Abstract
Peritoneal carcinomatosis (PC) of colorectal origin is associated with a poor prognosis. However, cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy is available for a selected group of PC patients, which significantly increases overall survival rates up to 30%. As a consequence, there is substantial room for improvement. Tumor targeting is expected to improve the treatment efficacy of colorectal cancer (CRC) further through 1) more sensitive preoperative tumor detection, thus reducing overtreatment; 2) better intraoperative detection and surgical elimination of residual disease using tumor-specific intraoperative imaging; and 3) tumor-specific targeted therapeutics. This review focuses, in particular, on the development of tumor-targeted imaging agents. A large number of biomarkers are known to be upregulated in CRC. However, to date, no validated criteria have been described for the selection of the most promising biomarkers for tumor targeting. Such a scoring system might improve the selection of the correct biomarker for imaging purposes. In this review, we present the TArget Selection Criteria (TASC) scoring system for selection of potential biomarkers for tumor-targeted imaging. By applying TASC to biomarkers for CRC, we identified seven biomarkers (carcinoembryonic antigen, CXC chemokine receptor 4, epidermal growth factor receptor, epithelial cell adhesion molecule, matrix metalloproteinases, mucin 1, and vascular endothelial growth factor A) that seem most suitable for tumor-targeted imaging applications in colorectal cancer. Further cross-validation studies in CRC and other tumor types are necessary to establish its definitive value.
Introduction
Patients with colorectal cancer (CRC) have an estimated 5-year survival, varying from approximately 90% in patients with stage I disease (Dukes A) to approximately 10% in patients with metastatic disease (Dukes D) [1]. Peritoneal carcinomatosis (PC) is a common form of end-stage colorectal cancer (CRC), affecting 10% to 15% of patients at the time of primary surgery and accounting for 25% to 35% of the recurrences of CRC [2]. PC has a median survival of 5 to 7 months without treatment [3–5].
Since the last decade, selected stage IV CRC patients with PC are treated with hyperthermic intraperitoneal chemotherapy (HIPEC). This procedure consists of flushing the intra-abdominal cavity with heated chemotherapy perioperatively after primary cytoreduction. HIPEC improves the median survival to 13 to 63 months, with a 5-year survival varying from 19% to 51% [6–10]. However, further improvement is still desirable.
A more extensive surgical cytoreduction is associated with an increase in survival [11,12]. Furthermore, because penetration of chemotherapeutic drugs into peritoneally located tumor tissue is only superficial (limited to 1–2 mm), optimal cytoreduction by removing all visible tumor noduli is an essential prerequisite for the HIPEC procedure [13–15].
The limited survival in stage IV CRC asks for a more vigorous approach to improve prognosis. Current research is mainly focused on tumor-targeted imaging and therapy for diagnosis, treatment, and follow-up because these are expected to yield tumor-specific and thus stronger diagnostic and therapeutic effects. Therefore, objective identification of suitable tumor biomarkers for diagnostic and therapeutic purposes seems appropriate. Furthermore, tumor-targeted imaging can aid in identification of metastatic disease and in detection of recurrent disease. In this review, we emphasize tumor-targeted imaging because targeted therapeutics demand an entirely different approach for a meta-analysis.
A large number of biomarkers have been reported to play an important role in CRC. However, a limited number of these markers are suitable for tumor targeting based on characteristics such as, for example, expression rates. In literature, few objective data on how to determine the suitability of a potential target are available. Therefore, we set out to design a novel scoring system for classification and selection of biomarkers for tumor targeting applications. CRC is used as a clinical example for development and initial testing of this novel scoring system. With the emphasis on diagnostic and intraoperative imaging, we identified the most promising markers for tumor targeting in CRC using the scoring system.
In conclusion, in this review, we provide an overview of potential biomarkers for tumor targeting in CRC, supported by a newly designed TArget Selection Criteria (TASC) scoring system.
Methods
Design of the TASC Scoring System
Seven most important target characteristics selected based on the literature were summarized and granted 0 to 6 points, in order of importance. Subsequently, the selection system was tested by scoring a number of random biomarkers. Cutoff values were determined, and the scores were slightly adjusted where necessary to assure realistic outcomes. Finally, the selection system was further validated by testing a wide spectrum of biomarkers based on a publication of Cardoso et al. [16].
Literature Search Methods
Cardoso et al. [16] presented a table of genes found to be upregulated in CRC compared with normal colon tissue, as confirmed in three or more articles. The initial literature search query was based on this extensive table of genes. In addition, based on this table, we analyzed all genes mentioned for overexpression of the related protein because protein expression is not always synchronously upregulated, using Swiss-Prot and PubMed from 1985 to May 2010 (Figure 1). Furthermore, we included a number of proteins that were not mentioned in the table of Cardoso et al. but were otherwise described in the literature to play a significant role in CRC.
Figure 1.
Selection of biomarkers upregulated in CRC.
Finally, a systematic search of literature was performed, with PubMed as the main database, using the following search terms: the name of the protein + “immunohistochemistry” + “colorectal cancer,” and the name of the protein + “imaging” + “colorectal cancer,” or variations of these terms, from 1985 to May 2010.
Target Selection: Introducing TASC
A tumor biomarker can be defined as a distinguishable component present on the tumor cell or secreted by a tumor cell to the surrounding stromal tissue. Such a biomarker is often a target in biologic interactions, e.g., the combination of CXC chemokine receptor 4 (CXCR4) as target of SDF-1. Alternatively, a biomarker can be used as a target for a synthetic substrate, which can be a single molecule, antibody, or others. Such a substrate can be conjugated to a diagnostic or imaging agent or a drug for clinical application purposes.
To our best knowledge, a scoring system to identify the most ideal target characteristics has never been explicitly described or developed or even validated. However, a number of favorable target features can be logically extracted from literature so far. On the basis of these characteristics, we propose a novel scoring system for target selection in particular for imaging purposes, the TASC.
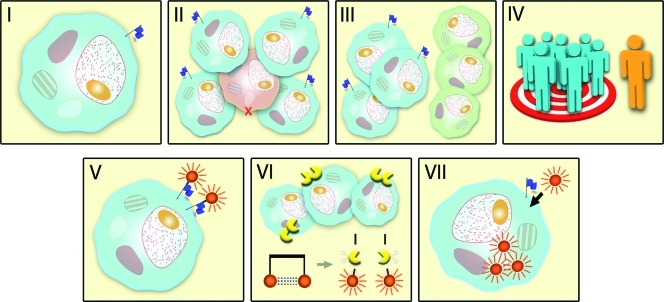
The TASC score is based on the seven most favorable target characteristics that are granted points if it applies to the marker (Table 1). These characteristics are as follows: I) extracellular biomarker localization, either on the cell membrane or in close proximity of the tumor cell; II) expression pattern; III) tumor-to-healthy tissue ratio (T/N); IV) percentage of positive tumors; V) reported successful use of the biomarker in in vivo imaging studies; VI) enzymatic activity; and VII) internalization (Figure 2).
Table 1.
The TASC.
| TASC Scoring System | |||
| Characteristics | Score | ||
| I | Extracellular protein localization | Bound to cell surface (receptor) | 5 |
| In close proximity of tumor cell | 3 | ||
| II | Diffuse up-regulation through tumor tissue | 4 | |
| III | T/N ratio > 10 | 3 | |
| IV | Percentage up-regulation in patients | >90% | 6 |
| 70%–90% | 5 | ||
| 50%–69% | 3 | ||
| 10%–49% | 0 | ||
| V | Previously imaged with success in vivo | 2 | |
| VI | Enzymatic activity | 1 | |
| VII | Internalization | 1 | |
| Total: maximum 22 | |||
| Potential target ≥ 18 | |||
A biomarker is granted points for seven factors (I–VII). A total score of 18 or higher indicates that the biomarker is potentially suitable for tumor-targeted imaging purposes.
Figure 2.
The TASC. The blue flag represents the selected biomarker. I. Extracellular localization of the biomarker, cell membrane-bound, or in close proximity of tumor cell. II. Diffuse up-regulation of the target throughout tumor tissue. III. T/N ratio greater than 10. Blue cells represent tumor cells; normal cells are green. IV. Up-regulation of the biomarker in most patients. V. A biomarker that has previously successfully been used in in vivo imaging studies. VI. Enzymatic activity facilitating the use of activatable probes. Shown are cleaving enzymes (yellow) that activate the imaging agent. VII. Internalization of probe for accumulation of imaging agent.
We will briefly explain these seven individual characteristics:
I—A target must be easily accessible by an agent, administered either systemically or intraperitoneally. For effective targeting, as little as possible barriers should be between the agent and its target. As a consequence, most conveniently, the marker is present on the cell surface. Alternatively, the expression of the target in the extracellular tumor matrix may also be adequate for imaging purposes. In our opinion, the extracellular localization of the target, either membrane-bound or near the tumor cell, is one of the most important factors and is therefore weighted substantially in the TASC system. Extra points are given to a cell membrane bound target because it is expected that membrane-bound targets more specifically emit signal from the tumor cell than soluble targets.
II—In the best scenario, the target is expressed by all tumor cells. However, in reality, this is very rare because cancer cells have the reputation of being heterogenic [17]. Also acceptable is a marker that is evenly distributed throughout the tumor tissue. High sensitivity to detect all tumor tissue is essential; therefore, this factor also has a significant power in TASC.
III—The expression of the biomarker should be minimal in normal tissue. In modalities like positron emission tomography (PET) and single photon emission computed tomography (SPECT) scanning, a tumor-to-healthy cell (T/N) ratio of greater than 10 is considered sufficient [18]. In fluorescence imaging, a minimal T/N ratio has not yet been described but is expected to be comparable to the previously mentioned modalities on the basis of its detection sensitivity and specificity.
IV—It is highly preferable that the use of a particular biomarker is of value for large patient populations rather than only small groups of “special” patients. Overexpression of the target in most patients increases clinical applicability of a tumor-targeted agent.
V—Previous use of a biomarker in in vivo imaging indicates suitability of the marker for imaging purposes in other diseases; in this case, CRC.
VI—Although not an absolute condition for a target, (extracellular) enzymatic activity in and around the tumor tissue offers the possibility of applying locally activated imaging agents, so-called smart probes, increasing the signal-to-background ratio [19].
VII—It is reported in the literature that internalization of the probe-target complex in the tumor can lead to intracellular accumulation of the imaging agent, which improves the signal and leads to a more optimal T/N ratio [20]. For this reason, internalization is granted points in the selection criteria.
Selecting a target that meets up to all of these conditions is challenging. In most cases, it is not necessary to meet all criteria.
