Abstract
Using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to monitor vascular changes induced by sunitinib within a murine xenograft kidney tumor, we previously determined a dose that caused only partial destruction of blood vessels leading to “normalization” of tumor vasculature and improved blood flow. In the current study, kidney tumors were treated with this dose of sunitinib to modify the tumor microenvironment and enhance the effect of kidney tumor irradiation. The addition of soy isoflavones to this combined antiangiogenic and radiotherapy approach was investigated based on our studies demonstrating that soy isoflavones can potentiate the radiation effect on the tumors and act as antioxidants to protect normal tissues from treatment-induced toxicity. DCE-MRI was used to monitor vascular changes induced by sunitinib and schedule radiation when the uptake and washout of the contrast agent indicated regularization of blood flow. The combination of sunitinib with tumor irradiation and soy isoflavones significantly inhibited the growth and invasion of established kidney tumors and caused marked aberrations in the morphology of residual tumor cells. DCE-MRI studies demonstrated that the three modalities, sunitinib, radiation, and soy isoflavones, also exerted antiangiogenic effects resulting in increased uptake and clearance of the contrast agent. Interestingly, DCE-MRI and histologic observations of the normal contralateral kidneys suggest that soy could protect the vasculature of normal tissue from the adverse effects of sunitinib. An antiangiogenic approach that only partially destroys inefficient vessels could potentially increase the efficacy and delivery of cytotoxic therapies and radiotherapy for unresectable primary renal cell carcinoma tumors and metastatic disease.
Introduction
The advent of targeted therapies has changed the paradigm of systemic therapy for advanced metastatic renal cell carcinoma (RCC). Extensive research is focusing on drugs that target both the tumor cells and the tumor vasculature to inhibit processes in the tumor microenvironment that stimulate tumor growth. The incidence of RCC has increased in recent years with approximately 58,240 new cases each year in the United States. The disease is responsible for an estimated 13,040 deaths each year [1]. Nearly half of the patients present with localized disease that can be treated by surgical removal [2]. However, one third of the patients have metastatic disease at first presentation, and 20%to 30%of the patients treated for localized RCC subsequently develop metastatic disease which frequently involves the lungs [2,3]. Metastatic RCC disease has been resistant to chemotherapy and radiotherapy with a patient median survival of only 8 to 11 months [2,3]. This poor responsiveness could be due to the high vascularity of RCC tumors, which is structurally and functionally abnormal consisting of enlarged, disorganized, and leaky vessels. These vascular features cause impaired blood supply, decreased oxygen, and interstitial hypertension in tumors compromising the delivery and efficacy of cytotoxic agents and radiotherapy [4,5].
Recent treatments with new antiangiogenic drugs to disrupt tumor vasculature have shown significant responses and improved survival in RCC patients with metastatic disease [6–10]. Among others, small-molecule receptor tyrosine kinases (RTKs) inhibitors target the receptors of angiogenic factors including the vascular endothelial growth factor (VEGF) produced by tumor cells and associated stromal cells [11,12]. The RTK inhibitors sunitinib and axitinib inhibit the signal transduction induced byVEGF binding to VEGF receptors (VEGFR). Sunitinib also targets signaling of additional RTKs including platelet-derived growth factor receptor (PDGFR), KIT, and FLT3 in mouse xenograft models [11,13,14], whereas axitinib is a more selective RTK inhibitor of all three VEGF receptors VEGFR-1, -2, and -3 [15,16]. We have previously shown that sunitinib exhibits direct antitumor activity by inhibiting VEGFR-2 and PDGFR-β RTKs that are expressed on human KCI-18 RCC cancer cells, a cell line established from an RCC tumor specimen in our laboratory [11,17]. These RTKs are probably involved in signaling for cancer cell proliferation as shown in other studies [13,14]. Sunitinib also exhibits antiangiogenic activity by inhibition of signaling through VEGFR-2 and PDGFR-β RTKs expressed on endothelial or stromal cells [18,19].
Sunitinib has been approved by the US Food and Drug Administration in January 2006 for RCC treatment based on significant clinical responses in metastatic sites and primary tumors and is currently used as a front-line standard of care in metastatic RCC [10,19]. Sunitinib increased the median survival to 28 months [10]; however, long-term control of the disease is still not achieved, and the therapy is limited by adverse effects of cardiotoxicity observed in some of the patients, probably because of alterations to normal vasculature [20,21]. Therefore, we used the KCI-18 orthotopic xenograft murine tumor model to investigate whether sunitinib can be used at lower and less toxic doses to regularize the vasculature, decrease tumor vessel leakiness and interstitial pressure, and improve the blood flow within the tumor [11]. Using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to image vascular changes induced by sunitinib within a KCI-18 kidney tumor in nude mice, we have previously shown that a suboptimal daily sunitinib dose of 20 mg/kg per day mildly affected normal vessels but caused better tumor perfusion and decreased vascular permeability, in agreement with histologic observations of thinning and regularization of tumor vessels [11]. These studies indicated that this dose and schedule of sunitinib, which caused only partial destruction of inefficient blood vessels, lead to “normalization” of tumor vasculature and improved the tumor blood flow [11]. We further demonstrated that scheduling chemotherapy with gemcitabine, at a time when the blood flow is improved by pretreatment with sunitinib, resulted in enhanced therapeutic response [12].
In the current studies, the combination of the antiangiogenic drug sunitinib with kidney tumor irradiation and soy isoflavones was investigated to induce greater tumor cell killing and prolong the therapeutic response. The rationale for selecting soy isoflavones as anticancer agents is based on our previous studies showing that these compounds cause tumor cell apoptosis and also sensitize cancer cells to radiation both in vitro and in vivo in preclinical orthotopic models of RCC and prostate cancer [17,22–27]. Soy isoflavones are safe natural anticancer agents, as demonstrated in clinical trials [28], and they can also act as antioxidants in normal tissues and protect them from treatment-induced toxicity. A recent clinical trial for prostate cancer patients showed that soy isoflavones pills, taken in conjunction with radiotherapy, reduced radiation toxicity to normal tissues including urinary, gastrointestinal, and sexual functions [29]. Therefore, we also tested whether soy isoflavones could enhance the therapeutic effect of sunitinib combined with radiotherapy while reducing toxicity to normal organs.
The combination of the antiangiogenic drug sunitinib at a suboptimal dose of 20 mg/kg per day with tumor irradiation and soy isoflavones significantly inhibited the growth and invasion of established kidney tumors and caused marked aberrations in the morphology of residual tumor cells. DCE-MRI studies demonstrated that the three modalities, sunitinib, radiation, and soy isoflavones, also exerted antiangiogenic effects resulting in increased uptake and clearance of the contrast agent. In addition, DCE-MRI and histologic observations of the normal contralateral kidneys suggest that soy could protect the vasculature of normal tissue from adverse effects of sunitinib.
