Abstract
Cocaine achieves its psychostimulant, reinforcing properties through selectively blocking dopamine transporters, and this neurobiological mechanism impedes the use of classical receptor-antagonist pharmacotherapies to outcompete cocaine at CNS sites. Passive immunization with monoclonal antibodies (mAb) specific for cocaine circumvents this problem as drug is sequestered in the periphery prior to entry into the brain. To optimize an immunopharmacotherapeutic strategy for reversing severe cocaine toxicity, the therapeutic properties of mAb GNC92H2 IgG were compared to those of its engineered formats in a mouse overdose model. Whereas the extended half-life of an IgG justifies its application to the prophylactic treatment of addiction, the rapid, thorough biodistribution of mAb-based fragments, including F(ab')2, Fab and scFv, may correlate to accelerated scavenging of cocaine and reversal of toxicity. To test this hypothesis, mice were administered the anti-cocaine IgG (180 mg/kg, i.v.) or GNC92H2-based agent after receiving an LD50 cocaine dose (93 mg/kg, i.p.), and the timeline of overdose symptoms was recorded. All formats lowered the rate of lethality despite the >100-fold molar excess of drug to antibody binding capacity. However, only F(ab')2-92H2 and Fab-92H2 significantly attenuated the progression of premorbid behaviors, and Fab-92H2 prevented seizure generation in a percentage of mice. The calculation of serum half-life of each format demonstrated that the pharmacokinetic profile of Fab-92H2 (elimination half-life, t1/2 ∼ 100 minutes) best approximated that of cocaine. These results not only confirm the importance of highly specific and tight drug binding by the mAb, but also highlight the benefit of aligning the pharmacokinetic and pharmacodynamic properties of the immunopharmacotherapeutic with the targeted drug.
Keywords: Cocaine, Drug Overdose, Immunopharmacotherapy, Vaccine, Passive Immunization, Monoclonal Antibody, Mice
1. Introduction1
Cocaine attenuates the clearance of dopamine from synapses through binding the dopamine transporter (Ritz et al., 1987). The net increase in dopaminergic transmission conveys the elevated pleasure or reward response, which explains the risk of long-term addiction as well as acute overdose (Withers et al., 1995). Despite a thorough understanding of the neurochemical basis for cocaine's physiological effects, an effective pharmacotherapy to treat cocaine addiction or overdose has yet to successfully advance through clinical trials (Karila et al., 2008). Currently prescribed therapeutics, which include small molecule modulators of dopaminergic signaling, non/specific monoamine reuptake inhibitors, N-methyl D-aspartate receptor antagonists/partial antagonists/blockers, σ receptor antagonists, ATP-sensitive potassium (KATP) channel openers, and specific classes of GABA receptor modulators, target the same neurocircuitry affected by cocaine rather than the drug molecule itself (Maurice et al., 2002; Reyes et al., 2007; Reyes et al., 2005; Sharkey et al., 1988). As a result, these pharmacotherapies come with an array of side-effects and oftentimes are unable to counteract all symptoms of cocaine use. By contrast, anti-cocaine active and passive vaccines suppress or eliminate the drug high without engaging the ‘middleman’, or the neurocircuitry activated during the drug high (Carrera et al., 1995; Carrera et al., 2001; Carrera et al., 2000; Carrera et al., 2005; Fox et al., 1996; Kantak et al., 2000; Mets et al., 1998; Norman et al., 2009; Norman et al., 2007; Redwan et al., 2003).
At physiological pH, the lipid solubility of cocaine ensures its prompt tissue penetration and the rapid development of its neuropharmacological effects; in humans, the initial, euphoric rush of a cocaine high stems from the rate at which blood cocaine levels spike and drive cocaine entry into the brain rather than the sheer magnitude of the brain cocaine content (Hearn, 1997). In mice, the widespread biodistribution of cocaine is achieved within minutes upon i.v. administration (distribution half-life, t1/2α = 1.9 min; elimination half-life, t1/2β = 26.1 min; volume of distribution at steady state, Vdss = 6.0 L/kg) (Benuck et al., 1987; Norman et al., 2007). Intraperitoneal injection grants 71% drug bioavailability, with the peak brain concentration attained within 5-15 minutes, and at an LD50 dose, overdose symptoms such as seizures appear within 3.4 min (Azar et al., 1998; Benuck et al., 1987; Carrera et al., 2005). It follows that an immunotherapeutic agent designed for overdose rescue must both function with comparable pharmacokinetics to the targeted drug (i.e. cocaine) and possess high drug affinity in order to interrupt cocaine influx into the CNS. With respect to the latter, mAb GNC92H2 (Kd = 2 nM; by KinExA (Ohmura et al., 2001) and mAb 2E2 (Kd = 4 nM (Paula et al., 2004)) already possess favorable binding kinetics, and thus further refinement of the mAb binding site is not anticipated to substantially improve the therapeutic utility of GNC92H2 for the indication of overdose reversal. With respect to the former, a few studies have explored the therapeutic benefit of short-acting monovalent antibody fragments over the whole IgG for rapid small-molecule detoxification (e.g. exogenous toxins, pharmaceutical and abused drugs) (Covell et al., 1986; Eddleston and Persson, 2003; Holliger and Hudson, 2005; Ochs and Smith, 1977). Of particular relevance, a comparison between digoxin-specific IgG and Fab fragments in the treatment of acute digoxin toxicity depicted the more rapid and less variable reversal of digoxin-induced cardiac arrhythmias and lethality by Fab infusion than by the equivalent dose of IgG (Lloyd and Smith, 1978). While Fab treatment promoted rapid urinary excretion of digoxin after an initial 10-fold spike in serum digoxin levels, IgG administration mediated a protracted increase in serum digoxin levels (∼50-fold) that appeared to slow digoxin metabolism and urinary exretion (Butler et al., 1977). This trend was observed for mAb-based treatment of acute toxicity from digitoxin, a structurally related cardiac glycoside that, unlike digoxin, displays high serum albumin binding and slower elimination via the liver (Ochs and Smith, 1977). From these studies, it was postulated that IgG-based therapies caused the increased sequestration of these small-molecule toxins in tissue stores, and that this event might subsequently lead to the gradual and potentially hazardous release of toxin back into circulation. In contrast, the rapid urinary excretion of protein-bound digitoxin under Fab treatment appeared to counteract the slow redistribution of toxin between brain, blood flow and tissue stores.
The aforementioned studies endorsed the use of Fab over IgG for immediate detoxification involving small-molecule toxins and, by extension, abused drugs. As a basic pharmacological principal, the free drug concentration in serum drives its biodistribution through different compartments (e.g. brain, blood, tissue). It has been established that the administration of Fab or IgG at an approximate mole-equivalent dose to the steady state drug concentration (Css) triggers serum drug levels to spike 10- to 100-fold above control levels, respectively (Butler et al., 1977; Valentine et al., 1994). In addition, while the serum drug concentration is equally partitioned between protein-bound and unbound states before mAb administration, both IgG and Fab mediate a dramatic shift in this equilibrium to favor nearly 100% drug-protein binding in serum. The disappearance of free drug in circulation upon immunopharmacotherapeutic treatment encourages the exodus of drug from brain and tissue stores. Interestingly, despite the significant drop in brain drug levels, the concentration of free drug in serum does not necessarily increase proportionately. In the case of PCP exposure, it was hypothesized that PCP metabolism may shift from nonrestrictive to restrictive-type metabolism after immunopharmacotherapeutic treatment so that free drug re-entering circulation is immediately cleared. The uncertain and pharmacologically complex effects of immunopharmacotherapy on drug disposition has not hindered the development and study of mAb-based medications for a varied array of exogenous small-molecules, including cardiac glycosides (e.g., digoxin (Butler et al., 1977), digitoxin (Ochs and Smith, 1977)), pharmaceutical drugs (e.g., desipramine (Lin et al., 1996; Pentel and Keyler, 1995), colchicines (Sabouraud et al., 1991; Urtizberea et al., 1991)), toxins (e.g., snake venom (Chippaux and Goyffon, 1998; Gutiérrez et al., 2003; Lomonte et al., 1996), polychlorobiphenyls (Keyler et al., 1994)), and a few abused drugs (e.g., most notably PCP-specific Fab (Owens and Mayersohn, 1986) and IgG (Valentine and Owens, 1996), but also nicotine-specific IgG (Keyler et al., 2005) and methamphetamine-specific IgG (Byrnes-Blake et al., 2003; McMillan et al., 2002) and scFv (Peterson et al., 2008)). Thus, the potential for smaller GNC92H2 formats, specifically F(ab')2-92H2, Fab-92H2 and scFv-92H2, to rapidly sequester cocaine and reverse acute toxicity seemed worthy of investigation.
It was previously conjectured that antibody-mediated small-molecule (i.e., drug) detoxification required an equivalent molar ratio between mAb binding capacity and the drug dose (Scherrmann et al., 1989). This guideline has been refuted for PCP and cocaine as the antagonism of drug effects by submolar mAb doses was preserved (Pitas et al., 2006). Nevertheless, the smaller size of Fab and scFv fragments lowers the protein load and infusion volume of a given molar mAb dose relative to the IgG (see Table 3, GNC92H2 format doses), an important consideration given the cardiovascular stress during cocaine overdose (Katz et al., 2007; Maraj et al., 2010). IgG-based therapies permit the recruitment of effector functions and long-term protection, but these traits are superfluous to this abuse scenario as well as directly responsible for the potential immunogenicity and limited biodistribution of the large Fc-containing IgG (Bird et al., 1988; Covell et al., 1986; Holton III et al., 1987). Previous research on desipramine toxicity, which involves a high molar dose of the toxin, revealed that mAb-mediated increases in desipramine-serum concentration were inversely correlated with the mAb distribution volume (i.e., svFv > Fab > F(ab')2 > IgG) (Keyler et al., 1995; Shelver et al., 1996). Similarly, immunopharmacotherapy with a PCP-binding Fab increased serum concentrations and renal elimination of PCP, thereby attenuating acute behavioral and physiological toxicity (Hardin et al., 1998; Valentine et al., 1996; Valentine and Owens, 1996). Herein, our study establishes mAb GNC92H2 fragments as more effective antidotes to severe cocaine toxicity relative to the IgG based on their superior capacity to redistribute cocaine from the brain to serum within the restricted timeframe of cocaine overdose.
Table 3.