A total score of 21 or 22 implies that a marker has a high potential for use as a target for imaging tracers in vivo. If a marker scores 18 or higher, it is considered to be a potential target. Markers with a score of less than 18 seem less suitable for targeted imaging modalities and require more research to evaluate their potential.
Possible Target Candidates in Colorectal Cancer
It is well known that it can be difficult to distinguish cancer cells from its normal surroundings because of the many similarities between malignant cells and normal cells. Furthermore, tumors are mutually heterogenic. However, what most cancer cells have in common and what separates them from normal cells is uncontrolled growth, resulting in a high nutritional uptake. An alternative property is the ability to invade normal tissue and metastasize. In this respect, it is not surprising that the potential targets presented in this review support these phenotypic characteristics. The biomarkers reported for CRC can roughly be divided into the following groups:
Proteins necessary for high cancer cell metabolism and proliferation rate: epidermal growth factor receptor (EGFR), folate receptor-alpha (FR-α), transforming growth factor (TGF), vascular endothelial growth factor (VEGF).
Proteins with regulatory functions in the extracellular matrix: carbonic anhydrase (CA) IX, collagen, matrix metalloproteinases (MMPs), osteonectin (SPARC).
Cell adhesion and signaling molecules: cadherin 3, carcinoembryonic antigen (CEA), CD44, CXCR4, epithelial cell adhesion molecule (EpCAM), integrins.
Cytokines/chemokines and their corresponding receptors, involved in metastasis: CXCR1, CXCR2, CXCR4, CXC chemokine ligands (CXCLs).
Miscellaneous: cathepsin, inducible nitric oxide synthase (iNOS), mucin 1 (Muc1), neutrophil gelatinase-associated lipocalin (NGAL) also called lipocalin-2 (LCN2), tumor-associated glycoprotein 72 (TAG-72).
These potential targets are summarized in Table 2. As is shown in this table, several potential target candidates can be identified; however, currently, a limited number of matching clinically approved agents are available for application in humans (Table 3).
Table 2.
Proteins Upregulated in Colorectal Cancer.
| TASC Item | I | I | II | III | IV | V | VI | VII | TASC Score | References |
| Extracellular: Membrane-bound or Secreted | In Close Proximity of Tumor Cell | Pattern of Up-regulation by Tumor Tissue | T/N Ratio | Percentage Patients with Positive Colorectal Tumors | Previously Imaged | Enzymatic Activity | Target-Mediated Internalization | |||
| CA IX | Membrane-bound | Yes | Focal as well as diffuse | High | ∼47% | Animal experiment [121,122] | Yes | Yes [123] | 11 | Niemela et al. [124], Kivela et al. [125], Saarnio et al. [126] |
| Cadherin 3 | Membrane-bound | Yes | Diffuse | High | Probably high; no percentage known | No | Not described | Not described | 15 | Imai et al. [127] |
| Cathepsin B, D | Mostly secreted; cathepsin B is partly membrane-bound | Mainly | Diffuse, but most expression at invasion front | High | ∼60% | Animal experiment [128–130] | Yes | Not described | 16 | Kaneko et al. [45], Kuester et al. [131], Emmert-Buck et al. [82] |
| CD44 | Membrane-bound | Yes | Diffuse | ∼1.4 | ∼50% | Animal experiment [132] | Not described | Yes [133] | 16 | Bendardaf et al. [134], Fernandez et al. [135] |
| CEA | Partly membrane-bound; partly secreted | Mainly | Diffuse, not homogenous | >60 | >90% | In patients [69,136,137] | Not described | Yes [138,139] | 19 | Li et al. [61], Kim et al. [62], Suwanagool et al. [63], Hamada et al. [64] |
| COL11A1 | Secreted | Yes | Unknown | Unknown | Unknown | No | Not described | Not described | 3 | Bowen et al. [140] |
| CXCL 1, 5 | Secreted | Unknown | Unknown | High | Unknown | No | Not described | Not described | 3 | Rubie et al. [179], Wen et al. [141] |
| CXCR1 | Membrane-bound | Yes | Diffuse, mainly in primary tumor | High | ∼55% | No | Not described | Yes [142,143] | 16 | Rubie et al. [30] |
| CXCR2 | Membrane-bound | Yes | Diffuse, mainly in primary tumor | High | ∼60% | No | Not described | Yes [142,143] | 16 | Rubie et al. [30] |
| CXCR4 | Membrane-bound | Yes | Diffuse, more expression in metastases | High | ∼70% | Animal experiment [32] | Not described | Yes [144] | 20 | Rubie et al. [30] |
| EGFR | Membrane-bound | Yes | Diffuse | Unknown, probably high | ∼80% | In patients [108] Animal experiment [111,114,145–147] | Not described | Yes [148] | 20 | Cunningham et al. [101], Goldstein et al. [102] |
| EpCAM | Membrane-bound | Yes | Diffuse | High (own data) | >79% | Animal experiment [149] | Not described | Yes [29] | 20 | Paret et al. [21], Xie et al. [22] |
| Folate receptor-α | Membrane-bound | Yes | Diffuse, little strong expression | High | ∼40% | In patients [150,151] | Not described | Yes [152] | 15 | Shia et al. [153] |
| Galectin 3 | Partly membrane-bound, partly secreted | Yes | Diffuse, but not homogenous | High | 65%–95% | Animal experiment [154,155] | Not described | Yes [156] | 13 | Paret et al. [21], Endo et al. [157], Tsuboi et al. [158] |
| iNOS | Mainly intracellular | Yes | Diffuse | High | ∼78% | Animal experiment [159] | Yes | Is already intracellular | 16 | Yu et al. [160], Zafirellis et al. [161] |
| Integrins | Membrane-bound | Yes | Diffuse | Unknown, but imaging T/N ratio >5 [188] | ∼60% | In patients [163–164] | Not described | Yes [165,166] | 15 | Fan et al. [167], Sipos et al. [168] |
| MMP1, 2, 3, 7, 9 | Mainly secreted | Yes | Diffuse | Moderate to high | 30%–95% | Animal experiment [88,89,91,169] | Yes | Not described | 18 | McKerrow et al. [79], Jeffery et al. [80], Madoz-Gurpide et al. [81], Kaneko et al. [45], Emmert-Buck et al. [82] |
| Muc1 | Membrane-bound | Yes | Diffuse, more expression in larger tumors and lymph node metastases | High | ∼50% | In patients [54–56,60] | Not described | Yes [170] | 18 | Kaneko et al. [45], Suzuki et al. [46] |
| NGAL (LCN2) | Secreted | Mainly | Diffuse | High | ∼75% | In vitro [171] | Not described | Not described | 15 | Conrotto et al. [171], Madoz-Gurpide et al. [81] |
| Osteonectin (SPARC) | Secreted | Yes | Diffuse | High | High, no percentage known | Animal experiment [172] | Not described | Not described | 17 | Madoz-Gurpide et al. [81] |
| TAG-72 | Partly membrane-bound; partly secreted | Mainly | Not diffuse | High | 46%–98% | In patients [173,174] | Not described | Not described | 11 | Loy et al. [175], Muraro et al. [176], Molinolo et al. [177] |
| TGFBI | Secreted | Mainly | Unknown | High | Unknown | No | Not described | Not described | 6 | Roessler et al. [178] |
| VEGF-A | Partly membrane-bound, partly secreted | Mainly | Diffuse, more expression in metastases | High | 56%–78% | In patients [40,44] | Not described | Not described | 17 | Cao et al. [38], Abdou et al. [39] |
Under “previously imaged” (item V), only the most advanced research is mentioned. For each biomarker, the final TASC score is given, based on the characteristics as explained in Table 1.
Table 3.
Clinically Approved Ligands for the Biomarkers Mentioned in Table 2.
| Target | Clinically Approved Ligand | In Clinical Trial |
| CEA | Arcitumomab, Altumomab | |
| CXCR4 | AMD3100 | BKT-140, AMD11070, MSX-122 |
| EGFR | Cetuximab, Panitumumab, Nimotuzumab | Necitumumab, Zalutumumab |
| EpCAM | Edrecolomab, Catumaxomab (anti-EpCAM x anti-CD3) | Adecatumumab, Tucotuzumab |
| Folate receptor-α | Folate | |
| Integrin | MoaB PF-04605412 (mAb against α5β1 integrin), Etaracizumab (mAb against αvβ3 integrin) | |
| Muc1 | Pemtumomab | 90Y-hPAM4 |
| TAG-72 | Anatumomab mafenatox, Minretumomab | |
| VEGF | Bevacizumab, Ranibizumab |
Some targets have T/N ratio of less than 10. However, it should be noted that some targets internalize the imaging agent more rapidly in tumor cells compared with normal cells [18].
This leads to an accumulation of the conjugated imaging agent, which may compensate the signal for the lower T/N ratio, as with FDG-PET imaging [18,20].
Which Biomarkers Meet the Targeting Criteria?
When applying the proposed TASC score (Table 1) to the biomarkers mentioned in Table 2, not all requirements can be objectified by data from literature. Most often, expression rates and pattern are unknown; therefore, it would be interesting to focus future research on target finding on these aspects. The following six targets have a score greater than 17 points and can therefore be considered most promising in CRC (Table 4): EpCAM (20 points), CXCR4 (20 points), Muc1 (18 points), MMPs (18 points), EGFR (20 points), and CEA (19 points). In this section, we discuss these targets in more detail, including the status of these biomarkers in targeted imaging.
Table 4.
The Biomarkers That Score 18 or More Points When Applying TASC.
| Biomarker | TASC Score |
| CXCR4 | 20 |
| EpCAM | 20 |
| EGFR | 20 |
| CEA | 19 |
| Muc1 | 18 |
| MMPs | 18 |
| VEGF-A | 17* |
These biomarkers are regarded most promising for tumor-targeted imaging in colorectal cancer.
VEGF-A scores 17 points but was nonetheless included based on the broad experience with this marker for imaging purposes.
VEGF-A scores 17 points, which implies less potential as a target. However, given the extensive experience in VEGF-A-targeted imaging, this biomarker was, nevertheless, considered to be promising and is therefore given attention in this section.
Epithelial Cell Adhesion Molecule
EpCAM is a cell surface receptor, which is involved in cell adhesion and is expressed on most epithelial cells. EpCAM is upregulated on several epithelial cancers, including CRC. The expression of EpCAM in CRC is more than 80% [21–23]. Paradoxically, a higher expression of EpCAM on tumor cells is associated with increased tumor cell migration [23]. Eder et al. [24] successfully imaged EpCAM-expressing tumors in mice, using an antibody fragment targeting EpCAM conjugated to radionuclide, for PET imaging.