Materials and Methods
Orthotopic KCI-18/IK RCC Tumor Model
The human RCC cell line designated KCI-18 was established in our laboratory from a primary renal tumor specimen obtained from a patient with papillary RCC (nuclear grade 3/4) [17]. Cells were cultured in Dulbecco modified Eagle medium with supplements and serially passaged in the kidney of nude mice in vivo to generate highly tumorigenic KCI-18/IK cell lines, as previously detailed [17]. KCI-18/IK cells were washed with Hank's balanced salt solution and subcapsularly injected at a concentration of 5 x 105 cells in 30 µl of Hank's balanced salt solution in the right kidney in 5- to 6-week-old female BALB/C nu/nu nude mice (Harlan, Indianapolis, IN) [17]. Mice were housed and handled under sterile conditions in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The animal protocol was approved by Wayne State University Animal Investigation Committee.
Reagents
Sunitinib (Pfizer, Inc, New York, NY) was prepared in a carboxymethyl cellulose suspension vehicle, at a dosage of 20 mg/kg per day (SU20) and given orally by gavage, once a day [11]. The soy isoflavones mixture G-4660 consisted of 83.3% genistein, 14.6% daidzein, 0.26% glycitein, with the remainder being carbohydrate (manufactured by Organic Technologies and obtained from the National Institutes of Health). The soy isoflavones mixture was dissolved in 0.1 M Na2CO3 and mixed with sesame seed oil at a 2:1 ratio just before treatment to facilitate gavage and avoid irritation of the esophagus by Na2CO3 [25]. Mice were treated with 1 mg/d of soy (50 mg/kg body weight per day) by gavage.
Tumor-Bearing Kidney Irradiation
Three anesthetized mice, in jigs, were positioned under a 6.4-mm lead shield with three cut outs in an aluminum frame mounted on the x-ray machine to permit selective irradiation of the right tumor-bearing kidneys in three mice at a time, as previously described [17]. The radiation dose to the kidney and the scattered dose to areas of the mouse outside the radiation field were carefully monitored. Photon irradiation was performed at a dose of 8Gy with a Siemens Stabilipan X-ray set (Siemens Medical Systems, Inc, Malvern, PA) operated at 250 kV, 15 mA with 1-mm copper filtration at a distance of 47.5 cm from the target.
Experimental Protocol
After injection of KCI-18/IK cells in the kidney, a few mice were killed at early time points to assess tumor growth before initiating treatment. The tumor-bearing kidneys were weighed and measured in three dimensions to calculate the volume using length x width x thickness x 0.5236 / 2. Small tumors were established by day 10, and measurements of tumor-bearing kidneys were 150 mm3 (SD, 7) volume and 186 mg (SD, 4) weight compared with normal kidney sizes of 125 mm3 (SD, 2), 148 mg (SD, 12) [11]. On day 10, mice were pretreated each day for 3 days with soy isoflavones first given at 1 mg and, 6 hours later, with sunitinib given at 20 mg/kg; both drugs were administered orally by gavage [11,17,25]. Radiation was administered on day 13 at 8 Gy and targeted to the area of the right tumor-bearing kidney [17]. Sunitinib and/or soy treatments were continued daily for the duration of the experiment. To assess the therapeutic response of kidney tumors to sunitinib, soy, and radiation, eight mice per experimental group were treated. Mice were killed by day 27 after tumor cell injection, when the tumor burden in control animals was large (>1.5 cm x 1 cm in size compared with 0.7 cm x 0.25 cm for normal kidney) to compare with tumor sizes in treated groups [11]. The tumor-bearing right kidneys and the contralateral left normal kidneys were resected and weighed [11,12].
Tissue Preparation for Histology
At the completion of experiments, mice were killed, and tumor-bearing kidneys, normal contralateral kidneys, and the lungs were resected and processed for histology. All tissues were fixed in 10% buffered formalin, embedded in paraffin, and sectioned [11]. Sections were stained with hematoxylin-eosin (H&E) or immunostained with anti-CD31 antibody (Ab) (Thermo Scientific, Fremont, CA) using an avidin-biotin-immunoperoxidase technique [11].
DCE-MRI Monitoring of Tumor Perfusion and Permeability in Kidney Tumors
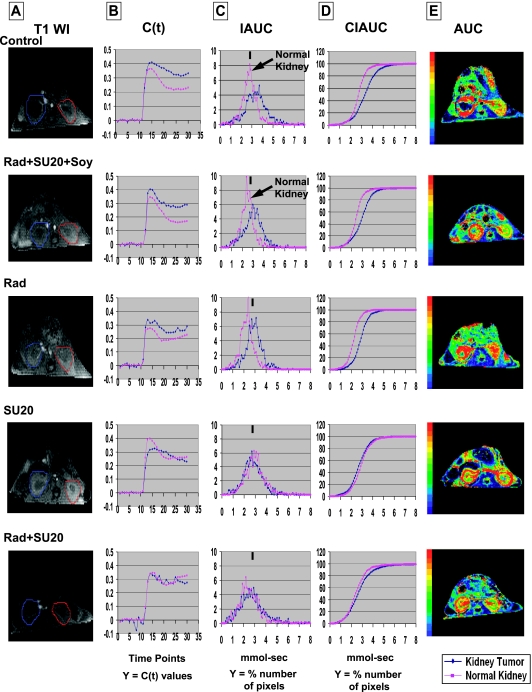
Mice treated with single or combined treatments of sunitinib, soy, and radiation were imaged by DCE-MRI, as previously detailed [11,12]. A catheter was inserted into mice tail vein and was attached to a syringe containing Gd-DTPA contrast agent (Berlex, Wayne, NJ). Anesthetized mice were positioned on a cradle heated by temperature-controlled water [11,12]. A 2-cm-diameter receive-only surface coil was placed over the tumor, and the cradle was placed inside an 11-cm-inner-diameter transmit-only volume coil for imaging using a Bruker Biospec AVANCE animal scanner (Bruker, Karlsruhe, Germany) equipped with a 4.7-T horizontal bore magnet and actively shielded gradients. Baseline imaging data of the kidneys were obtained using the short TR DCE scan for 30 time points (7 seconds between time points). On time point 10, 100 µl of Gd-DTPA (0.125 mmol/kg) was injected into the tail vein catheter. Then, imaging data were acquired for 20 more time points. The imaging parameters for this multislice two-dimensional gradient echo scan were as follows: repetition time = 54.7 milliseconds, echo time = 2.9 milliseconds, flip angle = 30°, field of view = 32 mm x 32 mm, slice thickness = 1.5 mm with 0.5-mm gap, matrix size = 128 x 128. Five slices were collected for each animal. Data were processed to determine changes in contrast agent uptake using the SPIN DCE software (Detroit, MI) [11,12]. For data analysis, the full kidney was selected as the region of interest (ROI) for the tumor-bearing kidney and the contralateral left normal kidney. A threshold was set at three times (3x) the noise to remove noise-only pixels in the image [11]. Gd concentrations [C(t)] in the tissue were calculated for all pixels in the ROI and for each time point [11]. Data from the C(t) curves were compiled for each pixel for nine time points (63 seconds) after Gd injection to create the initial area under the curve (IAUC). The CIAUC is the cumulative initial area under the curve of the IAUC histogram[11]. To evaluate the uptake, washout, and leakage of Gd into the tumor and surrounding kidney tissue, T1-weighted images and AUC parametric maps serve as a means to enhance tumor visibility, quantify Gd concentration, and monitor changes in vascular response.