Summary of ataxia and seizure severity scores in cocaine control and immunized mice upon injection of an LD50 cocaine dose. Data represents group mean ± S.E.M. of the maximum ataxia (0-5) or seizure (0-3) score attained by each subject within the 60-min observation period.
| Cocaine Alone | IgG | F(ab')2 | Fab | scFv | |
|---|---|---|---|---|---|
|
Dose (mol. Eq.)a |
93 mg/kg (0.2737) |
180 mg/kg (0.0024)b |
132 mg/kg (0.0024)b |
120 mg/kg (0.0024) |
60 mg/kg (0.0024) |
| Ataxia | 4.10 ± 0.21 | 3.83 ± .37 | 3.90 ± .41 | 2.09 ± 0.51 | 4.45 ± 0.37 |
| Seizure | 1.49 ± 0.18 | 1.06 ± 0.25 | 1.30 ± .47 | 0.46 ± 0.28 | 1.09 ± .37 |
Mole-equivalent dose, mmol/kg
Data adjusted for IgG and F(ab')2 bivalency: two cocaine binding sites per antibody molecule
2. General Methods
2.1 Cocaine Vaccines
2.1.1 Production and purification of anti-cocaine mAb GNC92H2 scFv
The preparation of scFv by this laboratory has been described previously (McKenzie et al., 2007; Meijler et al., 2005). Here, Escherichia coli TG1 cells (Stratagene; Santa Clara, CA) were used for over-expression of soluble scFv-92H2 protein with a C-terminal Flag-tag. To summarize, scFv-92H2 gene fragments were digested with Sfi I (Roche; San Francisco, CA), ligated into the expression vector pET-Flag (derived from pET-15b, Novagen; Gibbstown, NJ), and transformed into E. coli TG1 cells by electroporation. SOC medium (2% peptone, 0.5% yeast extract, 0.05% NaCl, 2.5 mM KCl, 10 mM MgCl2, 20 mM glucose) was added immediately after transformation. The cells were allowed to recover at 37 °C for 1 h, then plated onto Luria-Bertani (LB) agar plates containing carbenicillin (100 μg /mL) and incubated at 30 °C overnight. DNA sequencing was used to confirm the correct sequences. For overexpression, the purified pETFlag-scFv DNA from a single clone was again transformed into E. coli TG1 cells to prepare stock clones, and cells from a single clone were used to inoculate 6 L of SB (super broth: 3% peptone, 2% yeast extract, 1% MOPS) containing carbenicillin (100 g/mL). The cultures were incubated on a shaker (250 rpm) at 37 °C until an OD600 between 0.6 and 0.8 was reached. IPTG was added up to a final concentration of 1 mM, and the temperature was adjusted to 30 °C. The cultures were incubated overnight. The Flag-tagged scFvs were purified on anti-Flag M2 affinity agarose (Sigma-Aldrich; St. Louis, MO). After elution from the column (0.1 M glycine, pH 2.3) and neutralization with 1 M Tris (pH 9), the eluate was prepared for use in animal studies. Upon endotoxin removal (Endoclean™ #18603, BioVintage; San Diego, CA), scFv-92H2 protein solution was extensively dialyzed using Thermo Scientific Slide-A-Lyzer dialysis cassettes (MWCO 10-kDa, Pierce; Rockford, IL) into endotoxin-free 50 mM ammonium biocarbonate and lyophilized before storage. The production and purity of scFv-92H2 was verified by SDS-PAGE. Aliquots of the bacterial supernatant from the overexpression culture, FPLC-isolated anti-Flag M2 affinity column eluate, endotoxin-removed protein solution, dialyzed protein solution, and reconstituted protein for animal injection were collected. Both unreduced and reduced (addition of dithiothreitol, DTT) samples were denatured through boiling, and Nupage LDS (4X) sample buffer (Invitrogen; Carlsbad, CA) was added before sample analysis on a Nupage 4–12% Bis–Tris Gel (1.0×10 mm per well) with Benchmark prestained protein standard (Invitrogen). Bands were visualized by staining with Coomassie Blue. For animal studies, the protein was resuspended in an appropriate volume of sterile isotonic saline, and the final concentration measured by reading the absorbance at 280 nm. The cocaine binding-activity of scFv-92H2 was monitored after reconstitution via accessing GNC-BSA binding by enzyme-linked immunoabsorbant assay (ELISA).
2.1.2 Production and purification of anti-cocaine mAb GNC92H2 Fab and F(ab')2
GNC92H2 was previously identified as the mAb clone from GNC-KLH immunizations and hybridoma production with the most favorable overall properties of specificity and affinity for cocaine (isotype κγ2a, no cross reactivity with ecgonine or ecgonine methyl ester) (Carrera et al., 2000). The Fab fragments were isolated through papain (Sigma) digestion of the purified 92H2-IgG, followed by isolation of cleaved Fab-92H2 with Protein A chromatography (Thermo Fisher Scientific Inc.; Rockford, Il). Specifically, papain (10 μg per 1 mg IgG) was preactivated in Buffer A (100 mM sodium acetate, 1 mM EDTA, pH 5.5) supplemented with 1 mM cysteine and then added to the prepared IgG-92H2 solution (5 mg/ml dialyzed into Buffer A, prewarmed in 37 °C water bath). The optimal digestion time was determined through SDS-PAGE analysis of 20 μl aliquots, and the reaction was terminated through the addition of iodoacetamide (final concentration, 75 mM) and a 30-min incubation at room temperature. The digested sample was dialyzed into 1 M PBS, pH 7.4 prior to loading onto a Protein A column for removal of the uncut IgG, Fc, and Fab/c fragments. Additional purification steps included Protein G chromatography (Thermo Fisher Scientific Inc.; Rockford, Il) to remove unwanted enzyme, dialysis of Fab-containing fractions into 20 mM PB pH 7.0, and cation exchange (Mono S; Pharmacia, Sweden) chromatography. The progress of digestion reactions and the effectiveness of purification steps were monitored through SDS-PAGE of unreduced and reduced (addition of DTT) samples. The bivalent F(ab')2 fragments were generated in a similar manner except with pepsin digestion of the purified IgG-92H2. To determine the optimal reaction conditions, pilot digestions were performed in 0.2 M acetate buffer, pH 4 and 4.5, and aliquots were removed at multiple time points for monitoring of digestion progress. The reaction was terminated through the addition of 2 M Tris base, followed by dialysis, Protein A chromatography, and ion-exchange chromatography. Once obtained, the isolated Fab-92H2 and F(ab')2-92H2 were concentrated on a microdialysis/concentration unit (Amicon Corp.; Danvers, MA) with final protein concentrations measured via spectrophotometry. Both formats underwent endotoxin removal purification steps (BioVintage; purity confirmed with Limulus amebocyte lysate (LAL) testing) before use in animal studies.
2.1.3 Enzyme-Linked Immunosorbent Assay
Cocaine-binding activity of scFv-92H2 as well as GNC92H2 IgG, F(ab')2, Fab, and scFv antibody concentration in mouse blood samples were evaluated by ELISA. The cocaine hapten, termed GNC, was coupled to bovine serum albumin (BSA), and the GNC–BSA conjugate was applied to an ELISA plate (CoStar; 96-well, half-volume) at a concentration of 10 μg/ml in PBS at 37 °C for 1 h with shaking, with unmodified BSA serving as a negative control. After washing with distilled water ten times, the wells were blocked for 1 h at 37 °C with 50 μL of Blotto (5% QuikBlot powder in PBS). Mouse serum (25 μL of an initial 1:500 dilution in Blotto) was added to the first row and serially diluted down the plate. A quantification standard of mAb GNC92H2 dilution stock (IgG-92H2, F(ab')2-92H2, Fab-92H2, or scFv-92H2) covering a range of concentrations (0.005 to 5.0 μg/mL per well) was also plated in a column alongside sera samples, and plates were incubated for 1.5 h at 37 °C. After washing, 25 μL of a 1:5,000 dilution of a goat-anti-mouse IgG (heavy and light chain) horseradish peroxidase conjugate (Thermo Fisher Scientific Inc.; Waltham, MA), goat-anti-mouse IgG (Fab-specific) horseradish peroxidase conjugate (Sigma), or anti-Flag M2 horseradish peroxidase conjugate (Sigma) in Blotto was added to wells for a 1-hour incubation at 37 °C. The plate was developed with the colorimetric reagent tetramethyl-benzidine substrate (TMB, 50 μl/well, Pierce), quenched with an equal volume of 2 M H2SO4, and the absorbance at 450 nm measured on a 96-well ELISA plate reader.
2.2 Pharmacokinetic Studies with mAb GNC92H2
2.2.1 Sample preparation
Mice (n = 5) were administered mAb GNC92H2 IgG (180 mg/kg), F(ab')2 (132 mg/kg), Fab (120 mg/kg), or scFv (60 mg/kg) via a bolus infusion (< 1 min) through a polyethylene tube attached to the catheter on the animals' back. To obtain blood samples for mAb quantification, the mouse tail tip was amputated with a sterile scalpel blade, permitting 10 μl of blood to be collected at several time points using heparinized capillary pipette tips. Collections were made prior to mAb infusion and at time (t) = 3 or 5 min, 10 min, 15 min, 20 min, 30 min, 45 min, 60 min, 2 h, 3 h, 6 h, 24 h, 48 h, 72 h, 96 h, 7 d, and 10 d for all mAb formats. Additional weekly samples were collected for up to 4 weeks after mAb administration for mice infused with F(ab')2 and up to 8 weeks for mice infused with IgG. The blood was immediately placed in a 0.6-mL polypropylene microcentrifuge tube containing 40 μl of 0.1 M sodium citrate/0.1% sodium azide, pH 4.75. These samples were diluted 1:10 in sterile PBS in preparation for subsequent ELISA analysis of blood antibody concentration. Samples were stored at 4 °C for immediate analysis or -80 °C for long-term storage.
2.2.2 Half-life determination
ELISAs were conducted using IgG-92H2, F(ab')2-92H2, Fab-92H2, and scFv-92H2 stock dilutions in order to generate a standard curve for each mAb format and thus determine its concentration in sera samples for all collection time points. To approximate the time course of mAb elimination in vivo, the pharmacokinetic data of each GNC92H2 format were analyzed according to single and two-compartment models using standard volume of steady-state distribution values, followed by the application of goodness-of-fit and regression analysis to antibody concentration versus time data. Whereas the two-compartment model offers a better approximation in theory to the antibody distribution data, the improvement over the single-compartment model was often minimal relative to its additional complexity. Thus, calculations of elimination half-life reflect the application of the single compartment model to the terminal phase of antibody distribution data.
