Abstract
Objectives
To date a number of studies have examined the association between maternal weight and testicular cancer risk although results have been largely inconsistent. This systematic review and meta-analysis investigated the nature of this association.
Methods
Search strategies were conducted in Ovid Medline (1950—2009), Embase (1980—2009), Web of Science (1970—2009), and CINAHL (1937—2009) using keywords for maternal weight (BMI) and testicular cancer.
Results
The literature search produced 1,689 hits from which 63 papers were extracted. Only 7 studies met the pre-defined criteria. Random effects meta-analyses were conducted. The combined unadjusted OR (95% CI) of testicular cancer in the highest reported category of maternal BMI compared with the moderate maternal BMI was 0.82 (0.65 – 1.02). The Cochran’s Q P value was 0.83 and the corresponding I2 was 0%, both indicating very little variability among studies. The combined unadjusted OR (95% CI) for testicular cancer risk in the lowest reported category of maternal BMI compared to a moderate maternal BMI category was 0.92 (0.75 – 1.12). The Cochran’s Q P value was 0.05 and the corresponding I2 was 54%, indicating evidence of statistical heterogeneity. No association was observed when maternal BMI was treated as a continuous variable.
Conclusion
This meta-analysis, which included a small number of studies, showed an inverse association between high maternal BMI and testicular cancer risk of borderline statistical significance. Further primary studies with adjustment for appropriate confounders are required.
INTRODUCTION
In the United Kingdom, 2,109 new cases of testicular cancer were diagnosed in 2005 (Office for National Statistics; ISD Online; Welsh Cancer Intelligence; NICR 2008). With the introduction of combination chemotherapy in the 1970s, survival rates for testicular cancer have increased each year and the most recent population-based five-year survival rate for all patients registered in England and Wales was 98% (Coleman et al, 2004).
Testicular cancer has several distinct epidemiological features compared with other cancers. Firstly, it has an unusual age-distribution, occurring most commonly in young and middle-aged men. Secondly, for reasons as yet unknown, its incidence is rising, particularly in white Caucasian populations throughout the world (Horwich et al, 2006). Ninety-five percent of testicular tumours are germ-cell tumours (TGCTs), of which approximately 40–45% are seminomas and a similar percentage are nonseminomas (Bray, 2006). Nonseminomas tend to occur on average ten years earlier than seminomas and the incidence of nonseminomas peaks in the 20–35 age group while the incidence of seminomas peaks in the 25–45 age group.
The causes of testicular cancer are unclear, but both genetic and environmental factors most likely play a part. Established risk factors for TGCT include cryptorchidism, atrophy, inguinal hernia and infertility (Storgaard et al, 2006; Horwich et al, 2006). It is thought that TGCTs are initiated during foetal development, most likely in the first trimester, and progress to invasive cancer under the influence of adult hormones (Horwich et al, 2006; Oosterhuis et al, 2003). Therefore, several studies have investigated prenatal and perinatal exposures in relation to TGCT risk, although most of these analyses have included only a small number of cases. Research has also focused on maternal factors which could influence foetal development and it has been suggested that carcinoma in situ of the testis, a precursor of TGCT, has its origins in foetal life (Rorth et al, 2000) and that subnormal androgen exposure and/or increased oestrogen exposure are potentially important risk factors (Skakkebaek et al, 2001). Maternal weight influences the intrauterine hormonal milieu and, as obesity has been increasing in both males and females in western populations in recent decades, it is possible that trends in maternal weight might account for at least part of the increase in testicular cancer incidence seen in these countries (Godfrey et al, 2001). The aim of this study was to synthesise the evidence base for a relationship between maternal weight and testicular cancer risk in male offspring by systematic review and meta-analysis of the existing literature.
MATERIALS AND METHODS
Search Strategy
An electronic literature search was conducted using Ovid Medline (US National Library of Medicine, Bethesda, MD, USA)(1950—2009), Embase (Reed Elsevier PLC, Amsterdam, The Netherlands)(1980—2009), Web of Science (Thompson Reuters – New York, NY, USA)(1970—2009), and CINAHL (EBSCO Publishing, Ipswich, MA, USA)(1937—2009) on 13th March 2009. The search included the following keywords or medical subject heading (MeSH) terms, cancer of the testi(s), testi(s) cancer(s), testi(s) neoplasm(s), testicular neoplasm(s), testi(s) tumo(u)r, seminoma(s), testi(s) teratocarcinoma, or testicular germ cell tumo(u)r, and maternal weight, overweight, obesity, pregnancy, body mass index, BMI, adiposity, central adiposity, body composition, body fat, fat distribution, overweight mothers, obese mothers, maternal obesity, body weight, waist circumference, waist-hip-ratio, and WHR.
Selection Criteria
The titles and abstracts of identified articles were screened by three reviewers (SSA, MMC, LJM) to exclude those that were clearly irrelevant. The full text articles of potentially relevant studies were obtained and independently examined by two reviewers (SSA, MMC) to determine whether they met the criteria for inclusion in the review. To be included, observational studies (case-control or cohort) with testicular cancer as an outcome had to include an estimate of the association (odds ratio or relative risk) between maternal body mass index (BMI) or provide data from which this estimate could be calculated. Review publication types were removed but no language restriction was specified. The reference lists of all included articles were also examined to identify any other relevant studies that may have been missed.
Data extraction
From each article two reviewers (SSA, MMC) extracted the following information: year of publication, study design and location, sample size, case definitions, population demographics, exclusion criteria, time of BMI measurement in relation to relevant pregnancy, adjustments for confounders, and results from each study. The reviewers applied the Newcastle-Ottawa Quality Assessment Scale (NOS) (http://www.lri.ca) to all studies.
Statistical Analysis
The association between testicular cancer risk in sons and maternal BMI was summarised by recalculating an unadjusted odds ratio (OR) and standard error (SE) for testicular cancer in sons in the highest and lowest categories of maternal BMI compared to a moderate category. Unadjusted values for ORs and SE were calculated as no uniformity was observed among adjusted confounders within each study and also because the categories used to classify maternal BMI differed among studies – the reference (moderate) BMI category also varied among studies, consequently we recalculated the unadjusted ORs so that a similar reference category was used for all studies. The BMI data was stratified into three categories for each study as slightly different thresholds were used.
