Abstract
In a randomized trial of therapy for FMS-like tyrosine kinase-3 (FLT3) mutant acute myeloid leukemia in first relapse, 224 patients received chemotherapy alone or followed by 80 mg of the FLT3 inhibitor lestaurtinib twice daily. Endpoints included complete remission or complete remission with incomplete platelet recovery (CR/CRp), overall survival, safety, and tolerability. Correlative studies included pharmacokinetics and analysis of in vivo FLT3 inhibition. There were 29 patients with CR/CRp in the lestaurtinib arm and 23 in the control arm (26% vs 21%; P = .35), and no difference in overall survival between the 2 arms. There was evidence of toxicity in the lestaurtinib-treated patients, particularly those with plasma levels in excess of 20μM. In the lestaurtinib arm, FLT3 inhibition was highly correlated with remission rate, but target inhibition on day 15 was achieved in only 58% of patients receiving lestaurtinib. Given that such a small proportion of patients on this trial achieved sustained FLT3 inhibition in vivo, any conclusions regarding the efficacy of combining FLT3 inhibition with chemotherapy are limited. Overall, lestaurtinib treatment after chemotherapy did not increase response rates or prolong survival of patients with FLT3 mutant acute myeloid leukemia in first relapse. This study is registered at www.clinicaltrials.gov as #NCT00079482.
Introduction
Activating mutations of the receptor tyrosine kinase FMS-like tyrosine kinase-3 (FLT3) are detectable in the leukemia cells of up to 30% of patients with acute myeloid leukemia (AML).1,2 AML patients whose leukemia cells harbor FLT3 mutations, particularly those with an internal tandem duplication (ITD) mutation in the juxtamembrane domain, have a markedly increased rate of relapse and a shorter overall survival (OS) compared with similarly aged patients who lack such mutations.3–7 Several small molecule inhibitors of FLT3 kinase activity have been studied as single-agent therapy for FLT3 mutant AML.8–13 Most of these agents have led to transient reductions in peripheral blasts, and occasionally also bone marrow blast reductions, and more recently there are reports of more potent FLT3 inhibitors inducing remissions.12,14–16
Lestaurtinib (previously referred to as CEP-701) is an orally available indolocarbazole derivative that was originally identified as an inhibitor of the neurotropin receptor TrkA but was subsequently found to have potent in vitro activity against FLT3.17,18 The pharmacokinetics of lestaurtinib were originally studied in patients with solid tumors, and the drug was then evaluated in AML patients.19 In 2 separate phase 2 trials, lestaurtinib was generally well tolerated and was noted to induce reductions in peripheral blood and marrow blasts in patients harboring FLT3 activating mutations.9,20 The modest clinical activity in these trials was closely correlated with in vivo FLT3 inhibition. These early phase studies also established that lestaurtinib was highly bound in vivo to plasma protein, specifically α1-acid glycoprotein (AGP).
In vitro studies of lestaurtinib combined with chemotherapeutic agents demonstrated synergistic killing of FLT3 mutant AML cells.21 The most effective approach was to expose the cells to the inhibitor either concurrently or after chemotherapy. These results, together with the modest but reproducible clinical activity of lestaurtinib in FLT3 mutant AML, provided the rationale for combining this drug with a conventional chemotherapy regimen with the intent of improving outcomes for this difficult to treat population of patients. We report here the results of a multicenter randomized trial of lestaurtinib after salvage chemotherapy for FLT3 mutant AML patients in first relapse.
Methods
Patient eligibility
The Cephalon 204 trial enrolled patients age 18 years and older with confirmed AML and an FLT3 activating mutation (FLT3/ITD or FLT3/D835). FLT3 activating mutations were identified at participating institutions. There was no central reference laboratory for this parameter, but participating institutions were required to use methods conforming to published protocols.22 Patients were required to be in first relapse after a first remission of 1 to 24 months. They were required to have an Eastern Cooperative Oncology Group performance score of 0, 1, or 2. Patients with liver impairment (transaminases > 3× the upper limit of normal) or active infection were excluded, as were patients previously treated with FLT3 inhibitors.
The protocol was approved by the Institutional Review Board at all participating centers, and all patients signed an informed consent document in accordance with the Declaration of Helsinki.