2.3 Cocaine Overdose Model
2.3.1 Subjects
Male CD-1 mice (30 ± 5 g, n ≥ 10 for overdose experiments) purchased from the Scripps Breeding Facility and Charles River Laboratories (Wilmington, MA) were used. Animals were housed four per cage prior to catheter implantation in a 12:12-h light–dark cycle (lights off at 09:00 h). Water and food pellets were available ad libitum in their living cages. All the experiments described in this study were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health, and were approved by the Animal Care and Use Committee at The Scripps Research Institute. Every effort was made to reduce the number of animals used.
2.3.2 Catheter implantation
One week after arrival, animals were labeled, weighed, and subjected to intravenous catheterization. Methods for the preparation and surgical implantation of jugular vein catheters in mice are described in detail elsewhere (Ritz and George, 1993). Briefly, mice were placed under general anesthesia with isoflurane vapor mixture and then implanted with a silastic indwelling catheter (silastic tubing, id 0.3 mm, od 0.64 mm; 12 mm in length) in the right external jugular. Animals were surgically prepared via shaving the areas for incision and applying a 70% alcohol and povidone/iodine solution, and then incisions were made in the mid-scapular region as well as anteromedial to the right forearm above the external right jugular vein. The jugular catheter was passed subcutaneously to a dental cement encased polyethylene assembly housing a guide cannula (Plastic Products, C313G) mounted on the back of the animals. The other end of the catheter was passed subcutaneously from the dorsal incision to the ventral incision, and the silastic tubing inserted into the jugular vein, then tied gently with suture thread to the vein. The incisions were sutured closed, and the catheter was capped with a tygon stopper. The catheters were kept patent with daily flushings of heparinized saline (20 U/flush), and animals were housed singly during their full recovery (about 1 week) and up through drug administration, immunization, and behavioral testing procedures.
2.3.3 Cocaine treatment
Animals were administered a 50% lethal dose (LD50 = 93 mg/kg) of (-) cocaine hydrochloride (NIDA; Rockville, MD), which was dissolved in sterile 0.9% saline for i.p. injection at a volume of 10 ml/kg (Hearn et al., 1991). Upon receiving this LD50 cocaine injection, all immunized mice and cocaine alone controls were then transferred to individual locomotor activity cages for observation.
2.3.4 Passive immunization
The mAb-based agent or vehicle (sterile 0.9% saline) was administered via a bolus infusion through a 10-in.-long polyethylene tube attached to the catheter. This permitted the experimenter to deliver the mAb infusion during active recording of subject in the locomotor activity chamber without disrupting its behavior or full ambulatory range. The 30-min mAb pretreatment time point allowed the subject to recover from any stress related to the administration of a large bolus mAb infusion prior to cocaine injection. The 3-min post-cocaine treatment time point was chosen on the basis of previous work where the average onset of seizures, one predictor of lethality, occurred 3.4 minutes after drug injection (Carrera et al., 2005). The mAb binding capacity for cocaine remained constant across the different GNC92H2 format doses for the 3 min post-cocaine treatment, and this molar mAb dose represents less than 1/100th the molar amount of cocaine administered (LD50 dose = 93 mg/kg). Antibody doses were allotted in a between-subjects design.
2.3.5 Experiment 1: Prophylactic immunopharmacotherapy to prevent of cocaine-related toxicity
The purpose of this experiment was to provide a benchmark of mAb GNC92H2 efficacy in averting the onset of severe symptoms of cocaine toxicity and to which the therapeutic ability of antidotal passive immunization could be compared. The mAb GNC92H2 IgG format was tested at the lower dose level (IgG: 90 mg/kg) in our murine cocaine overdose model. Thirty minutes following i.v. infusion of IgG-92H2 or a vehicle saline solution, the LD50 cocaine dose was administered by i.p. injection, and test subjects were placed directly into locomotor activity cages for activity monitoring across the entire progression of cocaine-related symptoms. The smaller mAb fragments were not tested in this pretreatment model because their rapid elimination would directly counteract their application to prophylactic immunopharmacotherapy. Conversely, the 30-minute delay between immunization with IgG-92H2 and cocaine injection granted the thorough biodistribution of this mAb format prior to drug exposure, and thus this experiment was expected to reflect the maximum efficacy of an IgG-based therapeutic.
2.3.6 Experiment 2: Immunopharmacotherapeutic antidote to cocaine overdose
To evaluate the different capacity of each mAb GNC92H2 format to rescue mice from lethal overdose, subjects were immediately placed in individual locomotor activity cages after cocaine injection (i.p). Attached to the indwelling catheter, a 10 inch-long polyethylene tube permitted the passive vaccine to be administered (i.v.) 3 minutes after cocaine exposure without interfering with mouse behavioral monitoring. The behavioral response to cocaine toxicity was recorded in the same manner as experiment 1. Antibody formats were tested at the following doses: 180 mg/kg IgG-92H2, 132 mg/kg F(ab')2-92H2, 120 mg/kg Fab-92H2, 60 mg/kg scFv-92H2, all of which deliver 2.4 μmol/kg cocaine recognition sites. The constant molar dose of all GNC92H2 formats was adjusted based on the molecular weight of each format (e.g., IgG = 150 kDa, F(ab')2 = 110 kDa, Fab = 50 kDa, scFv = 25 kDa) and its antigen binding capacity (e.g., IgG, F(ab')2 are bivalent; Fab, scFv are monovalent).
2.3.7 Behavioral monitoring
Behavioral monitoring was conducted via the use of Plexiglas locomotor activity cages (42 × 22 × 20 cm). All mice underwent a brief cage habituation session 24 h prior to behavioral testing. On the test day, mice received an intraperitoneal injection (i.p.) of cocaine, immediately before their transfer to individual cages for 60-min observation sessions. Subjects' behavior was recorded by a “blind” observer for the time-of-onset, severity, and duration premorbid behaviors (e.g., hyperlocomotion, ataxia, Straub tail response, writhing, convulsions, or loss of righting posture for at least 5 s and/or clonic movements, agitation), seizures (e.g., tonic, clonic, wild-running clonis, tonic-clonic), and death. Specifically, each mouse received a binary score of present (1) or absent (0) for each symptom category (premorbid behavior, seizures, death) so as to permit the pooling of nominal data by group and its comparison to control group behavior using the χ2 test. From these data, the incidence of each overdose symptom within groups was computed as a percentage for ease of depiction in figures.
In addition, the severity of premorbid behaviors and of seizure activity was ranked within successive 3-min time intervals during the 60-min observation period. A scale developed by Sturgeon et al. was applied to the scoring of premorbid ataxia in mice (score 0: inactive; score 1 = unusual, awkward, and/or jerky movements with only occasional loss of balance; score 2 = awkward and jerky movements accompanied by a moderate rate of falling onto their sides but not onto their backs; score 3 = significant loss of balance and falling onto their sides and/or backs when attempting to move about the cage; score 4 = loss of righting ability, or severe impairment of the anti-gravitational reflex to the extent that mice are unable to move beyond a small area within the cage; score 5 = absence of overall movement except for twitching, head bobbing or writhing, and body rolling) (Sturgeon et al., 1979). The ataxia scores of mice in each treatment group were averaged (mean ± S.E.M.) within each time interval.
A similar ranking system was created for the assessment of seizure severity. Subjects were scored across successive 3-min intervals after cocaine injection based on the following scale: score 0 = absence of seizures; score 1 = short-lasting, mild seizures occasionally accompanied by loss of the righting reflex and with repeated bouts of convulsive activity absent or infrequent; score 2 = convulsive seizures accompanied by severe clonus (wild-running clonus) or rearing, and/or bouts of seizure trains that occur repeatedly or that cause fatal respiratory disorders given their violent nature. These ataxia and seizure ranking systems permitted the severity of overdose symptomology within all mAb treatment groups and the cocaine-alone control group to be compared by analysis of variance (ANOVA), repeated measures ANOVA, and Fisher's PLSD, or by the χ2 distribution for nominal data (e.g., the appearance of cocaine overdose symptomology).
2.4 Statistical Analysis
To measure the effect of GNC92H2 format treatment on cocaine-induced toxicity, the appearance of each overdose symptomology (premorbid behaviors, seizures, death) was assigned a value of incidence (present = 1, absent = 0) for each mouse regardless of the time of onset, and group data was analyzed via the χ2 distribution (p < 0.05). The theoretical frequencies for premorbid behaviors, seizures, and death were based on the cocaine alone group data. Whereas the χ2 test of goodness-of-fit was employed to examine the therapeutic benefit of mAb treatment, the χ2 test of independence or the Fisher's exact test was then used to illustrate differences between immunopharmacotherapeutic treatments in their capacity to alter cocaine-induced symptomology. Lastly, the times of onset of behavioral observations were used to score ataxia and seizure severity within successive 3-min time intervals (repeated measure for ANOVA), and group mean scores within these intervals were analyzed for parametricity (normal distribution, homogeneity of variance). If these conditions were met, significant differences were determined by analysis of variance (ANOVA) and post-hoc Student's t tests and Fisher's PLSD. Otherwise, data were analyzed using the non-parametric Mann-Whitney U statistic or the Kruskal-Wallis test. In addition, the maximum ataxia score and maximum seizure score attained within the 60-min session was identified for each subject, and group means for these scores were compared via the statistical tests listed above. Serum antibody levels were also compared by ANOVA (data not shown), and mAb format half-life was evaluated using the model-independent method based on the statistical-moment theory.
3. Results
3.1 Plasma Pharmacokinetics of the Anti-cocaine mAb GNC92H2 IgG, F(ab')2, Fab and scFv Formats
To determine the pharmacokinetic profiles of mAb GNC92H2 IgG, F(ab')2, Fab, and scFv distribution in mice, tail vein blood samples were collected immediately before immunization and then as early as 3 min after the i.v. infusion of GNC92H2. From the calculations of mean serum-mAb content at every time point, it was observed that the concentrations of each immunopharmacotherapeutic increased slightly across the first blood collection time points, with IgG-92H2 reaching a peak volume of distribution of Vd ∼ 0.126 ± 0.013 L/kg. The IgG-92H2 and F(ab')2-92H2 levels in sera remained elevated for approximately 1 hour before their gradual decline. The Fab-92H2 and scFv-92H2 fragments were rapidly eliminated from circulation. From these serum levels, the half-life of each format was estimated (Table 1). Gratifyingly, the in vivo pharmacokinetic profile of all GNC92H2 formats approximated the biodistribution data of other murine antibodies reported in the literature (Covell et al., 1986; Holliger and Hudson, 2005).