Random effects models were used to calculate pooled ORs. ORs with 95% confidence intervals were combined and then weighted to produce a pooled estimate. The I2 statistic estimates between-study heterogeneity that is not due to chance. An I2 value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity (Higgins et al, 2002). Begg’s funnel plots were produced and Egger’s test (Egger, 1997) was conducted to inspect potential small study bias. Further sensitivity analyses were conducted, whereby each study is omitted in turn. A summary OR per unit BMI (kg/m2) was calculated assuming a normal distribution across categories for each study (Greenland et al, 1992). Statistical analysis was conducted using Intercooled STATA (version 9.2, College Station, TX, USA).
RESULTS
Search Results
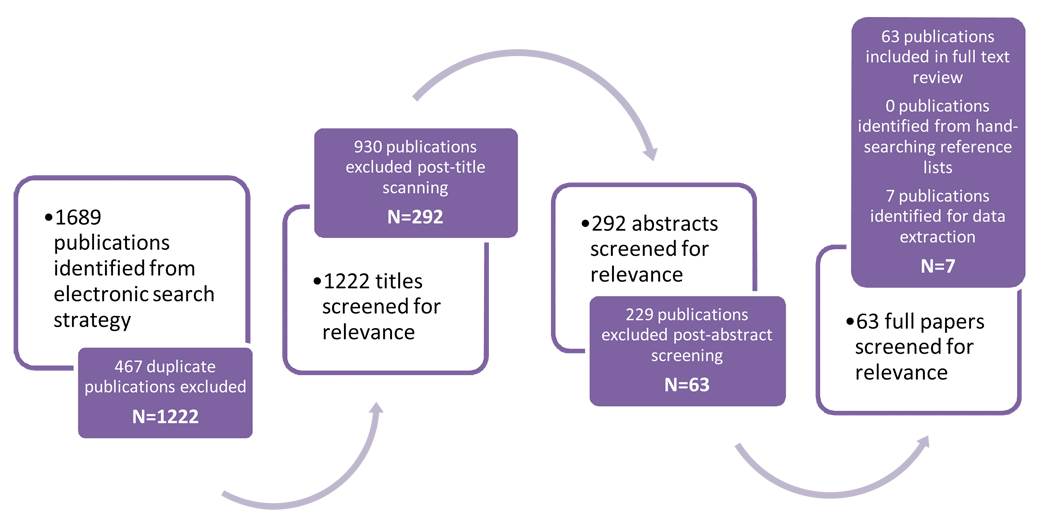
The search strategy results are shown in Figure 1. Seven studies met the pre-defined criteria (Pettersson et al, 2008; Sonke et al, 2007; Coupland et al, 2004; Weir et al, 2000; Moller et al, 1997; Mori et al, 1990; Depue et al, 1983). All seven studies were case-control studies, five population-based and two hospital-based. These seven studies included over 2,400 cases and 3,500 controls. Three studies were conducted in Europe (Sweden, Denmark, and United Kingdom), three in North America (Canada and United States) and one in Asia (Japan). All studies employed either self-reported or interviewer-administered questionnaires for the men diagnosed with testicular cancer and the controls.
Figure 1.
A flow diagram of study selection for maternal BMI and testicular cancer risk in sons.
One study (Moller et al, 1996) provided only three pre-pregnancy weight categories, which were used as substitutes for BMI categories (low [<55 kg], moderate [55–59 kg], high [>60 kg]), another study (Depue et al, 1983) provided three BMI categories (<19, 19–21, 22+), but the data were stratified into two categories for the purpose of this systematic review (low [<19] and moderate [>19]). A third study (Mori et al, 1988; Mori et al, 1990) involved a very small number of cases (37) and controls (37) and only provided two categories of BMI (high [>25] and moderate [<25]).
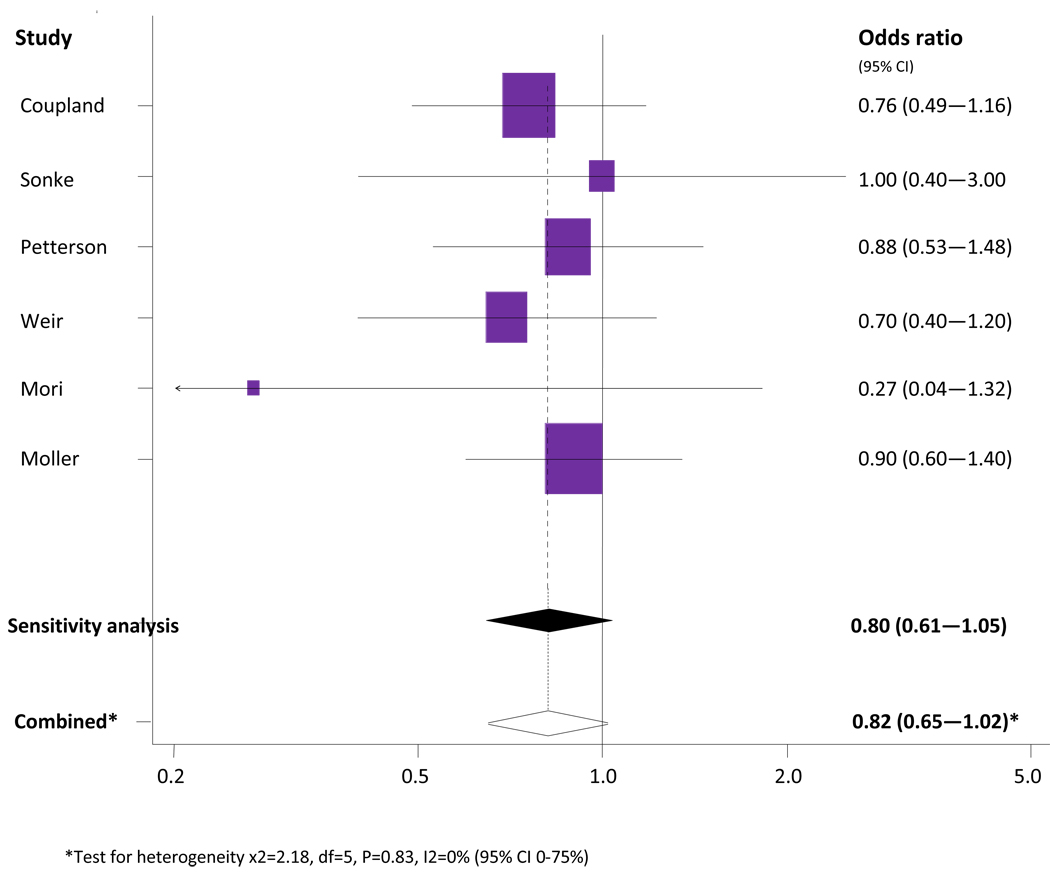
Meta-analysis of high maternal BMI versus moderate BMI
The association between high maternal BMI and testicular cancer risk in sons is shown in Figure 2. Six studies contributed data to the meta-analysis (Pettersson et al, 2008; Sonke et al, 2007; Coupland et al, 2004; Weir et al, 2000; Moller et al, 1997; Mori et al, 1990). The study by Depue et al. was not included in this meta-analysis as the re-categorised BMI data did not include a ‘high’ category.
