Abstract
Exposure and dose estimation are essential to understanding the etiology of environmentally linked childhood diseases. The behavior of resuspended particulate matter (PM) suggests that stationary measurements may underestimate household exposures in young children (ages 6 to 36 months). Because of the size and weight of the sampling equipment, use of personal samplers in this age group is either difficult or impossible. The Pre-toddler Inhalable Particulate Environmental Robotic (PIPER Mk IV) sampler has been developed to provide a surrogate method to ascertain personal exposures to PM for this age group.
As part of a study of childhood asthma, 55 homes in central New Jersey were tested. Simultaneous sampling for inhalable PM using stationary (110 cm height) and PIPER mobile sampler were carried out. In homes with bare floors (N=21), the absolute difference was 3.9 μg/m3 (S.E. = 3.01; p = 0.217) and relative difference (PIPER/Stationary) was 1.12 (linearized S.E. = 0.11). On carpets (N=34), the absolute difference was 54.1 μg/m3 (S.E. = 13.50; p = 0.0003), and the relative difference was 2.30 (linearized S.E. = 0.34). The results confirm the importance of understanding the personal dust cloud caused by children’s activity in a room, particularly when rugs or carpets are present.
Keywords: Dust, Children, Robot, Particulate Matter, Resuspension
Introduction
In the United States over the last decade concern has risen over the steady increase in the number of children who are diagnosed annually with asthma. The reported prevalence of asthma increased between 1980 and the mid 1990’s by 75% (1). According to the U.S. Centers for Disease Control and Prevention (1) as of 2008, 1.5 million preschoolers (birth - 4 years) and 8.7 million school-aged children (5 – 17 years) have ever been diagnosed with asthma. Almost half were reported to have had an episode or attack within the last year and the cost of treating those under age 18 years is currently estimated at $3.2 billion annually. All of these factors have led to research interest into possible environmental factors in both the origin and exacerbation of this condition. A variety of chemical and biological agents that can be present in and around the home have been identified as having a role in triggering asthma; these include second-hand smoke, pesticides, dusts, fungi, and other allergens (2–4). In the last decade there has been mounting evidence that acute asthma symptoms are not just elicited by allergens and pollutants, but that these agents may also play a significant role in the cause and/or development of asthma in early childhood (5–6). However, some studies have failed to find significant associations between indoor particulate matter (PM) levels and asthma (7) with previously existing methods of measuring dust exposure. While studies have examined the role of various environmental toxins in triggering respiratory symptoms in school age asthmatic children, relatively little research has focused on the youngest children (6 months to 3 years) and the role environmental exposures may play in the earliest stages of asthma development.
Exposures of young children (infants and toddlers) to inhalable PM (airborne particles with aerodynamic diameter approximately less than 100 μm) may be greater than that of adults since children’s floor activities can result in PM becoming resuspended in their breathing zone. The exposure issues dictate that understanding of the microenvironment between 0 and 1 meter above the floor is critical in appreciating the potential exposure and hazard of suspended PM to children. The ability to accurately measure exposures in the air space that is breathed by young children in this near-floor environment, and associated with their typical activities, is important in understanding the origin of asthma as well as asthma triggers in this age group. In this near floor environment otherwise settled dust particles are resuspended as a result of crawling, walking, and play activity on the floor. The greater the level of a child’s activity on the floor, the higher the level of resuspended PM in the dust cloud is to be expected.
An early prototype robotic sampler designed to estimate personal exposure of pre-toddlers (Pre-Toddler Inhalable Particulate Environmental Robotic - PIPER Mk I) observed significant increases of PM from floor motion (8). However, this device had significant limitations, including limited control of speed, fixed sampling height, and limited programming capabilities. The prototype was constructed to explore the magnitude of PM resuspension in the near floor environment and was never intended for extensive field deployment. The latest version of PIPER, the Mk IV, significantly advances the concept of using robotic sampling for exposure assessment and represents a quantum advance as a tool for the study of the near floor microenvironment in which children play. Its design, performance and the observed results are the subject of this manuscript.
