Abstract
Methamphetamine/polysubstance abuse in women of childbearing age is a major concern because of the potential long-term detrimental effects on the brain function of the fetus following in utero exposure. A battery of established tests, including the Wechsler Abbreviated Scale of Intelligence, Conners’ Continuous Performance Test II, Behavioral Rating Inventory of Executive Function, the CMS Family Pictures and Dot Location tests, the Spatial Span test from the WISC-IV-Integrated, and a recently developed spatial learning and memory measure (Memory Island), was used to assess the effects of prenatal drug exposure on neurobehavioral performance. Participants were 7 to 9 year old children from similar socioeconomic backgrounds who either had (N = 31) or had not (N = 35) been exposed to methamphetamine/polysubstance during pregnancy. Compared to unexposed children, exposed children showed pronounced elevations (i.e. more problems) in parental ratings of executive function, including behavioral regulation and metacognition. Exposed children also exhibited subtle reductions in spatial performance in the Memory Island test. In contrast, IQ, Spatial Span, Family Pictures, Dot Location, and vigilance performance was unaffected by prenatal drug exposure history. Thus, children of women who reported using methamphetamine and other recreational drugs during pregnancy showed a selective profile of abnormalities in parentally rated executive function.
Keywords: alcohol, learning, neuropsychology, nicotine
1. INTRODUCTION
A greater number of pregnant women receiving drug treatment services in the United States listed their primary drug as amphetamines than opiates, cocaine, marijuana, or even alcohol (Terplan et al. 2009). A longitudinal study showed more pregnancy complications including low maternal weight gain, increased premature delivery, and neonatal mortality of women who had been unable to discontinue amphetamine use during the first trimester, relative to those who had terminated use (Eriksson et al. 1981). By twelve months of age, these infants had been admitted more frequently to the hospital and exhibited abnormalities in emotional development (Billing et al. 1980). General IQ was slightly lower than the population average for other preschoolers (Billing et al. 1985) and the frequency of maternal amphetamine use was associated with aggression in eight-year-olds (Billing et al. 1994). Ten-year-old girls whose mothers used amphetamine were shorter and weighed less. Fourteen-year-olds of both sexes with a history of prenatal amphetamine exposure were three times as likely to be behind a grade in school relative to other children from the same area. However, a clear majority of women who used amphetamine during pregnancy also used alcohol and nicotine (Cernerud et al. 1996) and amphetamine may only be a component in the teratogenic profile for their offspring.
Relative to amphetamine, much less is known about the neuro-functional profile of children with a history of methamphetamine exposure. In utero methamphetamine exposure increased the risk for being born small for gestational age (Chomchai et al. 2004) and of having CNS birth defects (Forrester & Merz, 2007). Full-term neonates with a positive toxicology for methamphetamine exhibited a seven-fold increase in echoencephalographic abnormalities, including ventricular enlargement, hemorrhages, and elevated white-matter density (Dixon & Bejar, 1989). Neonates with a history of methamphetamine and other substance exposure exhibit a selective, but replicable, neurobehavioral profile (Lagasse et al. 2010; Smith et al. 2008) independent of maternal depression levels (Paz et al. 2009). Neurochemical abnormalites in frontal white matter were also documented among 3- to 4-year-old children (Chang et al. 2009). Eight-year-old children exposed to methamphetamine during at least two-trimesters of pregnancy had higher striatal creatine levels (Smith et al. 2001). Therefore, given the prevalence of methamphetamine use by women of reproductive age (Slutsker et al. 1993; Terplan et al. 2009), particularly among women that subsequently lose child custody (DHS, 2007) and increasing evidence of neurostructural alterations (Chang et al. 2004, 2009; Smith et al. 2001), the objective of this study was to examine behavioral performance among primary school children that had been exposed to methamphetamine during pregnancy.
2.0 MATERIAL & METHODS
2.1 Study Participants
Flyers posted at the Oregon Health Science University (OHSU) and in the Portland, Oregon, metro area were used to recruit exposed and unexposed children. Online advertisements for the study were placed on the OHSU study participant recruitment website, on Craigslist, and on the website for the Post-Adoption Resource Center of Oregon. Unexposed children were recruited from the same community based on similar household income levels during pregnancy and age as exposed children. The inclusion criteria for this study were that the potential participant be a 7- to 9-year-old boy or girl. This period was selected based on informal parental reports that difficulties in exposed children become most apparent after they start school, as well as prior experience with the test battery at this age (Piper et al. 2010). Child exclusion criteria were having been born more than five weeks premature; epilepsy, Tourette’s syndrome, cerebral palsy, a head injury or severe brain trauma, substantial visual impairments or any congenital abnormalities, all of which would interfere with cognitive assessments. The parents/guardians of all participants provided informed consent for participation in the study which had been approved by the Institutional Review Board of OHSU and carried out in accordance with the Code of Ethics of the World Medical Association. Parents/guardians also completed a medical release for their child. The OHSU electronic medical record database was examined to verify the exclusion criteria and prenatal drug exposure. The incentive for participation was a fifty-dollar gift certificate to a toy store (Toys-R-Us). A National Institute on Drug Abuse Certificate of Confidentiality was obtained which ensured the confidentiality of information regarding maternal substance use.
2.2 Behavioral Assessments
The children were tested during a session that averaged about 2 hours and 30 minutes. The sequence of tests was Dot location, Memory Island, Wechsler Abbreviated Scale of Intelligence, Conner’s Continuous Performance Test II, Family Pictures, and Spatial Span. The parents completed the Behavior Rating Inventory of Executive Function (BRIEF) as well as a maternal history questionnaire that determined demographics including whether the child had been diagnosed with Attention Deficit Hyperactivity Disorder (ADHD), ethnicity, income during pregnancy and currently (< 15K, 15–35K, or > 35 K/year) and identified the recreational and therapeutic drugs used during pregnancy. This battery has been employed previously (Piper et al. 2010) and each of these measures are described briefly below.