Figure 2.
Meta-analysis of high maternal BMI versus moderate BMI and testicular cancer risk in sons.
The combined unadjusted OR (95% CI) of testicular cancer in the highest reported category of maternal BMI compared with the moderate maternal BMI was 0.82 (0.65 – 1.02). Cochran’s Q test had a P value of 0.83 and the corresponding I2 was 0%, both indicating very little variability among studies that cannot be explained by chance (Figure 2). The statistical homogeneity was not changed even when two case-control studies (Mori et al, 1988; Moller et al, 1997) were excluded in a separate analysis. The results from the study by Mori were excluded as it was a small case-control study (37 cases and 37 controls), receiving a NOS quality score of 5/9 and in comparison with western populations, BMI of the Japanese population is typically lower (Japanese adult mean BMI levels of 22–23 kg/m2) (WHO 2009). The Moller study was excluded as it only contained three weight categories used as proxy BMI categories. The sensitivity analysis produced a combined unadjusted OR of 0.80 (0.61 – 1.05) and Cochran’s Q test had a P value of 0.10 with an I2 of 0%.
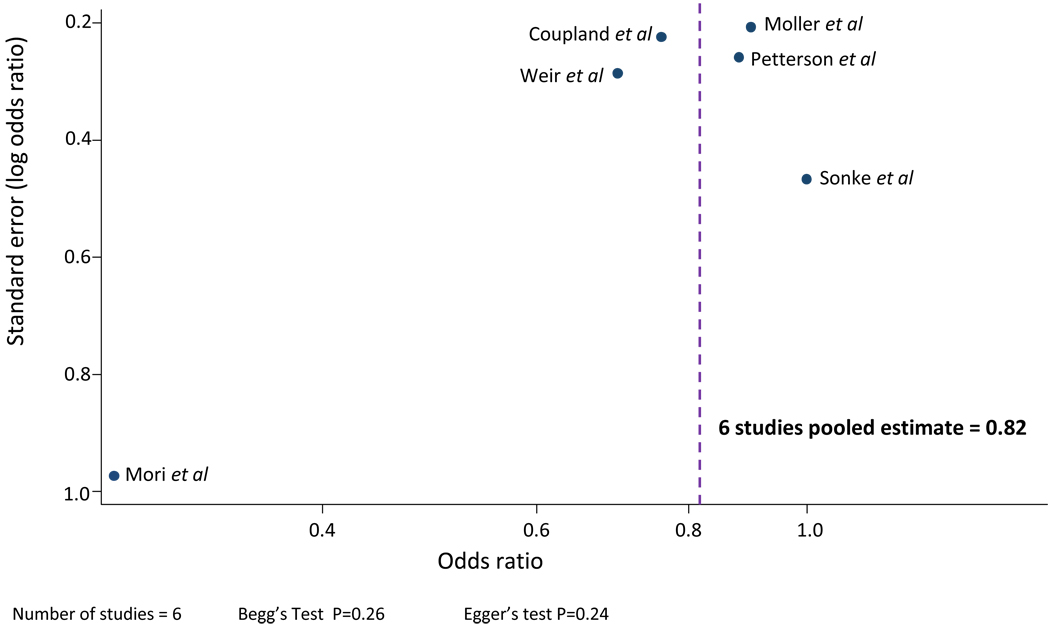
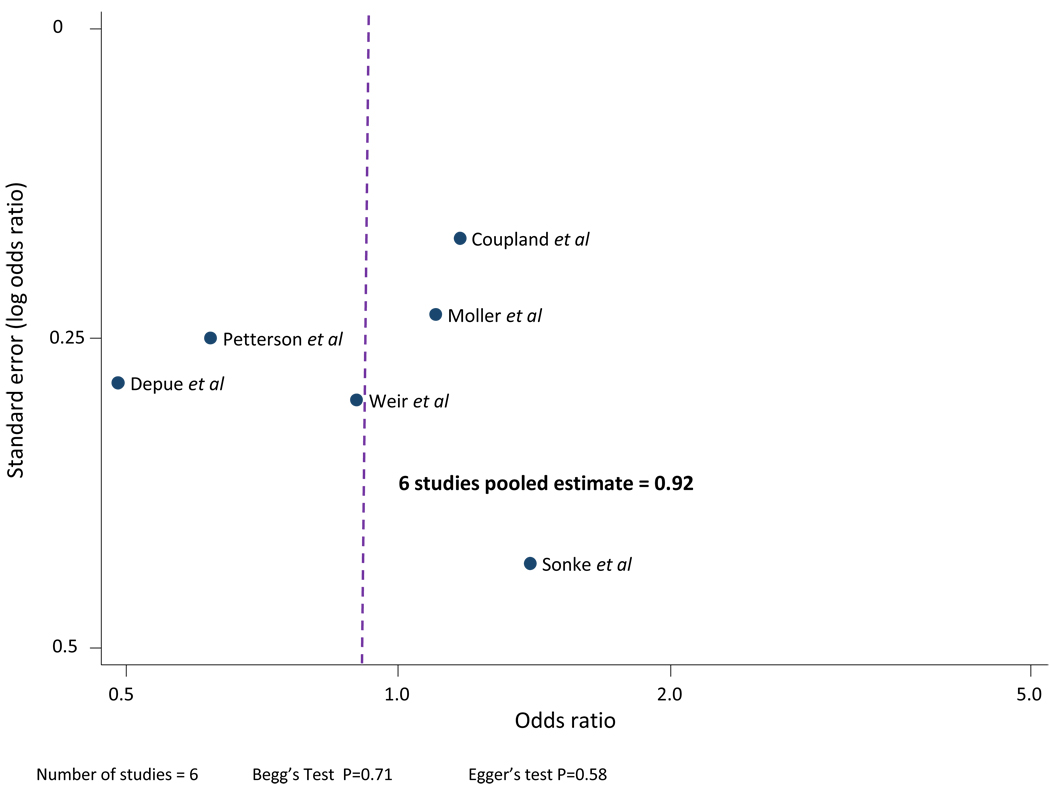
Publication bias is not present as the funnel plot appears symmetrical (Figure 3) and Begg’s and Egger’s tests were not statistically significant.