Experimental
Development of robotic surrogate personal sampler
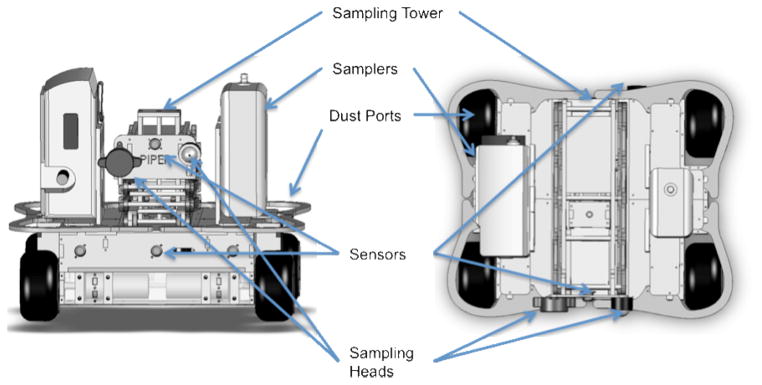
PIPER Mk IV is a four-wheeled robot with a variable height air-sampling tower. It was designed to be a simple, robust platform that could carry the personal sampling systems (i.e. sampling pumps, direct reading instruments, sampling heads) used on adults, but which cannot be employed with young children or toddlers. The PIPER Mk IV sampling system consists of the robot (Figure 1) and a laptop computer that the operator uses to program and monitor the robot. PIPER weighs 10 kg and is 41 cm wide, 35 cm long and 25 cm in height. It can carry up to two personal sampling systems with a total weight of ~ 3 kg and up to 25 cm in height, 18 cm in width and 9 cm in depth, which are secured by nylon web straps to the robot’s base. Room air may be sampled at any height from 20 to 100 cm by mounting the sampling head on PIPER’s telescoping tower. A range of size selective aerosol and bioaerosol samplers can be accommodated on the sampling tower by the use of snap on attachments that allow quick changes for evaluating particles of different types and size fractions. PIPER is autonomous and is capable of maneuvering in a typical home setting without operator intervention. The laptop contains the main PIPER software program and provides the software platform for the implementation of the child activity profiles, while all other functions are handled by on-board sensors and processors. Areas to be sampled can be delimited by infrared emitters that create virtual walls which PIPER can detect. This is particularly useful in rooms with multiple entryways and no doors, or to avoid falling down stairways. A unique design aspect of PIPER are the dust ports located above the four drive wheels (see Figure 1 – Top View). These ensure that dust resuspended by the rotation of the wheels is allowed to continue upward with minimal obstruction. This is important as there are no obstruction above a child’s feet.
Figure 1.
Computer-Aided Design – Computer-Aided Manufacturing (CAD/CAM) drawings of PIPER Mk IV sampling platform (Left: Front View, Right: Top View). In both views PEM Sampling Head (Left) Button Sampling Head (Right).
The activity profiles were created by carrying out a detailed quantitative analysis of 70 videorecordings (approximately 300 hours) of children at play in their homes. Video-recordings of all children who were 36 months of age and younger were scored for floor contact activity. The children’s activities were transcribed using VTD™ software (Virtual Timing Device; SamaSama Consulting). The software is a refinement of the VideoTraq software developed at Stanford University (9) and has been used to create the Micro-level Activity Time Series database (10). In order to characterize floor activities the standard template was modified to describe children’s activity in terms of location, movement, and posture. The location of the child was dichotomized to indoors and outdoors. The movement of the child was subjectively defined as still, slow, and fast. Four postures were used to define the child’s relative breathing zone while playing on the floor (on-floor play): lying (prone or supine), crawling, sitting/kneeling, and standing. Additionally, categories of not-in-view and off-floor play (e.g., sitting in a chair) were included in the posture selections to enable a complete transcription of the entire video-recorded period.