2.2.1 Behavior Rating Inventory of Executive Function (BRIEF)
The BRIEF is an 86-item parental questionnaire of executive functioning in the context of the child’s everyday activities. Behaviors in the last six months are rated as never, sometimes, or often a problem (1 to 3 points, respectively). The parents of children diagnosed with ADHD were instructed to, when possible, base their evaluations on those times when their child was medication-free. The 8 BRIEF scales form two dimensions of executive functioning (Metacognition and Behavioral Regulation) and a measure of overall executive functioning (Global Executive Composite). The Metacognition Index comprises the following categories: Initiate (e.g., has trouble coming up with ideas for what to do in play time), Working Memory (e.g., when given three things to do, remembers only the first or last), Plan/Organize, Organization of Materials, and Monitor scales. The Behavioral Regulation Index is composed of scales measuring Inhibit (e.g., interrupts others), Shift, and Emotional Control. Standardized T50 scores were calculated from raw scores based on age and sex norms. A T50 ≥ 65, i.e., 1.5 standard deviations above the mean, is interpreted as a clinically significant impairment (Goia et al. 2000). This parental rating instrument has been employed previously with non-biological (i.e. adoptive) parents (Groza et al. 2008; Jacobs et al. 2010; Lansdown et al. 2007). The BRIEF also includes an inconsistency and a negativity scale. A raw score of ≤6 on the inconsistency scale is deemed acceptable, 7–8 is questionable, and ≥ 9 is inconsistent. A negativity point was earned for each “often” rating on nine items (e.g., forgets what he/she was doing, says the same things over and over). A negativity score of ≤4 is acceptable, 5–6 is elevated, and ≥7 is highly elevated. The internal consistency of the BRIEF is quite good (Cronbach’s alpha = 0.98 in a clinical sample) and detailed convergent and discriminant data is available elsewhere (Goia et al. 2000).
2.2.2 Conners’ Continuous Performance Test (CPT)
The CPT II is a 14 min computerized assessment of vigilance where respondents press the space bar whenever any letter except ‘X’ is displayed on the computer screen. The primary response variables are response measures (mean hit reaction time, reaction time standard error (RT SE), and number of omission and commission errors), and signal detection (d’, the perceptual sensitivity to targets, and B, an index of a more risky response style) (Conners & Jeff, 1999).
2.2.3 Wechsler Abbreviated Scale of Intelligence (WASI)
The Vocabulary, Block Design, and Matrix Reasoning subtests were completed to assess performance relative to normative data (Psychological Corporation, 1999).
2.2.4 Family Pictures
In the Family Pictures visual recognition test (FPI and FPII; 30 minute interval), children are shown pictures of people in a particular scene and asked to remember everything they can about each scene (4 scenes total). Immediate recall is assessed by asking who was in the scene, where they were in the scene (based on a quadrant division of the scene), and a basic description of what they were doing in the scene (eating, gardening etc.). This provides an index of the processing, encoding, and recollection abilities of the child and requires verbal as well as visual processing (Cohen, 1997).
2.2.5 Dot Location Test
Participants are shown an array of dots over three trials and then a single distracter array is presented during this test. Subjects are then asked to recall the original dot array and the distracter array. After an interval of 5 min, the children are again asked to recall the original dot array. The objectives of this test are to detect changes between training and test images and correctly identifying training arrays after the presentation of distracter images. Overall, this provides a measure of the child’s ability to process, learn, and recall spatial location (Cohen, 1997).
2.2.6 Spatial Span
The spatial span provides a measure of visual-spatial working memory and is a subtest of the Wechsler Intelligence Scale for Children IV Integrated (Wechsler et al. 2004). The child watches an examiner tap a sequence of numbered cubes on the Spatial Span board (number side faces examiner) and then is asked to tap out the same sequence. The spatial span is discontinued if a subjects scores 0 on each of two trials of the same item. In the first test, the child must repeat the same order (Spatial Span Forward) and, in the second test, the order is reversed (Spatial Span Backward).
2.2.7 Memory Island
Memory Island is a test of spatial learning and memory requiring navigation developed for use with children (Piper et al. 2010). The virtual world simulates an island environment of 347 × 287 m comprising four quadrants, each containing a different target item. Testing equipment included a 48.3 cm Dell computer monitor and a Microsoft Sidewinder joystick to control the direction and speed of movement. The participants were first asked to navigate to a target location visibly marked with a flag adjacent to the target (visible trials) and then trained to navigate to a hidden target (i.e., no flag adjacent to the target) in four subsequent trials. The starting location for all trials was always the center of the island (X, Y coordinate 0, 0). The location of the hidden target, the sculpture, was kept constant for all participants (+285, −328). Twenty minutes following the last hidden target trial, the participant received a single 30-second probe trial (target removed) with the objective of finding the target. In each trial, movement of the participants is tracked and recorded in time-stamped coordinate files, which are used to calculate distance traveled (virtual units), latency to reach the target (seconds), and speed (virtual units/second). The percentage of time spent in the target relative to the non-target [(left + right + opposite)/3] quadrants was determined for the probe trial.
2.2.8 Parental Child Development Questionnaire
The biological mother or legal guardian completed 53-items about his or her child’s history (e.g., Is your child attending school at, above, or below age level? Is your child’s reading ability at, above, or below age level? Has your child’s biological father been involved in your child’s life?), demographics (e.g., What was your family income during the past 12 months/the year you were pregnant?), and maternal drug use during pregnancy (e.g., During your pregnancy, how many cigarettes did you smoke on an average day?). If information was unknown, particularly regarding the timing of substance exposure, this was recorded as such (this accounts for the lower N/group on selected measures in Table 1). Some adoptive parents also provided verification of prenatal drug exposure including photocopies of urine analyses, the child’s birth certificate, or maternal legal documentation.
Table 1.
