for Tim-1 seems to be stronger at this point, since a polymorphism in human Tim-1 (Fig. 1) is also associated with differential asthma risk (13). Intriguingly, this polymorphism is similar to one of the differences between Tim-1 from different mouse strains, i.e. an insertion/deletion in the mucin-like domain. Indeed, the presence of the insertion seems to confer a decreased risk for developing asthma, but only in individuals who are sero-positive for exposure to Hepatitis A virus (13). This finding suggests that polymorphisms in Tim-1 may contribute to asthma susceptibility because of direct or indirect effects on cellular interactions with HAV. Obviously, the connection to HAV cannot explain the results obtained with the congenic mouse strains that possess differential susceptibility to the OVA model of allergic asthma. Also, in mice, the longer mucin domain is found in the more atopic BALB/c strain, which is the opposite of the finding with human TIM-1. There is still a need for these issues to be addressed experimentally.
Around the same time that the DeKruyff group came upon the tim locus via a genetic approach, Kuchroo and colleagues were on the hunt for more specific markers of Th1 T cells, using an antibody generation approach. In 2002 they reported the generation of a monoclonal antibody that could recognize all Th1 T cell clones and de novo generated Th1 T cells, but not naïve T cells or Th2 T cell clones (14). When the antigen recognized by this antibody was identified, it was revealed to be murine Tim-3. Administration of Tim-3 antibody to mice exacerbated disease in the EAE model (14), the first indication that Tim-3 might negatively regulate Th1-dependent immune responses.
Stimulatory and co-stimulatory functions of Tim-1 on T cells
In 2005, several studies demonstrated that Tim-1 ligation can co-stimulate T cell proliferation and cytokine production (15–17). This was shown through the use of an agonistic antibody (17) and through transient overexpression (15). In addition, similar co-stimulatory function could be provided to T cells by interaction of Tim-1 with Tim-4, which is preferentially expressed by antigen presenting cells (16, 18). The Tim1:Tim4 interaction appears to occur mainly through the Ig domains of the two proteins (Fig. 2), although it may be further regulated by the mucin domains (19). Recent studies have suggested the existence of additional ligands for Tim-1, including the possibility that Tim-1 can homo-dimerize through a non-covalent interaction mediated by the Ig domain (discussed further below). The putative co-stimulatory function of Tim-1 appears to be more analogous to late-acting co-stimulatory molecules, such as OX-40, since Tim-1 is present at very low levels on naïve T cells, but is upregulated after activation (15–17). Also, Tim-4 itself may have one or more additional protein ligands. Thus, treatment of naïve T cells (which do not express detectable Tim-1) with a soluble Tim-4 fusion protein inhibited T cell activation, while the same treatment of pre-activated T cells enhanced activation (20). Although most studies have focused on the direct effects of TIM-1 ligation on effector T cells, agonistic TIM-1 antibodies may also enhance immune responses through inhibition of regulatory T cell function or generation (21).
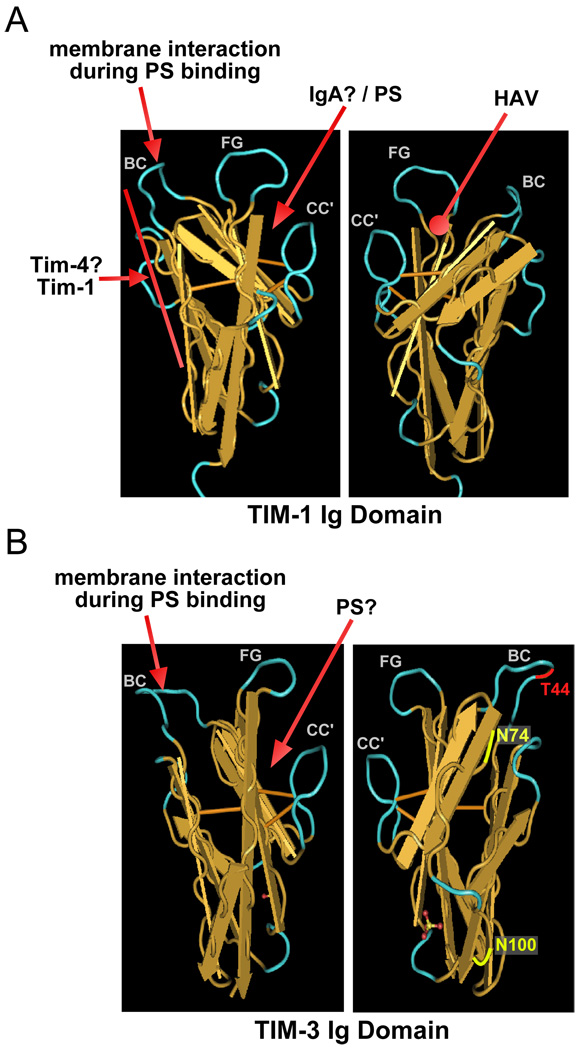
Figure 2. Reported ligand interactions with TIM-1 and TIM-3.