Analysis of Cell Survival In Vitro by Clonogenic Assay
The radiation dose and drug concentrations were determined based on our previous studies [11,23]. KCI-18 human RCC cells were pretreated for 24 hours with 1 µM sunitinib and/or 5 µM soy isoflavones and then irradiated with 3-Gy photons as previously described [11,23]. Single and combined treatments were tested. For comparison between each treatment group, the number of treated cells plated in the clonogenic assay was adjusted relative to untreated cells to predict a measurable survival fraction, as determined in pilot experiments. Cells were plated in a colony formation assay in triplicate wells of six-well plates at 500 cells per well for control; 1000 cells per well for sunitinib, soy, or radiation alone; 2000 cells per well for sunitinib + soy; 3000 cells for radiation + sunitinib, radiation + soy; and 4000 cells for radiation + sunitinib + soy [11,12,23]. The drugs were added to the cells in the colony plates, and cells were incubated for 10 days at 37°C in a 5% CO2/5% O2/90% N2 incubator. Colonies were fixed, stained, and counted as previously described [11]. The plating efficiency was calculated for each well by dividing the number of colonies by the original number of cells plated. The surviving fraction (SF) was normalized to control cell plating efficiency by dividing the plating efficiency of treated cells by that of control cells [11]. The plating efficiency was calculated for each well, and the surviving fraction was normalized to control cells [11].
Statistical Analysis
Evaluation of the shape of the frequency distribution of tumor weights indicated that a log transformation was required to meet the assumptions of normal theory tests. Linear models were used to assess the statistical significance of differences in tumor weight between experimental groups. Adjustment for multiple comparisons between treatments was made using Holm's procedure to protect against inflated type 1 errors [11,12].
Results
Enhanced Cytotoxic Effect of Sunitinib, Soy Isoflavones, and Radiation in KCI-18 Cells In Vitro
A dose of 1 µMsunitinib caused 37% inhibition in KCI-18 cell survival in a clonogenic assay (Table 1) [11]. This dose was selected to investigate whether cell killing is enhanced by the addition of soy and radiation. After pilot titration experiments, suboptimal doses of soy and radiation were tested alone and combined with sunitinib in a clonogenic assay to assess whether the combination is more effective at cell killing. A low dose of 5 µM of the mixture of soy isoflavones caused 25% (SD, 5) inhibition in cell survival but significantly increased the effect of sunitinib to 56% (SD, 2) inhibition (P < .002) compared with sunitinib alone (Table 1). Cells irradiated with 3-Gy photons showed a 56% (SD, 4) inhibition in cell growth that was further significantly enhanced to 74% to 80% (SD, 2.3) by cotreatment with sunitinib or soy isoflavones or both (P < .05; Table 1).
Table 1.
Inhibition of KCI-18 Cell Growth by Sunitinib Combined with Soy and Radiation In Vitro.
| Treatment | Survival Fraction (Mean ± SD) | % Inhibition (Mean ± SD) |
| Control | 1 ± 0 | 0 ± 0 |
| SU | 0.63 ± 0.04 | 37 ± 7 |
| Soy | 0.75 ± 0.07 | 25 ± 4 |
| SU + soy | 0.44 ± 0.02 | 56 ± 2 |
| Rad | 0.44 ± 0.04 | 56 ± 4 |
| Rad + SU | 0.26 ± 0.03 | 74 ± 3 |
| Rad + soy | 0.23 ± 0.02 | 77 ± 2 |
| Rad + SU + soy | 0.20 ± 0.02 | 80 ± 3 |
KCI-18 cells were treated with sunitinib at 1 µMor soy at 5 µM or both drugs for 24 hours, and then cells were irradiated with 3-Gy photons and plated in a colony formation assay for 10 days. The mean survival fraction (SF) was calculated from triplicate wells. The percent inhibition was calculated as follows: SF (control) - SF (treatment) / SF (control) x 100.
Enhanced Therapeutic Response of Kidney Tumors by Combined Sunitinib, Radiation, and Soy In Vivo
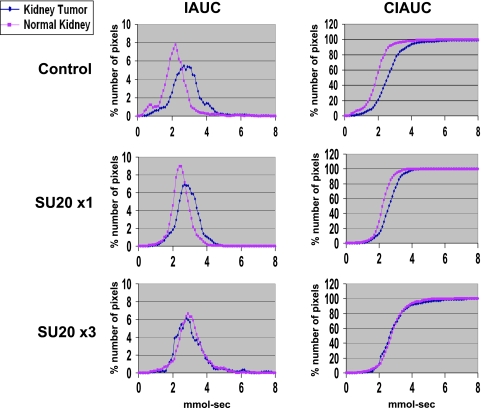
We previously showed that a dosage of 20 mg/kg per day sunitinib caused regularization of tumor vessels with improved tumor perfusion [11]; therefore, this dosage was selected for combination with radiotherapy. To schedule the combination therapy, regularization of tumor vessels was monitored by DCE-MRI of kidney tumor-bearing mice treated with sunitinib only. On day 10 after tumor implantation, mice with established kidney tumors were treated daily with sunitinib at 20 mg/kg per day (SU20) for 1 or 3 days and then imaged by DCE-MRI (Figure 1A). As previously observed, a slower clearance of Gd was observed in the tumor-bearing kidney, with the CIAUC curve shifting to the right of the normal kidney (Figure 1) [11]. After 1 day of SU20 treatment, a shift of the tumor-bearing kidney IAUC and CIAUC curves toward those of normal kidney was observed. However, the effect of a 3-day treatment with SU20 was more pronounced as the kidney tumor showed identical patterns of Gd uptake and clearance than those of the normal kidney. The IAUC and CIAUC histograms of the kidney tumor overlapped those of the normal kidney, indicating decreased Gd retention and improved tumor perfusion (Figure 1), as previously described [11].