Edrecolomab and catumaxomab are clinically approved antibodies directed at EpCAM (Table 3) and tested for therapeutic use. However, so far, no obvious therapeutic advantage has been reported for these agents [25–28]. To our best knowledge, these antibodies have not yet been used for in vivo imaging of EpCAM. When applying TASC to EpCAM; the total score of 20 points comes about as followed: EpCAM is cell membrane bound (5 points), diffusely upregulated (4 points), has a high T/N ratio (3 points), is upregulated in more than 79% of the CRC patients (5 points), has been previously imaged with success in vivo (2 points), and is able to internalize a compound (1 point) [21,22,29].
CXC Chemokine Receptor 4
CXCR4 is a cell surface receptor involved in homing of hemopoietic stem cells and lymphocytes to the bone marrow, but it is also associated with metastatic spread in several types of cancer, including CRC. CXCR4 is expressed in approximately 70% of the colorectal tumors [30].
Imaging of CXCR4 has recently attracted attention of many different research groups. Nimmagadda et al. [31,32] reported imaging of CXCR4 in tumor-bearing mice using a radionuclide-labeled anti- CXCR4 monoclonal antibody (mAb) using SPECT/CT scanning. Recently, the same group also succeeded in imaging CXCR4 expressing tumors in mice with the use of AMD3100, a clinically approved molecule that selectively binds to CXCR4 (Table 3), conjugated to a radionuclide [32]. AMD3100 is a clinically approved agent that is most promising in harvesting hemopoietic stem cells from the bone marrow. Alternatively, CXCR4-targeting peptides conjugated to a radionuclide or a fluorophore have been reported [33,34]. Misra et al. [35] labeled stromal-derived factor-1 alpha (SDF-1α), a ligand ofCXCR4, to a radionuclide for myocardial infarction imaging purposes.
When applying TASC, CXCR4 is granted 20 points based on its expression in CRC.
Vascular Endothelial Growth Factor-A
VEGF is an epithelial growth factor that is most extensively known for its ability to induce angiogenesis. Angiogenesis in turn is considered one of the primary markers in tumor diagnostics [36]. There are four VEGFs, namely VEGF-A, -B, -C, and -D. VEGF-A is the most important subtype. When tumor cells become hypoxic, VEGF-A expression is upregulated [36]. VEGF-A is partly membrane bound, but it also diffuses through the interstitial cell space. The latter potentially limits broader use as a target. However, the highest VEGF-A concentrations are observed close to the source of expression, inducing the creation of new blood vessels to the hypoxic tumor areas [37].
VEGF-A is upregulated in more than 56% to 78% of all colorectal tumors [38,39].
Multiple groups have successfully imaged VEGF-A expression in tumors induced in animals using a VEGF-A antibody conjugated to an imaging agent. Imaging has most commonly been performed with bevacizumab (Avastin; Roche), a clinically approved therapeutic anti-VEGF-A mAb, which was made suitable for imaging by conjugation to a radionuclide [40–44].
In a clinical imaging study, Scheer et al. [40] did not find a significant correlation between VEGF-A expression and a positive SPECT signal, which may imply that the used tracer was not specific enough. Furthermore, a study in melanoma patients with bevacizumab conjugated to a radionuclide by Nagengast et al. [44] yielded more promising results.
When applying TASC to VEGF-A, a total of 17 points are granted. This low score is mainly caused by the fact that the largest proportion of VEGF-A is not membrane bound and by the expression in a relatively low percentage of patients with CRC. However, because of the recent results in various imaging modalities, as described above, VEGF-A can be considered a potential target for future imaging purposes and is therefore worth to be included in this overview.
Mucin 1
Muc1 is a cell surface receptor that plays a role in protection and lubrication of epithelial surfaces in luminal structures. This receptor is also involved in signal transduction in cell adhesion and antiadhesion mechanisms. Overexpression of Muc1 is often found on malignant cells. In CRC, Muc1 is expressed on approximately 50% of the tumors [45,46].
Different groups have successfully imaged Muc1 in tumor-bearing mice using muc1-targeted monoclonal antibodies or aptamers conjugated to a radiopharmaceutical [47–53]. The use of monoclonal antibodies directed to Muc1 conjugated to a radionuclide has already been described in patients with bladder and pancreatic cancer [54–56]. Medarova et al. [57] described the use of a dual-modality imaging agent by conjugating a Muc1-targeting peptide to fluorophore Cy5.5 for fluorescence imaging and to iron oxide nanoparticles for magnetic resonance (MR) imaging. This probe was tested in mice bearing human pancreatic cancer with good imaging results for both modalities. Muguruma and Ito [58] proved the ability to endoscopically detect tumors by using a fluorescent antibody-based tracer targeting Muc1, in freshly resected specimens of gastric cancer. A different approach for tumor imaging is a two-step pretargeting technique using a bi-specific antibody. An antibody directed to both Muc1 and the used radiopharmaceutical is administered on which the radiopharmaceutical is administered subsequently. The radiopharmaceutical binding site of the circulating antibody can be blocked, thus yielding a higher tumor-to-background ratio [59]. Promising results were obtained in breast cancer patients with bispecific antibody-based PET scanning [60].
The total TASC score for Muc1 in CRC is 18 points.
Carcinoembryonic Antigen
CEA is a glycoprotein that plays a role in cell adhesion. In healthy adults, hardly any CEA is found; however, CEA is strongly expressed in CRC (>90%) [61–64] and is one of its best studied tumor markers. CEA is also measurable in blood, but by far, the highest concentration of CEA is found at the tumor site. CEA imaging using a CEA-directed antibody or antibody fragment conjugated to a radionuclide has extensively been described in animal studies and in patients, without showing disadvantages of having simultaneous high serum and tumor CEA levels [65–70]. Yazaki et al. [71] fused CEA-antibody fragments conjugated to a radionuclide to albumin for a more specific tumor uptake. Technetium 99m (99mTc) arcitumomab is a commercially available antibody fragment directed to CEA conjugated to 99mTc, which is used in the CEA scan. However, in comparison to FDG-PET, 99mTc arcitumomab offers little convincing advantages in the detection of CRC [72,73]. The use of CEA-antibody fragment-based radiotracers for guided surgery has also been described [68,70,74].
As well as inMuc1 targeting, the two-step pretargeting system using a bispecific antibody has been described in animal studies and in patients for CEA [75,76].
Few studies are available on fluorescence imaging for targeting of CEA. Fidarova et al. [77] described the use of an anti-CEA mAb conjugated to a fluorophore for the detection of metastatic CRC in mice. Kaushal et al. [78] showed the use of an anti-CEA mAb conjugated to a fluorophore, in intraoperative detection of colorectal tumor deposits, with good in vivo results.
When applying TASC to CEA in CRC, the total score is 19 points.
Matrix Metalloproteinases
MMPs are zinc- and calcium-dependent endoproteases that are upregulated in the tumor environment and are capable of degrading proteins in the extracellular matrix. MMPs are upregulated in 30% to 95% of colorectal tumors, depending on the type of MMP (Table 2) [45,79–82].
Several groups have targeted MMPs in vivo by using fluorescent or radiolabeled specific MMP-inhibitors [83–86]. One study reports using a radiolabeled mAb for in vivo targeting of MMP1, an MMP subtype [87]. Because MMPs have proteolytic activity, this target is ideal for activatable probes. The advantage of activatable probes is that they greatly reduce background signal. Several studies demonstrate the in vivo use of proteolytic beacon coupled to a fluorophore, which emits a signal after cleavage by MMP [88,89]. MMP sense is a commercially available MMP-dependent activatable fluorescent probe, successfully tested in in vivo models [90]. Veiseh et al. [91] describe the in vivo use of chlorotoxin, a small peptide derived from snake venom that interacts with MMP2, conjugated to the fluorophore Cy5.5, for potential intraoperative imaging. Lepage et al. synthesized a contrast agent containing gadolinium chelate, which is cleaved by MMP. On cleavage, this agent is less soluble in water and remains at the tumor site. Good in vivo results have been demonstrated for MR imaging using this protease-modulated contrast agent [92–94]. Aguilera et al. [95] developed activatable cell penetrating peptides (ACPPs) that enter the cell after cleavage by MMP. The ACPPs were labeled with Cy5.5 for fluorescence imaging, with gadolinium chelate for MR imaging, or with both for dual imaging [96]. These ACPPs were further improved by conjugation to large molecule dendrimers, which improved tumor uptake and thus the emitted signal [97,98].
MMPs granted an average of 18 points in CRC when applying TASC, depending on the subtype.
Epidermal Growth Factor Receptor
EGFR is a cell surface receptor involved in processes such as cell proliferation, differentiation, adhesion, and migration. EGFR is upregulated in different types of cancer, including skin, breast, ovary, bladder, prostate, kidney, head and neck, and non-small cell lung cancers [99,100]. In colorectal cancer, EGFR is upregulated in approximately 80% of the tumors [101,102].
EGFR has been extensively imaged by radionuclide- or fluorophore-conjugated antibodies. Most often, cetuximab, a clinically approved anti-EGFR antibody, is used [103–107]. In 1994, Dadparvar et al. [108] administered radionuclide-labeled anti-EGFR antibodies to patients with intracranial neoplasms for SPECT scanning. Although promising results were obtained, to our knowledge, no sequel was given to this radiopharmaceutical. Also, a few studies described the use of panitumumab in vivo, which is the second clinically approved antibody directed at EGFR [109,110]. Variants using antibody fragments or affibodies have been described in animal studies [111,112].
Alternatively, EGF, the natural ligand of EGFR, is also used in vivo as an imaging agent, conjugated to mainly fluorophores or quantum dots [113–115]. Goetz et al. [116] described a fluorescent anti-EGFR antibody capable of imaging human CRC tissue, which is not only successful in in vivo imaging results but also potentially useful in endoscopy. Hama et al. [117] described an alternative two-step pretargeting model, using nonfluorescent biotinylated cetuximab as first antibody, followed by a neutravidin-BODIPY-FL fluorescent conjugate. The latter binds to the first antibody by a neutravidin-biotin binding. The concept was tested in vivo in a PC model. A 10-fold signal amplification was found, leading to high tumor-to-background ratios and good detection of lesions as small as 0.8 mm.
The TASC score of EGFR in CRC adds up to 20 points.
Discussion
TASC needs to be validated in other cancer types and adjusted where necessary.
It should be pointed out that TASC is designed as a directive which can help gain objectivity and extra insight in target selection. Future validation studies and adjustments, to our opinion, will improve TASC to make it more broadly applicable to various types of cancer. Immunohistochemical analysis of collected tumor specimens is a relatively easy way to determine applicability of a target. In the case of a promising target, further validation is needed by testing a target-directed imaging probe in vitro, for proof of concept and specific binding, and, subsequently, in appropriate tumor mouse models in vivo.