Maternal substance-use during pregnancy and demographic information from unexposed and exposed groups.
| Unexposed | N | Exposed | Na | |
|---|---|---|---|---|
| Trimesters of methamphetamine use (0 to 3) | 0.0 (0.0) | 35 | 2.5 (0.3)*** | 23 |
| Nicotine (%) | 17.1% | 35 | 76.2%*** | 21 |
| Alcohol (%) | 11.4% | 35 | 71.4%*** | 21 |
| Marijuana (%) | 11.4% | 35 | 58.8%*** | 17 |
| Birth Weight (grams) | 3,279 (114) | 35 | 3,367 (194) | 19 |
| Income While Pregnant (% < 15,000/year) | 17.1% | 35 | 81.0%*** | 25 |
| Current Income (% > 35,000/year) | 51.4% | 35 | 60.0% | 25 |
| Maternal age at birth (of child) | 26.9 (1.1) | 35 | 29.8 (1.5) | 23 |
| Biological father involved in child’s life (%) | 85.7% | 35 | 44.8%*** | 29 |
| Number of siblings or other children in home | 2.7 (0.3) | 35 | 1.3 (0.3)** | 27 |
Note that the group size is ≠31 due to missing/unknown information;
p < .01;
p < .001.
2.3 Statistical analysis
All analyses were conducted using SPSS, version 16.0 with data expressed as mean (±SEM). Due to the number of neurobehavioral tests, a p < .01 was considered statistically significant although findings meeting more liberal alphas (p < .05) were noted for group (unexposed versus exposed) comparisons. If a group difference between exposed and unexposed groups was identified, exploratory subgroup analyses, e.g., exposed ADHD positive (+) versus exposed ADHD negative (−), were also completed. Continuous level data was examined using ANOVAs, with repeated measures (REM) as the within-subjects variable (where applicable) and Exposure as the between-groups factor. Categorical measures were analyzed with a χ2, or, if the N/cell was <5, Likelihood ratios. Pearson product moment correlations were completed to examine the association among measures.
3.0 RESULTS
Table 1 shows the demographics and the maternal substance-use habits during pregnancy. Mothers of methamphetamine-exposed children were of the same age and had a similar current household income as the mothers of the children in the unexposed group. In contrast, incomes were significantly more likely to be lower (<$15,000) during pregnancy for the mothers of the exposed cohort. As anticipated, mothers who used methamphetamine throughout their pregnancies were more likely to report using nicotine, alcohol, marijuana, and other drugs, primarily opiates, during this period.
Exposed (N=31) and unexposed (N=35) children did not differ in terms of gender, age, birth weight, or ethnicity (Table 2). Significantly more of the exposed children were rated as being behind similarly aged children in school. Approximately twice as many of the exposed group were behind their classmates in reading (p = .09). An ADHD diagnosis was four-fold more likely among exposed children. Notably, the majority of ADHD children in both groups were currently receiving medication for this condition at the time of testing (75% in the unexposed and 80% in the exposed group).
Table 2.
Demographic and academic characteristics of unexposed (N = 35) and exposed (N = 31) children.
| Unexposed | Exposed | |
|---|---|---|
| Age | 8.4 (0.2) | 8.1 (0.2) |
| Gender (% Female) | 42.9% | 53.3% |
| Ethnicity (% non-Caucasian) | 25.7% | 20.0% |
| Below Grade level (%) | 3.0% | 25.9%* |
| Below Reading level (%) | 20.6% | 38.5% |
| ADHD | 11.8% | 46.7%*** |
p < .05;
p < .005
3.1 Executive Function (BRIEF)
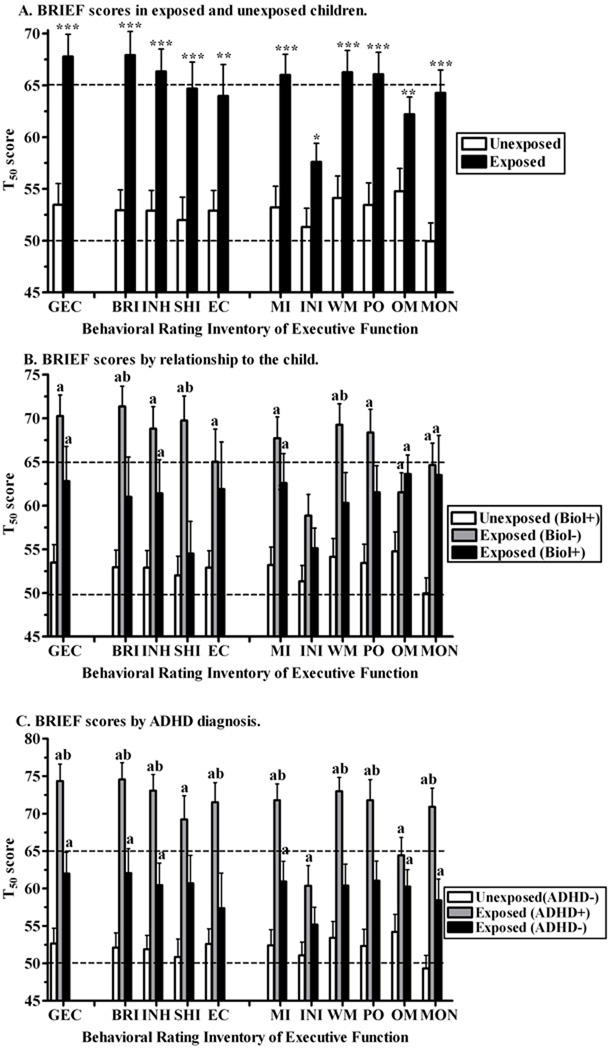
There was a pronounced difference in parental ratings of executive function. Unexposed children had ratings closely distributed around the population norm (t50 = 50). However the exposed mean was statistically increased (p < .01) for the Global Executive Composite, both indices, and all scales except the Initiate scale (Figure 1A). Note that the elevation in the exposed Global Executive Composite was still retained (p ≤ .001) with ADHD diagnosis (also described below), prenatal alcohol, nicotine, or marijuana included as covariates in the analysis.
Fig. 1.