Figure 3.
Funnel plot: test for publication bias in studies comparing high maternal BMI to moderate maternal BMI.
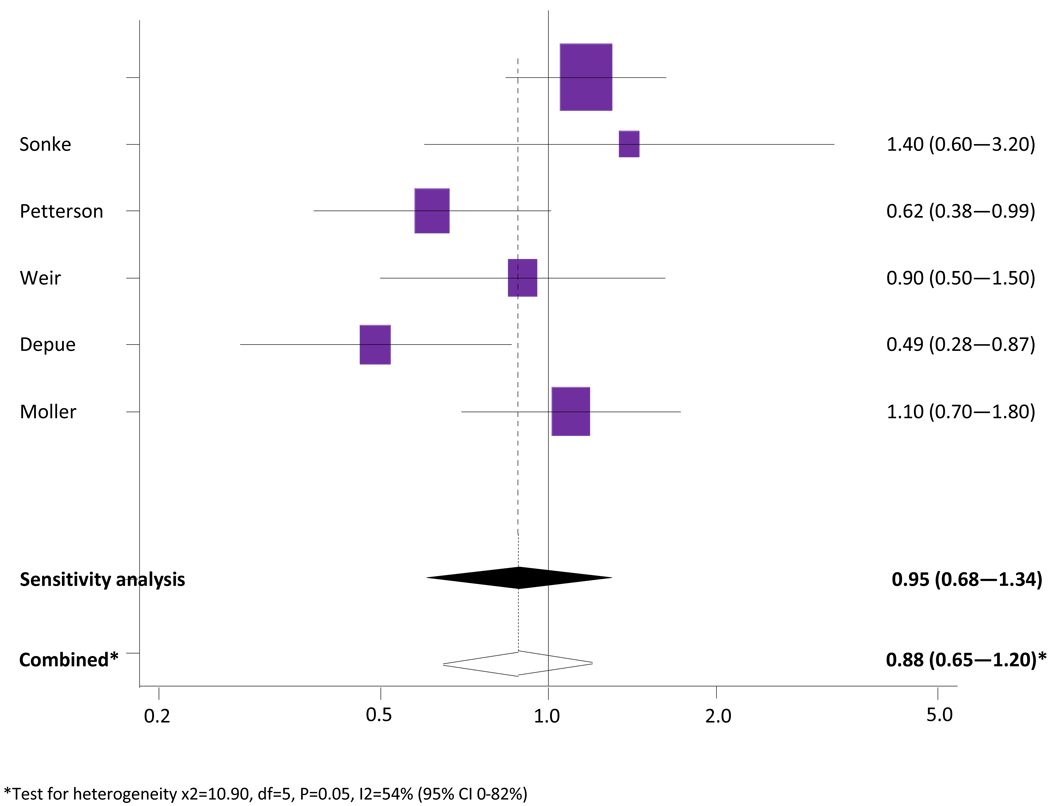
Meta-analysis of low maternal BMI versus moderate BMI
The association between low maternal BMI and testicular cancer risk in sons is shown in Figure 4. Six studies were included in the meta-analysis (Pettersson et al, 2008; Sonke et al, 2007; Coupland et al, 2004; Weir et al, 2000; Moller et al, 1997; Depue et al, 1983). One study (Mori et al, 1988; Mori et al, 1990) involved a very small number of cases (37) and controls (37) and provided two categories of BMI (high [>25] and moderate [<25]) and therefore was not included in this meta-analysis.
Figure 4.
Meta-analysis of low maternal BMI versus moderate BMI and testicular cancer risk in sons.
The combined unadjusted OR (95% CI) for testicular cancer in the lowest reported category of maternal BMI compared to a moderate maternal BMI was 0.92 (0.75 – 1.12). The Cochran’s Q test had a P value of 0.05 and the corresponding I2 was 54%, indicating evidence of statistical heterogeneity. The statistical heterogeneity was reduced when two case-control studies (Moller et al, 1997; Depue et al, 1983) were excluded in a separate analysis. The study by Moller was excluded as it only contained three weight categories used as proxy BMI categories. The study by Depue was excluded as it received a NOS quality score of 4/9. The sensitivity analysis produced a combined unadjusted OR of 0.95 (0.68 – 1.34) and Cochran’s Q test had a P value of 0.16 with an I2 of 42%.
Publication bias is not present as the funnel plot appears symmetrical (Figure 5) and Begg’s test and Egger’s tests were not statistically significant.
Figure 5.