Shown are the structures of the Tim-1 (A) and Tim-3 (B) IgV domains on two faces as visualized with Cn3D, using structural information from the public database, and described in published reports (41, 42). Residues highlighted in red and yellow in the right-hand panel of Tim-3 (B) indicate sites of N-linked glycosylation and possible galectin-9 binding (42). Arrows indicate the known locations of ligand interactions, based upon mutational, antibody blocking or structural studies. While the mucin domains of TIM proteins can contribute to ligand binding (19), this has not been characterized as well as interactions with the IgV domains. Ligands for which the precise interaction site has not been mapped are indicated in italics, with possible locations of interaction.
There has been some progress made in understanding the biochemical pathways downstream of Tim-1. The cytoplasmic tail of Tim-1 contains two tyrosines, one of which is contained within a sequence that makes it a likely site for phosphorylation. Thus, mutating tyrosine 276 within the cytoplasmic tail of murine Tim-1 impairs its ability to co-stimulate T cell activation in conjunction with TCR/CD28 ligation (15). Phosphorylation of Y276 functions in part by recruiting the p85 adaptor protein, through one or more of its SH2 domains (22). This leads to downstream activation of the serine/threonine kinase Akt (18, 22), which likely augments NFAT activity through inhibition of the NFAT inhibitory kinase GSK-3. Another downstream target for Tim-1 is the ERK MAP kinase, phosphorylation of which is augmented by Tim-1 ligation (18). In addition, Tim-1 signaling may intersect at a more proximal point with TCR signaling pathways. Thus, Tim-1 co-stimulation can increase tyrosine phosphorylation of the transmembrane adaptor protein LAT (18) and of the TCR-proximal tyrosine kinases ZAP-70 and Itk (23). At least in the case of human T cells, these observations are consistent with a report that Tim-1 may be in close association with the TCR/CD3 complex (23).
The ability of Tim-1 ligation by itself to initiate T cell activation has been observed with a single Tim-1 antibody – the monoclonal antibody 3B3 (see Table I) – while other Tim-1 antibodies and the ligand Tim-4 seem to only act as co-stimulatory agents (16–18). Intriguingly, there are at least two studies that have demonstrated differential effects of Tim-1 ligation, depending upon the antibody used. First, in work by Rennert and colleagues, two Tim-1 antibodies (1H8.2 and 5D1.1 –Table I) were shown to significantly enhance responses to model antigens, as determined by ex vivo re-stimulation or in vivo inflammation, while two other antibodies (3A2.5 and 4A2.2) attenuated inflammation (24). The mechanistic basis for the different effects seen with the activating vs. inhibitory Tim-1 antibodies is unclear, although it is intriguing that the activating antibodies in this case both recognized parts of the mucin domain, while the inhibitory antibodies recognized either the stalk or IgV domain (Table I). However, a different antibody to the Tim-1 IgV domain (1H9.9) had no obvious effects on inflammation (24). Similar observations were reported by Kuchroo and colleagues, who compared the 3B3 antibody (17) with another monoclonal antibody (RMT1-10). These two antibodies both recognize the IgV domain, and may in fact compete with each other. However, while 3B3 enhanced proliferation and Th1 cytokine production of antigen-primed T cells, RMT1-10 suppressed both proliferation and Th1 cytokine production (25). Also, in this same study 3B3 exacerbated, while RMT1-10 ameliorated, PLP-induced EAE. Although both antibodies were found to bind to the IgV domain, the 3B3 antibody had a significantly higher affinity for Tim-1 (25). It is not yet known how possible differences in signaling by these antibodies might translate into the different responses to them in vivo or in vitro.