Parental Behavioral Rating of Executive Function (±SEM) in prenatally unexposed and exposed children. GEC = Global Executive Composite (total score), BRI = Behavioral Regulation Index and component scales INH = Inhibit, SHI = Shift, EC = Emotional Control; MI = Metacognition Index and component scales INI = Initiate, WM = Working Memory, PO = Plan/Organize, OM = Organization of Materials, and MON = Monitor. Higher scores indicate more problems on that domain. A) All subjects (**p < .01; ***p < .0005 versus exposed). B) Sub-group analysis comparing ratings made by biological (+: mother, father, grandmother) versus non-biological (adoptive or foster parent) raters (ap < .05 versus unexposed, bp < .05 versus exposed, biological+ rater). C) Sub-group analysis comparing ratings by exposure and Attention Deficit Hyperactivity Disorder (+/−) diagnosis (ap < .05 versus unexposed/ADHD−, bp < .05 versus exposed/ADHD−).
Examination of individual scales revealed clinically significant elevations on the Inhibit, Working Memory, and Plan/Organize scales. For the unexposed group, all inventories were completed by a biological relative, almost always the mother (88.9%), with the remainder by a grand-parent (5.6%) or the father (5.6%). In contrast, for the exposed children, the majority of the inventories were completed by non-biological, i.e., adoptive or foster, parents (66.7%). Further examination was made to determine whether there was any difference based on who was completing the BRIEF (biological versus non-biological relative). Global Executive Composite scores were statistically elevated among exposed children, independent of the relationship of the rater. However, among exposed children, the non-biological raters indicated that their children had more problems on the Behavioral Regulation Index, especially the Shift and the Working Memory scales (Figure 1B).
Figure 1C shows statistically, but not clinically, significant elevations among exposed children that had not been diagnosed with ADHD. Exposed children that were ADHD− also had statistically significantly elevated BRIEF scores relative to ADHD− unexposed cohort. Further, the executive function profile was statistically significantly more problematic, generally by a full standard deviation, in exposed ADHD+ relative to exposed ADHD− children. Note that the ADHD+ unexposed condition was omitted for clarity because there were only four children in this group.
Negativity scale scores met the criteria for elevated more frequently in exposed children (Unexposed = 3.0%, Exposed = 20.0%, Likelihood ratio (1) = 4.97, p < .05). Additionally, among the exposed group, the non-biological ratings (Mean = 2.8±0.5) were more negative relative to those made by biological (Mean = 0.7±0.4) parents (t(27.5) = 3.21, p < .005). In contrast, exposed ADHD+ ratings (2.9±0.6) were not significantly higher than exposed ADHD− (1.4±0.5) ratings (t(28) = 1.95, p = .062). Inconsistency scale scores were acceptable for 94.6% of unexposed children and 96.7% of exposed children. There were no differences among exposed children on inconsistency ratings based on sub-groups (ADHD diagnosis or rater-relationships).
3.2 Vigilance (CPT)
The t-scores for reaction times, SE of RT, omissions, commissions, d’, and response style (B) did not differ by prenatal exposure history, indicating no effect on vigilance, impulsivity, or inattentiveness (Table 3). The percentage of each group with high commission errors (t50 ≥ 65) was not significantly more prevalent among exposed (21.4%) relative to unexposed children (5.9%, p = .07).
Table 3.
Neurobehavioral function in exposed and unexposed children.
| Unexposed | Exposed | |
|---|---|---|
| WASI (T-score) | ||
| Vocabulary | 51.0 (2.2) | 46.3 (1.7) |
| Matrix Reasoning | 53.8 (1.9) | 48.4 (2.2) |
| Block Design | 52.7 (2.2) | 50.0 (1.8) |
| IQ | 104.5 (3.7) | 95.1 (3.5) |
| Conners’ CPT (T-score) | ||
| Reaction Time | 51.4 (1.8) | 51.3 (2.0) |
| SE of Reaction Time | 56.1 (1.3) | 54.0 (2.2) |
| Omissions | 51.1 (1.5) | 53.0 (2.4) |
| Commissions | 53.0 (1.4) | 51.5 (1.6) |
| d’ | 52.6 (1.2) | 52.6 (1.6) |
| Response style (B) | 49.4 (1.2) | 49.8 (1.4) |
| Spatial Span (Raw Score) | ||
| Forward | 5.7 (0.3) | 5.3 (0.3) |
| Backward | 4.9 (0.4) | 4.5 (0.4) |
| Family Pictures (Percentile) | ||
| Immediate | 57.0 (5.3) | 46.5 (5.1) |
| Delayed | 52.6 (5.7) | 43.4 (5.1) |
| BRIEF (Raw Score) | ||
| Inconsistency | 3.4 (0.3) | 2.8 (0.4) |
| Negativity | 0.6 (0.2) | 2.1 (0.4)** |
p < .01
3.3 Intelligence (WASI)
Both groups had a 2-subtest IQ that was within the normal range. Although the exposed mean was over a half standard deviation lower, this difference did not fulfill the criteria for significance (p = .07). Similarly, group differences were non-significant on the Vocabulary (p = .09) and Matrix Reasoning (p = .07, Table 3) scales.
3.4 Visual/Verbal Memory (Family Pictures)
A mixed Interval (Immediate versus Delayed) by Exposure ANOVA identified only an Interval effect (F(1,56) = 5.6, p < .05, Table 3).
3.5 Spatial Cognition (Dot Location)
A mixed Delay (Short vs. Long)×Exposure ANOVA revealed a main effect of Delay (F(1,61) = 16.38, p ≤ .0005), but not of exposure (data not shown).
3.6 Spatial Working Memory (Spatial Span)
An Order (Forward vs. Backward)×Exposure mixed ANOVA identified the anticipated Order effect (F(1,61) = 16.4, p ≤ .0005), but no significant effect of exposure (Table 3).