Table 1.
The pharmacokinetic profiles of mAb GNC92H2 formats.
| t1/2 (mean ± S.E.M.) | Kd (μM) | |
|---|---|---|
| cocaine | 26.1 min a | --- |
| IgG | 12.1 ± 1.70 d | 0.2 - 0.002 b |
| F(ab')2 | 302 ± 21.6 min | nd c |
| Fab | 73.9 ± 11.5 min | 0.1 |
| scFv | 17.9 ± 1.79 min | 2 - 0.4d |
The binding affinity of GNC92H2 IgG for cocaine was measured via several methods: by equilibrium dialysis (Kd = 200 nM), by competition ELISA for cocaine (Kd,app ∼ 13 nM), and by the KinExA system (Kd = 2 nM). Competition ELISA provides a semi-quantitative estimation of of antibody binding affinity for an antigen, whereas KinExA (Sapidyne Instruments Inc.; (Ohmura et al., 2001)) measures the Kd, Kon, and Koff binding constants in the solution phase to characterize bimolecular binding events. It thereby avoids the mass transport limitations and mobility effects inherent to traditional methods that measure binding events between a solution phase and a solid phase, and thus provides the most accurate Kd measure. This method was not used to characterize the other GNC92H2 fragments, whereas binding affinities of the IgG, Fab, and scFv formats were all estimated via equilibrium dialysis.
Not determined.
92H2-scFv, isolated and purified via immobilized metal affinity chromatography and high performance liquid chromatography, or via immunoaffinity purification (Moss et al.,2003).
3.2 Effect of Pretreatment with Anti-cocaine mAb GNC92H2 IgG on the Outcome of Acute Cocaine Toxicity
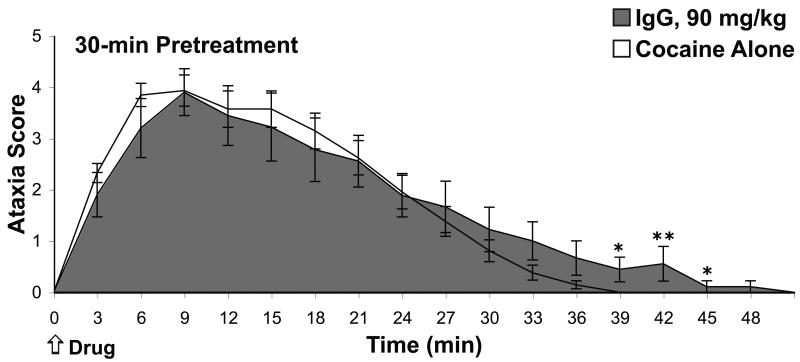
Mice were immunized with IgG-92H2 (90 mg/kg) 30 minutes prior to cocaine exposure in order to confirm our previous results on IgG-92H2-mediated prevention of lethality and to conduct detailed recordings on overdose symptomology. The analysis of behavioral data of immunized and cocaine alone groups revealed that this IgG-92H2 dose had the capacity to attenuate severe cocaine-induced toxicity; lethality was averted in a significant percentage of immunized mice (χ2 = 8.14, p < 0.005). All subjects demonstrated hyperactivity, increased exploratory behavior, and the initial progression into ataxic behaviors that directly follow cocaine injection. The time-of-onset, duration, and severity of seizure generation showed greater inter-subject variation, and thus any treatment-dependent variation between groups lacked statistical significance. Approximately half of the cocaine alone mice succumbed to cocaine toxicity, as expected for an LD50 drug dose. Whereas a higher percentage of immunized mice survived the LD50 cocaine injection, the duration of premorbid behaviors was protracted in the immunized group. This was evidenced by the statistically significant difference in ataxic behavior in cocaine alone versus immunized mice in observation intervals extending past the 36-min time point (Fig. 1, Student's t test: p < 0.05, 36-45 min post-cocaine).
Figure 1.
Therapeutic benefit of prophylactic immunization against cocaine. A) Cocaine-induced ataxia upon pretreatment with mAb GNC92H2 IgG (90 mg/kg) 30 minutes prior to drug administration. The ataxia ranking system, which was adapted from the 0-5 scale proposed by Sturgeon et al., involved assigning a single score (0, 1, 2, 3, 4, 5) to each mouse based on the highest level of ataxic behavior displayed by that subject within a 3-min observation period. Data (mean ± S.E.M.) for cocaine alone mice (unshaded line) and immunized mice (shaded line) represent the ataxia score average of mice in a treatment group for each successive 3-min time interval after i.p. injection of cocaine at time = 0 min. *p < 0.05, ** p < 0.005 for the Student's t test comparison of control versus immunized groups in designated time intervals.
3.3 Antidotal Immunization with mAb GNC92H2-based Agents
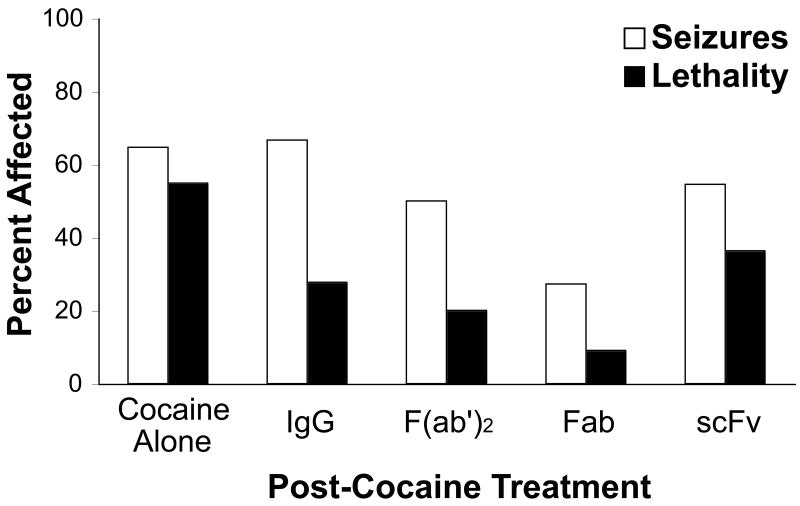
The mAb pretreatment experiment established the capacity of IgG-92H2 to bind cocaine in vivo and prevent cocaine-induced lethality. Of greater clinical interest was the potential for administration of an anti-cocaine immunopharmacotherapeutic to reverse a potentially lethal overdose after the appearance of the outward signs of acute toxicity. The overall therapeutic effect of GNC92H2-based treatment was examined in the mouse overdose model through computing the percentage of mice within each group that developed symptoms of cocaine toxicity (Fig. 2). The appearance and severity of ataxia, seizures, and lethality as an endpoint, were recorded across 3-min observation intervals for statistical analysis (Table 2). Gratifyingly, the efficacy of IgG-92H2 as a therapeutic for cocaine overdose extends beyond its prophylactic benefit. Antidotal delivery of IgG-92H2 (180 mg/kg) conferred a 50% reduction in the incidence of lethality relative to the cocaine alone group. However, neither the appearance of premorbid behaviors nor seizure generation was significantly altered by the IgG format of this immunopharmacotherapy (Fig. 2).
Figure 2.
Protection against cocaine-induced toxicity by passive immunization with mAb GNC92H2-based immunopharmacotherapeutics. Treatments (Cocaine Alone: 10 mL/kg saline, IgG: 180 mg/kg, F(ab')2: 132 mg/kg, Fab: 120 mg/kg, or scFv: 60 mg/kg; i.v.) were administered 3 minutes after cocaine injection (93 mg/kg, i.p.). Data are expressed as a percentage of mice in each treatment group that experiences seizures or lethality from an LD50 cocaine dose.
Table 2.
Statistical analysis of changes to cocaine-induced symptomology by 3-min post-treatment with mAb GNC92H2 IgG, F(ab')2, Fab, or scFv.