Funnel plot: test for publication bias in studies comparing low maternal BMI to moderate maternal BMI.
Meta-analysis of testicular cancer risk per unit increase in maternal BMI
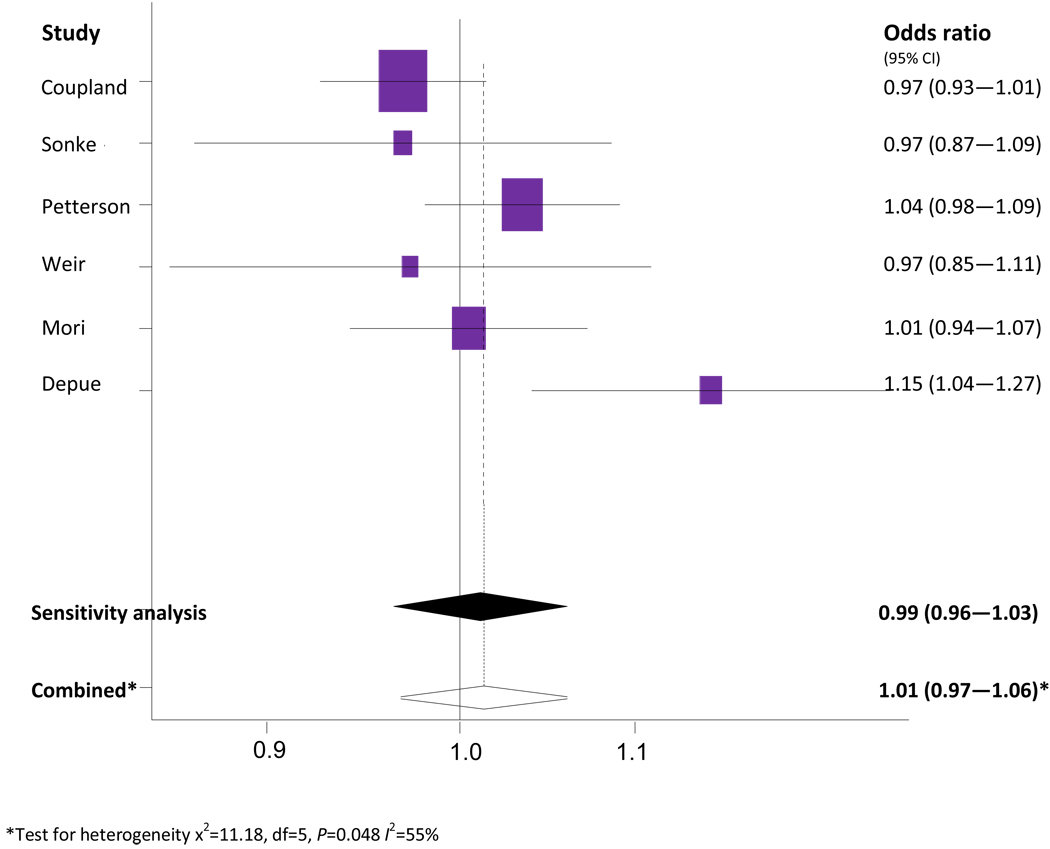
The odds of testicular cancer per unit increase in maternal BMI are shown in Figure 6. Six studies contributed to the analysis (Pettersson et al, 2008; Sonke et al, 2007; Coupland et al, 2004; Weir et al, 2000; Mori et al, 1990; Depue et al, 1983). The study by Moller et al (Moller et al, 1996) could not be included in this analysis because it reported weight and not BMI.
Figure 6.
Meta-analysis of testicular cancer risk per unit increase in maternal BMI.
The combined unadjusted OR (95% CI) of testicular cancer risk per unit increase in maternal BMI was 1.01 (0.97 – 1.06). The Cochran’s Q test had a P value of 0.048 and the corresponding I2 was 55% indicating evidence of statistical heterogeneity. The statistical heterogeneity was reduced when two case-control studies (Mori et al, 1988; Depue et al, 1983) were excluded in a separate analysis. The Mori study was excluded based on the small study size and difference in mean BMI as Asian countries tend to be lower than European and North American countries. The study by Depue was excluded as it received a NOS quality score of 4/9. The sensitivity analysis produced a combined unadjusted OR of 0.99 (0.96 – 1.03) and Cochran’s Q test had a P value of 0.30 with an I2 of 0%.
DISCUSSION
This is the first systematic review of the literature on maternal BMI and testicular cancer (TC) risk in male offspring. The pooled estimate was based on data from over 2,400 cases and 3,500 controls obtained from six case-control studies and indicates that high compared with normal maternal BMI is associated with a decrease in testicular cancer risk (OR = 0.82; 95% CI 0.65—1.02), although the results were only borderline statistically significant (p=?). There was no evidence to suggest an association for low compared with normal maternal BMI and TC risk in offspring.
Maternal BMI is positively associated with offspring birth weight (Hindmarsh et al, 2008) and high birth weight has recently been shown to be positively, not inversely, associated with testicular cancer risk; Ramlau-Hansen (2009) reported an incidence rate ratio of 1.6 (95% CI 1.0–2.4) for men born with a high birth weight (>4,150 g) compared to those of normal birth weight. However, the issue of birth weight and testicular cancer is contentious as different studies and differing meta-analyses have shown conflicting findings (Michos et al 2007; Cook et al 2007; Akre et al 2008). It would be cicumspect to conclude that an association between these two variables remains unsubstantiated, although no studies to date have shown that a high birth weight is associated with a reduced risk of testicular cancer and therefore the inverse association between maternal BMI and TC risk seen in this meta-analysis is unlikely to be mediated by birth weight.