Treatment plan
Salvage chemotherapy was administered according to the duration of first remission. Patients with a first remission lasting from 1 to 6 months (31-180 days) received MEC, consisting of mitoxantrone 8 mg/m2 per day, etoposide 100 mg/m2, and cytarabine 1000 mg/m2 per day all intravenously on days 1 to 5. Patients whose first remission lasted from 6 to 24 months received HiDAC, consisting of cytarabine 1500 mg/m2 daily on days 1 to 5. For patients on the lestaurtinib arm, lestaurtinib was administered at a dose of 80 mg orally twice daily (12 hours between doses), beginning 2 days after the completion of chemotherapy (day 7). Patients were randomized before initiation of chemotherapy. Bone marrow aspirates and biopsies were obtained on or within 2 days of day 15 of the start of treatment (aplasia assessment). If the marrow cellularity was less than or equal to 5%, observation (control arm) or lestaurtinib was continued. On recovery of peripheral blood counts, or on day 42 if count recovery had not occurred by then, the bone marrow aspirate and biopsy were repeated (outcome assessment). If the bone marrow on day 15 revealed a cellularity of 20% or greater and at least a 50% reduction in blasts, patients received a second course of the same chemotherapy, with or without lestaurtinib (which had begun on day 7) per their previous randomization. If the day 15 marrow showed persistent leukemia with no reduction in blast percentage from baseline, or progressive leukemia, patients were removed from the protocol. In responding patients randomized to receive lestaurtinib, lestaurtinib was continued for up to day 112. If lestaurtinib was judged to be of ongoing clinical benefit, patients could continue to receive lestaurtinib indefinitely through enrollment on an extension protocol.
Patients randomized to the control arm received chemotherapy only, with the regimen based on remission duration. Control patients were eligible for crossover if they achieved a partial response as revealed by the day 42 marrow assessment.
Lestaurtinib was supplied as a clear yellow oral solution at a concentration of 25 mg/mL in a 1:1 mixture of polysorbate 80 NF and propylene glycol USP. The drug was diluted in juice before administration.
Outcomes
The primary efficacy measure was complete remission (CR) or complete remission except for incomplete platelet recovery (CRp) based on the International Working Group criteria.23 The key secondary endpoint was OS. Other endpoints included partial remission and assessment of safety and tolerability of lestaurtinib administered after chemotherapy. For the safety and tolerability assessment, the National Cancer Institute Common Toxicity Criteria Version 3.0 were used. Determination of remission status or response was performed at the participating institutions, with central review of source documents.
Pharmacokinetics
Plasma concentrations of lestaurtinib were determined on day 1 of the first course of chemotherapy, on day 15 (± 2 days) of every course of chemotherapy, and on the day of outcome assessment.
Plasma samples were analyzed for concentrations of lestaurtinib by the Department of Drug Safety and Disposition of Cephalon, Inc, using a validated high-performance liquid chromatography method. The method used liquid-liquid extraction of lestaurtinib and an internal standard (KT-252a) from 0.1 mL of human plasma into a mixture of ethyl acetate/methylene chloride 4:1 (volume/volume) followed by reversed-phase chromatography on a Hypersil BDS phenyl column (2.1 × 150 mm; 5-μm particle size; Thermo Fisher Scientific) maintained at 35°C. Chromatography was isocratic, with a mobile phase consisting of a 70:30 (vol/vol) mixture of water/acetonitrile. The eluate was monitored by fluorescence detection with an excitation wavelength of 303 nm and an emission wavelength of 403 nm. Quantification was based on a 1/(χ2)-weighted linear regression analysis of the peak height ratios of lestaurtinib to the internal standard versus nominal concentration, from extracted human plasma calibration standards. The quantifiable range of the assay was from 5 to 1000 ng/mL of human plasma. Within-run and between-run coefficients of variation over the assay range during method validation were less than or equal to 6.5% and less than or equal to 9.9%, respectively. Within-run accuracy and between-run accuracy were 94.9% to 113% and 101% to 104%, respectively.
Correlative studies
For patients randomized to receive lestaurtinib after chemotherapy, whole-blood samples drawn into heparin vacuum tubes were obtained within one week before starting chemotherapy (“Pretreatment”), and at the same time points at which the trough pharmacokinetic samples were obtained (12 hours after the most recent dose at the time of aplasia assessment, and 12 hours after the most recent dose at the time of outcome assessment). The samples were packed on cold packs and shipped via overnight carrier to a central laboratory in Baltimore, MD. Samples collected within the United States or Canada typically arrived within 48 hours of collection, whereas international samples arrived 48 to 72 hours after collection. On arrival in the central laboratory, plasma was separated by centrifugation and stored frozen at −80°C until use. All samples were assayed for FLT3 plasma inhibitory activity (PIA), FLT3 ligand (FL) levels, and AGP levels within 120 days of collection.