| Symptomology | Group | χ2 Test of Goodness-of-Fit | χ2 Test of Independence |
|---|---|---|---|
| Death (alive or dead) |
IgG | χ2 = 5.349, p < 0.05 | (all groups) χ2 = 11.979 p < 0.05 |
| F(ab')2 | χ2 = 4.92, p < 0.05 | ||
| Fab | χ2 = 9.324, p < 0.005 | ||
| scFv | nsa | ||
| Seizure Score (0,1,2,3) |
IgG | χ2 = 19.74, p < 0.0005 | (all groups) ns |
| F(ab')2 | ns | ||
| Fab | χ2 = 8.866, p < 0.05 | ||
| scFv | ns | ||
| Ataxia Score (0,1,2,3,4,5) |
IgG | ns | (all groups) χ2 = 40.769 p < 0.005 |
| F(ab')2 | χ2 = 11.623, p < 0.05 | ||
| Fab | χ2 = 73.686, p < 0.0001 | ||
| scFv | ns | ||
ns: not significant
F(ab')2-92H2, Fab-92H2, and scFv-92H2 were administered at doses equivalent to the full IgG in binding capacity for cocaine (Table 3). It was hypothesized that both the more rapid biodistribution and lower protein dose of mAb fragments would enhance their therapeutic ability relative to the full immunoglobulin in the scenario of overdose rescue. Relative to the 54% lethality mediated by cocaine alone and the 28% lethality within the IgG-92H2 group, the LD50 dose of cocaine was 20%, 9%, and 36% lethal in the F(ab')2-92H2, Fab-92H2 and scFv-92H2 treatment groups, respectively. Both the F(ab')2-92H2 and Fab-92H2 offered significant protection against death from cocaine overdose, as assessed by the χ2 test of goodness-of-fit (Table 2). The different mAb GNC92H2-based treatments were indistinguishable by the χ2 test of independence (IgG, F(ab')2, Fab, and scFv: χ2 = 2.49, p > 0.40), however the pair-wise comparison between the rate of lethality in the cocaine alone group with each mAb-treated group depicted the superior ability of Fab-92H2 to mediate overdose rescue (IgG-92H2: χ2 = 3.92, p < 0.05; F(ab')2-92H2: χ2 = 4.06, p < 0.05; Fab-92H2: χ2 = 7.63, p < 0.01)
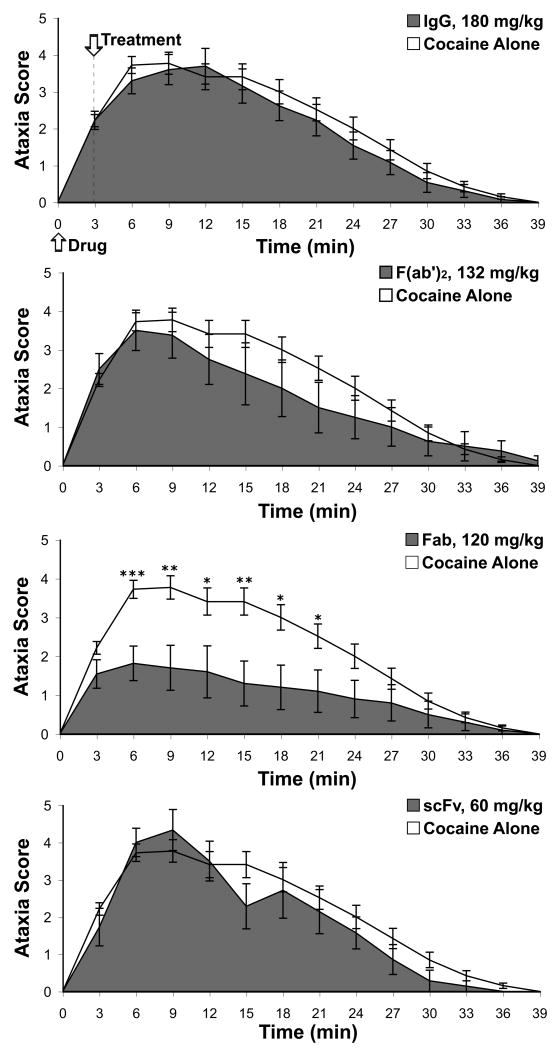
As with the immunopharmacotherapeutic protection against lethality, specific mAb formats showed different capacities to attenuate the progression of overdose symptomology. IgG-92H2 treatment did not significantly alter the incidence of premorbid behaviors in comparison to cocaine alone subjects, while all mice within F(ab')2-92H2 and scFv-92H2 groups displayed premorbid behaviors. Of all GNC92H2-based treatments, Fab-92H2 served as the best antidote. The initial hyperactivity and slight ataxia failed to transition to the severe overdose symptomology in a significant number of mice within this group relative to the cocaine alone group as indicated by the repeated measures ANOVA (time interval) of ataxia severity [F(1, 34) = 4.783, p < 0.05].
3.4 Effect of the Anti-cocaine mAb GNC92H2 IgG, F(ab')2, Fab and scFv Formats on Cocaine-induced Premorbid Behaviors and Seizure Generation
To compare the utility of each GNC92H2 format in alleviating the outward signs of cocaine toxicity, both the severity of ataxic symptoms and of seizures were scored (Table 3). The general time course of ataxia includes circling, brief spurts of locomotor stimulation and depression, and the deterioration in motor coordination within the first few minutes post-cocaine. Then, the ataxic behavior may transition to head-weaving, loss of righting, jerky or convulsive movements, full body contortions (head and body rolls, arched back, lying prone, paw peddling) and finally a loss of the anti-gravitational reflex with movements limited to convulsions. At this point, a subset of intoxicated mice has begun to experience seizures, with an average onset at 3.4 min post-cocaine for the LD50 dose. The ataxic behavior often alternated with seizure activity for several minutes before death or gradual recovery. In the latter case, the progression of ataxic symptoms was often repeated in reverse order.
To detect changes in the severity of premorbid behaviors by immunization, the ataxia scores for all treated mice across successive 3-min intervals were analyzed statistically by ANOVA and by the unpaired Student's t test for comparisons between two groups within specific intervals. A one-way ANOVA of ataxia scores confirmed that there was no significant difference in the onset of cocaine toxicity among different treatment groups during the 3-min time period preceding passive vaccination [F(4,96) = 1.211, p > 0.05] (see Fig. 3). However, the acute development of ataxic symptoms was modulated by treatment, as illustrated by the one-way ANOVA for each 3-min time interval (3-6 min: [F(4,96) = 4.140, p < 0.005], 6-9 min [F(4,73) = 3.552, p < 0.05], 9-12 min: [F(4,62) = 2.478, p = 0.05], 12-15 min [F(4,60) = 2.780, p < 0.05]). The overall severity of ataxia significantly varied based on treatment, as depicted by a one-way ANOVA between groups for the highest ataxia score experienced by each mouse over the entire observation period [F(4,96) = 4.469, p < 0.005]. Fisher's PLSD was implemented for pair-wise comparisons between treatment groups, and it demonstrated that only Fab-92H2 treatment significantly lessoned the profile of cocaine-induced ataxia relative to the ataxic behavior in the cocaine-alone mice (p < 0.0001). Similarly, this pair-wise statistical analysis confirmed the superiority of Fab-92H2 over all other therapeutic treatments in terms of rescue from cocaine-induced ataxia (Fab vs. IgG: p < 0.005, Fab vs. F(ab')2: p < 0.01, and Fab vs. scFv: p < 0.001).
Figure 3.
Effect of GNC92H2-based immunopharmacotherapeutics on cocaine-induced ataxia. Subjects were administered an LD50 cocaine dose by i.p. injection at time = 0 min, upon which each subject was transferred to a locomotor activity cage for behavioral monitoring. The assigned mAb treatment (top graph: IgG, 180 mg/kg; middle graphs: F(ab')2, 132 mg/kg and Fab, 120 mg/kg; bottom graph: scFv, 60 mg/kg) or vehicle (sterile 0.9% saline) was infused through the jugular vein catheter at time = 3-4 min. Ataxia (0-5 scale) was scored for each subject within successive 3-min intervals for the 39-min observation period, and group scores were averaged within each 3-min interval (mean ± S.E.M.) for comparison of ataxic symptoms between the cocaine alone control (unshaded line) and immunized groups (shaded line). *p < 0.05, ** p < 0.005 for the Student's t test comparison of cocaine alone versus immunized groups within designated time intervals.
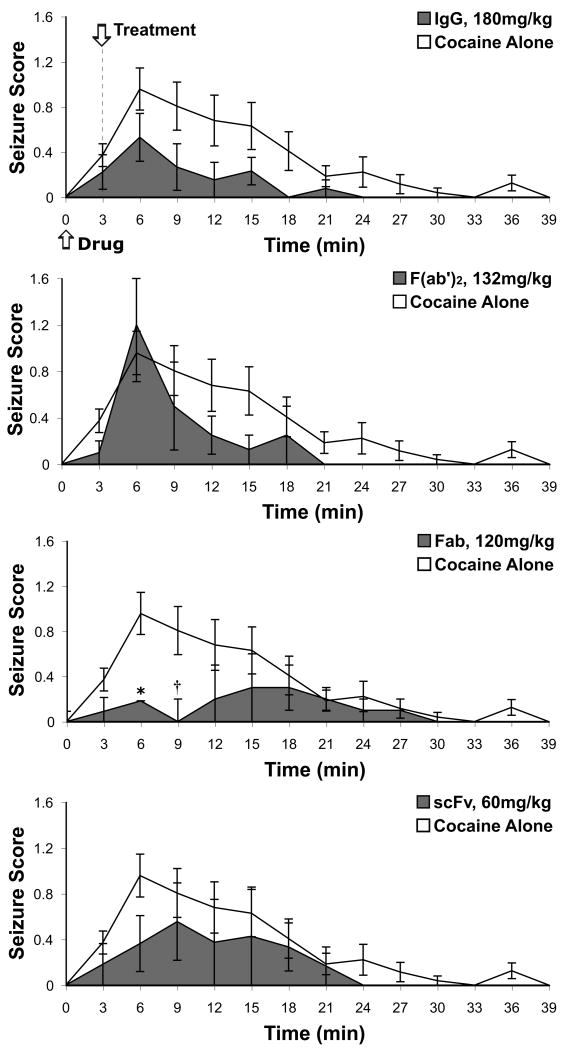
Passive immunization with Fab-92H2 3 minutes following cocaine injection was the only mAb GNC92H2-based treatment to stem seizure generation (Fig. 4). Whereas 65% of drug alone mice exhibited seizure activity as a result of acute cocaine toxicity, only 27% of Fab-92H2-immunized mice developed seizures (Fig. 2), a level of protection that was statistically significant by χ2 analysis (seizure occurrence: yes/no, χ2 = 6.749, p < 0.01; seizure severity: 0-3, χ2 = 8.866, p < 0.05). This contrasted with the greater than 50% of mice in IgG-92H2, F(ab')2-92H2, and scFv-92H2 treatment groups exhibiting seizures. Moreover, analyses by χ2 tests of goodness-of-fit for the appearance and severity of seizures of each mAb-treated group (Table 3), in which seizure generation within the cocaine-alone group was treated as the expected values, demonstrated that IgG-92H2 administration lessoned seizure severity but failed to block their occurrence (seizure severity: 0-3, χ2 = 19.74, p < 0.0005; seizure appearance: yes = 1, no = 0, χ2 = 0.03, p > 0.5). Administration of F(ab')2-92H2 or scFv-92H2 did not have a statistically relevant effect on seizure symptomology relative to administration of the saline vehicle in cocaine-alone mice.
Figure 4.
Time-dependent severity of cocaine-induced seizures in cocaine alone and immunized groups. An LD50 dose cocaine was i.p. injected in mice at the start (time = 0 min) of behavioral monitoring, and 3 minutes into the observation period, the designated mAb treatment (top graph: IgG, 180 mg/kg; middle graphs: F(ab')2, 132 mg/kg; Fab, 120 mg/kg; bottom graph: scFv, 60 mg/kg) or vehicle (Cocaine Alone: sterile 0.9% saline) was infused through the indwelling jugular vein catheter. Seizure activity (0-3 scale) was scored for each mouse across successive 3-min intervals and values for each time interval represent group mean scores ± S.E.M. for cocaine alone control (unshaded line) and immunized (shaded line) groups. *p < 0.05, † p = 0.055 for the Student's t test comparison of control versus immunized groups within specific time intervals.