The activity data were divided into six age and gender specific groups, ages 6 months to 1 year, > 1 to 2 years, and > 2 years to 3 years of age. The mean percentage of time spent by children in each category of activity (unique combination of position and speed) and the mean duration of the activity were used to create a unique behavioral profile for each age and gender (e.g., Age 6 months to 1 year Boy, etc.). Actual breathing zone heights, corresponding to age specific postural positions, and speeds were derived for each age and gender from the published literature (11–12). This data was then entered into an activity file which can be accessed by the PIPER program. These activity profiles direct PIPER to vary its speed from being stationary to a maximum of 65 cm/sec simulating the pace of children in different activities. Additionally these profiles direct the sampling height by raising or lowering the sampling tower from a minimum height of 20 cm above the floor to a maximum of 84 cm to simulate different breathing zone heights for different ages and postures. This allows the speed and breathing zone of children of ages 6 months to 3 years to be simulated. The order of instructions is generated by a probability algorithm, derived from the quantitative analysis of the videorecordings and based upon the percent of time that a child would spend in the selected activity for that age and gender specific. The software interface on the laptop incorporates pull down menus for operation to select the activity profiles.
Household Sampling
Environmental sampling of PM was carried out in 55 homes in central New Jersey. Households were recruited through two central New Jersey pediatric clinics as part of an ongoing NIEHS funded study (ES014717) of early childhood dust exposures and asthma. Eligible homes contained children between the ages of 6 months and 4 years of age. Since health and household data were collected as part of the asthma study, informed consents which had been reviewed and approved by the UMDNJ-IRB were obtained.
The room in the home that was selected for sampling was based upon the mother’s reporting of the child’s primary play area. The type of flooring in the sampled area was recorded (bare or carpet/rug). All heating or air conditioning systems present in the home were allowed to be left running during the course of the testing. Air samples for PM were collected using Leland Legacy pumps (SKC Inc. Eighty Four, PA) running at ~ 10.0 l/min, drawing room air through 25 mm Teflon filters with 3.0 μm pore size mounted in an SKC Button Aerosol Sampler (13) which collects inhalable particles (< 100 μm aerodynamic diameter). All filters were allowed to equilibrate in a weighing room with controlled temperature (21°C) and relative humidity (30–40%) for a minimum of 72 hours prior to weighing. Filters were weighed using a Mettler Toledo MT5 Microbalance, weights were recorded and filters then placed in individual containers until loaded into the samplers prior to commencement of air sampling. One SKC Button Sampling head was mounted on a tripod at a fixed height of 110 cm (14–15), while another was attached to the sampling tower on PIPER. Tubes of equal lengths were attached from the pumps to the Button Samplers on both the stationary tripod and PIPER. Flow rates for both pumps were tested before and after all sampling with a TSI Series 4000 Flowmeter and flow rates recorded. Temperature and relative humidity were also recorded.
During the testing in each home 30 minutes were allowed to elapse after setting up the stationary tripod and PIPER, in order to provide for dust settling in the room prior to the start of air sampling. Subsequently 90 minutes of sampling of the room air by both the stationary sampler and PIPER was commenced. PIPER was programmed to run one of its 6 activity profiles, both fixed and PIPER mounted sampling pumps were then started and PIPER was set in motion and air samples were collected. For the first 30 minutes PIPER collected samples for qualitative analysis of mold (not described in this paper) and for an additional 60 minutes PIPER collected samples for quantitative analysis of PM only. The stationary sampling for PM was carried out for 90 minutes. PIPER was in operation over the entire 90 minutes (except for ~ 2–3 minutes changing sampling heads) the stationary sampler was in operation. The longer sampling time for the stationary sampler was done in order to increase the collected mass on the stationary filters. Each pump had its flow rate checked and recorded after completion of each sampling run. Fields blanks were included for quality control purposes. After sample collection, filters were then removed from the button sampler, replaced in their containers and returned to the same weighing room to equilabrate at the same temperature and humidity for a minimum of 72 hours prior to reweighing with the same microbalance. All field blanks (N=17, 15% of samples) were weighed and the mean difference in pre and post weights was less than 0.001 μg (S.D. = 0.003). PM concentrations were computed from the collected mass of the weighed samples and the calculated volume of air sampled. All data were verified and entered into a computer database.