A possible explanation for the inverse association between maternal BMI and TC risk in offspring in this meta-analysis could be confounding by parity, maternal age, or birth order. For example, it is known that multiparous women are more likely to be overweight than nulliparous women (Arroya et al, 1995; Brown et al, 1992) and that increased parity is associated with a decreased risk of TC in offspring compared with nulliparous women (Sabroe and Olsen, 1998; Wanderas et al, 1998; Westergaard et al, 1998; Prener et al, 1992). A number of studies have investigated the link between maternal age and TC risk in offspring (Ramlau-Hansen et al, 2009; Coupland et al, 2004; Dieckmann et al, 2001) and all have reported an inverse association. Several studies have also reported an inverse association between birth order and TC risk (Cook et al, 2009; Richiardi et al, 2004; Coupland et al, 2004; Sabroe and Olsen, 1998; Westergaard et al, 1998). In previous studies, maternal age and birth order have been interpreted mainly as proxies for foetal exposure to maternal hormones because higher levels of oestrogens have been measured during first compared with subsequent pregnancies (Bernstein et al, 1986; Panagiotopoulou et al, 1990; Key et al, 1996). According to the circulating oestrogen hypothesis (Sharpe et al 1993; Depue et al, 1983), increasing levels of exposure to endogenous oestrogen in foetal life increases the risk of testicular cancer. A review of published epidemiologic studies on male reproductive disorders and indicators of prenatal maternal oestrogens concluded that the studies support the hypothesis that higher prenatal oestrogen exposure is associated with testicular cancer (Storgaard et al, 2006). Confounding by parity, maternal age or birth order may therefore explain the apparent inverse association between maternal weight and testicular cancer risk seen in this meta-analysis.
The results of this study should be interpreted with caution as there are several limitations. Firstly, a small number of studies met the inclusion criterion; however the majority of studies included were of high quality. Secondly, studies included in the meta-analysis were also of case-control design and it is possible that the results of the individual studies have been affected by recall bias due to the retrospective recording of maternal BMI. Thirdly, measurement error may have also been an issue as mothers self-reported their weight and height (Stunkard et al, 1981; Palta et al, 1982). Another potential limitation is the pooling of unadjusted estimates in the meta-analyses. However pooling unadjusted estimates is the recommended method, in contrast to combining adjusted estimates or artificially adjusted estimates, where there is variability among adjusted factors within studies (Greenland et al, 1992).
CONCLUSION
This is the first meta-analytic review of maternal weight in relation to testicular cancer. The meta-analysis provides some evidence that higher pre-pregnancy maternal weight may be associated with a decrease in testicular cancer risk in male offspring.
Further larger epidemiological studies are required that differentiate between seminomas and non-seminomas, include better measures of maternal obesity (such as the waist hip ratio, weight gain during pregnancy etc.) and which examine the association between maternal BMI adjusted for important confounders such as birth weight, birth order, maternal age and parity.
Table 1.
Characteristics of studies included in systematic review of maternal BMI and risk of testicular cancer in sons
| Study-year- location |
Study design |
Cases & source |
Controls & source |
Matching cases/controls (Frequency or Individually) |
Maternal BMI assessment |
Newcastle-Ottawa Quality scale score (NOS) |
Moderate BMI group (kg/m2) |
|---|---|---|---|---|---|---|---|
| Pettersson et al (2008) Sweden | Population-based case-control | 293 Retrieved from Swedish Cancer Register | 861 First three men born at the same hospital after a case | No | Pre-pregnancy BMI Collected during antenatal period in Swedish Medical Birth Register | 8/9 | 20–24 |
| Sonke et al (2007) United States | Hospital-based case-control | 144 Retrieved from University Cancer Centre and Tumour Registry | 86 Cases nominated 4+ cancer- free friends that were of same race | No | Pre-pregnancy BMI Self-reported/ Interviewed on phone | 7/9 | 18.5–24.9 |
| Coupland et al (2004) United Kingdom | Population-based case-control | 794 Retrieved from major cancer treatment centres and regional cancer registries | 794 Selected from GP list where case registered | Individually-matched by age | Pregnancy BMI Self-reported/ collected from postal questionnaire | 7/9 | 20–25 |
| Weir et al (2000) Canada | Population-based case-control | 502 Retrieved from Ontario Cancer Registry | 975 Selected from Ontario census data | No | Pre-pregnancy BMI Self-reported/ interviewed on phone | 7/9 | 18–25 |
| Moller et al (1997) Denmark | Population-based case-control | 296 Retrieved from Danish Cancer Registry | 287 Selected from Danish Central Person Register | Frequency-matched by year of birth | Pre-pregnancy BMI Self-reported/ collected from postal questionnaire | 8/9 | 55–59 kg*** |
| Mori et al (1990) Japan | Hospital-based case-control | 37 Retrieved from urological ward records of nine hospitals | 37 Selected from address registers at five public health centres | Individually-matched by year of birth | Pre-pregnancy BMI Self-reported/ collected from postal questionnaire | 5/9 | <25 |
| Depue et al (1983) United States | Population-based case-control | 108 Retrieved from population-based cancer registry | 108 Selected from same neighbourhoods as cases | Individually-matched by year of birth and race | Pre-pregnancy BMI Self-reported/ interviewed on phone | 4/9 | ≥19 |
References
- Akre O, Ekbom A. Similar at a glance, but not the same. Int J Cancer. 2008;123(6):1480. doi: 10.1002/ijc.23669. [comment to Michos et al 2007 paper] [DOI] [PubMed] [Google Scholar]
- Arroyo P, Avila-Rosas H, Fernandez V, Casanueva E, Galvan D. Parity and the prevalence of overweight. Int J Gynaecol Obstet. 1995;48(3):269–272. doi: 10.1016/0020-7292(94)02284-6. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Depue RH, Ross RK, Judd HL, Pike MC, Henderson BE. Higher maternal levels of free estradiol in first compared to second pregnancy: early gestational differences. J Natl Cancer Inst. 1986;76:1035–1039. [PubMed] [Google Scholar]