3.7 Spatial Learning & Memory (Memory Island)
Data were analyzed separately for the visible, hidden, and probe trials. During the four visible trials, a mixed Trial by Exposure ANOVA on speed revealed an effect of Trial (F(2.2,120.5) = 13.2, p ≤ .0005) and a Trial×Exposure interaction (F(2.2,120.5) = 3.2, p <.05). Exposed children moved slower on the first visible trial (Exposed = 8.4 + 0.4, Unexposed = 9.5 + 0.3 virtual units/sec, t(56) = 2.21, p < .05) but no exposure effects were noted during the hidden trials.
In the spatial memory (probe) trial, unexposed children searched significantly more in the quadrant that previously contained the target (45.1 ± 6.2%) relative to the non-target quadrants (18.3 ± 2.7%, t(31) = 3.25, p < .005). In contrast, the exposed group did not (Target = 37.8 ± 6.7, Non-target = 20.7 ± 2.2, t(25)=1.93, p = .065).
3.8 Correlations
Table 4 shows the correlations among the neurobehavioral indices. There were strong intra-test associations among measures on the BRIEF, WASI, Continuous Performance Test, Spatial Span, and Family Pictures. Significant, but low, negative correlations were noted between the BRIEF and Family Pictures and moderate correlations were identified with the WASI and Spatial Span. Children that spent more time in the target quadrant during Memory Island probe trial also showed better performance on the WASI.
Table 4.
Correlations among neurobehavioral measures. BRIEF = Behavioral Rating Inventory of Executive Function; GEC = Global Executive Composite; BRI = Behavioral Rating Index; MI = Metacognition Index; WASI = Weschsler Abbreviated Scale of Intelligence; MR = Matrix Reasoning; BD = Block Design; CPT = Connor’s Continuous Performance Test; RT = Reaction Time; SE = Standard Error; SS = Spatial Span; FP = Family Pictures; and MI = Memory Island.
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. BRIEF: GECs | +1.00 | ||||||||||||||
| B. BRIEF: BRIs | +0.94c | +1.00 | |||||||||||||
| C. BRIEF: MIs | +0.97c | +0.82c | 1.00 | ||||||||||||
| D. WASI: IQs | −0.21 | −0.18 | −0.22 | +1.00 | |||||||||||
| E. WASI: Vocabs | −0.23 | −0.24 | −0.21 | +0.85c | +1.00 | ||||||||||
| F. WASI: MRs | −0.17 | −0.10 | −0.20 | +0.85c | +0.45c | +1.00 | |||||||||
| G. WASI: BDs | +0.21 | −0.19 | −0.22 | +0.77c | +0.77c | +0.57c | +1.00 | ||||||||
| H. CPT: RTs | −0.08 | −0.10 | −0.07 | −0.07 | +0.06 | −0.15 | +0.13 | +1.00 | |||||||
| I. CPT: SE of RTs | +0.01 | −0.03 | +0.01 | −0.23 | −0.08 | −0.25 | +0.10 | +0.70 | +1.00 | ||||||
| J. CPT: Ommissions | +0.05 | +0.04 | +0.05 | −0.25 | −0.12 | −0.25 | +0.01 | +0.54c | +0.80c | +1.00 | |||||
| K. SS: Forward | −0.07 | −0.07 | −0.06 | +0.61c | +0.43b | +0.56c | +0.42a | −0.30a | −0.32a | −0.31a | +1.00 | ||||
| L. SS: Backward | −0.18 | −0.15 | −0.19 | +0.54c | +0.42b | +0.46c | +0.51c | −0.21 | −0.27a | −0.24 | +0.64c | +1.00 | |||
| M. FP: Immediates | −0.24 | −0.26a | −0.21 | +0.19 | +0.34a | +0.00 | +0.14 | −0.04 | −0.13 | −0.22 | +0.27a | +0.21 | +1.00 | ||
| N. FP: Delayeds | −0.31a | −0.30a | −0.29a | +0.23 | +0.29a | +0.12 | +0.21 | +0.05 | −0.13 | +0.14 | +0.30a | +0.26 | +0.85c | +1.00 | |
| O. MI: Target | −0.23 | −0.20 | −0.24 | +0.43b | +0.40b | 0.34b | +0.37b | −0.14 | −0.23 | −0.21 | +0.17 | +0.19 | +0.16 | +0.14 | +1.00 |
p < .05,
p < .005,
p < .0005
4.0 DISCUSSION
Neurobehavioral assessments of school-aged children with a history of prenatal methamphetamine, nicotine, and alcohol exposure revealed pronounced dysfunction in executive function and a slight reduction in spatial memory. Parental ratings were clinically significant on the Global Executive Composite as well as both the Behavioral Regulation and Metacognition Indexes, which indicate substantial difficulties in everyday tasks, including working memory, impulse control, setting goals, flexibility, and emotional control, both at home and at school. As prenatal amphetamine exposure was also associated with an increased likelihood of being behind a grade in school (Cernerud et al. 1996), the identified impairments are likely contributing factors to the academic success of children so exposed. Although the ratings of exposed children that were and were not living with a biologically related family member were both elevated, there was also some evidence to indicate that the executive function profile was more problematic when the BRIEF was completed by a non-biologically related caregiver. One possibility is that the children placed in the care of an adoptive parent are more severely affected by their early developmental history and show more difficulties with behavioral regulation and working memory. This could result from a heavier pattern of drug abuse among women that subsequently lose child custody. A second possibility is that adoptive parents have a tendency to rate their children’s behavior more extremely than do biological parents. Importantly, the BRIEF has been frequently employed in other research with adopted children (Groza et al. 2004; Jacobs et al. 2010; Lawson et al. 2007). For example, Lawson and colleagues (2007) obtained BRIEF ratings of adoptive children made by their parents and their teachers. Although no formal statistical comparison was made in this report, examination of the means reveals that scores were generally concordant across raters for girls, but higher (more problematic) for boys for parental ratings. Further, the higher negativity ratings of children with a methamphetamine history, particularly those made by the non-biologically related parents, would support the second explanation. The elevated negativity is also cause for some concern as maternal methamphetamine use may result in a stigmatization of these children which could diminish parental expectations.