Figure 1.
DCE-MRI of early vascular changes induced by sunitinib. Mice bearing established kidney tumors were treated with sunitinib at 20 mg/kg per day for 1 day (SU20 x1) and on a daily basis for 3 days (SU20 x3) and imaged by DCE-MRI.
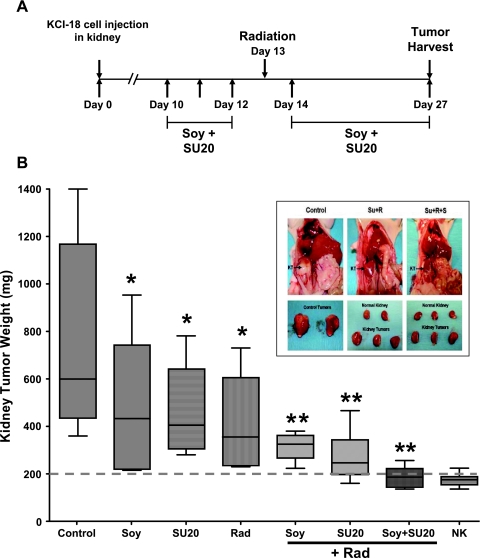
On the basis of these data demonstrating vascular regularization by DCE-MRI after three daily doses of SU20 treatment, irradiation (Rad) of the tumor-bearing kidney was administered at that time point, on day 13, as shown in Figure 2A. We had shown that pretreatment with soy potentiates the effect of radiation in kidney and prostate tumors; therefore, soy treatment was also initiated on days 10 to 12 before radiation. Both SU20 and soy treatments were continued daily after radiation up to day 27 when mice were killed to assess tumor response (Figure 2B). Compared with control kidney tumors (mean ± SD weight, 771 ± 413 mg), treatments with soy alone (P = .03), SU20 alone (P = .04), and radiation alone (P = .008) caused a significant decrease of tumor growth, but these tumors were still very large with a mean weight range of 400 to 470 mg (Figure 2B). However, the combinations of soy + Rad, SU20 + Rad, and SU20 + Soy + Rad caused a much greater and significant inhibition of tumor growth with weights in the range of 190 to 320 mg compared with control mice (P < .001; Figure 2B). Although combining the three modalities soy, sunitinib, and radiation was borderline significant to two modalities (P = .06), it still resulted in a more consistent and almost 97%complete inhibition of tumor growth, with a mean tumor weight of 187 ± 46 mg compared with 174 ± 25 mg of the normal kidneys nonbearing tumors. The combination of soy, SU20, and radiation narrowed the range of tumor-bearing kidney sizes compared with SU20 + Rad or Soy + Rad, and these values were very close and not statistically different from those of normal kidneys (NK) (P = .77; see broken line at 200 mg in Figure 2B), suggesting dramatic tumor growth inhibition. Indeed, mice treated with SU20 + Rad + Soy had very small tumor-bearing kidneys (KT) that approximated the size and appearance of normal kidneys compared with the large tumors in control mice (Figure 2B, inset). No signs of toxicity were observed in these mice.
Figure 2.
KCI-18 kidney tumor response to sunitinib combined with radiation and soy. (A) Treatment schedule for combination therapy. Mice bearing established kidney tumors were pretreated with sunitinib at 20 mg/kg per day (SU20) and/or soy at 50 mg/kg per day for 3 days, on days 10 to 12 after KCI-18 cell injection in the kidney. Then, mice received an 8-Gy radiation to the tumor-bearing kidney. Sunitinib and/or soy were continued daily for up to 27 days. (B) Response of tumor-bearing kidneys to single and combined therapy. On day 27, tumor-bearing kidneys and contralateral normal kidneys were resected and weighed. The weights of the tumor-bearing kidneys and their median are reported for eight mice per group treated with vehicle (control) or SU20 or soy (Soy) or radiation (Rad) as single modalities and radiation combined with SU20, soy, or both (*P < .05, **P < .001 compared with control mice). The normal contralateral kidney weights (NK) are shown. Inset shows the size of kidney tumors before and after resection of control mice; mice treated with radiation and SU20; and mice treated with radiation, SU20, and soy (Su + R + S) compared with normal kidney size.
In Situ Effects of Sunitinib, Radiation, and Soy on KTs
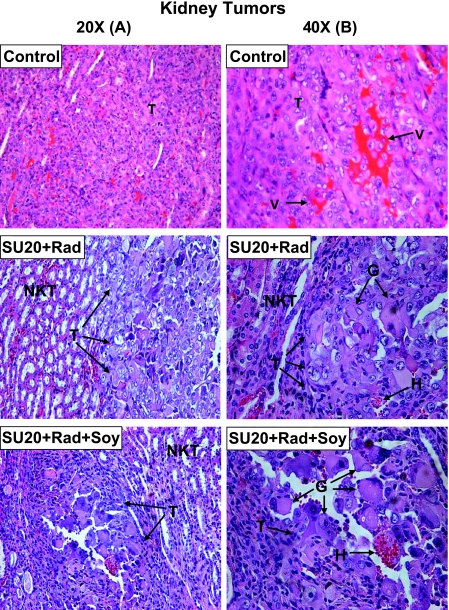
Histologic samples of the kidney tumors obtained from mice treated with SU20, soy, and radiation were processed for histology and H&E staining. Kidney tumors from control mice consisted of cells with large pleomorphic nuclei and were highly vascularized with a sinusoidal vascular pattern and abnormal enlarged vessels (Figure 3, A and B) [11]. After SU20 and kidney tumor irradiation, small nodules of tumor were seen surrounded by normal kidney tissue consisting of typical tubules and glomeruli (Figure 3A). These tumor nodules contained atypical giant tumor cells or detached rhabdoid cells with large vacuoles (Figure 3B), which are characteristic of radiation-induced alterations, as previously described [17]. Kidney tumors treated with SU20, tumor irradiation, and soy isoflavones showed comparable findings with very small remaining tumor nodules surrounded by normal kidney tissue (Figure 3A). The residual tumor areas had hemorrhages and consisted mostly of detached rhabdoid cells with large vacuoles and atypical giant cells with pleomorphic nuclei often undergoing degenerative changes (Figure 3B). These data are indicative of slow death due to alterations in cell division at the level of cytokinesis, as previously described [22,25]. These changes are similar to those previously observed in KCI-18 kidney tumors treated with genistein and radiation and therefore reflect further tumor destruction by soy and radiation [17].