Expression of a target may depend on tumor stage. For example, CXCR4, EGFR, and VEGF are associated with more advanced tumor stages and metastasis in CRC [118–120]. However, MUC1 is also generally expressed in T1 CRC tumors [46]. Therefore, such a target may also be of value in early CRC detection.
Conclusions
In PC of colorectal origin, tumor-targeted imaging may yield better diagnostic and therapeutic results. A large number of tumor biomarkers are upregulated in CRC. However, there is no objective system for selecting their clinical applicability in targeted imaging applications. In this review, we introduce a novel scoring system for target selection for imaging purposes, the TASC. When applying TASC to biomarkers for CRC, we found that the most potent targets for imaging are CXCR4, VEGF-A, Muc1, MMPs, EGFR, EpCAM, and CEA based on their scoring. Clearly, the ideal target for imaging purposes does not exist; moreover, by using the TASC system, we propose a novel guideline in tumor targeting for selecting appropriate targets for imaging purposes.
Acknowledgments
The authors thank R.G. Pleijhuis for the artwork design.
References
- 1.American Cancer Society, author. Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2008. [Google Scholar]
- 2.Knorr C, Reingruber B, Meyer T, Hohenberger W, Stremmel C. Peritoneal carcinomatosis of colorectal cancer: incidence, prognosis, and treatment modalities. Int J Colorectal Dis. 2004;19(3):181–187. doi: 10.1007/s00384-003-0524-x. [DOI] [PubMed] [Google Scholar]
- 3.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63(2):364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Culliford AT, Brooks AD, Sharma S, Saltz LB, Schwartz GK, O'Reilly EM, Ilson DH, Kemeny NE, Kelsen DP, Guillem JG, et al. Surgical debulking and intraperitoneal chemotherapy for established peritoneal metastases from colon and appendix cancer. Ann Surg Oncol. 2001;8(10):787–795. doi: 10.1007/s10434-001-0787-9. [DOI] [PubMed] [Google Scholar]
- 7.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203(6):878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goere D, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 9.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 11.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12(1):65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 12.Vaira M, Cioppa T, D'Amico S, de Marco G, D'Alessandro M, Fiorentini G, De Simone M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo. 2010;24(1):79–84. [PubMed] [Google Scholar]
- 13.de Bree E, Rosing H, Michalakis J, Romanos J, Relakis K, Theodoropoulos PA, Beijnen JH, Georgoulias V, Tsiftsis DD. Intraperitoneal chemotherapy with taxanes for ovarian cancer with peritoneal dissemination. Eur J Surg Oncol. 2006;32(6):666–670. doi: 10.1016/j.ejso.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Howell SB. Pharmacologic principles of intraperitoneal chemotherapy for the treatment of ovarian cancer. Int J Gynecol Cancer. 2008;18(suppl 1):20–25. doi: 10.1111/j.1525-1438.2007.01099.x. [DOI] [PubMed] [Google Scholar]
- 15.Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol. 1990;25(6):389–394. doi: 10.1007/BF00686048. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso J, Boer J, Morreau H, Fodde R. Expression and genomic profiling of colorectal cancer. Biochim Biophys Acta. 2007;1775(1):103–137. doi: 10.1016/j.bbcan.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Bertagnolli MM. The forest and the trees: pathways and proteins as colorectal cancer biomarkers. J Clin Oncol. 2009;27(35):5866–5867. doi: 10.1200/JCO.2009.24.8013. [DOI] [PubMed] [Google Scholar]
- 18.Sega EI, Low PS. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008;27(4):655–664. doi: 10.1007/s10555-008-9155-6. [DOI] [PubMed] [Google Scholar]
- 19.Elias DR, Thorek DL, Chen AK, Czupryna J, Tsourkas A. In vivo imaging of cancer biomarkers using activatable molecular probes. Cancer Biomark. 2008;4(6):287–305. doi: 10.3233/cbm-2008-4602. [DOI] [PubMed] [Google Scholar]
- 20.Pauwels EK, Ribeiro MJ, Stoot JH, McCready VR, Bourguignon M, Maziere B. FDG accumulation and tumor biology. Nucl Med Biol. 1998;25(4):317–322. doi: 10.1016/s0969-8051(97)00226-6. [DOI] [PubMed] [Google Scholar]
- 21.Paret C, Hildebrand D, Weitz J, Kopp-Schneider A, Kuhn A, Beer A, Hautmann R, Zoller M. C4.4A as a candidate marker in the diagnosis of colorectal cancer. Br J Cancer. 2007;97(8):1146–1156. doi: 10.1038/sj.bjc.6604012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie X, Wang CY, Cao YX, Wang W, Zhuang R, Chen LH, Dang NN, Fang L, Jin BQ. Expression pattern of epithelial cell adhesion molecule on normal and malignant colon tissues. World J Gastroenterol. 2005;11(3):344–347. doi: 10.3748/wjg.v11.i3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong A, Eck SL. EpCAM: a new therapeutic target for an old cancer antigen. Cancer Biol Ther. 2003;2(4):320–326. doi: 10.4161/cbt.2.4.451. [DOI] [PubMed] [Google Scholar]
- 24.Eder M, Knackmuss S, Le Gall F, Reusch U, Rybin V, Little M, Haberkorn U, Mier W, Eisenhut M. 68Ga-labelled recombinant antibody variants for immuno-PET imaging of solid tumours. Eur J Nucl Med Mol Imaging. 2010;37(7):1397–1407. doi: 10.1007/s00259-010-1392-6. [DOI] [PubMed] [Google Scholar]
- 25.Fields AL, Keller A, Schwartzberg L, Bernard S, Kardinal C, Cohen A, Schulz J, Eisenberg P, Forster J, Wissel P. Adjuvant therapy with the monoclonal antibody Edrecolomab plus fluorouracil-based therapy does not improve overall survival of patients with stage III colon cancer. J Clin Oncol. 2009;27(12):1941–1947. doi: 10.1200/JCO.2008.18.5710. [DOI] [PubMed] [Google Scholar]
- 26.Hartung G, Hofheinz RD, Dencausse Y, Sturm J, Kopp-Schneider A, Dietrich G, Fackler-Schwalbe I, Bornbusch D, Gonnermann M, Wojatschek C, et al. Adjuvant therapy with edrecolomab versus observation in stage II colon cancer: a multicenter randomized phase III study. Onkologie. 2005;28(6–7):347–350. doi: 10.1159/000084595. [DOI] [PubMed] [Google Scholar]
- 27.Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J, Barone C, Fountzilas G, Riess H, Moylan E, et al. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet. 2002;360(9334):671–677. doi: 10.1016/S0140-6736(02)09836-7. [DOI] [PubMed] [Google Scholar]
- 28.Sebastian M, Kiewe P, Schuette W, Brust D, Peschel C, Schneller F, Ruhle KH, Nilius G, Ewert R, Lodziewski S, et al. Treatment of malignant pleural effusion with the trifunctional antibody catumaxomab (Removab) (anti-EpCAM x Anti-CD3): results of a phase 1/2 study. J Immunother. 2009;32(2):195–202. doi: 10.1097/CJI.0b013e318195b5bb. [DOI] [PubMed] [Google Scholar]
- 29.Litvinov SV, Bakker HA, Gourevitch MM, Velders MP, Warnaar SO. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes Commun. 1994;2(5):417–428. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- 30.Rubie C, Kollmar O, Frick VO, Wagner M, Brittner B, Graber S, Schilling MK. Differential CXC receptor expression in colorectal carcinomas. Scand J Immunol. 2008;68(6):635–644. doi: 10.1111/j.1365-3083.2008.02163.x. [DOI] [PubMed] [Google Scholar]
- 31.Nimmagadda S, Pullambhatla M, Pomper MG. Immunoimaging of CXCR4 expression in brain tumor xenografts using SPECT/CT. J Nucl Med. 2009;50(7):1124–1130. doi: 10.2967/jnumed.108.061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmagadda S, Pullambhatla M, Stone K, Green G, Bhujwalla ZM, Pomper MG. Molecular imaging of CXCR4 receptor expression in human cancer xenografts with [64Cu]AMD3100 positron emission tomography. Cancer Res. 2010;70(10):3935–3944. doi: 10.1158/0008-5472.CAN-09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishizawa K, Nishiyama H, Oishi S, Tanahara N, Kotani H, Mikami Y, Toda Y, Evans BJ, Peiper SC, Saito R, et al. Fluorescent imaging of high-grade bladder cancer using a specific antagonist for chemokine receptor CXCR4. Int J Cancer. 2010;127(5):1180–1187. doi: 10.1002/ijc.25145. [DOI] [PubMed] [Google Scholar]
- 34.Hanaoka H, Mukai T, Tamamura H, Mori T, Ishino S, Ogawa K, Iida Y, Doi R, Fujii N, Saji H. Development of a 111In-labeled peptide derivative targeting a chemokine receptor, CXCR4, for imaging tumors. Nucl Med Biol. 2006;33(4):489–494. doi: 10.1016/j.nucmedbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Misra P, Lebeche D, Ly H, Schwarzkopf M, Diaz G, Hajjar RJ, Schecter AD, Frangioni JV. Quantitation of CXCR4 expression in myocardial infarction using 99mTc-labeled SDF-1α. J Nucl Med. 2008;49(6):963–969. doi: 10.2967/jnumed.107.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002;29(6 suppl 16):3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]
- 37.Guba M, Seeliger H, Kleespies A, Jauch KW, Bruns C. Vascular endothelial growth factor in colorectal cancer. Int J Colorectal Dis. 2004;19(6):510–517. doi: 10.1007/s00384-003-0576-y. [DOI] [PubMed] [Google Scholar]
- 38.Cao D, Hou M, Guan YS, Jiang M, Yang Y, Gou HF. Expression of HIF-1α and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432. doi: 10.1186/1471-2407-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdou AG, Aiad H, Asaad N, Abd El-Wahed M, Serag El-Dien M. Immunohistochemical evaluation of vascular endothelial growth factor (VEGF) in colorectal carcinoma. J Egypt Natl Canc Inst. 2006;18(4):311–322. [PubMed] [Google Scholar]