Statistical analysis for data collected in homes was carried out utilizing Stata Version 11.1 Statistical Software (StataCorp, College Station, TX). Univariate analyses for data in the homes included mean (both arithmetic and geometric), median and an indepth analysis of the distribution of observed PM values. Paired t-tests were computed for absolute differences between stationary and PIPER samples for both bare and carpeted flooring and “p” values and 95% confidence intervals were calculated against the null hypotheses. Both paired and unpaired ratios (relative difference) between stationary and PIPER samples were computed for both bare and carpeted flooring and ratios and linearized standard errors and 95% confidence intervals computed.
Results and Discussion
The mass of inhalable PM collected on filters ranged from 2 to 164 μg (mean 35.5 μg; median 29.0 μg) for the stationary sampler and 4 to 226 μg (mean 43.2 μg; median 29.0 μg) for the PIPER sampler. The overall observed arithmetic and geometric means of the inhalable PM concentrations collected on the filters from the stationary sampler were 37.4 and 31.3 μg/m3 respectively (range 2.2 to 136.5 μg/m3; median 32.2 μg/m3). On the PIPER sampler the overall observed arithmetic and geometric mean of the inhalable PM concentrations were 72.3 and 52.6 μg/m3 respectively (range 6.6 to 378.6 μg/m3; median 51.6 μg/m3).
The concentrations of inhalable PM as measured by stationary sampling and by PIPER sampling are presented in Table 1, stratified by homes with bare and carpeted/rug flooring. The stratified distribution of PM concentrations was evaluated and was found to be approximately log normal. However for convenience of interpretation, data are presented without transformation as no appreciable effect on any statistical determinations between the transformed and non-transformed data was observed.
Table 1.
Concentration (μg/m3) of inhalable particulate in 55 homes by type of flooring.
| Sampling Method by Floor Covering | N | Arithmetic Mean | Geometric Mean | Median | Min. | Max. | Std. Dev. |
|---|---|---|---|---|---|---|---|
| Stationary | |||||||
| Bare Floor | 21 | 30.7 | 28.0 | 25.1 | 10.9 | 60.9 | 13.5 |