The PIA assay was performed as described.24 Briefly, frozen plasma samples were thawed and clarified by centrifugation at 16 000g for 2 minutes. All assays described herein were performed within 120 days of collection. For each time point, 2 × 106 TF/ITD cells (human AML expressing a FLT3/ITD construct) were incubated with 1 mL plasma at 37°C for 1 hour. The cells were washed twice with ice-cold phosphate-buffered saline and then lysed. After immunoblotting for phosphorylated FLT3, densitometric analysis was performed on the bands and the PIA for each plasma sample was calculated by expressing the density of its corresponding band as a percentage of the density of the baseline band (which was arbitrarily set at 100%). The PIA assay was performed in triplicate for each plasma time point, and the results were averaged.
FL concentrations in plasma samples were determined using an ELISA kit obtained from R&D Systems. AGP was assayed using an immunodiffusion assay kit obtained from Kent Laboratories.
Statistical considerations
Patients were stratified between the 2 treatment arms by duration of remission status and by age (< 50 years vs ≥ 50 years). Patients were assessed for an outcome of CR/CRp as defined by the International Working Group criteria.23 The CR/CRp rates between the 2 arms were compared using the Mantel-Haenszel χ2 test, using both age group and duration of first remission as stratification variables. The sample size of 220 was planned to yield a target power of 80%, based on an assumed odds ratio of 2.44, and a 2-sided α of 5%. The study was also powered for the key secondary endpoint, OS. The study met the criteria to continue accrual after a planned interim analysis for futility after enrollment of 110 patients. Patients initially randomized to the control arm who crossed over to receive lestaurtinib remained in the control arm for evaluation of results.
Results
Patients
Between January 2004 and December 2008, 428 patients were screened for eligibility. Of these, 188 did not meet inclusion criteria, 8 had exclusion criteria, 6 withdrew consent, and 2 had disease progression. The remaining 224 patients were enrolled and randomized at 53 different institutions. The data lock was on June 12, 2009. Of those randomized, 220 actually received treatment, with 4 dying of disease progression (3 in the control arm and one in the lestaurtinib arm) before beginning therapy. Table 1 lists the baseline characteristics of those enrolled. As expected, the large majority of patients had the FLT3/ITD mutation and those with D835 point mutations were equally distributed between the 2 treatment arms. There were more patients less than 60 years of age in the control arm compared with the lestaurtinib arm.
Table 1.
Patient characteristics
| Parameter | Chemotherapy only | Chemotherapy + lestaurtinib |
|---|---|---|
| Patients, n | 112 | 112 |
| Median age, y (range) | 54 (21-79) | 59 (20-81) |
| < 50 y, n | 34 | 33 |
| < 60 y, n | 79 | 64 |
| Male/female, n | 53/59 | 50/62 |
| FAB type, no. | ||
| M0 | 2 | 4 |
| M1 | 14 | 7 |
| M2 | 15 | 13 |
| M4 | 26 | 20 |
| M5 | 6 | 10 |
| M6 | 0 | 0 |
| M7 | 0 | 0 |
| Unknown | 49 | 58 |
| Duration of first CR, n (%) | ||
| 1-6 months | 53 (47) | 53 (47) |
| ≥ 6 months | 59 (53) | 59 (53) |
| FLT3 mutation, n | ||
| ITD only | 97 | 101 |
| D835 only | 8 | 9 |
| ITD and D835 | 6 | 2 |
| Not confirmed* | 1 | 0 |
FAB indicates French-American-British classification.
For a single patient, the FLT3 mutation status was not confirmed on central review.
Outcome
Of the 220 patients receiving therapy on this trial, 111 were on the lestaurtinib arm, 106 of whom received at least 1 week of lestaurtinib. For these 106 patients, the median number of days of lestaurtinib treatment was 38 (range, 8-202 days). A total of 15 patients on the lestaurtinib arm received the drug after outcome assessment on the extension protocol for a median of 43 days (range, 4-255 days). Of the 109 patients on the control arm, only 7 crossed over to receive lestaurtinib after the outcome assessment, whereas an additional 30 patients would eventually be treated with lestaurtinib by enrolling on the extension protocol. On the control arm, the reasons for discontinuing therapy before completion were adverse events (AEs, 7%), disease progression (DP, 48%), and lack of response (19%). On the lestaurtinib arm, the reasons for discontinuing were AE (26%), DP (34%), and lack of response (6%). On the control arm, therefore, 73 of 109 patients (67%) discontinued therapy before completion for either disease progression or lack of response compared with 45 of 111 patients (41%) on the lestaurtinib arm (P < .001; Fisher exact test).