4. Discussion
Our laboratory and others have reported on the generation of cocaine-binding and cocaine-degrading mAbs to prevent lethality upon binge exposure (Briscoe et al., 2001; Carrera et al., 2005; Mets et al., 1998). However, we wished to extend the therapeutic utility of immunopharmacotherapeutic treatments beyond the protection from terminal overdose to the attenuation of other medically hazardous symptoms of acute cocaine toxicity. The time course of cocaine intoxication, during which mice experience a peak brain concentration 5-15 minutes after i.p. injection, implies that a potential pharmacotherapy must dampen and/or reverse the influx of cocaine into the CNS and the resultant central stimulatory effects within a narrow timeframe (Benuck et al., 1987). We hypothesized that the scFv, Fab and F(ab')2 formats derived from the mAb GNC92H2 scaffold might better mirror the rapid kinetic profile (distribution t1/2α = 1.9 min, elimination t1/2β = 26.1 min) and widespread tissue distribution (Vdss = 6.0 L/kg) of cocaine in vivo and thus achieve greater success than the IgG in cocaine detoxification (Norman et al., 2007).
In this study, the antibody-based manifolds were created using GNC92H2 as a template so as to preserve its pharmacodynamic properties, including its high binding affinity for cocaine (Kd = 2 nM, as determined via KinExA). In previous work, the Kd values of the GNC92H2 IgG and Fab formats for cocaine and its major metabolites were estimated through competition equilibrium dialysis using radiolabeled ligands (cocaine, benzoylecgonine, ecgonine methyl ester, and ecgonine 3H-labeled at the N-methyl position). Both formats exhibited superior affinity and specificity for cocaine (Kd ∼ 10-7 M) relative to the major nonpsychoactive metabolites (benzoylecgonine: Kd ∼ 10-5 M, ecgonine methyl ester: Kd ∼ 10-3 M, ecgonine: Kd ≫ 10-3 M) (Larsen et al., 2001). Likewise, immunoaffinity-purified scFv-92H2 displayed a Kd ∼ 10-7 M (Moss et al., 2003).
Previously, we conducted a study on cocaine overdose reversal through passive immunization with IgG-92H2, however given the precedence for mouse age, sex, and strain to exert a measurable effect on cocaine pharmacokinetics and behavioral response (Azar et al., 1998; McCarthy et al., 2004; Visalli et al., 2005), the experiments for IgG-92H2 pretreatment (90 mg/kg) and 3-min post-treatment (180 mg/kg) relative to cocaine injection were repeated to establish a benchmark of IgG-92H2 efficacy. Pretreatment results for the percentage of mice exhibiting each symptomology paralleled past data. However, the scoring of ataxic behavior across successive 3-min intervals revealed a statistically significant phenomenon in the recovery of immunized mice. The overall premorbid behaviors of immunized and cocaine alone groups were indistinguishable in the short-term, but the mild ataxia, which follows the acute, severe symptoms of toxicity, subsided more quickly in unimmunized mice. The prolonged ataxia that was observed under IgG-92H2 pretreatment may suggest a slow release of cocaine from tissue or IgG-92H2-bound stores and its subsequent re-distribution between plasma and brain.
The different GNC92H2 formats were then evaluated under our murine overdose model, and the ability of these immunopharmacotherapeutics to serve as antidotes for acute cocaine toxicity was compared against IgG-92H2. From this current study emerged preclinical evidence that smaller mAb constructs hold the greatest therapeutic potential for this scenario of cocaine abuse. Whereas all GNC92H2 formats except scFv-92H2 prevented lethality in a significant proportion of subjects when the immunopharmacotherapeutic was delivered three minutes after cocaine exposure, Fab-92H2 and to a lesser extent, F(ab')2-92H2 ameliorated other symptoms of severe toxicity.
The pharmacodynamic properties of smaller mAb fragments pose two distinct advantages over the IgG with respect to counteracting excessive cocaine levels. Their shorter half-lives reflect their immediate but extensive extravascular distribution as well as their rapid elimination via renal catabolism. Whereas IgG distributes to a volume equivalent to the plasma volume, F(ab')2 and Fab antibody formats have been shown to disperse throughout 2× to 4-5× this volume, respectively, highlighting the extent to which either penetrates superficial and deep tissue (Chippaux and Goyffon, 1998). Anti-drug passive vaccines are effective therapeutics because they shift the gradient of drug molecules from the brain to plasma, and as a result, the drug Vdss approaches that of the administered antibody (i.e., the plasma volume for IgG-based vaccines). Antibody fragments sequester their drug antigen in tissue as well as blood, and thus they tend to mediate a lesser rise in plasma drug concentration than their respective full IgG. As a corollary, the rate of cocaine redistribution between brain, blood and tissue, which is expected to vary with mAb format distribution, may contribute to the unique therapeutic properties of each GNC92H2-based vaccine.
Immunopharmacotherapeutic use of mAbs against PCP, methamphetamine and nicotine has documented this antibody-mediated drug redistribution and attributed it to the equimolar binding of drug molecules by the available antigen-binding sites of the mAb as opposed to antibody-mediated changes in drug metabolism (Byrnes-Blake et al., 2003; Keyler et al., 2005; Laurenzana et al., 2003; Proksch et al., 2000; Roiko et al., 2009; Valentine and Owens, 1996). By contrast, immunization with a smaller antibody fragment may affect the t1/2β of small-molecule drugs when the antigen-bound immunopharmacotherapeutic agent is within the molar weight threshold for renal filtration and elimination through urine. For example, digitoxin urinary excretion increased upon treatment with anti-digitoxin Fab but decreased below control levels upon use of the IgG (Ochs and Smith, 1977). Even though methamphetamine renal clearance remained unchanged by the anti-methamphetamine scFv6H4 and the rate of digoxin elimination slowed with anti-digoxin Fab treatment, the anti-PCP Fab appeared to accelerate urinary excretion of PCP and partially block PCP metabolism in treated rats (Butler et al., 1977; Peterson et al., 2008; Valentine et al., 1994). Thus, we hypothesized that both the greater tissue penetration of Fab-92H2 and F(ab')2-92H2, which augments the peripheral sink for cocaine upon mAb-mediated drug redistribution, and the direct urinary excretion of cocaine-bound Fab-92H2 would favor a greater efflux of cocaine from of the CNS compartment. The heightened pressure on the blood-brain cocaine gradient by mAb-mediated drug sequestration in the blood and periphery provides a pharmacodynamic explanation for the ability of a submolar mAb dose to clear enough circulating drug molecules to mediate overdose rescue and for the superior therapeutic efficacy of Fab-based versus IgG-based cocaine vaccines.
Although Fab-92H2 was delivered several minutes into the development of acute cocaine intoxication, post-treatment significantly counteracted the standard progression of premorbid symptoms and future seizure generation. Premorbid behaviors, seizures and lethality were present in 82%, 27%, and 9% of Fab-92H2-treated mice, respectively, versus the corresponding rates of 92%, 65%, and 55% observed in cocaine-alone mice. Whereas the suppression of seizures was not significant in treatment groups administered IgG-92H2, F(ab')2-92H2, or scFv-92H2, the percentage of mice displaying seizures in the cocaine-alone group was markedly lower here in comparison to previous studies performed in this laboratory using different mouse strains (Carrera et al., 2005). This seemingly higher seizure threshold in CD-1 mice would offset any vaccine-mediated percent reduction in seizure activity reported in this study and thus diminish the perceived antidotal value of these GNC92H2-based therapeutics.
Even though the biodistribution and elimination of the F(ab')2 format is more akin to the IgG than smaller fragments, F(ab')2-92H2 profits over Fab-92H2 and scFv-92H2 in that its bivalency guarantees the preservation of its stability and antigen affinity. It is crucial that any vaccine retains its full binding activity at high concentrations prior to use and upon its injection as a cocaine antidote. In addition, the longer half-life of F(ab')2-92H2 permits drug scavenging for several hours to days, thus granting continued protection from cocaine exposure as drug reenters circulation from lipid-rich tissue stores. These benefits notwithstanding, F(ab')2-92H2 demonstrated a diminished capacity to reverse cocaine overdose relative to Fab-92H2 under this acute model. The severity of ataxic behavior was significantly attenuated, but the F(ab')2-derived reductions in seizure generation and severity were statistically insignificant (Table 2).
scFv-92H2 conferred the least protection from cocaine-induced symptomology relative to other mAb GNC92H2 constructs in the overdose rescue paradigm. Its half-life of t1/2 ∼ 18 min represents an extension over those reported in the literature for other murine scFv monomers (t1/2 < 10 min). Multivalent scFv species possess higher residence times in serum and tissue due to their greater stability and decreased first-pass renal elimination, and indeed, both the 15-residue linker connecting the VH and VL domains of scFv-92H2, and the high protein concentration required for passive immunization (> 5 mg/ml) favor the formation of diabodies and higher molecular weight multimers (Kortt et al., 1994; Moss et al., 2003; Redwan et al., 2003; Shelver et al., 1996). For drug detoxification, the multivalent derivatives originating from the monovalent anti-methamphetamine scFv (15-residue linker) promoted the redistribution of methamphetamine to the plasma compartment for several hours (Peterson et al., 2008). This in vivo persistence and antigen binding activity of scFv multimers relative to scFv monomers (monovalent t1/2Z ∼ 5.8 min, multivalent t1/2Z ∼ 228 min) may prove advantageous to their clinical use in immunotherapy.
However, scFv-92H2 demonstrated suboptimal therapeutic efficacy in this overdose model relative to Fab-92H2 despite the improvement in its pharmacokinetic profile via dimerization, which was depicted in half-life data and in previous in vitro studies (Moss et al., 2003). Several seemingly minor factors may have contributed to the decreased efficacy of scFv-92H2 treatment, including: 1. the formation of aggregates, which may diminish the cocaine binding capacity of a given mAb dose, 2. the documented tendency of scFv fragments to possess a lower affinity for antigen in comparison to the larger, stabilized formats, and 3. the rapid elimination of a large fraction of the original scFv dose. With respect to the latter, monomeric scFv undergoes first-pass renal excretion soon after i.v. infusion. We have hypothesized that the 18-min half-life, which was approximated via a single-exponential rather than a bi-exponential curve, is biased by the longer residence times of multimeric species, and that the initial clearance of monomers may lower the effective scFv dose to sub-therapeutic levels. The steepness of the cocaine dose-response curve in mice (Bedford et al., 1982; Hearn et al., 1991) implies that the elimination or inactivation of a small fraction of the scFv dose could have a detectable consequence on its efficacy. Also, scFv fragments possess the most rapid and extensive distribution out of vascular space into superficial and deep tissue relative to the Fab, F(ab')2, and IgG formats (Milenic et al., 1991; Shelver et al., 1996). Even though this characteristic permits scFv-92H2 to sequester cocaine within tissue, the proportionate decreases in free and mAb-bound cocaine in serum may be accompanied by a slower redistribution of cocaine out of the brain. The re-emergence of ataxic behaviors (Fig. 3) and severe cocaine toxicity (Fig. 2, 4) at approximately 12 min after scFv infusion may suggest that scFv levels dropped precipitously or that scFv differentially modulated cocaine redistribution from vasculature and tissue stores to brain.