| Rug/Carpet | 34 | 41.5 | 33.6 | 32.9 | 2.2 | 136.5 | 27.2 |
| PIPER | |||||||
| Bare Floor | 21 | 34.6 | 30.4 | 37.7 | 6.6 | 63.3 | 15.8 |
| Rug/Carpet | 34 | 95.6 | 73.8 | 68.4 | 23.7 | 378.6 | 85.5 |
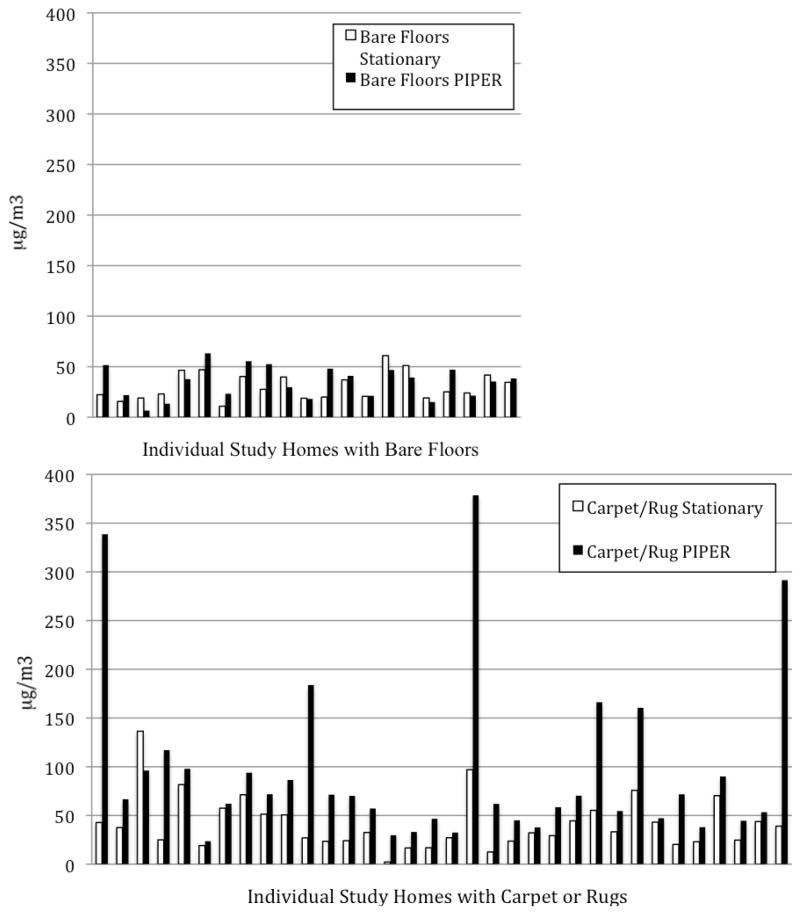
A comparison of the individual concentrations for both PIPER and the stationary sampler, stratified by type of flooring, are presented for each home in Figure 2. The paired t-test results between PIPER collected and stationary samples for homes with bare floors were minimally different with arithmetic and geometric mean differences of 3.9 and 2.4 μg/m3 respectively (arith. mean; p = 0.11). However in homes with carpets or area rugs, arithmetic and geometric mean differences for PIPER collected versus stationary samples were 54.1 and 40.2 μg/m3 respectively and the PIPER samples were statistically significantly higher (arith. mean; p = 0.0003).
Figure 2.
Comparison of PM measurements (μg/m3) using stationary general area and PIPER sampling for individual homes with bare floors (top - x axis, N=21) and carpets and rugs (bottom – x axis, N=34).
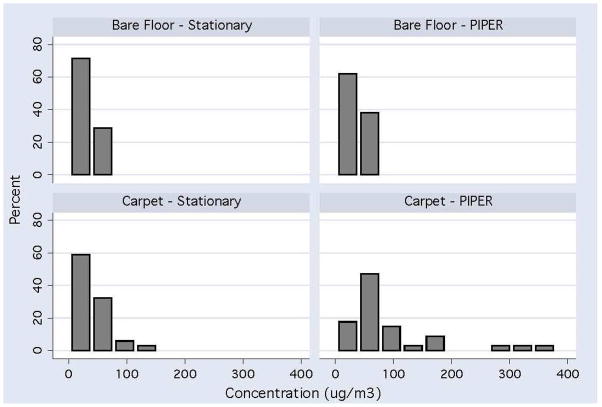
Relative differences (ratios) in PM concentrations for both paired and unpaired analyses were computed and stratified by floor covering. The reason both were computed is that the large variation in ratios observed in individual homes (see Figure 2) may have slightly skewed the results higher in the paired analysis. The relative difference (PIPER/Stationary = RD = ratio) for both unpaired and paired analyses between PIPER and stationary samplers were RD = 1.13 and 1.22 respectively for homes with bare floors (95% CI 0.92 – 1.34 and 0.95 – 1.48) with a range of 0.35 to 2.40. In homes with carpets or area rugs the RD = 2.3 and 2.9 respectively (95% CI 1.61 – 3.00 and 1.95 – 3.78) with a range of from 0.70 to 13.4. The comparison of the distribution of PM concentrations between stationary sampling and PIPER sampling are presented in Figure 3.
Figure 3.
Distribution Concentration of PM Levels (μg/m3) Comparing Stationary Sampling versus PIPER Sampling in 34 Homes with Rug or Carpeted Flooring and 21 Homes with Bare Floors in the Primary Play Area.