Figure 3.
Histology of kidney tumors from mice treated with sunitinib, radiation and soy. Kidney tumors from control mice or mice treated with SU20 and kidney tumor irradiation (SU20 + Rad) and SU20 combined with radiation and soy (SU20 + Rad + Soy), obtained on day 27 from experiments described in Figure 2, were processed for histology and H&E staining. The main findings were labeled on the prints with T for tumor, V for vessels, NKT for normal kidney tissue, H for hemorrhages, and G for giant cells. A lower magnification 20x is shown in panel A and a highermagnification of 40x is shown in panel B to emphasize the findings. Control untreated tumors consisted of tumor cells with large pleomorphic nuclei, were highly vascularized with a sinusoidal vascular pattern of abnormal enlarged dilated vessels (A, B). Kidney tumors treated with SU20 + Rad showed small tumor nodules consisting of atypical giant tumor cells or detached rhabdoid cells with large vacuoles (B). These nodules were surrounded by normal kidney tissue (NKT) consisting of typical tubules and glomeruli (A). Small remaining tumor nodules treated with SU20 + Rad + Soy consisted mostly of abnormal degenerating giant tumor cells including focal hemorrhages and were surrounded by normal kidney tissues (A, B).
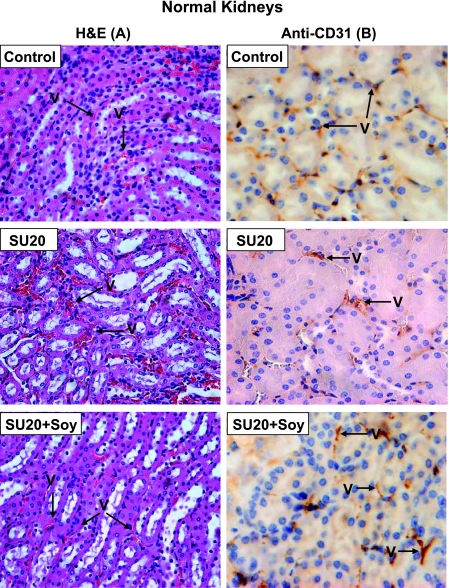
The systemic effects of SU20 and soy isoflavones on the vasculature of normal tissues were studied on the contralateral left normal kidney not bearing a tumor, which was not irradiated. The normal kidney tissue sections shown in Figure 4 were obtained from untreated mice; mice treated with SU20 + Rad (labeled SU20 in Figure 4) or SU20 + Soy + Rad (labeled SU20 + Soy in Figure 4) only reflect the alterations induced by SU20 or soy isoflavones because they were not in the field of radiation. The H&E observations (Figure 4A) were confirmed by anti-CD31 immunostaining of endothelial cells (Figure 4B). Compared with thin and regular vessels in normal kidneys of control mice, the normal kidney vessels of mice treated with SU20 showed mild dilatation, both by H&E and immunostaining with anti-CD31 (Figure 4, A and B), as previously seen for SU20 (Figure 4) [11]. The vessels of normal left kidneys from mice treated with SU20 and soy isoflavones looked thinner and more regular, as confirmed by anti-CD31 staining (Figure 4, A and B).
Figure 4.
Histology of normal kidneys from mice treated with sunitinib and soy. The normal contralateral kidneys from control mice or mice treated with SU20 and SU20 combined with soy (SU20 + Soy), obtained on day 27 from experiments described in Figure 2, were processed for histology and H&E staining (A) and anti-CD31 immunostaining (B). Only alterations induced by SU20 or soy can be observed in these left kidneys because they were not in the field of radiation. The normal kidney from control mice showed intact, regular, and thin blood vessels (A, B). The vessels in normal kidneys of mice treated with SU20 showed mild dilatation (A, B). In contrast, the vessels of normal left kidneys treated with systemic SU20 and soy looked thinner and more regular (A) as confirmed by anti-CD31 staining of endothelial cells (B).
DCE-MRI Evaluation of Vascular Changes Induced by Sunitinib, Soy, and Radiation Treatments in Kidney Tumors
Established KCI-18 kidney tumors were pretreated with SU20 and soy isoflavones on days 10 to 12. On day 13, the right kidneys bearing KCI-18 tumors were irradiated with 8 Gy. On days 14, 15, and 16, sunitinib and soy treatments were resumed. On day 17, i.e., 4 days after irradiation and six daily treatments with sunitinib and soy, mice were imaged by DCE-MRI (T1-weighted images shown in Figure 5A), before and after Gd injection (Figure 5A–E). Data from C(t) curves (Figure 5B) of the kinetics of Gd concentrations were compiled for nine time points after Gd contrast injection to create IAUC (Figure 5C) and CIAUC (Figure 5D) histograms. Images of the first nine time points after Gd injection were selected for constructing IAUC and CIAUC histograms because MR images showed early and rapid drastic changes in Gd uptake and clearance within these time intervals, in treated tumors compared with control tumors and relative to normal kidneys. Control tumors showed slower Gd clearance and a shift of IAUC and CIAUC curves to the right of the normal kidney curves. No uptake of Gd in the core of the kidney tumor was seen in AUC parametric maps (Figure 5E). Kidney tumors treated with radiation alone or combined with SU20 and soy showed a pattern of uptake and clearance of Gd in C(t) curves parallel to that of normal kidney with improved kinetics of Gd clearance (Figure 5B). A shift to the left in IAUC histograms and a narrower distribution compared with that of control tumors indicated an improved Gd uptake and clearance (Figure 5C), as confirmed by Gd tumor uptake in AUC parametric maps (Figure 5E ). Treatment with SU20 alone or combined with radiation showed IAUC and CIAUC patterns of kidney tumors overlapping with those of normal kidneys in these mice, confirming the effect of SU20 on regularization of blood flow in kidney tumors (Figure 5, B–D). However, as previously observed, SU20 caused vascular changes in the normal left kidney visualized as a wider IAUC peak and a shift to the right of the IAUC curve compared with that seen in the normal kidney of control mice. This effect was also seen in the left normal kidney (nonirradiated) of mice treated with SU20 and radiation, thus reflecting only SU20 treatment of normal vessels (Figure 5C). Interestingly, when mice were treated with both systemic soy and SU20 treatments in addition to the right kidney tumor irradiation, the left normal kidney exhibited a normal uptake, and clearance of Gd contrast agent similar to that of the normal kidney of control mice or mice treated with radiation only (Figure 5C, see arrow in the IAUC curve). The wider distribution of IAUC and the shift of IAUC to the right seen with SU 20 alone were not observed when soy was added.
Figure 5.