- Bray F. Do Testicular Seminoma and Nonseminoma Share the Same Etiology? Evidence from an Age-Period-Cohort Analysis of Incidence Trends in Eight European Countries. Cancer Epidemiol Biomarkers Prev. 2006;15(4):652–658. doi: 10.1158/1055-9965.EPI-05-0565. [DOI] [PubMed] [Google Scholar]
- Brown JE, Kaye SA, Folsom AR. Parity-related weight change in women. Int J Obes Relat Metab Disord. 1992;16(9):627–631. [PubMed] [Google Scholar]
- Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337(19):1403. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- Brown LM, Pottern LM, Hoover RN. Prenatal and perinatal risk factors for testicular cancer. Cancer Res. 1986;46(9):4812–4816. [PubMed] [Google Scholar]
- Chia VM, Li Y, Goldin LR, Graubard BI, Greene MH, Korde L, Rubertone MV, Erickson RL, McGlynn KA. Risk of cancer in first- and second-degree relatives of testicular germ cell tumor cases and controls. Int J Cancer. 2009;124:952–957. doi: 10.1002/ijc.23971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Risk of childhood germ cell tumors in association with parental smoking and drinking. Cancer. 2005;103(5):1064–1071. doi: 10.1002/cncr.20894. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Rachet B, Woods LM, Mitry E, Riga M, Cooper N, Quinn MJ, Brenner H, Estève J. Trends in socioeconomic inequalities in cancer survival in England and Wales up to 2001. Brit J Cancer. 2004;90(7):1367–1373. doi: 10.1038/sj.bjc.6601696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MB, Richiardi L, McGlynn KA. Birth weight and risk of testicular cancer [Letter to Editor] Int J Cancer. 2007;122(4):957. doi: 10.1002/ijc.23129. [DOI] [PubMed] [Google Scholar]
- Coupland CA, Forman D, Chilvers CE, Davey G, Pike MC, Oliver RT. Maternal risk factors for testicular cancer: a population-based case-control study (UK) Cancer Cause Control. 2004;15(3):277–283. doi: 10.1023/B:CACO.0000024257.49409.1f. [DOI] [PubMed] [Google Scholar]
- Depue RH, Pike MC. Estrogen exposure during gestation and risk of testicular cancer. J Natl Cancer I. 1983;71(6):1151–1155. [PubMed] [Google Scholar]
- Egger M, Davey SG, Schneider M. Bias in meta-analysis detected by simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavaghan DJ, Moore AR, McQay HJ. An evaluation of homogeneity tests in meta-analysis in pain using simulations of patient data. Pain. 2000;85:415–424. doi: 10.1016/S0304-3959(99)00302-4. [DOI] [PubMed] [Google Scholar]
- Giwercman A, Grindsted J, Hansen B, Jensen OM, Skakkebaek NE. Testicular cancer risk in boys with maldescended testis: a cohort study. J Urol. 1987;138:1214–1216. doi: 10.1016/s0022-5347(17)43553-1. [DOI] [PubMed] [Google Scholar]
- Greenland S, Longnecker M. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ. Fetal Programming and Adult Health. Public Health Nutr. 2001;4(2B):611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- Hasle H, Mellemgaard A, Nielsen J, Hansen J. Cancer incidence in men with Klinefelter syndrome. Br J Cancer. 1995;71:416–420. doi: 10.1038/bjc.1995.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BE, Benton B. Risk factors for cancer of the testis in young men. Int J Cancer. 1979;23(5):598–602. doi: 10.1002/ijc.2910230503. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hindmarsh PC, Geary MP, Rodeck CH, Kingdom JC, Cole TJ. Factors predicting ante- and postnatal growth. Pediatr Res. 2008;63:99–102. doi: 10.1203/PDR.0b013e31815b8e8f. [DOI] [PubMed] [Google Scholar]
- Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367(9512):754–765. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- ISD Online Information and Statistics Division. Scotland: NHS; 2008. [Google Scholar]
- Jensen MS, Toft G, Thulstrup AM, Bonde JP, Olsen J. Cryptorchidism according to maternal gestational smoking. Epidemiology. 2007;18:220–225. doi: 10.1097/01.ede.0000254061.90686.9f. [DOI] [PubMed] [Google Scholar]
- Kaijser M. Maternal lung cancer and testicular cancer risk in the offspring. Cancer Epidemiol Biomarkers Prev. 2003;12(7):643–646. [PubMed] [Google Scholar]
- Key T, Bull D, Ansell P, Brett A, Clark G, Moore J, Chilvers C, Pike MC. A case-control study of cryptorchidism and matemal hormone concentrations in early pregnancy. Br J Cancer. 1996;73:698–701. doi: 10.1038/bjc.1996.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Am J Obstet Gynecol. 2001;185:845–849. doi: 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- Lutke Holzik MF, Rapley EA, Hoekstra HJ, Sleijfer DT, Nolte IM, Sijmons RH. Genetic predisposition to testicular germ-cell tumours. Lancet Oncol. 2004;5(6):363–371. doi: 10.1016/S1470-2045(04)01493-7. [DOI] [PubMed] [Google Scholar]
- McCarthy A, Hughes R, Tilling K, Davies D, Smith GD, Ben-Shlomo Y. Birth weight, postnatal, infant and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly growth study. Am J Clin Nutr. 2007;86:907–913. doi: 10.1093/ajcn/86.4.907. [DOI] [PubMed] [Google Scholar]
- Michos A. Birth weight and the risk of testicular cancer: A meta-analysis. Int J Cancer. 2007;121:1123–1131. doi: 10.1002/ijc.22771. [DOI] [PubMed] [Google Scholar]
- Moller H, Skakkebaek NE. Risks of testicular cancer and cryptorchidism in relation to socio-economic status and related factors: case-control studies in Denmark. Int J Cancer. 1996;66(3):287–293. doi: 10.1002/(SICI)1097-0215(19960503)66:3<287::AID-IJC2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Moller H, Skakkebaek NE. Testicular cancer and cryptorchidism in relation to prenatal factors: case-control studies in Denmark. Cancer Cause Control. 1997;8(6):904–912. doi: 10.1023/a:1018472530653. [DOI] [PubMed] [Google Scholar]
- Mori M, Davies TW. Maternal factors of testicular cancer: a case-control study in Japan. Jpn J Clin Oncol. 1990;20(1):72–77. [PubMed] [Google Scholar]
- Mori M, Miyake H. A case-control study of testicular cancer. Hinyokika Kiyo - Acta Urol Jpn. 1988;34(5):832–838. [PubMed] [Google Scholar]
- Moss AR, Osmond D. Hormonal risk factors in testicular cancer. A case-control study. Am J Epidemiol. 1986;124(1):39–52. doi: 10.1093/oxfordjournals.aje.a114369. [DOI] [PubMed] [Google Scholar]
- Cancer Incidence and Mortality in Northern Ireland; Northern Ireland Cancer Registry. 2008
- Office for National Statistics, Cancer Statistics registrations: Registrations of cancer diagnosed in 2005, England. London: National Statistics; 2008 Series MB1 no.35.