The results of this study suggest that reliance on stationary general area sampling to characterize household exposure to PM may systematically underestimate children’s exposure. However, accurately characterizing indoor air quality is essential since Americans, and children in particular, spend up to 90% of their time indoors (16). Assessing exposure to indoor airborne contaminants and asthmagens can provide valuable information about the role of contaminants in asthma development (7, 17–18). Exposure assessment in asthma studies has in general relied on two methods of sample collection: vacuum dust samples and stationary general area air sampling. Meanwhile studies in older children have suggested the importance of the “personal dust cloud” and its role in asthma, which suggests that personal monitoring is more relevant than general area monitoring (19–21) in characterizing the “true” level of exposure. Representative air sampling within the personal dust cloud is therefore critical for asthma research. The comparison of particle concentration in settled dust with that in personal air samples found that different asthmagens resuspend at different rates (22) and thus relying on surface sampling might not be an accurate metric for what is actually inhaled. However, the use of personal sampling to investigate exposures in the youngest children, those in the first years of life is problematic since placing sampling pumps on them is just not a realistic or ethical option. First, the weight of the equipment (1–1.5 kg) is quite significant next to that of a child particularly those under one year of age. Second is the propensity for young children to put objects in their mouths. Yet these are the children who are at the greatest risk for inhalation of resuspended floor dust because of their time spent playing on the floor (23).
Ample studies have demonstrated that personal sampling of adults is more informative in understanding exposure to PM than general area monitoring (24–25). Unfortunately, for children from birth to age 3 there is little information regarding indoor PM, let alone the specific issue of exposure to these size fractions. While some research has concluded that exposure of infants to PM can be adequately characterized by single stationary fixed height samplers (26), Edwards (27), has shown that any activity in a room results in the coarser particles being resuspended from the floor. In this study we observed a mean increase of 2 to 3-fold in inhalable PM as measured by PIPER compared to stationary measurements and up to a ten-fold higher concentration in some individual homes (Figure 2) when carpets or rugs were present. It is also important to note that while stationary area measurements rarely exceeded 100 μg/m3, the level of inhalable PM in a significant number of carpeted rooms frequently exceeded this value, with some homes approaching 400 μg/m3 (Figure 3). Even though this has been previously recognized as an issue in indoor PM (28–29) the role of carpets and rugs and how they contribute to PM in the near-floor microenvironment requires further study.
The main motivation for the creation of PIPER was to examine the role of near floor activity on the resuspension of particulate matter and potential exposure of very young children. Since children do not need to participate in such a robot based study, use of the robotic platfrom to estimate exposures avoids ethical complications such as lead to the cancellation of U.S.E.P.A. CHEERS study (30). This cancellation has raised concern about the future ability of researchers to directly study and characterize children’s exposure to toxins in homes, because of the ethical considerations involved. The PIPER Mk IV makes use of extensive videorecordings of children at play to approximate the movement patterns that create a personal cloud and examine how measurements in this cloud depart from general area measurements of exposure. The observed concentration of airborne PM collected with PIPER demonstrate that samples taken in this cloud are higher on average than those taken in the same room at the same time by a stationary sampler at a sampling height of 110 cm. The disparity of the concentration distribution is evident in Figure 3 and suggests that stationary sampling in the home underestimates exposure in a room as compared to what would be experienced if the dust was being disturbed. Disturbance and resultant resuspension of housedust is likely to occur when children are engaged in any activities on the floor. The filter data from PIPER sampling demonstrate that when there are disturbances in the home environment from floor activity significant increases in PM mass concentrations are observed. Further that these PM increases are on average statistically significantly over two-fold higher, in areas with rugs or carpets, compared to stationary measurements. While it is true that PIPER’s mechnical action is different from a child at play, we have attempted to simulate their activity level and their changing breathing zone heights as closely as possible. PIPER’s movements are directed by our quantitive analysis of children at play in their homes. This allows it to simulate the disturbance of particles settled on play surfaces. It is therefore likely that the cloud of resuspended PM created by PIPER is a better approximation of the dust cloud and thus a more relavant estimate of the potential for inhalation exposures of young children as compared to stationary general area monitoring. Future studies to validate PIPER in children ages 2 to 3 years are planned where lighter sampling pumps can be worn for limited amounts of time by children in their home and the results directly compared to PIPER equipped with the same devices.