DCE-MRI imaging of vascular changes induced by sunitinib, radiation and soy in KCI-18 kidney tumors. Separate experimental groups of three mice per group were treated with vehicle only (control), SU20, Rad, and Soy. Mice bearing established kidney tumors were pretreated with sunitinib at 20 mg/kg per day and soy at 50 mg/kg per day for 3 days, on days 10 to 12 after KCI-18 cell injection in the kidney. On day 13, the right kidneys bearing KCI-18 tumors were irradiated with 8 Gy. SU20 and soy were continued daily on days 14, 15, and 16, and on day 17, mice were imaged by DCE-MRI and data were analyzed. (A) T1-weighted images (T1 WI): Baseline images before Gd contrast agent injection. The full kidney was selected as the ROI for the tumor-bearing kidney (blue contour on left of T1-weighted image) and the contralateral normal kidney (red contour on right of T1-weighted image). (B) C(t) kinetics of Gd contrast uptake and clearance: The first 10 time points represent baseline data. Gd was injected at time point 10, and images were collected for 20 more time points. (C) IAUC graphs: Data from the C(t) curves were compiled for nine time points (63 seconds) after Gd injection to draw IAUC63. The small black bar indicates the peak position of normal kidney in control mice, and this can be used as a reference for curve shifting in normal kidneys and kidney tumors after treatment. (D) CIAUC graphs: CIAUC graphs were derived from IAUC curves. In B, C, and D graphs, blue lines are for kidney tumors and pink lines are for normal kidneys. Data from a representative mouse from each treatment group are presented. (E) AUC parametric map: Parametric color maps were constructed based on uptake and concentration of Gd in the tissue, represented by the colors blue, green, yellow, and red with gradual increase of Gd from lowest values (0 for blue) to highest values (1 for red). This scale represents a normalized ratio obtained by dividing each AUC value in an individual structure by the maximum AUC value in the overall image. The tumor-bearing kidney is on the left, and the normal contralateral kidney is on the right of the MR images. The color coding in the kidneys is shown for integrated AUC.
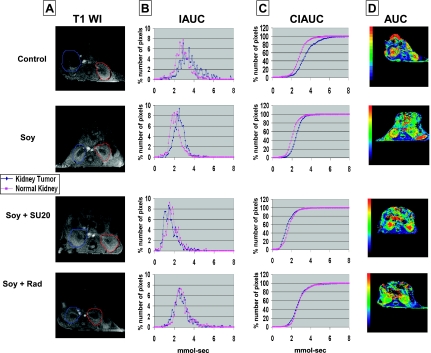
To determine further the effect of soy on vessels of normal tissues and tumors, mice treated with soy alone and combined either with SU20 or radiation were imaged by DCE-MRI. The IAUC and CIAUC shift relative to normal kidney curves was reduced by soy treatment alone (Figure 6, B and C), which also caused an increased uptake of Gd contrast in the tumor observed in AUC parametric maps, which is indicative of soy mediating a regularizing effect on tumor vasculature (Figure 6D). The left normal kidney showed a normal pattern of Gd uptake and clearance. Soy combined with SU20 induced vascular changes in the kidney tumor comparable to those observed with SU20 alone with a shift of IAUC and CIAUC curves toward those of the normal kidney (Figure 6, B and C). However, in contrast to SU20 alone, the pattern of Gd uptake and clearance in the left normal kidney of Soy + SU20 (Figure 6B) resembled that of control mice or soy-only-treated mice, confirming that soy regularized Gd flow in the normal kidney and mitigated the vascular changes induced by SU20 alone. Vascular regularization visualized by overlapping and shifted curves was also observed in the kidney tumors of mice treated with soy and radiation, suggesting that soy and radiation regularized the tumor vasculature (Figure 6, B and C). These findings are in agreement with the AUC parametric maps showing Gd uptake in tumors. Although a representative mouse is shown for each treatment, presented in Figures 5 and 6, these data were reproduced and consistent in additional mice and two series of independent experiments.
Figure 6.
DCE-MRI imaging of vascular changes induced by soy combined with sunitinib or radiation in KCI-18 kidney tumors. In separate experiments, mice bearing established kidney tumors were treated with soy, soy + SU20, or soy + Rad using the schedule and doses described in Figure 5. Mice were imaged by DCE-MRI on day 17. (A) T1-weighted images (T1 WI): Baseline images before Gd contrast agent injection as described in Figure 5. (B) IAUC graphs: Data from the C(t) curves were compiled for nine time points after Gd injection to draw IAUC63. (C) CIAUC graphs: CIAUC curves were derived from IAUC curves. In A, B, and C panels, blue lines are for kidney tumors and pink lines are for normal kidneys. (D) AUC parametric map: Parametric color maps were constructed as described in Figure 5. The tumor-bearing kidney is on the left and the normal contralateral kidney is on the right of the MR images. The color coding in the kidneys is shown for integrated AUC. Data from a representative mouse from each treatment group are presented.
To compare the patterns of Gd uptake in tumor-bearing kidneys versus normal kidneys, R50 values were derived from the CIAUC curves for both kidney tumors and normal kidneys (Figures 5 and 6). The R50 (median) values correspond to the concentration of Gd at which 50% of the pixels have been included [11]. R50 values of KTs were then normalized to the R50 values of NKs for each mouse, as detailed previously [11,12]. The means and SDs of normalized R50 values (NR50) for four mice per treatment group are presented in Table 2. These quantitative data confirm that mice treated with SU20 alone and SU20 combined with soy or radiation had NR50 values near zero (P < .005 compared with control) owing to similar distributions of IAUCand CIAUC curves for KT and NK as seen in Figures 5 and 6. Interestingly, soy combined with radiation also had low values of NR50 (P < .05 compared with control) because they both destroy some of the tumor vasculature causing normalization as visualized by overlapping IAUC and CIAUC curves of KT and NK (Figure 6). Soy alone or radiation alone had greater NR50 values comparable to those of control mice (P > .05), although they affected tumor vasculature but probably not enough to get normalization (Figures 5 and 6). When SU20 was combined with radiation and soy, soy mitigated the effect of SU20 on normal kidney vessels, which now show a normal pattern of uptake and clearance of Gd. All three modalities regularize the uptake and clearance of Gd with a narrower IAUC distribution, increased clearance but still do not overlap with the normal kidney resulting in a greater NR50 value (Table 2 and Figure 5).
Table 2.