In addition to identifying Fab-92H2 as an optimal antidote to cocaine overdose, the application of engineered mAb constructs to rapid small molecule detoxification illustrates a manner in which to circumvent many of the perceived weaknesses of anti-drug vaccines. First, to attain a molar equivalence between antibody binding capacity and the molar drug dose, the target concentration of circulating antibodies must be exceedingly high. With respect to active vaccination strategies, an anti-cocaine vaccine has yet to elicit consistently high antibody titers in clinical trials (Martell et al., 2009). Likewise, the requisite dose of a passive vaccine carries a risk of adverse effects from the protein load of the IgG infusion. The presence of the Fc constant region in IgG-based agents, which augments their size relative to mAb fragments, is both superfluous and potentially detrimental in anti-drug immunopharmacotherapy. Whereas its physiological purpose consists of initiating immune effector functions upon antigen binding, the Fc component of IgG-based therapies contributes to their extended half-life and heightened stability. Neither of these functions is required in the scenario of overdose reversal, and indeed, the slow elimination of anti-drug IgGs may cause the passive vaccine to become immunogenic.
As alternatives to the IgG, anti-PCP Fab and anti-methamphetamine scFv have been examined in drug detoxification strategies, but in contrast to overdose reversal, testing was conducted using comparatively low, non-lethal drug doses (Peterson et al., 2008; Valentine et al., 1996). Also, subject survival did not hinge on the time-sensitive scavenging of circulating drug or on the delivery of an exceedingly high mAb dose. However, these studies validated the feasibility of using anti-drug mAb fragments to sequester drug in vivo through confirming the absence of Fab-induced renal toxicity and the preservation of scFv binding activity via multimer formation. Our current investigation establishes the therapeutic utility of different engineered GNC92H2 manifolds within a specific drug abuse scenario. To achieve accelerated drug clearance, smaller mAb constructs possess a pharmacokinetic advantage over the IgG and may prove therapeutically beneficial in the treatment of other time-sensitive indications. In sum, our results endorse the case-specific optimization of mAb formats as a route toward developing superior pharmacotherapies.
Acknowledgments
This work received financial support from the Skaggs Institute for Chemical Biology, the National Institute on Drug Abuse [DA 21939 to J.B.T., DA 08590 to K.D.J.], and NIH Blueprint for Neurosciences Research [NS057096 to A.J.R.]. We gratefully acknowledge Dr. Lisa Eubanks (The Scripps Research Institute) for technical assistance in the expression and purification of scFv-92H2, and Dr. Tobin Dickerson (The Scripps Research Institute) for insightful discussions.
Footnotes
Abbreviations: mAb: monoclonal antibody, IgG: immunoglobulin G, Fab: Fab antibody fragment, scFv: single-chain antibody fragment, VH and VL: antibody heavy- and light-chain variable domains, GNC-BSA: GNC hapten conjugated to bovine serum albumin carrier protein, i.v.: intravenous, Cocaine Alone (group): mouse group administered vehicle saline (i.v.) and LD50 dose cocaine (i.p.), IgG-92H2: GNC92H2 IgG-based passive vaccine, F(ab')2-92H2: GNC92H2-F(ab')2-based passive vaccine, FAB-92H2: GNC92H2-Fab-based passive vaccine, scFv-92H2: GNC92H2 scFv-based passive vaccine, ELISA: enzyme-linked immunosorbent assay, Kd: dissociation constant, t1/2 : elimination half-life, Vdss: volume of distribution at steady state, Css: concentration at steady state, ANOVA: analysis of variance, Fisher's PLSD: Fisher's protected least significant difference, CNS: central nervous system, PCP: phencyclidine, NIDA: National Institute on Drug Abuse
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer B. Treweek, Email: treweek@scripps.edu.
Amanda J. Roberts, Email: aroberts@scripps.edu.
Kim D. Janda, Email: kdjanda@scripps.edu.
References
- 1.Azar MR, Acar N, Erwin VG, Barbato GF, Morse AC, Heist CL, Jones BC. Distribution and clearance of cocaine in brain is influenced by genetics. Pharmacol Biochem Behav. 1998;59:637–640. doi: 10.1016/s0091-3057(97)00471-1. [DOI] [PubMed] [Google Scholar]
- 2.Bedford JA, Turner CE, Elsohly HN. Comparative lethality of coca and cocaine. Pharmacol Biochem Behav. 1982;17:1087–1088. doi: 10.1016/0091-3057(82)90499-3. [DOI] [PubMed] [Google Scholar]
- 3.Benuck M, Lajtha A, Reith ME. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243:144–149. [PubMed] [Google Scholar]
- 4.Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science. 1988;242:423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 5.Briscoe RJ, Jeanville PM, Cabrera C, Baird TJ, Woods JH, Landry DW. A catalytic antibody against cocaine attenuates cocaine's cardiovascular effects in mice: a dose and time course analysis. Int Immunopharmacol. 2001;1:1189–1198. doi: 10.1016/s1567-5769(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 6.Butler VPJ, Schmidt DH, Smith TW, Haber E, Raynor BD, Demartini P. Effects of sheep digoxin-specific antibodies and their Fab fragments on digoxin pharmacokinetics in dogs. J Clin Invest. 1977;59:345–359. doi: 10.1172/JCI108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, Owens SM. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol. 2003;461:119–128. doi: 10.1016/s0014-2999(03)01313-x. [DOI] [PubMed] [Google Scholar]
- 8.Carrera MRA, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 9.Carrera MRA, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci USA. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrera MRA, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection against relapse in a rat model. Proc Natl Acad Sci USA. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrera MRA, Trigo JM, Wirsching P, Roberts AJ, Janda KD. Evaluation of the anticocaine monoclonal antibody GNC92H2 as an immunotherapy for cocaine overdose. Pharmacol Biochem Behav. 2005;81:709–714. doi: 10.1016/j.pbb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Chippaux JP, Goyffon M. Venoms, antivenoms and immunotherapy. Toxicon. 1998;36:823–846. doi: 10.1016/s0041-0101(97)00160-8. [DOI] [PubMed] [Google Scholar]
- 13.Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab')2, and Fab' in mice. Cancer Res. 1986;46:3969–3978. [PubMed] [Google Scholar]
- 14.Eddleston M, Persson H. Acute plant poisoning and antitoxin antibodies. J Toxicol Clin Toxicol. 2003;41:309–315. doi: 10.1081/clt-120021116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, Gefter ML, Exley MA, Swain PA, Briner TJ. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez JM, León G, Lomonte B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin Pharmacokinet. 2003;42:721–741. doi: 10.2165/00003088-200342080-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hardin JS, Wessinger WD, Proksch JW, Owens SM. Pharmacodynamics of a monoclonal antiphencyclidine Fab with broad selectivity for phencyclidine-like drugs. J Pharmacol Exp Ther. 1998;285:1113–1122. [PubMed] [Google Scholar]
- 18.Hearn WL. Cocaine: Pharmacokinetics, Metabolism, and Brain Distribution. In: Nahas GG, Burks TF, editors. Drug Abuse in the Decade of the Brain. Amsterdam: IOS Press; 1997. pp. 183–190. [Google Scholar]
- 19.Hearn WL, Rose S, Wagner J, Ciarleglio A, Mash DC. Cocaethylene is more potent than cocaine in mediating lethality. Pharmacol Biochem Behav. 1991;39:531–533. doi: 10.1016/0091-3057(91)90222-n. [DOI] [PubMed] [Google Scholar]
- 20.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 21.Holton OD, III, Black CD, Parker RJ, Covell DG, Barbet J, Sieber SM, Talley MJ, Weinstein JN. Biodistribution of monoclonal IgG1, F(ab')2, and Fab' in mice after intravenous injection. Comparison between anti-B cell (anti-Lyb8.2) and irrelevant (MOPC-21) antibodies. J Immunol. 1987;139:3041–3049. [PubMed] [Google Scholar]
- 22.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology. 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 23.Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lépine JP. New treatments for cocaine dependence: A focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 24.Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6:257–259. doi: 10.1016/j.autrev.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Keyler DE, Goon DJ, Shelver WL, Ross CA, Nagasawa HT, St Peter JV, Pentel PR. Redistribution and enhanced urinary excretion of 2,2′,4,4′,5,5′-hexachlorobiphenyl (HCB) in rats using HCB-specific IgG and Fab fragments. Biochem Pharmacol. 1994;48:767–773. doi: 10.1016/0006-2952(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 26.Keyler DE, Le Couteur DG, Pond SM, St Peter JV, Pentel PR. Effects of specific antibody Fab fragments on desipramine pharmacokinetics in the rat in vivo and in the isolated, perfused liver. J Pharmacol Exp Ther. 1995;272:1117–1123. [PubMed] [Google Scholar]
- 27.Keyler DE, Roiko SA, Benlhabib E, LeSage MG, St Peter JV, Stewart S, Fuller S, Le CT, Pentel PR. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose- and affinity-response relationships. Drug Metab Dispos. 2005;33:1056–1061. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- 28.Kortt AA, Malby RL, Caldwell JB, Gruen LC, Ivancic N, Lawrence MC, Howlett GJ, Webster RG, Hudson PJ, Colman PM. Recombinant anti-sialidase single-chain variable fragment antibody. Characterization, formation of dimer and higher-molecular-mass multimers and the solution of the crystal structure of the single-chain variable fragment/sialidase complex. Eur J Biochem. 1994;221:151–157. doi: 10.1111/j.1432-1033.1994.tb18724.x. [DOI] [PubMed] [Google Scholar]