The mean time to response assessment was 41 days in the control arm and 43 days in the lestaurtinib arm. In the control arm, 86% of patients were assessed for remission within the protocol-designated window period of 42 plus or minus 2 days, compared with 88% of lestaurtinib patients. Delays in assessment were the result of patients on both arms receiving a second course of chemotherapy based on the day 15 bone marrow. Table 2 details the primary outcome of remission for the 2 arms, by intention-to-treat analysis. There was no evidence of a difference in remission rate for the lestaurtinib arm compared with the control arm, either overall or in the subgroups defined by duration of first remission or age. For patients with a D835 mutation only, 5 of 9 achieved a CR/CRp in the lestaurtinib arm, compared with 2 of 8 in the control arm (P = .6).
Table 2.
Primary outcome
| Parameter | Chemotherapy only, n (%) | Chemotherapy + lestaurtinib, n (%) | P |
|---|---|---|---|
| No. of total patients | 112 | 112 | > .99 |
| CR | 13 (12) | 19 (17) | .25 |
| CRp | 10 (9) | 10 (9) | > .99 |
| Total CR/CRp | 23 (21) | 29 (26) | .35 |
| First remission 1-6 mo, CR/CRp | 6 (11) | 10 (19) | .42 |
| First remission ≥ 6 mo, CR/CRp | 17 (29) | 19 (32) | .84 |
| < 50 y of age, CR/CRp | 4 (12) | 9 (27) | .21 |
| ≥ 50 y of age, CR/CRp | 19 (24) | 20 (25) | > .99 |
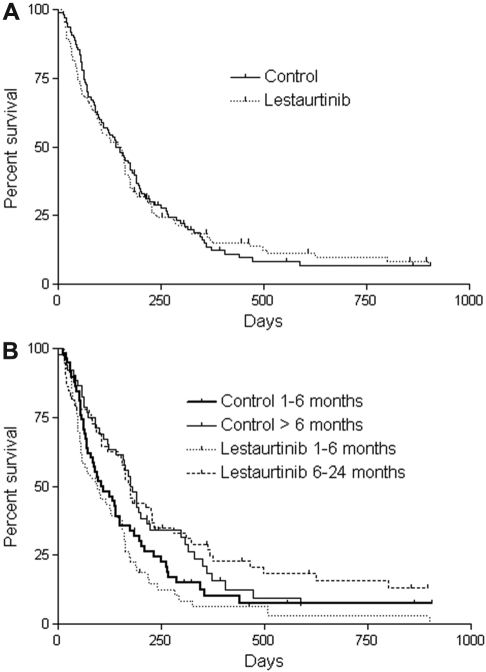
As of June 2009, 15 patients in the control group and 12 patients in the lestaurtinib group were still alive. Twenty-two patients in each arm eventually received an allogeneic stem cell transplantation. There was no difference in OS (by intention-to-treat analysis) between the lestaurtinib arm and control arm, either for all patients (Figure 1A) or according to duration of first remission (Figure 1B). For the survival data shown in Figure 1, patients were censored at the time of transplantation. There was no difference seen when the analysis was performed without censoring for transplant (not shown).
Figure 1.
Overall survival. (A) Kaplan-Meier estimates of OS from the time of randomization for patients who were randomized to the lestaurtinib arm compared with the control arm. Patients who received an allogeneic transplant were censored at the date of the allogeneic transplantation. (B) OS by duration of first remission. Kaplan-Meier estimates of OS from the time of randomization for patients whose first remission lasted from 1 to 6 months, and for patients whose first remission lasted more than 6 months. Patients who received an allogeneic transplant were censored at the date of the allogeneic transplantation.
Pharmacokinetics
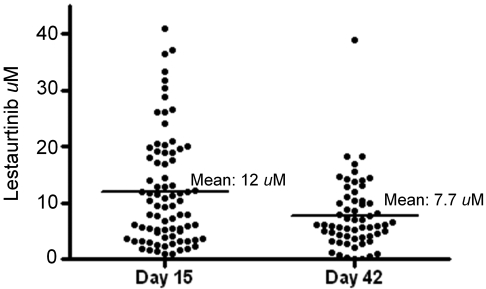
Plasma samples obtained from whole blood drawn 12 hours after lestaurtinib administration at the aplasia and outcome assessments (in most cases, on or around days 15 and 42, respectively) were analyzed for lestaurtinib concentration for 79 of the 106 patients on the lestaurtinib arm who received at least 7 days of lestaurtinib (the remainder were rendered unusable by the shipping process). The results of this analysis are plotted in Figure 2. There was a broad range of concentrations, particularly on day 15, when concentrations ranged from less than 1μM to more than 40μM. The variation at day 42 was less than on day 15, but the mean level had fallen from 12μM to 7.7μM. Lestaurtinib is known to be metabolized by cytochrome P450 enzymes (notably CYP3A4),19 and this decrease in steady-state levels over time may be a reflection of liver enzyme induction.