NR50 Quantitation of DCE-MRI Data for KCI-18 Kidney Tumors.
| Treatment | *NR50 [KT vs NK] (Mean ± SD) |
| Control | 0.22 ± 0.08 |
| SU | 0.01 ± 0.05 |
| SU + soy | -0.08 ± 0.07 |
| Rad + SU | 0.01 ± 0.04 |
| Rad + SU + soy | 0.21 ± 0.03 |
| Soy | 0.18 ± 0.08 |
| Rad | 0.19 ± 0.09 |
| Rad + soy | 0.08 ± 0.09 |
To calculate NR50, the R50 values for KTs and NKs were derived from CIAUC curves, which correspond to the Gd concentration at which 50% of the pixels have been included. R50 values of KT were normalized to R50 values of NK and represent *NR50 [KT vs NK] calculated as [R50KT - R50NK] / R50NK for each mouse. The mean NR50 for four mice per group is shown with SD.
Discussion
Antiangiogenic therapy causing excessive vascular regression could compromise the delivery of drugs or oxygen in the tumor and disrupt the vessels of normal tissues. A combined approach consisting of antiangiogenic drugs to disrupt the tumor vasculature, given in conjunction with radiation and cytotoxic agents to kill tumor cells, could be more effective at preventing progression and/or recurrence of tumors in metastatic RCC. The key to its success is to determine optimal scheduling and dosing of antiangiogenic therapy with radiation and/or cytotoxic agents for taking advantage of a time point in which normalization of the structure of blood vessels caused by antiangiogenic therapy will facilitate access of drugs and oxygen to tumor cells [30]. The goals of the current studies were to monitor partial destruction of tumor vasculature to improve the blood flow with sunitinib and schedule radiotherapy combined with soy isoflavones for RCC murine xenograft kidney tumors.
DCE-MRI was selected to detect early changes in the tumor induced by sunitinib as it measures a combination of tumor perfusion and vessel permeability and allows the detection of early changes in tumor vascularity induced by treatment with antiangiogenic drugs [31]. In the current study, DCE-MRI showed that at least three consecutive daily treatment with SU20 were sufficient to induce vascular changes of decreased Gd retention and improved tumor perfusion in KCI-18 kidney tumors, which were comparable to those of the normal contralateral kidney. On the basis of these findings indicating normalization of blood vessels, radiation was administered to the kidney tumor after three daily treatments of sunitinib. The effect of combined sunitinib and radiation on inhibition of kidney tumor growth was enhanced compared with each modality alone. Pretreatment with soy isoflavones also significantly increased tumor growth inhibition by radiation as previously observed with pure genistein, which is the active compound of soy [17]. Pretreatment with sunitinib and soy for 3 days followed by tumor irradiation and continued daily treatment with soy and sunitinib was particularly effective, causing almost complete inhibition of tumor growth. This effect was consistent in all mice treated, and the size and shape of the tumor-bearing kidneys were comparable to those of the normal contralateral kidneys. In agreement with our gross observations, only small residual tumor nodules surrounded by normal kidney tissue were histologically observed. These tumors showed a high frequency of abnormal giant tumor cells with degenerative changes in their cytoplasm and nuclei, indicative of cell death processes, as previously described [17]. In mice treated with sunitinib and radiation, the nonirradiated normal left kidney showed mild dilatation of normal vessels, reflecting only sunitinib-induced vascular effects, as previously observed [11]. Interestingly, addition of soy to sunitinib seems to protect the vessels from dilatation because the vessels in the normal kidney tissue looked thinner and more regular akin to those observed in control mice. Previous studies indicated that soy isoflavones inhibited apoptosis in endothelial cells and have shown benefits for the prevention of cardiovascular disease [32].
A direct and enhanced cytotoxic effect sunitinib, radiation, and soy on KCI-18 cells was mediated in vitro, as shown in a clonogenic assay, which could contribute to the enhanced therapeutic effect mediated in vivo in kidney tumors by these three modalities. In addition to the histologic findings in kidney tumors, DCE-MRI studies confirmed that all three modalities also exert antiangiogenic effects and affect the vascular properties of tumors. Kidney tumors treated with radiation alone showed an improved uptake and clearance of Gd compared with control untreated tumors, which was confirmed by better tumor perfusion seen in AUC parametric maps. These DCE-MRI data indicate that radiation induces vascular changes in tumors, probably by killing excess endothelial cells and disrupting blood vessels that are histologically visualized as hemorrhages. The pattern of Gd uptake and clearance in kidney tumors treated with SU20 and radiation was similar to that of tumors treated with SU20 alone with IAUC curves overlapping those of the normal left kidney [11]. These data confirm the effect of SU20 on regularization of blood flow in kidney tumors [11]. Interestingly, treatment of tumors with soy isoflavones alone also caused dramatic vascular changes regularizing Gd uptake and clearance and improving tumor perfusion akin to that observed in the normal kidney. A similar effect was observed when soy isoflavones were combined with radiation or sunitinib. These data suggest that soy isoflavones play an antiangiogenic role in tumors by disrupting tumor vasculature, corroborating our previous findings on VEGF inhibition in vitro [26]. Soy isoflavones' antiangiogenic effect has been documented in other studies in vivo including our studies in prostate tumors and RCC tumors [17,22,25]. AUC parametric maps showed increased perfusion in tumors treated with sunitinib, radiation, and soy isoflavones, suggesting that these three modalities induce significant vascular changes in the tumor environment. Our studies also confirm that DCE-MRI can detect early vascular changes in kidney tumors induced by sunitinib, radiation, soy isoflavones, and the combination of these modalities.
DCE-MRI monitoring of vascular changes induced by soy isoflavones in the normal left kidney revealed that soy does not affect the vascular flow compared with normal untreated kidney from control mice. It is interesting to note that unlike the mild vascular changes observed with SU20 systemic treatment in the left kidneys, mice receiving both systemic SU20 and soy isoflavones exhibited a normal pattern of Gd uptake and clearance akin to that of normal untreated kidney from control mice. Thus, addition of soy isoflavones to SU20 regularized Gd uptake and clearance in normal kidney and mitigated the vascular changes induced by SU20 alone. These results are in agreement with our in situ histologic observations of thinner and regular vessels. These novel findings suggest that sunitinib-induced vascular changes in normal kidney tissues can be attenuated by simultaneous treatment with soy isoflavones, giving support to our hypothesis that soy could reduce sunitinib-induced vascular damage in normal tissues.