Table I.
Selected antibodies to mouse and human TIM-1 that have been functionally characterized in the literature. Unless otherwise stated, the antibodies recognize murine Tim-1.
| Tim-1 Antibodies | Epitope | Functional Effects | Refs. |
|---|---|---|---|
| 3B3 | IgV | Activation; co-stimulation | (17) |
| 1H8.2 | Mucin/stalk (BALB/c only) |
Increased Th2 | (24) |
| 5D1.1 | Mucin/stalk (BALB/c only) |
Increased Th2 | (24) |
| 3A2.5 | Stalk | Decreased Th2 | (24) |
| 4A2.2 | IgV | Decreased Th2 | (24) |
| 1H9.9 | IgV | No apparent effects | (24) |
| RMT1-10* | IgV | Inhibition of T cell responses | (25, 50, 51) |
| 3D1 (human) | IgV | Blocks HAV and PS binding | (43, 52) |
This Tim-1 antibody should not be confused with a commercially available antibody with the same name
Thus, although a substantial amount of data are consistent with a co-stimulatory function for Tim-1, the findings with different Tim-1 antibodies discussed above suggest that under some circumstances Tim-1 may also be capable of inhibiting T cell activation. Such activity could be mediated by Tim-1 binding to a distinct (non-Tim4) ligand, or possibly by binding to the known ligand Tim-4, under different conditions of cellular activation or differentiation.
Inhibition of T cell responses by Tim-3
As stated above, initial studies of Tim-3 in murine EAE suggested that it is a negative regulator of Th1 immune responses (14). This interpretation was consistent with subsequent studies where a soluble Tim3-Ig fusion protein enhanced proliferation and cytokine production by primed T cells and prevented the induction of tolerance by soluble antigen (26). A genetic deficiency for Tim-3 expression also impaired the induction of tolerance (26). Similar conclusions were reached with a transfer model of autoimmune diabetes, in that treatment with either an antibody to Tim-3 (Table II) or a Tim3-Ig fusion protein could exacerbate disease (27). In this same study, it was also shown that blocking Tim-3 interaction with its putative ligands could prevent the induction of tolerance by the combined treatment of donor-specific transfusion (DST) and anti-CD40L antibody. Thus, putative blocking of Tim3:Tim3L interactions enhanced the development of autoimmune disease and inhibited the induction of tolerance. Consistent with this model, up-regulation of Th1 immune responses by blocking Tim-3 may result in an accompanying decrease in Th2 responses (28).
Table II.
Selected antibodies to mouse and human TIM-3 that have been functionally characterized in the literature. Unless otherwise stated, the antibodies recognize murine Tim-3.
| Tim-3 Antibodies | Epitope | Functional Effects | Refs. |
|---|---|---|---|
| 8B.2C12 | ? (BALB/c ≫ BL/6) | Increased Th1 | (14) |
| 5D12 | IgV | Increased Th1 | (36) |
| 8H7 | ? | Increased Th1 / Decreased Th2 | (27, 28) |
| RMT3-23 | ? | Increased Th1 | (30) |
| 2E2 (human) | ? | Reversal of CD8+ exhaustion (HIV) Increased Th1/Th17 cytokines |
(33, 34 |
| 1G5 (human) | ? | Reversal of T cell non- responsiveness (HCV) |
(35) |
The findings of the above blocking studies are consistent with the discovery by Kuchroo and colleagues of the first ligand for Tim-3, the β-galactoside binding protein galectin-9 (Fig. 2). Thus, addition of galectin-9 to Th1 T cells caused a rapid and atypical cell death (29). Similarly, in vivo administration of galectin-9 caused down-regulation of Th1-dependent immune responses. In addition to causing effector T cell death, galectin-9 treatment may also lead to an increase in regulatory T cells, as recently demonstrated in a model of viral immunopathology (30). One challenge to interpreting these experiments is the fact that galectin-9 (and indeed all galectins) can have pleiotropic effects, through binding to multiple proteins with β-galactoside modifications (31). In the case of galectin-9, this includes known binding to CD44 (31).