Exposed children exhibited very subtle deficits in spatial function as measured by Memory Island. Exposed children navigated around the virtual island more slowly on the first target visible trial. Interestingly, a prior study determined that speed on this trial shows a marked progression with age (Piper, Acevedo, McGinness, & Raber, unpublished observations). This finding, in conjunction with earlier investigations that revealed abnormalities copying a geometric pattern (Chang et al. 2004, 2009), suggests prenatal exposure may cause developmental delays in visual-motor integration. With the exception of this transient speed difference, performance was generally similar for both groups during the eight consecutively conducted visible and hidden trials of Memory Island, indicating that spatial learning was unaffected. However, unlike the unexposed group, exposed children did not spend significantly more time in the target relative to non-target quadrants during the probe trial which is indicative of a reduction in spatial memory. Early developmental exposure to recreational drugs alters dendritic morphology in the hippocampus (González-Burgos et al. 2006; Roy & Sabherwal, 1998; Williams et al. 2004), a structure that is integral to spatial function (Bird & Burgess, 2008). This finding with Memory Island extends upon a large body of preclinical research with early developmental methamphetamine exposure and water-maze assessments in adulthood (Golub et al. 2005; Siegel et al. 2010; Williams et al. 2003) and highlights the utility of a translational approach.
An interesting and novel finding from this investigation was that the frequency of an ADHD diagnosis was four-fold more common in the exposed group. Prior cross-sectional studies have identified as much as a three-fold increase in the rates of ADHD in children exposed prenatally to alcohol or nicotine, and this association appears to be dose dependent (Banerjee et al. 2005; Huizink & Mulder, 2006). It should also be emphasized that the parents and guardians of children diagnosed with ADHD were instructed to, if possible, base their evaluations on those times when their child was not under the influence of any cognitive enhancing drugs. The vast majority of the participants diagnosed with ADHD had received methylphenidate or other similar drug therapies prior to behavioral testing in this investigation. The question of whether pharmacotherapies for ADHD can improve neurobehavioral function, particularly spatial performance, has received some attention. Methylphenidate-naïve children diagnosed with ADHD showed an improvement in visual-spatial working memory and on some measures of motor speed following acute methylphenidate (Kempton et al. 1999). Similarly, amphetamine increased the spatial span in children with ADHD (Alloway et al. 2009). Exposed ADHD+ children did slightly, albeit non-significantly, better on the Memory Island probe trial than did exposed ADHD− children. Together, the ability of ADHD medications to improve neurobehavioral function in exposed children warrants additional research.
Although maternal ratings revealed clear abnormalities in executive function, several behavioral assessments that might be anticipated to corroborate this dysfunction (e.g., CPT, Dot Location, Spatial Span, or WASI) were unaffected. The present data suggests that some elements of the more global experiences of the parent ratings may be manifested in their child’s behavior in the virtual world created by Memory Island. Further, our qualitative impression is that some of the exposed children were much more challenging to test (e.g., requiring more breaks), especially in the latter half of the 2.5 hour session, which provides some verification for the parental BRIEF reports. Additional evidence and detailed explanations for the previously documented disassociation between laboratory based measures and parent ratings of executive function may be found elsewhere (Bodnar et al. 2007; Mcauley et al. 2010).
The postnatal environment of a child living with a biological mother who uses methamphetamine is likely to be non-optimal (Messina et al. 2010). Women who use drugs during pregnancy have high rates of psychiatric disorders as well as histories of emotional and/or sexual abuse, and their home environments often include domestic violence (Datner et al. 2007; Kissin et al. 2001; Medrano et al. 1999; Paz et al. 1999). Any one of these factors could adversely influence the cognitive development of a child. Animal researchers can easily cross-foster exposed pups onto non-methamphetamine–treated dams (Hrubá et al. 2009). A somewhat analogous practice is also occurring clinically as approximately two-thirds of the exposed children were currently residing in homes that did not include the biological mother. Clearly, the existence of group differences between exposed and unexposed children on ratings of executive function, largely independent of the rater, indicates that the prenatal environment may be a key determinant of these outcomes. As it has been estimated that as many as half of the children that entered the foster care system in the state of Oregon have a parent who uses methamphetamine (Oregon DHS, 2007), these findings may be particularly relevant for foster-care providers and adoptive families. Although the inclusion of adopted children in this study offers some pronounced limitations (e.g. note the smaller N on some measures in Table 1 where selected information on maternal substance use was unavailable from the adoptive mother), the inclusion of children living with both adoptive and biological parents is, overall, a novel aspect and strength of this report. Alternatively, children that are subsequently adopted may be subject to several influences (e.g. prenatal stress, neglect/abuse) in conjunction with maternal substance abuse which could adversely impact neurodevelopment. It should therefore be emphasized that prior investigations of executive function in adoptive children have been completed. Despite the many challenges facing adoptive children, there is often a tremendous resilience as well. Only 11% of international adoptees met the criteria for problems in executive function by the age of four (Jacobs et al. 2010). One could conceivably argue the key time period in prior investigations of methamphetamine exposed children (Chang et al. 2004, 2009) could be postnatal, rather than prenatal. Overall, incorporation of adopted children that are living in homes that are more similar to unexposed children, although raising some complexities, also allows for greater specificity of the origins of any group differences and provides an important contribution to the larger literature on early developmental methamphetamine (Golub et al. 2005; Lagasse et al. 2010; Lu et al. 2009; Meyer & Piper, 2010; Smith et al. 2001, 2006, 2008; Sowell et al. 2010).