- 40.Scheer MG, Stollman TH, Boerman OC, Verrijp K, Sweep FC, Leenders WP, Ruers TJ, Oyen WJ. Imaging liver metastases of colorectal cancer patients with radiolabelled bevacizumab: lack of correlation with VEGF-A expression. Eur J Cancer. 2008;44(13):1835–1840. doi: 10.1016/j.ejca.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Stollman TH, Scheer MG, Leenders WP, Verrijp KC, Soede AC, Oyen WJ, Ruers TJ, Boerman OC. Specific imaging of VEGF-A expression with radiolabeled anti-VEGF monoclonal antibody. Int J Cancer. 2008;122(10):2310–2314. doi: 10.1002/ijc.23404. [DOI] [PubMed] [Google Scholar]
- 42.Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, Brouwers AH, van Dongen GA, Perk LR, Lub-de Hooge MN. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J Nucl Med. 2007;48(8):1313–1319. doi: 10.2967/jnumed.107.041301. [DOI] [PubMed] [Google Scholar]
- 43.Nayak TK, Garmestani K, Baidoo KE, Milenic DE, Brechbiel MW. PET imaging of tumor angiogenesis in mice with VEGF-A targeted (86)Y-CHX-A″-DTPA-bevacizumab. Int J Cancer. 2011;128:920–926. doi: 10.1002/ijc.25409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagengast WB, Lub-de Hooge MN, van Straten EME, Brouwers AH, den Dunnen WFA, de Jong JR, Hollema H, Dierckx RA, Mulder NH, de Vries EG, et al. VEGF-SPECT with 111In-bevacizumab in stage III/IV melanoma patients [thesis] Groningen, the Netherlands: Rijksuniversiteit Groningen; 2009. pp. 80–90. [Google Scholar]
- 45.Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, Arihiro K, Shimamoto F, Chayama K. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol. 2007;13(28):3829–3835. doi: 10.3748/wjg.v13.i28.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki H, Shoda J, Kawamoto T, Shinozaki E, Miyahara N, Hotta S, Iizuka Y, Nakahara A, Tanaka N, Yanaka A, et al. Expression of MUC1 recognized by monoclonal antibody MY.1E12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. Clin Exp Metastasis. 2004;21(4):321–329. doi: 10.1023/b:clin.0000046133.35133.cc. [DOI] [PubMed] [Google Scholar]
- 47.Okarvi SM, Jammaz IA. Design, synthesis, radiolabeling and in vitro and in vivo characterization of tumor-antigen- and antibody-derived peptides for the detection of breast cancer. Anticancer Res. 2009;29(4):1399–1409. [PubMed] [Google Scholar]
- 48.Pieve CD, Perkins AC, Missailidis S. Anti-MUC1 aptamers: radiolabelling with (99m)Tc and biodistribution in MCF-7 tumour-bearing mice. Nucl Med Biol. 2009;36(6):703–710. doi: 10.1016/j.nucmedbio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Salouti M, Rajabi H, Babaei MH, Rasaee MJ. Breast tumor targeting with (99m)Tc-HYNIC-PR81 complex as a new biologic radiopharmaceutical. Nucl Med Biol. 2008;35(7):763–768. doi: 10.1016/j.nucmedbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Matsumura K, Niki I, Tian H, Takuma M, Hongo N, Matsumoto S, Mori H. Radioimmunoscintigraphy of pancreatic cancer in tumor-bearing athymic nude mice using (99m)technetium-labeled anti-KL-6/MUC1 antibody. Radiat Med. 2008;26(3):133–139. doi: 10.1007/s11604-007-0207-6. [DOI] [PubMed] [Google Scholar]
- 51.Borbas KE, Ferreira CS, Perkins A, Bruce JI, Missailidis S. Design and synthesis of mono- and multimeric targeted radiopharmaceuticals based on novel cyclen ligands coupled to anti-MUC1 aptamers for the diagnostic imaging and targeted radiotherapy of cancer. Bioconjug Chem. 2007;18(4):1205–1212. doi: 10.1021/bc0700741. [DOI] [PubMed] [Google Scholar]
- 52.Simms MS, Murray A, Denton G, Scholfield DP, Price MR, Perkins AC, Bishop MC. Production and characterisation of a C595 antibody-99mTc conjugate for immunoscintigraphy of bladder cancer. Urol Res. 2001;29(1):13–19. doi: 10.1007/s002400000147. [DOI] [PubMed] [Google Scholar]
- 53.Hughes OD, Bishop MC, Perkins AC, Frier M, Price MR, Denton G, Smith A, Rutherford R, Schubiger PA. Preclinical evaluation of copper-67 labelled anti-MUC1 mucin antibody C595 for therapeutic use in bladder cancer. Eur J Nucl Med. 1997;24(4):439–443. doi: 10.1007/BF00881818. [DOI] [PubMed] [Google Scholar]
- 54.Hughes OD, Perkins AC, Frier M, Wastie ML, Denton G, Price MR, Denley H, Bishop MC. Imaging for staging bladder cancer: a clinical study of intravenous 111indium-labelled anti-MUC1 mucin monoclonal antibody C595. BJU Int. 2001;87(1):39–46. doi: 10.1046/j.1464-410x.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 55.Hughes OD, Bishop MC, Perkins AC, Wastie ML, Denton G, Price MR, Frier M, Denley H, Rutherford R, Schubiger PA. Targeting superficial bladder cancer by the intravesical administration of copper-67-labeled anti-MUC1 mucin monoclonal antibody C595. J Clin Oncol. 2000;18(2):363–370. doi: 10.1200/JCO.2000.18.2.363. [DOI] [PubMed] [Google Scholar]
- 56.Gold DV, Cardillo T, Goldenberg DM, Sharkey RM. Localization of pancreatic cancer with radiolabeled monoclonal antibody PAM4. Crit Rev Oncol Hematol. 2001;39(1-2):147–154. doi: 10.1016/s1040-8428(01)00114-7. [DOI] [PubMed] [Google Scholar]
- 57.Medarova Z, Pham W, Kim Y, Dai G, Moore A. In vivo imaging of tumor response to therapy using a dual-modality imaging strategy. Int J Cancer. 2006;118(11):2796–2802. doi: 10.1002/ijc.21672. [DOI] [PubMed] [Google Scholar]
- 58.Muguruma N, Ito S. Labeled anti-mucin antibody detectable by infrared-fluorescence endoscopy. Cancer Biomark. 2008;4(6):321–328. doi: 10.3233/cbm-2008-4604. [DOI] [PubMed] [Google Scholar]
- 59.Schuhmacher J, Klivenyi G, Kaul S, Henze M, Matys R, Hauser H, Clorius J. Pretargeting of human mammary carcinoma xenografts with bispecific anti-MUC1/anti-Ga chelate antibodies and immunoscintigraphy with PET. Nucl Med Biol. 2001;28(7):821–828. doi: 10.1016/s0969-8051(01)00246-3. [DOI] [PubMed] [Google Scholar]
- 60.Schuhmacher J, Kaul S, Klivenyi G, Junkermann H, Magener A, Henze M, Doll J, Haberkorn U, Amelung F, Bastert G. Immunoscintigraphy with positron emission tomography: gallium-68 chelate imaging of breast cancer pretargeted with bispecific anti-MUC1/anti-Ga chelate antibodies. Cancer Res. 2001;61(9):3712–3717. [PubMed] [Google Scholar]
- 61.Li M, Li JY, Zhao AL, He JS, Zhou LX, Li YA, Gu J. Comparison of carcinoembryonic antigen prognostic value in serum and tumour tissue of patients with colorectal cancer. Colorectal Dis. 2009;11(3):276–281. doi: 10.1111/j.1463-1318.2008.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JC, Gong G, Roh SA, Park KC. Carcinoembryonic antigen gene and carcinoembryonic antigen expression in the liver metastasis of colorectal carcinoma. Mol Cells. 1999;9(2):133–137. [PubMed] [Google Scholar]
- 63.Suwanagool P, Fujimori T, Maeda S. Value of tissue carcinoembryonic antigen in patients with colorectal carcinoma. Asian Pac J Allergy Immunol. 1990;8(1):33–37. [PubMed] [Google Scholar]
- 64.Hamada Y, Yamamura M, Hioki K, Yamamoto M, Nagura H, Watanabe K. Immunohistochemical study of carcinoembryonic antigen in patients with colorectal cancer. Correlation with plasma carcinoembryonic antigen levels. Cancer. 1985;55(1):136–141. doi: 10.1002/1097-0142(19850101)55:1<136::aid-cncr2820550121>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 65.Cai W, Olafsen T, Zhang X, Cao Q, Gambhir SS, Williams LE, Wu AM, Chen X. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J Nucl Med. 2007;48(2):304–310. [PubMed] [Google Scholar]
- 66.Sundaresan G, Yazaki PJ, Shively JE, Finn RD, Larson SM, Raubitschek AA, Williams LE, Chatziioannou AF, Gambhir SS, Wu AM. 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med. 2003;44(12):1962–1969. [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda A, Shimada H, Okazumi S, Imaseki H, Suzuki T, Ochiai T, Miyoshi T, Seito T. Preclinical assessment and pilot study using anti-CEA monoclonal antibody 1B2 for colorectal carcinoma imaging. Hepatogastroenterology. 2008;55(88):2054–2058. [PubMed] [Google Scholar]
- 68.Lechner P, Lind P, Snyder M, Haushofer H. Probe-guided surgery for colorectal cancer. Recent Results Cancer Res. 2000;157:273–280. doi: 10.1007/978-3-642-57151-0_24. [DOI] [PubMed] [Google Scholar]
- 69.Wong JY, Chu DZ, Williams LE, Yamauchi DM, Ikle DN, Kwok CS, Liu A, Wilczynski S, Colcher D, Yazaki PJ, et al. Pilot trial evaluating an 123I-labeled 80-kilodalton engineered anticarcinoembryonic antigen antibody fragment (cT84.66 minibody) in patients with colorectal cancer. Clin Cancer Res. 2004;10(15):5014–5021. doi: 10.1158/1078-0432.CCR-03-0576. [DOI] [PubMed] [Google Scholar]
- 70.Kim JC, Roh SA, Koo KH, Ryu JH, Hong HK, Oh SJ, Ryu JS, Kim HJ, Bodmer WF. Enhancement of colorectal tumor targeting using a novel biparatopic monoclonal antibody against carcinoembryonic antigen in experimental radioimmunoguided surgery. Int J Cancer. 2002;97(4):542–547. doi: 10.1002/ijc.1630. [DOI] [PubMed] [Google Scholar]
- 71.Yazaki PJ, Kassa T, Cheung CW, Crow DM, Sherman MA, Bading JR, Anderson AL, Colcher D, Raubitschek A. Biodistribution and tumor imaging of an anti-CEA single-chain antibody-albumin fusion protein. Nucl Med Biol. 2008;35(2):151–158. doi: 10.1016/j.nucmedbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Libutti SK, Alexander HR, Jr, Choyke P, Bartlett DL, Bacharach SL, Whatley M, Jousse F, Eckelman WC, Kranda K, Neumann RD, et al. A prospective study of 2-[18F] fluoro-2-deoxy-D-glucose/positron emission tomography scan, 99mTc-labeled arcitumomab (CEA-scan), and blind second-look laparotomy for detecting colon cancer recurrence in patients with increasing carcinoembryonic antigen levels. Ann Surg Oncol. 2001;8(10):779–786. doi: 10.1007/s10434-001-0779-9. [DOI] [PubMed] [Google Scholar]