As with TIM-1, the in vivo function of TIM-3 appear to be more complex than initial reports suggested. Thus, two studies have now demonstrated that treatment of mice with galectin-9 also promotes the generation and/or survival of regulatory T cells, at the expense of pro-inflammatory Th17 T cells (30, 32). In addition, both of these studies provided direct evidence for the suppression of Th17 development by galectin-9, while one also showed that de novo generation of regulatory T cells was enhanced by galectin-9 (30). TIM-3 also appears to negatively regulate the development and/or survival of human Th17 T cells, since an antagonistic TIM-3 antibody can enhance the production of IL-17 by human T cells (33).
Perhaps the most exciting recent development regarding TIM protein function is the association of TIM-3 with the phenomenon of “immune exhaustion.” Thus, in addition to the previously described subset of PD-1-expressing non-responsive T cells in individuals with chronic HIV infection, there is a largely non-overlapping population of CD8+ T cells that express TIM-3 (34). Expression of TIM-3 correlates with disease progression, and is associated with lack of activation potential. Most strikingly, soluble TIM3-Ig or a putative blocking antibody (Table II) for TIM-3 can partially reverse the activation defect of these cells. TIM-3 appears to function via a distinct mechanism to PD-1, as blocking both pathways led to a cooperative or synergistic rescue of T cell activation (34). Upregulation of TIM-3 on both CD4+ and CD8+ exhausted T cells was also recently reported in patients with chronic hepatitis C virus (HCV) infection (35). As in the case of HIV infection, blocking the interaction of TIM-3 with its ligands partially rescued the functionality of such cells. It remains to be seen if a similar role exists for TIM-3 in other settings of infection or cancer where chronic stimulation may result in T cell exhaustion.
Positive regulation of T cell responses by Tim-3
While it is clear that Tim-3 can negatively regulate Th1 immune responses, Tim-3 appears to also act as a positive regulator of T cell responses, at least under some circumstances. Such effects may be indirect in part, i.e. through effects on antigen presenting cells (APC). Tim-3 is constitutively expressed by some monocytic cells, including dendritic cells (DC) and microglia, and ligation of Tim-3 on these cells can increase their expression of co-stimulatory receptors and cytokines (36). Several studies have also provided evidence for positive effects of Tim-3 on T cells themselves, particularly in responses to tumors. Injection of Tim-3+ Th1 T cells into SJL mice inhibited the growth of spontaneous or transplanted B cell lymphomas (37). Additionally, expression of a naturally occurring soluble form of Tim-3 inhibited the responses of mice to B16 melanoma cells. This inhibitory effect was even observed when purified T cells were stimulated in vitro with antibodies to CD3 and CD28, arguing against an indirect effect on APC’s (38). In vivo administration of galectin-9 increased the number of Tim-3+ CD8+ T cells and enhanced the killing of Meth-A sarcoma cells in syngeneic mice, although in this case at least part of the effect of galectin-9 was due to enhanced APC function (39).
It is still not clear precisely how the positive and negative effects of Tim-3 might balance out one another in vivo. Given the constitutive expression of Tim-3 on APC and late upregulation on effector T cells, it is possible that Tim-3 enhances T cell activation and/or differentiation during the early phase of an immune response, while acting to help terminate a response at a later time. However, it cannot be ruled out that ligation of Tim-3 on T cells by different ligands or under certain activation conditions may lead to direct augmentation of T cell activation.