Some clear limitations of this cross-sectional investigation as well as future directions should be noted. First, because women who use methamphetamine during pregnancy tend to concurrently abuse other recreational drugs (Chang et al. 2004, 2009; Paz et al. 2009; Smith et al. 2001, 2006, 2008), it is exceedingly difficult, if not impossible, to obtain a sample of women who use methamphetamine exclusively. Other substances, including recreational drugs and/or the chemicals used in manufacturing methamphetamine (Messina et al. 2010) acting alone or in combination with methamphetamine, or early developmental factors might have contributed to the neurobehavioral profile observed. Children with Fetal Alcohol Syndrome show pronounced impairments in both executive function and spatial learning and memory in a virtual water-maze (Hamilton et al. 2003, Schonfeld et al. 2006). However, the present study did not include children with either mental retardation or Fetal Alcohol Syndrome. Although not yet a common practice in this research area (Chang et al. 2004, 2009; Dixon & Bejar, 1989; Lagasse et al. 2010; Paz et al. 2009; Smith et al. 2001, 2006, 2008), based on the present outcomes, more reports that include a separate group of polysubstance users who do not abuse methamphetamine are warranted (Lu et al. 2009; Sowell et al. 2010; Roussotte et al. 2010) as, without this separate group, the present data only implies that methamphetamine is a component teratogen in these children. As women that exclusively use methamphetamine during pregnancy are virtually non-existent and standard research ethics preclude controlled maternal administration, preclinical studies (Golub et al. 2005; Siegel et al. 2010; Slamberová et al. 2005; Williams et al. 2003, 2004) are crucial as such reports will allow more satisfactory inferences regarding causality and specificity.
Second, children with an ADHD diagnosis and who received drug therapies were included in the sample. It is tempting to speculate, as some parents did, that methylphenidate and other cognitive enhancing drugs provide clear benefits for these children. Therefore, it is possible that stimulant medications obscured some behavioral deficits that would have been identified in a drug-free state. A within-subjects design in which exposed children are assessed on and off drug therapy is needed to evaluate this hypothesis. Notably, an earlier cross-sectional report excluded children with ADHD and, in general, obtained complementary results (Chang et al. 2004). The length of testing (2.5 hours) may have resulted in fatigue and, thus, may not accurately assess the children's cognitive abilities. Additional research is ongoing with a more focused emphasis on objective measures of executive function (e.g. with the Tower of London test).
Third, and perhaps foremost, the majority of exposed children were not currently living with their biological mothers. In these instances, retrospective identification (which always much be interpreted cautiously even when completed by the biological mother) of methamphetamine use during pregnancy came from other sources including the biological father, grandparents, laboratory analyses, maternal reports contained within the medical records obtained from the antenatal period, birth certificates, or other legal documents from the mother’s incarceration or custody hearing. More specifically, urine analysis positive for amphetamines for either the biological mother during pregnancy or the neonate was available for 52.6% (10/19) of adopted participants. Although inclusion of both ADHD+ and adopted children increases the generalizability of results, detailed information about the precise timing and extent of maternal drug use was unavailable for some adopted children (note reduced N on some measures on Table 1 due to unavailable information). On the other hand, as such a sizeable number of children exposed prenatally to methamphetamine in Oregon are adopted (Oregon DHS, 2007), we strongly believe that these families are worthy of systematic investigation while simultaneously keeping in mind the substantial caveats this group presents. Additional neurobehavioral studies of adoptive children without a prenatal exposure history are needed and are ongoing. Finally, the current study did not evaluate or screen for attachment issues which may be a concern in this population. As the living situations of exposed children were often rather complex, e.g., sometimes involving multiple transitions between foster care placements, adoptive parents, grandparents, as well as the biological mother, a larger sample will be needed to provide subgroup analyses to further determine the extent that an optimal postnatal context can mitigate against the sequelae of the prenatal history.
In conclusion, this study, in conjunction with many others, shows that there may be persistent neurological consequences of in utero methamphetamine exposure (Chang et al. 2004; 2009; Chomchai et al. 2004, Lagasse et al. 2004; Smith et al. 2001, 2006, 2008). This underlines the need for greater educational strategies to minimize maternal stimulant abuse and further development of specially tailored child interventions for neurobehavioral remediation in children exposed in utero to recreational drugs including methamphetamine.
Acknowledgements
Role of Funding Sources: The sponsors of this study had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report, or in the decision to submit the paper for publication.
This research was supported by the National Institute of Drug Abuse (L30 DA027582-01, T32 DA07262 and 1P50DA018165), the National Institute of Environmental Health Sciences (T32 ES007060), a Public Health Service Grant (1 UL1 RR024120-01 & UL1 RR024140), the Ellison Medical Foundation (AG-NS-0201), and the Clinical Research Enhancement Fund (90120298). Anthony Bader, Hilary Gray, Paige Cooper and Collin Clifford provided technical assistance. Finally, we acknowledge Bob Ritchie of the Ponce School of Medicine/RCMI Publications Office (Grant #5G12RR003050-25) for his help with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: OHSU receives a modest one-time fee to cover licensing costs from laboratories that want to use the Memory Island software.