- Oosterhuis JW, Looijenga LH. Current views on the pathogenesis of testicular germ cell tumours and perspectives for future research: highlights of the 5th Copenhagen Workshop on Carcinoma in situ and Cancer of the Testis. Apmis. 2003;111(1):280–289. doi: 10.1034/j.1600-0463.2003.1110131.x. [DOI] [PubMed] [Google Scholar]
- Panagiotopoulou K, Katsouyanni K, Petridou E, Garas Y, Tzonou A, Trichopoulos D. Matemal age, parity, and pregnancy estrogens. Cancer Causes Control. 1990;1:119–124. doi: 10.1007/BF00053162. [DOI] [PubMed] [Google Scholar]
- Pettersson A. Women smoking and testicular cancer: One epidemic causing another? Int J Cancer. 2004;109(6):941–944. doi: 10.1002/ijc.20088. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Richiardi L. Gestational hypertension, preeclampsia, and risk of testicular cancer. Cancer Res. 2008;68(21):8832–8836. doi: 10.1158/0008-5472.CAN-08-2309. [DOI] [PubMed] [Google Scholar]
- Prener A, Hsieh CC, Engholm G, Trichopoulos D, Jensen O. Birth order and risk of testicular cancer. Cancer Causes Control. 1992;3:265–272. doi: 10.1007/BF00124260. [DOI] [PubMed] [Google Scholar]
- Ramlau–Hansen CH, Olesen AV, Parner ET, Sørensen HT, Olsen J. Perinatal markers of estrogen exposure and risk of testicular cancer: follow-up of 1,333,873 Danish males born between 1950 and 2002. Cancer Causes Control. 2009;20(9):1587–1592. doi: 10.1007/s10552-009-9403-2. [DOI] [PubMed] [Google Scholar]
- Richiardi L. Body Size at Birth and Adulthood and the Risk for Germ-cell Testicular Cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(7):669–673. [PubMed] [Google Scholar]
- Rorth M, Rajpert-De Meyts E, Andersson L, Dieckmann KP, Fossa SD, Grigor KM, Hendry WF, Herr HW, Looijenga LH, Oosterhuis JW, Skakkebaek NE. Carcinoma in situ in the testis. Scand J Urol Nephrol Suppl. 2000;205:166–186. doi: 10.1080/00365590050509896. [DOI] [PubMed] [Google Scholar]
- Sabroe S, Olsen J. Perinatal correlates of specific histological types of testicular cancer in patients below 35 years of age: a case-cohort study based on midwives' records in Denmark. Int J Cancer. 1998;78:140–143. doi: 10.1002/(sici)1097-0215(19981005)78:2<140::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. The 'oestrogen hypothesis' – where do we stand now? Int J Androl. 2003;26:2–15. doi: 10.1046/j.1365-2605.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Sonke GS, Chang S. Prenatal and perinatal risk factors and testicular cancer: a hospital-based case-control study. Oncology Research. 2007;16(8):383–387. doi: 10.3727/000000006783980928. [DOI] [PubMed] [Google Scholar]
- Storgaard L, Bonde JP. Male reproductive disorders in humans and prenatal indicators of estrogen exposure. A review of published epidemiological studies. Reprod Toxicol. 2006;21(1):4–15. doi: 10.1016/j.reprotox.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720–726. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Pike MC. Risk of testicular cancer in cohort of boys with cryptorchidism. BMJ. 1997;314:1507–1511. doi: 10.1136/bmj.314.7093.1507. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ, De Stavola BL, Swanwick MA, Maconochie NE. Risks of breast and testicular cancers in young adult twins in England and Wales: evidence on prenatal and genetic aetiology. Lancet. 1997;350:1723–1728. doi: 10.1016/s0140-6736(97)05526-8. (b) [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, De Stavola BL, Swanwick MA, Mangtani P, Maconochie NE. Risk factors for testicular cancer: a case-control study in twins. Br J Cancer. 1999;80:1098–1102. doi: 10.1038/sj.bjc.6690470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir HK, Marrett LD. Pre-natal and peri-natal exposures and risk of testicular germ-cell cancer. International Journal of Cancer. 2000;87(3):438–443. doi: 10.1002/1097-0215(20000801)87:3<438::aid-ijc20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Westergaard T, Andersen PK, Pedersen JB, Frisch M, Olsen JH, Melbye M. Testicular cancer risk and maternal parity: a population-based cohort study. Br J Cancer. 1998;77:1180–1185. doi: 10.1038/bjc.1998.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanderas EH, Grotmol T, Fossa SD. Maternal health and pre- and perinatal characteristics in the etiology of testicular cancer: a prospective population- and register-based study on Norwegian males born between 1967 and 1995. Cancer Causes Control. 1998;9:475–486. doi: 10.1023/a:1008857702380. [DOI] [PubMed] [Google Scholar]
- Welsh Cancer Intelligence and Surveillance Unit. Cancer Incidence in Wales; 2008. [Google Scholar]
- [Accessed 15 June 2009];Geneva: World Health Organization; WHO Chronic disease information sheets: Obesity and overweight. 2009 http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/index.html.
- Winkvist A, Stenlund H, Hakimi M, Nurdiati D, Dibley M. Weight – gain patterns from pre-pregnancy until delivery among women in central Java, Indonesia. Am J Clin Nutr. 2002;75:1072–1077. doi: 10.1093/ajcn/75.6.1072. [DOI] [PubMed] [Google Scholar]