Signaling by TIM-3
At this point, relatively little is known about how TIM-3 might transmit signals that either inhibit T cell activation, cause cell death or even augment T cell activation or effector function, all functions discussed above. The cytoplasmic tail of TIM-3 is considerably more complex than that of TIM-1. While TIM-1 contains one tyrosine that is a good candidate for phosphorylation, TIM-3 possesses five such tyrosines. At least one of these tyrosines (Y265 in human TIM-3, corresponding to Y256 in mouse Tim-3) can be phosphorylated, at least in HEK 293 cells (40), which may be mediated by the Tec family tyrosine kinase Itk. It is not known how tyrosine phosphorylation of TIM-3 might regulate its function in T cells or other cell types in which it is active. However, it has been reported that antibody-mediated crosslinking of Tim-3 elicits different patterns of tyrosine phosphorylation in murine Th1 T cells and dendritic cells (36). Identification of these substrates may lead to a greater understanding of Tim-3 function. Finally, Tim-3 ligation can enhance activation of MAP kinase and NF-κB pathways (36), although the role of these pathways in mediating TIM-3 function has yet to be determined.
Structural insights into TIM protein function
The IgV domains of all four TIM proteins have been subjected to structural analysis. The structures of the murine Tim-1 and Tim-2 Ig domains were the first to be reported (41). Perhaps the most interesting revelations about Tim-1 and Tim-2 revealed by these structures were the findings regarding dimerization of the proteins. Thus, the Ig domain of Tim-1 crystallized in antiparallel dimers, suggestive of trans homo-dimerization of Tim-1 molecules expressed on different cells (Fig. 2A) (41). Biochemical experiments confirmed that Tim-1 can homo-dimerize, and that high affinity binding requires both the Ig and mucin domains (41). Although Tim-2 Ig domain crystals also contained dimers, these were very different from those observed with Tim-1. Specifically, the parallel dimers of Tim-2 that formed in the crystals were suggestive of cis homo-dimerization that would occur between molecules expressed on the same cell (41).
Detailed study of the Tim-3 Ig domain (Fig. 2B) also revealed some surprises about this member of the TIM family. Thus, bacterially derived tetramers of Tim-3 could stain the surface of numerous leukocytes, including T cells, B cells macrophages and DC’s (42). Since such recombinant Tim-3 would not be glycosylated, there must be one or more non-galectin-9 ligand(s) for Tim-3 on the surface of leukocytes. The identity of such a ligand or ligands is not known. Although PS can be transiently expressed on the surface of activated immune cells, the fact that Tim-3 uniformly stains freshly isolated murine leukocytes would seem to rule out PS as the ligand in this case.
TIM’s as ligands for phosphatidylserine
One of the hallmark events associated with apoptosis is the translocation of phosphatidylserine (PS) to the outside of the plasma membrane, exposing it to phagocytic cells that have specific receptors for PS (43). This results in the recognition and phagocytosis of the apoptotic cell. Several papers that appeared in the last few years have demonstrated a role for TIM family proteins in the recognition of PS and phagocytosis of apoptotic cells. Initially, it was shown that Tim-1 and Tim-4 can bind to PS and, if expressed by a phagocytic cell, could mediate phagocytosis (44, 45). These findings were confirmed by a structural study, in which the Tim-4 Ig domain was co-crystallized with PS (46). Binding of PS to Tim-4 occurred mainly in a pocket between the CC’ and FG loops (Fig. 2A); intriguingly, the FG loop, which contains aromatic residues, appears to coordinate with both the fatty acid chains of the PS and the plasma membrane of the target cell on which the PS is present (46). Similar activity is also possessed by Tim-3 (Fig. 2B), and phagocytosis of apoptotic cells mediated by Tim-3 can result in cross-presentation of antigens to CD8+ T cells (47). Tim-2 appears to be the only member of the family that does not mediate binding of PS (45). Since Tim-1 and Tim-3 can be expressed by T cells, it remains to be seen what the effects are of PS binding to these Tim’s on T cells, which are not phagocytic. In addition the role of TIM proteins in normal clearance of apoptotic cells during development, tissue injury and inflammation is not yet clear.