The combination of sunitinib with tumor irradiation and soy isoflavones showed a dramatic inhibition of tumor growth, disruption of vasculature, and tumor cell destruction in kidney tumors. The mechanisms of interaction between antiangiogenic agents and ionizing radiation could involve interactions between tumor stroma and vasculature and the tumor cells [33,34]. Combining radiotherapy with angiogenic inhibitors showed increased antitumor efficacy in animal tumor models owing to increased toxicity to endothelial cells and tumor vasculature. Inhibition of VEGFR-2 signaling by RTK inhibitors or anti-VEGFR-2 Abs enhanced response to radiotherapy in subcutaneous tumors in animal tumor models [35,36]. The role of hypoxia in radioresistance of tumors and angiogenesis could involve poor tumor oxygenation but also up-regulation of hypoxia-inducible factor (HIF-1α) as a cellular response to stress and damage induced by radiation [34]. Although radiation causes vascular damage in tumors, we and others have shown that radiation activates survival pathways in cancer cells including upregulation of HIF-1α and NF-κB activities, which are both transcription factors responsible for VEGF gene transcription [24,26]. This could trigger a vicious cycle of de novo angiogenesis in residual tumor areas in which tumor cells were not destroyed by radiation, leading to tumor recurrence. We have previously demonstrated that soy isoflavones inhibit up-regulation of HIF-1α and NF-βB induced by irradiation, and we surmise that this mechanism could further enhance the antitumor response of kidney tumors as suggested by our studies.
We conclude that an antiangiogenic approach, which only partially destroys inefficient vessels, could potentially increase the efficacy and delivery of cytotoxic therapies and radiotherapy for unresectable primary RCC tumors and metastatic disease.
Acknowledgments
The authors thank Yimin Shen, Hao Zhang, Amit Patel, and Sanket Gujarathi for excellent technical assistance. The authors also thank Andre Konski and Ulka Vaishampayan (Karmanos Cancer Institute) for stimulating discussions on clinical translation issues.
Footnotes
This study was supported by Pfizer Grant IIR No. GA61818Z 9, Karmanos Cancer Institute Pilot Project Grant and the Fund for Cancer Research (all to G.G. Hillman).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 3.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Jr, Munshi N, Crawford ED. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Basappa NS, Elson P, Golshayan AR, Wood L, Garcia JA, Dreicer R, Rini BI. The impact of tumor burden characteristics in patients with metastatic renal cell carcinoma treated with sunitinib. Cancer. 2010 doi: 10.1002/cncr.25713. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Rixe O, Rini B. Renal cell carcinoma: ten years of significant advances. Target Oncol. 2010;5:73–74. doi: 10.1007/s11523-010-0150-9. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI. New strategies in kidney cancer: therapeutic advances through understanding the molecular basis of response and resistance. Clin Cancer Res. 2010;16:1348–1354. doi: 10.1158/1078-0432.CCR-09-2273. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillman GG, Singh-Gupta V, Zhang H, Al-Bashir AK, Katkuri Y, Li M, Yunker CK, Patel AD, Abrams J, Haacke EM. Dynamic contrast-enhanced magnetic resonance imaging of vascular changes induced by sunitinib in papillary renal cell carcinoma xenograft tumors. Neoplasia. 2009;11:910–920. doi: 10.1593/neo.09618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillman GG, Singh-Gupta V, Al-Bashir AK, Zhang H, Yunker CK, Patel AD, Sethi S, Abrams J, Haacke EM. Dynamic contrast-enhanced magnetic resonance imaging of sunitinib-induced vascular changes to schedule chemotherapy in renal cell carcinoma xenograft tumors. Transl Oncol. 2010;3:293–306. doi: 10.1593/tlo.10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 14.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–478. [PubMed] [Google Scholar]
- 15.Kelly RJ, Rixe O. Axitinib—a selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Target Oncol. 2009;4:297–305. doi: 10.1007/s11523-009-0126-9. [DOI] [PubMed] [Google Scholar]
- 16.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 17.Hillman GG, Wang Y, Che M, Raffoul JJ, Yudelev M, Kucuk O, Sarkar FH. Progression of renal cell carcinoma is inhibited by genistein and radiation in an orthotopic model. BMC Cancer. 2007;7:4. doi: 10.1186/1471-2407-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang D, Ding Y, Li Y, Luo WM, Zhang ZF, Snider J, Vandenbeldt K, Qian CN, Teh BT. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70:1053–1062. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and plateletderived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 20.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;9:1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 21.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 22.Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M, Joiner MC, Marples B, Forman JD, Sarkar FH. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3:1271–1279. [PubMed] [Google Scholar]
- 23.Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-κB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raffoul JJ, Banerjee S, Singh-Gupta V, Knoll ZE, Fite A, Zhang H, Abrams J, Sarkar FH, Hillman GG. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67:2141–2149. doi: 10.1158/0008-5472.CAN-06-2147. [DOI] [PubMed] [Google Scholar]
- 25.Raffoul JJ, Banerjee S, Che M, Knoll ZE, Doerge DR, Abrams J, Kucuk O, Sarkar FH, Hillman GG. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007;120:2491–2498. doi: 10.1002/ijc.22548. [DOI] [PubMed] [Google Scholar]
- 26.Singh-Gupta V, Zhang H, Banerjee S, Kong D, Raffoul JJ, Sarkar FH, Hillman GG. Radiation-induced HIF-1α cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int J Cancer. 2009;124:1675–1684. doi: 10.1002/ijc.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh-Gupta V, Zhang H, Yunker CK, Ahmad Z, Zwier D, Sarkar FH, Hillman GG. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: potentiation of radiotherapy. Pharm Res. 2010;27:1115–1127. doi: 10.1007/s11095-010-0107-9. [DOI] [PubMed] [Google Scholar]
- 28.Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. 2006;89:1121–1134. [PubMed] [Google Scholar]
- 29.Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, Cher ML, Andic F, Rossi PJ, Kucuk O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62:996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 31.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 32.Rimbach G, Boesch-Saadatmandi C, Frank J, Fuchs D, Wenzel U, Daniel H, Hall WL, Weinberg PD. Dietary isoflavones in the prevention of cardiovascular disease—a molecular perspective. Food Chem Toxicol. 2008;46:1308–1319. doi: 10.1016/j.fct.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Griffin RJ, Williams BW, Wild R, Cherrington JM, Park H, Song CW. Simultaneous inhibition of the receptor kinase activity of vascular endothelial, fibroblast, and platelet-derived growth factors suppresses tumor growth and enhances tumor radiation response. Cancer Res. 2002;62:1702–1706. [PubMed] [Google Scholar]
- 34.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957–1971. [PubMed] [Google Scholar]
- 35.Dicker AP, Williams TL, Grant DS. Targeting angiogenic processes by combination rofecoxib and ionizing radiation. Am J Clin Oncol. 2001;24:438–442. doi: 10.1097/00000421-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Kozin SV, Boucher Y, Hicklin DJ, Bohlen P, Jain RK, Suit HD. Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res. 2001;61:39–44. [PubMed] [Google Scholar]