- 73.Willkomm P, Bender H, Bangard M, Decker P, Grunwald F, Biersack HJ. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of the recurrence of colorectal carcinoma. J Nucl Med. 2000;41(10):1657–1663. [PubMed] [Google Scholar]
- 74.Mayer A, Tsiompanou E, O'Malley D, Boxer GM, Bhatia J, Flynn AA, Chester KA, Davidson BR, Lewis AA, Winslet MC, et al. Radioimmunoguided surgery in colorectal cancer using a genetically engineered anti-CEA single-chain Fv antibody. Clin Cancer Res. 2000;6(5):1711–1719. [PubMed] [Google Scholar]
- 75.Sharkey RM, Cardillo TM, Rossi EA, Chang CH, Karacay H, McBride WJ, Hansen HJ, Horak ID, Goldenberg DM. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11(11):1250–1255. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 76.Aarts F, Boerman OC, Sharkey RM, Hendriks T, Chang CH, McBride WJ, Bleichrodt RP, Oyen WJ, Goldenberg DM. Pretargeted radioimmunoscintigraphy in patients with primary colorectal cancer using a bispecific anticarcinoembryonic antigen CEA x anti-di-diethylenetriaminepentaacetic acid F(ab′)2 antibody. Cancer. 2010;116(4 suppl):1111–1117. doi: 10.1002/cncr.24799. [DOI] [PubMed] [Google Scholar]
- 77.Fidarova EF, El Emir E, Boxer GM, Qureshi U, Dearling JL, Robson MP, Bergent RH, Trott KR, Pedley RB. Microdistribution of targeted, fluorescently labeled anti-carcinoembryonic antigen antibody in metastatic colorectal cancer: implications for radioimmunotherapy. Clin Cancer Res. 2008;14(9):2639–2646. doi: 10.1158/1078-0432.CCR-07-2031. [DOI] [PubMed] [Google Scholar]
- 78.Kaushal S, McElroy MK, Luiken GA, Talamini MA, Moossa AR, Hoffman RM, Bouvet M. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12(11):1938–1950. doi: 10.1007/s11605-008-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKerrow JH, Bhargava V, Hansell E, Huling S, Kuwahara T, Matley M, Coussens L, Warren R. A functional proteomics screen of proteases in colorectal carcinoma. Mol Med. 2000;6(5):450–460. [PMC free article] [PubMed] [Google Scholar]
- 80.Jeffery N, McLean MH, El Omar EM, Murray GI. The matrix metalloproteinase/tissue inhibitor of matrix metalloproteinase profile in colorectal polyp cancers. Histopathology. 2009;54(7):820–828. doi: 10.1111/j.1365-2559.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 81.Madoz-Gurpide J, Lopez-Serra P, Martinez-Torrecuadrada JL, Sanchez L, Lombardia L, Casal JI. Proteomics-based validation of genomic data: applications in colorectal cancer diagnosis. Mol Cell Proteomics. 2006;5(8):1471–1483. doi: 10.1074/mcp.M600048-MCP200. [DOI] [PubMed] [Google Scholar]
- 82.Emmert-Buck MR, Roth MJ, Zhuang Z, Campo E, Rozhin J, Sloane BF, Liotta LA, Stetler-Stevenson WG. Increased gelatinase A (MMP-2) and cathepsin B activity in invasive tumor regions of human colon cancer samples. Am J Pathol. 1994;145(6):1285–1290. [PMC free article] [PubMed] [Google Scholar]
- 83.Breyholz HJ, Wagner S, Faust A, Riemann B, Holtke C, Hermann S, Schober O, Schafers M, Kopka K. Radiofluorinated pyrimidine-2,4,6-triones as molecular probes for noninvasive MMP-targeted imaging. Chem Med Chem. 2010;5(5):777–789. doi: 10.1002/cmdc.201000013. [DOI] [PubMed] [Google Scholar]
- 84.Oltenfreiter R, Staelens L, Lejeune A, Dumont F, Frankenne F, Foidart JM, Slegers G. New radioiodinated carboxylic and hydroxamic matrix metalloproteinase inhibitor tracers as potential tumor imaging agents. Nucl Med Biol. 2004;31(4):459–468. doi: 10.1016/j.nucmedbio.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Sprague JE, Li WP, Liang K, Achilefu S, Anderson CJ. In vitro and in vivo investigation of matrix metalloproteinase expression in metastatic tumor models. Nucl Med Biol. 2006;33(2):227–237. doi: 10.1016/j.nucmedbio.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Faust A, Waschkau B, Waldeck J, Holtke C, Breyholz HJ, Wagner S, Kopka K, Schober O, Heindel W, Schafers M, et al. Synthesis and evaluation of a novel hydroxamate based fluorescent photoprobe for imaging of matrix metalloproteinases. Bioconjug Chem. 2009;20(5):904–912. doi: 10.1021/bc8004478. [DOI] [PubMed] [Google Scholar]
- 87.Temma T, Sano K, Kuge Y, Kamihashi J, Takai N, Ogawa Y, Saji H. Development of a radiolabeled probe for detecting membrane type-1 matrix metalloproteinase on malignant tumors. Biol Pharm Bull. 2009;32(7):1272–1277. doi: 10.1248/bpb.32.1272. [DOI] [PubMed] [Google Scholar]
- 88.McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, Matrisian LM. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem J 377(pt. 2004;3):617–628. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scherer RL, Van Saun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nano-beacon. Mol Imaging. 2008;7(3):118–131. [PMC free article] [PubMed] [Google Scholar]
- 90.von Burstin J, Eser S, Seidler B, Meining A, Bajbouj M, Mages J, Lang R, Kind AJ, Schnieke AE, Schmid RM, et al. Highly sensitive detection of early-stage pancreatic cancer by multimodal near-infrared molecular imaging in living mice. Int J Cancer. 2008;123(9):2138–2147. doi: 10.1002/ijc.23780. [DOI] [PubMed] [Google Scholar]
- 91.Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67(14):6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 92.Jastrzebska B, Lebel R, Therriault H, McIntyre JO, Escher E, Guerin B, Paquette B, Neugebauer WA, Lepage M. New enzyme-activated solubility-switchable contrast agent for magnetic resonance imaging: from synthesis to in vivo imaging. J Med Chem. 2009;52(6):1576–1581. doi: 10.1021/jm801411h. [DOI] [PubMed] [Google Scholar]
- 93.Lebel R, Jastrzebska B, Therriault H, Cournoyer MM, McIntyre JO, Escher E, Neugebauer W, Paquette B, Lepage M. Novel solubility-switchable MRI agent allows the noninvasive detection of matrix metalloproteinase-2 activity in vivo in a mouse model. Magn Reson Med. 2008;60(5):1056–1065. doi: 10.1002/mrm.21741. [DOI] [PubMed] [Google Scholar]
- 94.Lepage M, Dow WC, Melchior M, You Y, Fingleton B, Quarles CC, Pepin C, Gore JC, Matrisian LM, McIntyre JO. Noninvasive detection of matrix metalloproteinase activity in vivo using a novel magnetic resonance imaging contrast agent with a solubility switch. Mol Imaging. 2007;6(6):393–403. [PubMed] [Google Scholar]
- 95.Aguilera TA, Olson ES, Timmers MM, Jiang T, Tsien RY. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integr Biol (Camb) 2009;1(5–6):371–381. doi: 10.1039/b904878b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb) 2009;1(5–6):382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M, Ellies LG, Tsien RY. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA. 2010;107(9):4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci USA. 2010;107(9):4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kari C, Chan TO, Rocha de Quadros M, Rodeck U. Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res. 2003;63(1):1–5. [PubMed] [Google Scholar]
- 100.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 101.Cunningham MP, Essapen S, Thomas H, Green M, Lovell DP, Topham C, Marks C, Modjtahedi H. Coexpression of the IGF-IR, EGFR and HER-2 is common in colorectal cancer patients. Int J Oncol. 2006;28(2):329–335. [PubMed] [Google Scholar]
- 102.Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001;92(5):1331–1346. doi: 10.1002/1097-0142(20010901)92:5<1331::aid-cncr1455>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 103.Aerts HJ, Dubois L, Hackeng TM, Straathof R, Chiu RK, Lieuwes NG, Jutten B, Weppler SA, Lammering G, Wouters BG, et al. Development and evaluation of a cetuximab-based imaging probe to target EGFR and EGFRvIII. Radiother Oncol. 2007;83(3):326–332. doi: 10.1016/j.radonc.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 104.Milenic DE, Wong KJ, Baidoo KE, Ray GL, Garmestani K, Williams M, Brechbiel MW. Cetuximab: preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm. 2008;23(5):619–631. doi: 10.1089/cbr.2008.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nayak TK, Regino CA, Wong KJ, Milenic DE, Garmestani K, Baidoo KE, Szajek LP, Brechbiel MW. PET imaging of HER1-expressing xenografts in mice with 86Y-CHX-A″-DTPA-cetuximab. Eur J Nucl Med Mol Imaging. 2010;37(7):1368–1376. doi: 10.1007/s00259-009-1370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ping Li W, Meyer LA, Capretto DA, Sherman CD, Anderson CJ. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother Radiopharm. 2008;23(2):158–171. doi: 10.1089/cbr.2007.0444. [DOI] [PubMed] [Google Scholar]
- 107.Barrett T, Koyama Y, Hama Y, Ravizzini G, Shin IS, Jang BS, Paik CH, Urano Y, Choyke PL, Kobayashi H. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007;13(22 pt 1):6639–6648. doi: 10.1158/1078-0432.CCR-07-1119. [DOI] [PubMed] [Google Scholar]