- 29.Larsen NA, Zhou B, Heine A, Wirsching P, Janda KD, Wilson IA. Crystal structure of a cocaine-binding antibody. J Mol Biol. 2001;311:9–15. doi: 10.1006/jmbi.2001.4839. [DOI] [PubMed] [Google Scholar]
- 30.Laurenzana EM, Byrnes-Blake KA, Milesi-Hallé A, Gentry WB, Williams DK, Owens SM. Use of anti-(+)-methamphetamine monoclonal antibody to significantly alter (+)-methamphetamine and (+)-amphetamine disposition in rats. Drug Metab Dispos. 2003;31:1320–1326. doi: 10.1124/dmd.31.11.1320. [DOI] [PubMed] [Google Scholar]
- 31.Lin G, Pentel PR, Shelver WL, Keyler DE, Ross CA, Hieda Y, Flickinger MC, Pennell CA, Murtaugh MP. Bacterial expression and characterization of an anti-desipramine single-chain antibody fragment. Int J Immunopharmacol. 1996;18:729–738. doi: 10.1016/s0192-0561(97)85555-5. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd BL, Smith TW. Contrasting rates of reversal of digoxin toxicity by digoxin-specific IgG and Fab fragments. Circulation. 1978;58:280–283. doi: 10.1161/01.cir.58.2.280. [DOI] [PubMed] [Google Scholar]
- 33.Lomonte B, León G, Hanson LA. Similar effectiveness of Fab and F(ab')2 antivenoms in the neutralization of hemorrhagic activity of Vipera berus snake venom in mice. Toxicon. 1996;34:1197–1202. doi: 10.1016/0041-0101(96)00079-7. [DOI] [PubMed] [Google Scholar]
- 34.Maraj S, Figueredo VM, Lynn Morris D. Cocaine and the heart. Clin Cardiol. 2010;33:264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treament of cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Arch Gen Psych. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy LE, Mannelli P, Niculescu M, Gingrich K, Unterwald EM, Ehrlich ME. The distribution of cocaine in mice differs by age and strain. Neurotoxicol Teratol. 2004;26:839–848. doi: 10.1016/j.ntt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 38.McKenzie KM, Mee JM, Rogers CJ, Hixon MS, Kaufmann GF, Janda KD. Identification and characterization of single chain anti-cocaine catalytic antibodies. J Mol Biol. 2007;365:722–731. doi: 10.1016/j.jmb.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMillan DE, Hardwick WC, Li M, Owens SM. Pharmacokinetic antagonism of (+)-methamphetamine discrimination by a low-affinity monoclonal anti-methamphetamine antibody. Behav Pharmacol. 2002;13:465–473. doi: 10.1097/00008877-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Meijler MM, Kaufmann GF, Qi L, Mee JM, Coyle AR, Moss JA, Wirsching P, Matsushita M, Janda KD. Fluorescent cocaine probes: A tool for the selection and engineering of therapeutic antibodies. J Am Chem Soc. 2005;27:2477–2484. doi: 10.1021/ja043935e. [DOI] [PubMed] [Google Scholar]
- 41.Mets B, Winger G, Cabrera C, Seo S, Jamdar S, Yang G, Zhao K, Briscoe R, Almonte R, Woods JH, Landry DW. A catalytic antibody against cocaine prevents cocaine's reinforcing and toxic effects in rats. Proc Natl Acad Sci USA. 1998;95:10176–10181. doi: 10.1073/pnas.95.17.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milenic DE, Yokota T, Filpula DR, Finkelman MA, Dodd SW, Wood JF, Whitlow M, Snoy P, Schlom J. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991;51:6363–6371. [PubMed] [Google Scholar]
- 43.Moss JA, Coyle AR, Ahn JM, Meijler MM, Offer J, Janda KD. Tandem IMAC-HPLC purification of a cocaine-binding scFv antibody. J Immunol Methods. 2003;281:143–148. doi: 10.1016/j.jim.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Norman AB, Norman MK, Buesing WR, Tabet MR, Tsibulsky VL, Ball WJ. The effect of a chimeric human/murine anti-cocaine monoclonal antibody on cocaine self-administration in rats. J Pharmacol Exp Ther. 2009;328:873–881. doi: 10.1124/jpet.108.146407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman AB, Tabet MR, Norman MK, Buesing WR, Pesce AJ, Ball WJ. A chimeric human/murine anticocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J Pharmacol Exp Ther. 2007;320:145–153. doi: 10.1124/jpet.106.111781. [DOI] [PubMed] [Google Scholar]
- 46.Ochs HR, Smith TW. Reversal of Advanced Digitoxin Toxicity and Modification of Pharmacokinetics by Specific Antibodies and Fab Fragments. J Clin Invest. 1977;60:1303–1313. doi: 10.1172/JCI108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohmura N, Lackie SJ, Saiki H. An immunoassay for small analytes with theoretical detection limits. Anal Chem. 2001;73:3392–3399. doi: 10.1021/ac001328d. [DOI] [PubMed] [Google Scholar]
- 48.Owens SM, Mayersohn M. Phencyclidine-specific Fab fragments alter phencyclidine disposition in dogs. Drug Metab Dispos. 1986;14:52–58. [PubMed] [Google Scholar]
- 49.Paula S, Tabet MR, Farr CD, Norman AB, Ball WJJ. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 50.Pentel PR, Keyler DE. Drug-specific antibodies as antidotes for tricyclic antidepressant overdose. Toxicol Lett. 1995;82-83:801–806. doi: 10.1016/0378-4274(95)03596-6. [DOI] [PubMed] [Google Scholar]
- 51.Peterson EC, Laurenzana EM, Atchley WT, Hendrickson HP, Owens SM. Development and preclinical testing of a high-affinity single-chain antibody against (+)-methamphetamine. J Pharmacol Exp Ther. 2008;325:124–133. doi: 10.1124/jpet.107.134395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pitas G, Laurenzana EM, Williams DK, Owens SM, Gentry WB. Anti-phencyclidine monoclonal antibody binding capacity is not the only determinant of effectiveness, disproving the concept that antibody capacity is easily surmounted. Drug Metab Dispos. 2006;34:906–912. doi: 10.1124/dmd.105.005934. [DOI] [PubMed] [Google Scholar]
- 53.Proksch JW, Gentry WB, Owens SM. Anti-phencyclidine monoclonal antibodies provide long-term reductions in brain phencyclidine concentrations during chronic phencyclidine administration in rats. J Pharmacol Exp Ther. 2000;292:831–837. [PubMed] [Google Scholar]
- 54.Redwan e-RM, Larsen NA, Zhou B, Wirsching P, Janda KD, Wilson IA. Expression and characterization of a humanized cocaine-binding antibody. Biotechnol Bioeng. 2003;82:612–618. doi: 10.1002/bit.10598. [DOI] [PubMed] [Google Scholar]
- 55.Reyes S, Kane GC, Miki T, Seino S, Terzic A. KATP channels confer survival advantage in cocaine overdose. Mol Psychiatry. 2007;12:1060–1061. doi: 10.1038/sj.mp.4002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reyes S, Zingman L, Kane GC, Yamada S, Miki T, Seino S, Terzic A. Therapeutic benefit of a KATP-channel opening drug in cocaine toxicity. Clin Pharmacol Ther. 2005;77:P99–P99. [Google Scholar]
- 57.Ritz MC, George FR. Cocaine-induced seizures and lethality appear to be associated with distinct central nervous system binding sites. J Pharmacol Exp Ther. 1993;264:1333–1343. [PubMed] [Google Scholar]
- 58.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 59.Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol Biochem Behav. 2009;93:105–111. doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabouraud A, Urtizberea M, Grandgeorge M, Gattel P, Makula ME, Scherrmann JM. Dose-dependent reversal of acute murine colchicine poisoning by goat colchicine-specific Fab fragments. Toxicology. 1991;68:121–132. doi: 10.1016/0300-483x(91)90015-s. [DOI] [PubMed] [Google Scholar]
- 61.Scherrmann JM, Terrien N, Urtizberea M, Pierson P, Denis H, Bourre JM. Immunotoxicotherapy: present status and future trends. Clin Toxicol. 1989;27:1–35. doi: 10.3109/15563658909038567. [DOI] [PubMed] [Google Scholar]
- 62.Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- 63.Shelver WL, Keyler DE, Lin G, Murtaugh MP, Flickinger MC, Ross CA, Pentel PR. Effects of recombinant drug-specific single chain antibody Fv fragment on [3H]-desipramine distribution in rats. Biochem Pharmacol. 1996;51:531–537. doi: 10.1016/0006-2952(95)02233-3. [DOI] [PubMed] [Google Scholar]
- 64.Sturgeon RD, Fessler RG, Meltzer HY. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur J Pharmacol. 1979;59:169–179. doi: 10.1016/0014-2999(79)90279-6. [DOI] [PubMed] [Google Scholar]
- 65.Urtizberea M, Sabouraud A, Cano N, Grandgeorge M, Rouzioux JM, Scherrmann JM. Reversal of murine colchicine toxicity by colchicine-specific Fab fragments. Toxicol Lett. 1991;58:193–198. doi: 10.1016/0378-4274(91)90173-4. [DOI] [PubMed] [Google Scholar]
- 66.Valentine JL, Arnold LW, Owens SM. Anti-phencyclidine monoclonal Fab fragments markedly alter phencyclidine pharmacokinetics in rats. J Pharmacol Exp Ther. 1994;269:1079–1085. [PubMed] [Google Scholar]
- 67.Valentine JL, Mayersohn M, Wessinger WD, Arnold LW, Owens SM. Antiphencyclidine monoclonal Fab fragments reverse phencyclidine-induced behavioral effects and ataxia in rats. J Pharmacol Exp Ther. 1996;278:709–716. [PubMed] [Google Scholar]
- 68.Valentine JL, Owens SM. Antiphencyclidine monoclonal antibody therapy significantly changes phencyclidine concentrations in brain and other tissues in rats. J Pharmacol Exp Ther. 1996;278:717–724. [PubMed] [Google Scholar]
- 69.Visalli T, Turkall R, Abdel-Rahman MS. Gender differences in cocaine pharmacokinetics in CF-1 mice. Toxicol Lett. 2005;155:35–40. doi: 10.1016/j.toxlet.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Withers NW, Pulvirenti L, Koob GF, Gillin JC. Cocaine abuse and dependence. J Clin Psychopharmacol. 1995;15:63–78. doi: 10.1097/00004714-199502000-00010. [DOI] [PubMed] [Google Scholar]