References
- Alloway TP, Gathercole SE, Holmes JE, Place M, Elliott JG, Hilton K. The diagnostic utility of behavioral checklists in identifying children with ADHD and children with working memory deficits. Child Psychiatry Hum Dev. 2009;40:353–366. doi: 10.1007/s10578-009-0131-3. [DOI] [PubMed] [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96:1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negl. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Larsson G, Zetterström RR. Amphetamine addiction and pregnancy. III. One year follow-up of children. Acta Paediatr. Scand. 1980;69:675–680. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Steneroth G, Zetterström R. Pre-school children of amphetamine-addicted mothers. I. Somatic and psychomotor development. Acta Paediatr Scand. 1985;74:179–184. doi: 10.1111/j.1651-2227.1985.tb10946.x. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Bodnar LE, Prahme MC, Cutting LE, Denckla MB, Mahone EM. Construct validity of parent ratings of inhibitory control. Child Neuropsychol. 2007;13:345–362. doi: 10.1080/09297040600899867. [DOI] [PubMed] [Google Scholar]
- Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85:204–208. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, et al. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chomchai C, Na Manorom N, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Cohen CJ. Children’s Memory Scale: Stimulus Book One. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Conners CK, Jeff JL. ADHD in adults and children: The latest assessment and treatment strategies. Kansas City, MO: Compact Clinicals; 1999. [Google Scholar]
- Datner EM, Wiebe DJ, Brensinger CM, Nelson DB. Identifying pregnant women experiencing domestic violence in an urban emergency department. J Interpers Violence. 2007;22:124–135. doi: 10.1177/0886260506295000. [DOI] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: Incidence and clinical correlates. J Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Larsson G, Zetterström R. Amphetamine addiction and pregnancy. II. Pregnancy, delivery, and the neonatal period. Socio-medical aspects. Acta Obstet Gynecol Scand. 1981;60:253–259. doi: 10.3109/00016348109158127. [DOI] [PubMed] [Google Scholar]
- Forrester MB, Merz RD. Risk of selected birth defects with prenatal illicit drug use, Hawaii, 1986–2002. J Toxicol Environ Health A. 2007;70:7–18. doi: 10.1080/15287390600748799. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavioral Rating Inventory of Executive Function: Professional Manual. Lutz FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Golub M, Costa L, Crofton K, Frank D, Fried P, Gladen G, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of amphetamine and methamphetamine. Birth Defects Res B Dev Reprod Toxicol. 2005;74:471–584. doi: 10.1002/bdrb.20048. [DOI] [PubMed] [Google Scholar]
- González-Burgos I, Alejandre-Gómez M, Olvera-Cortés ME, Pérez-Vega MI, Evans S, Feria-Velasco A. Prenatal-through-postnatal exposure to moderate levels of ethanol leads to damage on the hippocampal CA1 field of juvenile rats: A stereology and Golgi study. Neurosci Res. 2006;56:400–408. doi: 10.1016/j.neures.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Groza V, Ryan SD, Thomas S. Institutionalization, Romanian adoptions, and executive functioning. Child Adoles Social Work Journal. 2000;25:185–204. [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hrubá L, Schutová B, Pometlová M, Slamberová R. Does cross-fostering modify the prenatal effect of methamphetamine on learning of adult male rats? Prague Med Rep. 2009;110:191–200. [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Jacobs E, Miller LC, Tirella LG. Developmental and behavioral performance of internationally adopted preschoolers: a pilot study. Child Psychiatry Hum Dev. 2010;41:15–29. doi: 10.1007/s10578-009-0149-6. [DOI] [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med. 1999;29:527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- Kissin WB, Svikis DB, Morgan GD, Haug NA. Characterizing pregnant drug-dependent women in treatment and their children. J Subst Abuse Treat. 2001;21:27–34. doi: 10.1016/s0740-5472(01)00176-3. [DOI] [PubMed] [Google Scholar]
- Lagasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, et al. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol. 2010 doi: 10.1016/j.ntt.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdown R, Burnell A, Allen M. Is it that they won't do it, or is it that they can't? Executive functioning and children who have been fostered and adopted. Adoption & Fostering Journal. 2007;31:44–53. [Google Scholar]
- Lu LH, Johnson A, O'Hare ED, Bookheimer SY, Smith LM, O'Connor MJ, et al. Effects of prenatal methamphetamine exposure on verbal memory revealed with functional magnetic resonance imaging. J Dev Behav Pediatr. 2009;30:185–192. doi: 10.1097/DBP.0b013e3181a7ee6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcauley T, Chen S, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J Int Neuropsychol Soc. 2010;16:495–505. doi: 10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- Medrano MA, Zule WA, Hatch J, Desmond DP. Prevalence of childhood trauma in a community sample of substance-abusing women. Am J Drug Alcohol Abuse. 1999;25:449–462. doi: 10.1081/ada-100101872. [DOI] [PubMed] [Google Scholar]
- Messina N, Marinelli-Casey P, West K, Rawson R. Children exposed to methamphetamine use and manufacture. Child Abuse Negl. 2007 doi: 10.1016/j.chiabu.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Piper BJ. Developmental neurotoxicology of abused drugs. In: Gupta RC, editor. Reproductive & Developmental Toxicology. Amsterdam: Elsevier; 2010. [Google Scholar]
- Oregon Department of Human Services. Drug abuse impact on foster care in Oregon. Annual Child Welfare Report. 2007 [Google Scholar]
- Paz MS, Smith LM, LaGasse LL, Derauf C, Grant PR, Shah A, et al. Maternal depression and neurobehavior in newborns prenatally exposed to methamphetamine. Neurotoxicol Teratol. 2009;31:177–182. doi: 10.1016/j.ntt.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Craytor MJ, Murray PW, Raber J. The use and validation of the spatial navigation Memory Island test in primary school children. Behav Brain Res. 2010;210:257–262. doi: 10.1016/j.bbr.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. WASI Stimulus Booklet. San Antonio, TX: Harcourt Brace; 1999. [Google Scholar]
- Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O'Connor MJ, et al. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: The effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TX, Sabherwal U. Effects of gestational nicotine exposure on hippocampal morphology. Neurotoxicol Teratol. 1998;20:465–473. doi: 10.1016/s0892-0362(97)00137-2. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychol. 2006;12:439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Siegel J, Craytor M, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav Pharmacol. 2010;21:602–614. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberová R, Pometlová M, Syllabová L, Mancusková M. Learning in a place navigation task, not the new-learning task, is altered by prenatal methamphetamine exposure. Dev Brain Res. 2005;157:217–219. doi: 10.1016/j.devbrainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Slutsker L, Smith R, Higgingson G, Fleming D. Recognizing illicit drug use by pregnant women: Reports from Oregon birth attendants. Am J Public Health. 1993;83:61–64. doi: 10.2105/ajph.83.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria M, et al. The infant development, environment, and lifestyle study: Effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol. Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Leow AD, Bookheimer SY, Smith LM, O'Connor MJ, Kan E, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci. 2010;30:3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 4th Edition–Integrated: Technical and Interpretive Manual. San Antonio, TX: PsychCorp; 2004. Wechsler Intelligence Scale for Children. [Google Scholar]
- Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 2004;19:3165–3170. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]