TIM protein effects on other immune cell types
While the TIM’s have been most extensively studied in the immune system for regulation of T cell function, these proteins can regulate myeloid cell function as well. As discussed above, the function of APC’s such as dendritic cells and microglia can be enhanced by Tim-3 ligation. Another myeloid cell type in which TIM function has been characterized is the mast cell. Thus, mouse bone-marrow derived mast cells express both Tim-1 and Tim-3 (48). Treatment of mouse mast cells with recombinant Tim-4, presumably through Tim-1 ligation, increased the production of several cytokines in response to IgE and antigen (48). A polyclonal antibody to Tim-3 could also enhance mast cell production of type 2 cytokines, in conjunction with IgE/antigen. However neither treatment had any effect on mast cell degranulation (48). Given the central role of mast cells in atopy, these findings warrant further study, as they may shed further light on the question of how Tim-1 and Tim-3 polymorphisms contribute to Th1/Th2 skewing. Finally Tim-1 is expressed at low levels on murine B cells (16, 17), while Tim-3 is not detectable (14). The potential function of Tim-1 on B cells has not yet been reported.
Conclusions
The expression of TIM proteins by activated T cells in various pathogenic settings has opened up the possibility that targeting these proteins may inhibit or augment immune responses, depending upon the clinical situation. Clearly, in vitro and murine in vivo studies have revealed that targeting TIM protein function can modulate immune responses. There are still limited in vitro studies with human cells, but these have also shown promise, particularly in the case of TIM-3, as discussed above. Modulating the function of TIM proteins through traditional protein drug approaches, i.e. use of blocking antibodies or soluble receptors, may be complicated by the fact that TIM:ligand interactions are somewhat promiscuous (Fig. 2). Also, the ability of different TIM antibodies or even concentrations of ligand to differentially affect activation of different subsets of T cells (and possibly other cell types), is a potential concern for future use in patients. A cautionary tale in this regard is the recent clinical experience with a super-agonist monoclonal antibody specific for CD28. Thus, while such antibodies functioned in pre-clinical models to preferentially expand regulatory T cells, at the expense of effector T cells, they were actually quite effective at activating memory/effector T cells in humans, resulting in a potentially fatal cytokine storm (49).
Regarding the normal functions of TIM-1 and TIM-3 in immunity, it will be necessary to more clearly define the requirements for binding of each putative and confirmed ligand, as well as to determine the identity of specific ligands (if any) for the mucin domains of these proteins. It will also be important to obtain a better understanding of the effects of TIM-1 and TIM-3 ligation on downstream signaling pathways that either enhance or inhibit leukocyte activation.
References
- 1.Chakravarti S, Sabatos CA, Xiao S, Illes Z, Cha EK, Sobel RA, Zheng XX, Strom TB, Kuchroo VK. Tim-2 regulates T helper type 2 responses and autoimmunity. J Exp Med. 2005;202:437–444. doi: 10.1084/jem.20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knickelbein JE, de Souza AJ, Tosti R, Narayan P, Kane LP. Cutting edge: inhibition of T cell activation by TIM-2. J Immunol. 2006;177:4966–4970. doi: 10.4049/jimmunol.177.8.4966. [DOI] [PubMed] [Google Scholar]
- 3.Rennert PD, Ichimura T, Sizing ID, Bailly V, Li Z, Rennard R, McCoon P, Pablo L, Miklasz S, Tarilonte L, Bonventre JV. TIM-2 gene-deficient mice reveal a novel mechanism for the regulation of TH2 immune responses and airway inflammation. J Immunol. 2006 doi: 10.4049/jimmunol.177.7.4311. In press. [DOI] [PubMed] [Google Scholar]
- 4.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 5.Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, Bonventre JV, Jaber BL. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14:423–431. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone SM. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. Embo J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 8.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 9.Locksley RM, Heinzel FP, Holaday BJ, Mutha SS, Reiner SL, Sadick MD. Induction of Th1 and Th2 CD4+ subsets during murine Leishmania major infection. Res Immunol. 1991;142:28–32. doi: 10.1016/0923-2494(91)90007-6. [DOI] [PubMed] [Google Scholar]
- 10.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 12.Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.McIntire JJ, Umetsu SE, Macaubas C, Hoyte EG, Cinnioglu C, Cavalli-Sforza LL, Barsh GS, Hallmayer JF, Underhill PA, Risch NJ, Freeman GJ, DeKruyff RH, Umetsu DT. Hepatitis A virus link to atopic disease. Nature. 2003;425:576. doi: 10.1038/425576a. [DOI] [PubMed] [Google Scholar]