- 108.Dadparvar S, Krishna L, Miyamoto C, Brady LW, Brown SJ, Bender H, Slizofski WJ, Eshleman J, Chevres A, Woo DV. Indium-111-labeled anti-EGFr-425 scintigraphy in the detection of malignant gliomas. Cancer. 1994;73(3 suppl):884–889. doi: 10.1002/1097-0142(19940201)73:3+<884::aid-cncr2820731320>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 109.Nayak TK, Garmestani K, Baidoo KE, Milenic DE, Brechbiel MW. Preparation, biological evaluation, and pharmacokinetics of the human anti-HER1 monoclonal antibody panitumumab labeled with 86Y for quantitative PET of carcinoma. J Nucl Med. 2010;51(6):942–950. doi: 10.2967/jnumed.109.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niu G, Li Z, Xie J, Le QT, Chen X. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J Nucl Med. 2009;50(7):1116–1123. doi: 10.2967/jnumed.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gong H, Kovar J, Little G, Chen H, Olive DM. In vivo imaging of xenograft tumors using an epidermal growth factor receptor-specific affibody molecule labeled with a near-infrared fluorophore. Neoplasia. 2010;12(2):139–149. doi: 10.1593/neo.91446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, Duan H, Ni C, Yuan Q, Adams G, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5(2):235–243. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diagaradjane P, Orenstein-Cardona JM, Colon-Casasnovas NE, Deorukhkar A, Shentu S, Kuno N, Schwartz DL, Gelovani JG, Krishnan S. Imaging epidermal growth factor receptor expression in vivo: pharmacokinetic and biodistribution characterization of a bio-conjugated quantum dot nanoprobe. Clin Cancer Res. 2008;14(3):731–741. doi: 10.1158/1078-0432.CCR-07-1958. [DOI] [PubMed] [Google Scholar]
- 114.Ke S, Wen X, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, Li C. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63(22):7870–7875. [PubMed] [Google Scholar]
- 115.Manning HC, Merchant NB, Foutch AC, Virostko JM, Wyatt SK, Shah C, McKinley ET, Xie J, Mutic NJ, Washington MK, et al. Molecular imaging of therapeutic response to epidermal growth factor receptor blockade in colorectal cancer. Clin Cancer Res. 2008;14(22):7413–7422. doi: 10.1158/1078-0432.CCR-08-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goetz M, Ziebart A, Foersch S, Vieth M, Waldner MJ, Delaney P, Galle PR, Neurath MF, Kiesslich R. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology. 2010;138(2):435–446. doi: 10.1053/j.gastro.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 117.Hama Y, Urano Y, Koyama Y, Choyke PL, Kobayashi H. Activatable fluorescent molecular imaging of peritoneal metastases following pretargeting with a biotinylated monoclonal antibody. Cancer Res. 2007;67(8):3809–3817. doi: 10.1158/0008-5472.CAN-06-3794. [DOI] [PubMed] [Google Scholar]
- 118.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11(5):1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 119.Antonacopoulou AG, Tsamandas AC, Petsas T, Liava A, Scopa CD, Papavassiliou AG, Kalofonos HP. EGFR, HER-2 and COX-2 levels in colorectal cancer. Histopathology. 2008;53(6):698–706. doi: 10.1111/j.1365-2559.2008.03165.x. [DOI] [PubMed] [Google Scholar]
- 120.Nakasaki T, Wada H, Shigemori C, Miki C, Gabazza EC, Nobori T, Nakamura S, Shiku H. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69(4):247–254. doi: 10.1002/ajh.10061. [DOI] [PubMed] [Google Scholar]
- 121.Ahlskog JK, Schliemann C, Marlind J, Qureshi U, Ammar A, Pedley RB, andNeri D. Human monoclonal antibodies targeting carbonic anhydrase IX for the molecular imaging of hypoxic regions in solid tumours. Br J Cancer. 2009;101(4):645–657. doi: 10.1038/sj.bjc.6605200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dubois L, Lieuwes NG, Maresca A, Thiry A, Supuran CT, Scozzafava A, Wouters BG, Lambin P. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother Oncol. 2009;92(3):423–428. doi: 10.1016/j.radonc.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 123.Chrastina A, Zavada J, Parkkila S, Kaluz S, Kaluzova M, Rajcani J, Pastorek J, Pastorekova S. Biodistribution and pharmacokinetics of 125I-labeled monoclonal antibody M75 specific for carbonic anhydrase IX, an intrinsic marker of hypoxia, in nude mice xenografted with human colorectal carcinoma. Int J Cancer. 2003;105(6):873–881. doi: 10.1002/ijc.11142. [DOI] [PubMed] [Google Scholar]
- 124.Niemela AM, Hynninen P, Mecklin JP, Kuopio T, Kokko A, Aaltonen L, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, et al. Carbonic anhydrase IX is highly expressed in hereditary nonpolyposis colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1760–1766. doi: 10.1158/1055-9965.EPI-07-0080. [DOI] [PubMed] [Google Scholar]
- 125.Kivela AJ, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila TS, et al. Differential expression of cytoplasmic carbonic anhydrases, CA I and II, and membrane-associated isozymes, CA IX and XII, in normal mucosa of large intestine and in colorectal tumors. Dig Dis Sci. 2001;46(10):2179–2186. doi: 10.1023/a:1011910931210. [DOI] [PubMed] [Google Scholar]
- 126.Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastorekova S, Pastorek J, Kairaluoma MI, Karttunen TJ. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153(1):279–285. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Imai K, Hirata S, Irie A, Senju S, Ikuta Y, Yokomine K, Harao M, Inoue M, Tsunoda T, Nakatsuru S, et al. Identification of a novel tumor-associated antigen, cadherin 3/P-cadherin, as a possible target for immunotherapy of pancreatic, gastric, and colorectal cancers. Clin Cancer Res. 2008;14(20):6487–6495. doi: 10.1158/1078-0432.CCR-08-1086. [DOI] [PubMed] [Google Scholar]
- 128.Alencar H, Funovics MA, Figueiredo J, Sawaya H, Weissleder R, Mahmood U. Colonic adenocarcinomas: near-infrared microcatheter imaging of smart probes for early detection—study in mice. Radiology. 2007;244(1):232–238. doi: 10.1148/radiol.2441052114. [DOI] [PubMed] [Google Scholar]
- 129.Mahmood U, Tung CH, Bogdanov A, Jr, Weissleder R. Near-infrared optical imaging of protease activity for tumor detection. Radiology. 1999;213(3):866–870. doi: 10.1148/radiology.213.3.r99dc14866. [DOI] [PubMed] [Google Scholar]
- 130.Funovics MA, Weissleder R, Mahmood U. Catheter-based in vivo imaging of enzyme activity and gene expression: feasibility study in mice. Radiology. 2004;231(3):659–666. doi: 10.1148/radiol.2313030831. [DOI] [PubMed] [Google Scholar]
- 131.Kuester D, Lippert H, Roessner A, Krueger S. The cathepsin family and their role in colorectal cancer. Pathol Res Pract. 2008;204(7):491–500. doi: 10.1016/j.prp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 132.Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31(1):106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 133.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10(5):590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bendardaf R, Algars A, Elzagheid A, Korkeila E, Ristamaki R, Lamlum H, Collan Y, Syrjanen K, Pyrhonen S. Comparison of CD44 expression in primary tumours and metastases of colorectal cancer. Oncol Rep. 2006;16(4):741–746. [PubMed] [Google Scholar]
- 135.Fernandez JC, Vizoso FJ, Corte MD, Gava RR, Corte MG, Suarez JP, Garcia-Muniz JL, Garcia-Moran M. CD44s expression in resectable colorectal carcinomas and surrounding mucosa. Cancer Invest. 2004;22(6):878–885. doi: 10.1081/cnv-200039658. [DOI] [PubMed] [Google Scholar]
- 136.Moffat FL, Jr, Pinsky CM, Hammershaimb L, Petrelli NJ, Patt YZ, Whaley FS, Goldenberg DM. Clinical utility of external immunoscintigraphy with the IMMU-4 technetium-99m Fab′ antibody fragment in patients undergoing surgery for carcinoma of the colon and rectum: results of a pivotal, phase III trial. The Immunomedics Study Group. J Clin Oncol. 1996;14(8):2295–2305. doi: 10.1200/JCO.1996.14.8.2295. [DOI] [PubMed] [Google Scholar]
- 137.Gu J, Zhao J, Li Z, Yang Z, Zhang J, Gao Z, Wang Y, Xu G. Clinical application of radioimmunoguided surgery in colorectal cancer using 125I-labeled carcinoembryonic antigen-specific monoclonal antibody submucosally. Dis Colon Rectum. 2003;46(12):1659–1666. doi: 10.1007/BF02660772. [DOI] [PubMed] [Google Scholar]
- 138.Ford CH, Tsaltas GC, Osborne PA, Addetia K. Novel flow cytometric analysis of the progress and route of internalization of a monoclonal anti-carcinoembryonic antigen (CEA) antibody. Cytometry. 1996;23(3):228–240. doi: 10.1002/(SICI)1097-0320(19960301)23:3<228::AID-CYTO6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 139.Stein R, Juweid M, Mattes MJ, Goldenberg DM. Carcinoembryonic antigen as a target for radioimmunotherapy of human medullary thyroid carcinoma: antibody processing, targeting, and experimental therapy with 131I and 90Y labeled MAbs. Cancer Biother Radiopharm. 1999;14(1):37–47. doi: 10.1089/cbr.1999.14.37. [DOI] [PubMed] [Google Scholar]
- 140.Bowen KB, Reimers AP, Luman S, Kronz JD, Fyffe WE, Oxford JT. Immunohistochemical localization of collagen type XI α1 and α2 chains in human colon tissue. J Histochem Cytochem. 2008;56(3):275–283. doi: 10.1369/jhc.7A7310.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wen Y, Giardina SF, Hamming D, Greenman J, Zachariah E, Bacolod MD, Liu H, Shia J, Amenta PS, Barany F, et al. GROα is highly expressed in adenocarcinoma of the colon and down-regulates fibulin-1. Clin Cancer Res. 2006;12(20 pt 1):5951–5959. doi: 10.1158/1078-0432.CCR-06-0736. [DOI] [PubMed] [Google Scholar]
- 142.Rose JJ, Foley JF, Murphy PM, Venkatesan S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J Biol Chem. 2004;279(23):24372–24386. doi: 10.1074/jbc.M401364200. [DOI] [PubMed] [Google Scholar]
- 143.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170(6):2904–2911. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 144.Futahashi Y, Komano J, Urano E, Aoki T, Hamatake M, Miyauchi K, Yoshida T, Koyanagi Y, Matsuda Z, Yamamoto N. Separate elements are required for ligand-dependent and -independent internalization of metastatic potentiator CXCR4. Cancer Sci. 2007;98(3):373–379. doi: 10.1111/j.1349-7006.2007.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu N, Cai G, Ye W, Wang X, Li Y, Zhao P, Zhang A, Zhang R, Cao B. Molecular imaging application of radioiodinated anti-EGFR human Fab to EGFR-overexpressing tumor xenografts. Anticancer Res. 2009;29(10):4005–4011. [PubMed] [Google Scholar]