PIPER offers three important advantages in estimating children’s exposure to PM. First unlike stationary monitoring, PIPER by its motion through the environment has been shown to resuspend settled particles. Second PIPER, unlike a child, provides a totally reproducible level of activity allowing for the direct comparison of levels of resuspension of PM between different locales and conditions. Third, PIPER may be used to evaluate environments regardless of the level of toxicant without rasing any ethical concerns about children’s exposure. By employing PIPER or similar devices researchers may be able to more precisely characterize young children’s exposures as they play on the floor inside their home. This information may be key in achieving a better understanding of the role of early childhood exposure to airborne pollutants in the etiology of asthma. It may also increase our general understanding of the personal dust cloud that children generate and are exposed to while at play.
Acknowledgments
Funding for this research was provided by NIEHS through the Center for Environmental Exposures and Disease: P30ES005022: H. Zarbl, PI, and R01ES014717: S. Shalat, PI and Support from EOHSI.
Literature Cited
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2008. Vital Health Stat. 2009;10(244):1–81. [PubMed] [Google Scholar]
- 2.Delfino RJ, Quintana PJ, Floro J, Gastanaga VM, Samimi BS, Kleinman MT, Liu LJ, Bufalino C, Wu CF, McLaren CE. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004;112(8):932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pino P, Walter T, Oyarzun M, Villegas R, Romieu I. Fine particulate matter and wheezing illnesses in the first year of life. Epidemiology. 2004;15(6):702–708. doi: 10.1097/01.ede.0000142153.28496.d0. [DOI] [PubMed] [Google Scholar]
- 4.Douwes J, Pearce N. Invited commentary: is indoor mold exposure a risk factor for asthma? Am J Epidemiol. 2003;158(3):203–206. doi: 10.1093/aje/kwg149. [DOI] [PubMed] [Google Scholar]
- 5.Brussee JE, Smit HA, van Strien RT, Corver K, Kerkhof M, Wijga AH, Aalberse RC, Postma D, Gerritsen J, Grobbee DE, et al. Allergen exposure in infancy and the development of sensitization, wheeze, and asthma at 4 years. J Allergy Clin Immunol. 2005;115(5):946–952. doi: 10.1016/j.jaci.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Pearce N, Douwes J, Beasley R. Is allergen exposure the major primary cause of asthma? Thorax. 2000;55(5):424–431. doi: 10.1136/thorax.55.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, McCormack MC, Williams DL, Breysse PN. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007;115(11):1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalat SL, Lioy PJ, Schmeelck K, Mainelis G. Improving estimation of indoor exposure to inhalable particles for children in the first year of life. J Air Waste Manag Assoc. 2007;57(8):934–939. doi: 10.3155/1047-3289.57.8.934. [DOI] [PubMed] [Google Scholar]
- 9.Zartarian VG, Ferguson AC, Ong CG, Leckie JO. Quantifying videotaped activity patterns: video translation software and training methodologies. J Expo Anal Environ Epidemiol. 1997;7(4):535–542. [PubMed] [Google Scholar]
- 10.Ferguson AC, Canales RA, Beamer P, Auyeung W, Key M, Munninghoff A, Lee KT, Robertson A, Leckie JO. Video methods in the quantification of children’s exposures. J Expo Sci Environ Epidemiol. 2006;16(3):287–298. doi: 10.1038/sj.jea.7500459. [DOI] [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention. National Center for Health Statistics. http://www.cdc.gov/growthcharts/clinical_charts.htm#Summary.