- 14.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 15.de Souza AJ, Oriss TB, O'Malley K, Ray A, Kane LP. TIM-1 is expressed on in vivo-activated T cells and provides a co-stimulatory signal for T cell activation. Proc Natl Acad Sci U S A. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, Dekruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 17.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, Dekruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, Greenfield EA, Anderson AC, Sobel RA, Hafler DA, Strom TB, Kuchroo VK. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706–4713. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilker PR, Sedy JR, Grigura V, Murphy TL, Murphy KM. Evidence for carbohydrate recognition and homotypic and heterotypic binding by the TIM family. Int Immunol. 2007;19:763–773. doi: 10.1093/intimm/dxm044. [DOI] [PubMed] [Google Scholar]
- 20.Mizui M, Shikina T, Arase H, Suzuki K, Yasui T, Rennert PD, Kumanogoh A, Kikutani H. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int Immunol. 2008;20:695–708. doi: 10.1093/intimm/dxn029. [DOI] [PubMed] [Google Scholar]
- 21.Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, Alexopoulos S, Oukka M, Umetsu DT, DeKruyff RH, Kuchroo V, Zheng XX, Strom TB. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza AJ, Oak JS, Jordanhazy R, Dekruyff RH, Fruman DA, Kaneb LP. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J Immunol. 2008;180:6518–6526. doi: 10.4049/jimmunol.180.10.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binne LL, Scott ML, Rennert PD. Human TIM-1 Associates with the TCR Complex and Up-Regulates T Cell Activation Signals. J Immunol. 2007;178:4342–4350. doi: 10.4049/jimmunol.178.7.4342. [DOI] [PubMed] [Google Scholar]
- 24.Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, Walsh M, Li Z, Zafari M, Dobles M, Tarilonte L, Miklasz S, Majeau G, Godbout K, Scott ML, Rennert PD. Epitope-dependent effect of anti-murine TIM-1 monoclonal antibodies on T cell activity and lung immune responses. J Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]
- 25.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, Gutierrez C, Moll T, Sobel RA, Umetsu DT, Yagita H, Akiba H, Strom T, Sayegh MH, DeKruyff RH, Khoury SJ, Kuchroo VK. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutierrez-Ramos JC, Coyle AJ, Strom TB. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 28.Kearley J, McMillan SJ, Lloyd CM. Th2-driven, allergen-induced airway inflammation is reduced after treatment with anti-Tim-3 antibody in vivo. J Exp Med. 2007;204:1289–1294. doi: 10.1084/jem.20062093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 30.Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators. J Immunol. 2009;182:3191–3201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovich GA, Toscano MA. Turning 'sweet' on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 32.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, Nishi N, Yamauchi A, Katoh S, Matsukawa A, Kuchroo V, Hirashima M. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 37.Simmons WJ, Koneru M, Mohindru M, Thomas R, Cutro S, Singh P, Dekruyff RH, Inghirami G, Coyle AJ, Kim BS, Ponzio NM. Tim-3+ T-bet+ tumor-specific Th1 cells colocalize with and inhibit development and growth of murine neoplasms. J Immunol. 2005;174:1405–1415. doi: 10.4049/jimmunol.174.3.1405. [DOI] [PubMed] [Google Scholar]
- 38.Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, Xiao H, Han LF, Feng ZH. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176:1411–1420. doi: 10.4049/jimmunol.176.3.1411. [DOI] [PubMed] [Google Scholar]
- 39.Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, Nagahata S, Hirabayashi J, Kuchroo VK, Yamauchi A, Hirashima M. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol. 2008;181:7660–7669. doi: 10.4049/jimmunol.181.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 41.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T Cell Immunoglobulin Mucin Receptors 1 and 2 Reveal Mechanisms for Regulation of Immune Responses by the TIM Receptor Family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, Zencheck WD, Lary JW, Cole JL, Deng H, Xiao H, Dilorenzo TP, Allison JP, Nathenson SG, Almo SC. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Tibrewald N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 Glycoproteins Bind Phosphatidylserine and Mediate Uptake of Apoptotic Cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T Cell Immunoglobulin Mucin Protein 4 Show a Metal-Ion- Dependent Ligand Binding Site where Phosphatidylserine Binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and the cross-presentation. Blood. 2009 doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 48.Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]