- 146.Wang K, Wang K, Li W, Huang T, Li R, Wang D, Shen B, Chen X. Characterizing breast cancer xenograft epidermal growth factor receptor expression by using near-infrared optical imaging. Acta Radiol. 2009;50:1095–1103. doi: 10.3109/02841850903008800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miao Z, Ren G, Liu H, Jiang L, Cheng Z. Small-animal PET imaging of human epidermal growth factor receptor positive tumor with a (64)Cu labeled Affibody protein. Bioconjug Chem. 2010;21:947–954. doi: 10.1021/bc900515p. [DOI] [PubMed] [Google Scholar]
- 148.Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chimeric human-murine monoclonal antibody. Drugs Today (Barc) 2005;41(2):107–127. doi: 10.1358/dot.2005.41.2.882662. [DOI] [PubMed] [Google Scholar]
- 149.Eder M, Knackmuss S, Le Gall F, Reusch U, Rybin V, Little M, Haberkorn U, Mier W, Eisenhut M. 68GA-labelled recombinant antibody variants for immuno-PET imaging of solid tumours. Eur J Nucl Med Mol Imaging. 2010;37(7):1397–1407. doi: 10.1007/s00259-010-1392-6. [DOI] [PubMed] [Google Scholar]
- 150.Fisher RE, Siegel BA, Edell SL, Oyesiku NM, Morgenstern DE, Messmann RA, Amato RJ. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J Nucl Med. 2008;49(6):899–906. doi: 10.2967/jnumed.107.049478. [DOI] [PubMed] [Google Scholar]
- 151.Matteson EL, Lowe VJ, Prendergast FG, Crowson CS, Moder KG, Morgenstern DE, Messmann RA, Low PS. Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical FolateScan. Clin Exp Rheumatol. 2009;27(2):253–259. [PMC free article] [PubMed] [Google Scholar]
- 152.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56(8):1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 153.Shia J, Klimstra DS, Nitzkorski JR, Low PS, Gonen M, Landmann R, Weiser MR, Franklin WA, Prendergast FG, Murphy L, et al. Immunohistochemical expression of folate receptor a in colorectal carcinoma: patterns and biological significance. Hum Pathol. 2008;39(4):498–505. doi: 10.1016/j.humpath.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 154.Deutscher SL, Figueroa SD, Kumar SR. Tumor targeting and SPECT imaging properties of an (111)In-labeled galectin-3 binding peptide in prostate carcinoma. Nucl Med Biol. 2009;36(2):137–146. doi: 10.1016/j.nucmedbio.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 155.Kumar SR, Deutscher SL. 111In-labeled galectin-3-targeting peptide as a SPECT agent for imaging breast tumors. J Nucl Med. 2008;49(5):796–803. doi: 10.2967/jnumed.107.048751. [DOI] [PubMed] [Google Scholar]
- 156.Furtak V, Hatcher F, Ochieng J. Galectin-3 mediates the endocytosis of β-1 integrins by breast carcinoma cells. Biochem Biophys Res Commun. 2001;289(4):845–850. doi: 10.1006/bbrc.2001.6064. [DOI] [PubMed] [Google Scholar]
- 157.Endo K, Kohnoe S, Tsujita E, Watanabe A, Nakashima H, Baba H, Maehara Y. Galectin-3 expression is a potent prognostic marker in colorectal cancer. Anticancer Res. 2005;25(4):3117–3121. [PubMed] [Google Scholar]
- 158.Tsuboi K, Shimura T, Masuda N, Ide M, Tsutsumi S, Yamaguchi S, Asao T, Kuwano H. Galectin-3 expression in colorectal cancer: relation to invasion and metastasis. Anticancer Res. 2007;27(4B):2289–2296. [PubMed] [Google Scholar]
- 159.Moriyama Y, Moriyama EH, Blackmore K, Akens MK, Lilge L. In vivo study of the inflammatory modulating effects of low-level laser therapy on iNOS expression using bioluminescence imaging. Photochem Photobiol. 2005;81(6):1351–1355. doi: 10.1562/2005-02-28-RA-450. [DOI] [PubMed] [Google Scholar]
- 160.Yu JX, Cui L, Zhang QY, Chen H, Ji P, Wei HJ, Ma HY. Expression of NOS and HIF-1α in human colorectal carcinoma and implication in tumor angiogenesis. World J Gastroenterol. 2006;12(29):4660–4664. doi: 10.3748/wjg.v12.i29.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zafirellis K, Zachaki A, Agrogiannis G, Gravani K. Inducible nitric oxide synthase expression and its prognostic significance in colorectal cancer. APMIS. 2010;118(2):115–124. doi: 10.1111/j.1600-0463.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 162.Hu G, Lijowski M, Zhang H, Partlow KC, Caruthers SD, Kiefer G, Gulyas G, Athey P, Scott MJ, Wickline SA, et al. Imaging of Vx-2 rabbit tumors with α(nu)β3-integrin-targeted 111In nanoparticles. Int J Cancer. 2007;120(9):1951–1957. doi: 10.1002/ijc.22581. [DOI] [PubMed] [Google Scholar]
- 163.Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ, Peschel C, Lordick F, Schwaiger M. Comparison of integrin αVβ3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49(1):22–29. doi: 10.2967/jnumed.107.045864. [DOI] [PubMed] [Google Scholar]
- 164.Posey JA, Khazaeli MB, Del Grosso A, Saleh MN, Lin CY, Huse W, LoBuglio AF. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti-αvβ3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm. 2001;16(2):125–132. doi: 10.1089/108497801300189218. [DOI] [PubMed] [Google Scholar]
- 165.Edwards WB, Akers WJ, Ye Y, Cheney PP, Bloch S, Xu B, Laforest R, Achilefu S. Multimodal imaging of integrin receptor-positive tumors by bioluminescence, fluorescence, gamma scintigraphy, and single-photon emission computed tomography using a cyclic RGD peptide labeled with a near-infrared fluorescent dye and a radionuclide. Mol Imaging. 2009;8(2):101–110. [PMC free article] [PubMed] [Google Scholar]
- 166.Gaietta G, Redelmeier TE, Jackson MR, Tamura RN, Quaranta V. Quantitative measurement of α6β1 and α6β4 integrin internalization under cross-linking conditions: a possible role for α6 cytoplasmic domains. J Cell Sci. 1994;107(pt 12):3339–3349. doi: 10.1242/jcs.107.12.3339. [DOI] [PubMed] [Google Scholar]
- 167.Fan LF, Dong WG, Jiang CQ, Xia D, Liao F, Yu QF. Expression of putative stem cell genes Musashi-1 and β1-integrin in human colorectal adenomas and adenocarcinomas. Int J Colorectal Dis. 2010;25(1):17–23. doi: 10.1007/s00384-009-0791-2. [DOI] [PubMed] [Google Scholar]
- 168.Sipos B, Hahn D, Carceller A, Piulats J, Hedderich J, Kalthoff H, Goodman SL, Kosmahl M, Kloppel G. Immunohistochemical screening for β6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45(3):226–236. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 169.Bremer C, Bredow S, Mahmood U, Weissleder R, Tung CH. Optical imaging of matrix metalloproteinase-2 activity in tumors: feasibility study in a mouse model. Radiology. 2001;221(2):523–529. doi: 10.1148/radiol.2212010368. [DOI] [PubMed] [Google Scholar]
- 170.Hisatsune A, Kawasaki M, Nakayama H, Mikami Y, Miyata T, Isohama Y, Katsuki H, Kim KC. Internalization of MUC1 by anti-MUC1 antibody from cell membrane through the macropinocytotic pathway. Biochem Biophys Res Commun. 2009;388(4):677–682. doi: 10.1016/j.bbrc.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 171.Conrotto P, Roesli C, Rybak J, Kischel P, Waltregny D, Neri D, Castronovo V. Identification of new accessible tumor antigens in human colon cancer by ex vivo protein biotinylation and comparative mass spectrometry analysis. Int J Cancer. 2008;123(12):2856–2864. doi: 10.1002/ijc.23861. [DOI] [PubMed] [Google Scholar]
- 172.Kelly KA, Waterman P, Weissleder R. In vivo imaging of molecularly targeted phage. Neoplasia. 2006;8(12):1011–1018. doi: 10.1593/neo.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Volpe CM, Abdel-Nabi HH, Kulaylat MN, Doerr RJ. Results of immunoscintigraphy using a cocktail of radiolabeled monoclonal antibodies in the detection of colorectal cancer. Ann Surg Oncol. 1998;5(6):489–494. doi: 10.1007/BF02303640. [DOI] [PubMed] [Google Scholar]
- 174.Doerr RJ, Abdel-Nabi H, Baker JM, Steinberg S. Detection of primary colorectal cancer with indium 111 monoclonal antibody B72.3. Arch Surg. 1990;125(12):1601–1605. doi: 10.1001/archsurg.1990.01410240083016. [DOI] [PubMed] [Google Scholar]
- 175.Loy TS, Nashelsky MB. Reactivity of B72.3 with adenocarcinomas. An immunohistochemical study of 476 cases. Cancer. 1993;72(8):2495–2498. doi: 10.1002/1097-0142(19931015)72:8<2495::aid-cncr2820720830>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 176.Muraro R, Frati L, Bei R, Ficari F, Valli C, French D, Mammarella S, Caramia F, Fegiz G, Mariani-Costantini R. Regional heterogeneity and complementation in the expression of the tumor-associated lycoprotein 72 epitopes in colorectal cancer. Cancer Res. 1991;51(19):5378–5383. [PubMed] [Google Scholar]
- 177.Molinolo A, Simpson JF, Thor A, Schlom J. Enhanced tumor binding using immunohistochemical analyses by second generation anti-tumor-associated glycoprotein 72 monoclonal antibodies versus monoclonal antibody B72.3 in human tissue. Cancer Res. 1990;50(4):1291–1298. [PubMed] [Google Scholar]
- 178.Roessler M, Rollinger W, Palme S, Hagmann ML, Berndt P, Engel AM, Schneidinger B, Pfeffer M, Andres H, Karl J, et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin Cancer Res. 2005;11(18):6550–6557. doi: 10.1158/1078-0432.CCR-05-0983. [DOI] [PubMed] [Google Scholar]
- 179.Rubie C, Frick VO, Wagner M, Schuld J, Graber S, Brittner B, Bohle RM, Schilling MK. ELR+CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. doi: 10.1186/1471-2407-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]