- 12.Cavagna GA, Franzetti P, Fuchimoto T. The mechanics of walking in children. J Physiol. 1983;343:323–339. doi: 10.1113/jphysiol.1983.sp014895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aizenberg V, Reponen T, Grinshpun SA, Willeke K. Performance of Air-O-Cell, Burkard, and Button Samplers for total enumeration of airborne spores. Am Indus Hyg Aassoc J. 2000;61(6):855–864. doi: 10.1080/15298660008984598. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Environmental Protection Agency. Air Quality Criteria for Particulate Matter. EPA; Washington, DC: 1994. EPA/600/P-99/002aF, EPA/600/P-99/002bF. [Google Scholar]
- 15.U.S. Environmental Protection Agency. Air Quality Criteria for Particulate Matter (Final Report, April 1996) EPA; Washington, D.C: 1996. EPA 600/P-95/001. [Google Scholar]
- 16.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 17.McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, Williams DL, Buckley TJ, Eggleston PA, Diette GB. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106(2):148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, Eggleston P, Diette GB. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117(2):294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozkaynak H, Xue J, Spengler J, Wallace L, Pellizzari E, Jenkins P. Personal exposure to airborne particles and metals: results from the Particle TEAM study in Riverside, California. J Expo Anal Environ Epidemiol. 1996;6(1):57–78. [PubMed] [Google Scholar]
- 20.Yip FY, Keeler GJ, Dvonch JT, Robins TG, Parker EA, Israel BA, Brakefield-Caldwell W. Personal exposures to particulate matter among children with asthma in Detroit, Michigan. Atmospheric Environment. 2004;38(31):5227–5236. doi: 10.1289/ehp.02110s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinovitch N, Liu AH, Zhang L, Rodes CE, Foarde K, Dutton SJ, Murphy JR, Gelfand EW. Importance of the personal endotoxin cloud in school-age children with asthma. J Allergy Clin Immunol. 2005;116(5):1053–1057. doi: 10.1016/j.jaci.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Raja S, Xu Y, Ferro AR, Jaques PA, Hopke PK. Resuspension of indoor aeroallergens and relationship to lung inflammation in asthmatic children. Environ Int. 2010;36(1):8–14. doi: 10.1016/j.envint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Cohen Hubal EA, Sheldon LS, Burke JM, McCurdy TR, Berry MR, Rigas ML, Zartarian VG, Freeman NC. Children’s exposure assessment: a review of factors influencing Children’s exposure, and the data available to characterize and assess that exposure. Environ Health Perspect. 2000;108(6):475–486. doi: 10.1289/ehp.108-1638158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, Spektor DM, Korn L, Winer AM, Kwon J, et al. Relationships of Indoor, Outdoor, and Personal Air (RIOPA). Part I. Collection methods and descriptive analyses. Res Rep Health Eff Inst. 2005;(130 Pt 1):1–107. discussion 109–127. [PubMed] [Google Scholar]
- 25.Janssen NA, Hoek G, Brunekreef B, Harssema H, Mensink I, Zuidhof A. Personal sampling of particles in adults: relation among personal, indoor, and outdoor air concentrations. Am J Epidemiol. 1998;147(6):537–547. doi: 10.1093/oxfordjournals.aje.a009485. [DOI] [PubMed] [Google Scholar]
- 26.Jones J, Stick S, Dingle P, Franklin P. Spatial variability of particulates in homes: implications for infant exposure. Sci Total Environ. 2007;376(1–3):317–323. doi: 10.1016/j.scitotenv.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 27.Edwards RD, Yurkow EJ, Lioy PJ. Seasonal deposition of housedusts onto household surfaces. Sci Total Environ. 1998;224(1–3):69–80. doi: 10.1016/s0048-9697(98)00348-9. [DOI] [PubMed] [Google Scholar]
- 28.Ferro AR, Kopperud RJ, Hildemann LM. Elevated personal exposure to particulate matter from human activities in a residence. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S34–40. doi: 10.1038/sj.jea.7500356. [DOI] [PubMed] [Google Scholar]
- 29.Ferro AR, Kopperud RJ, Hildemann LM. Source strengths for indoor human activities that resuspend particulate matter. Environ Sci Technol. 2004;38(6):1759–1764. doi: 10.1021/es0263893. [DOI] [PubMed] [Google Scholar]
- 30.Barrett JR. DOUBLE PROTECTION: reaching accord on the ethical conduct of child observational research. Environ Health Perspect. 2009;117(8):A354–357. doi: 10.1289/ehp.117-a354. [DOI] [PMC free article] [PubMed] [Google Scholar]