Figure 2.
Steady-state plasma lestaurtinib levels. Individual plasma lestaurtinib levels determined 12 hours after the most recent dose on or within 2 days of day 15 (aplasia assessment) and day 42 (outcome assessment). The horizontal lines indicate the mean levels for each time point.
Safety and toxicity
There were no significant differences in the frequency of AEs between the 2 arms (Table 3). However, 24% of patients on the lestaurtinib arm discontinued their planned therapy before completion because of AEs, whereas only 7% of the control arm patients did so. The frequency of serious AEs was also more common in the lestaurtinib arm (55% vs 45%) on the control arm, mostly because of a higher incidence of infection (32% vs 21%, respectively). There were 13 deaths (12%) by day 30 on the lestaurtinib arm (10 sepsis, 1 myocardial infarction, 1 multiorgan failure, and 1 cerebellar toxicity), compared with 7 deaths (6%) by day 30 of treatment on the control arm (3 sepsis, 2 multiorgan failure, 1 cardiac arrest, and 1 progressive disease).
Table 3.
Safety parameters
| Safety parameter | Chemotherapy only, n (%) | Chemotherapy + lestaurtinib, n (%) | P |
|---|---|---|---|
| Death within 30 days of start of treatment | 7/109 (6) | 13/111 (12) | .24 |
| Grade 3 or 4 AE | 101/109 (93) | 104/111 (94) | .8 |
| Serious AE | 49/109 (45) | 61/111 (55) | .18 |
P values were calculated using Fisher exact test.
AE indicates adverse event.
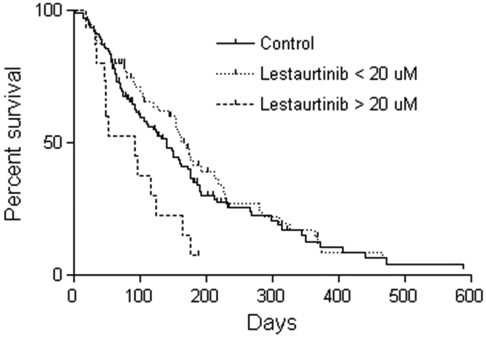
Of the 79 patients in whom lestaurtinib levels were measured at the aplasia assessment, 15 had plasma concentrations more than 20μM. This level was higher than anticipated based on data from the phase 1/2 trial, in which trough levels for patients receiving 60 mg twice daily were 4.4 ± 4.0μM.9 In the phase 1/2 trial, 20μM was the highest plasma level observed in any patient; therefore, no safety data were available for levels greater than 20μM.9 Given the multitargeting potential of lestaurtinib, this suggested one reason for the observed increases in toxicity. Figure 3 shows the OS for these 15 patients with high levels of lestaurtinib compared with the control group and to the other 65 patients with lestaurtinib levels less than 20μM. The median survival for the 15 patients with lestaurtinib levels more than 20μM was 92 days compared with 139 days for the control patients (P = .01) and 169 days for the 65 patients with lestaurtinib levels less than 20μM (P = .002). Although no specific toxicity was obviously increased in the patients with high lestaurtinib levels, there was a trend for increased infectious deaths (6 of 15 deaths because of infection vs 10 of 54 deaths because of infection in the high vs low lestaurtinib patients, respectively; P = .1). Overall, therefore, there was a suggestion of increased toxicity experienced by patients on the lestaurtinib arm, possibly related to elevated plasma drug levels.
Figure 3.
OS according to plasma level of lestaurtinib. At the aplasia assessment, 15 of 79 patients from whom lestaurtinib levels were measured had levels in excess of 20μM. Shown are Kaplan-Meier estimates of OS from the time of randomization for these 15 patients (dashed line) compared with the other 64 patients on the lestaurtinib arm (dotted line) and the control arm patients (solid line). The median survival for those patients with lestaurtinib levels more than 20μM is significantly different from both the other lestaurtinib patients (P = .002) and the control patients (P = .01).
Pharmacodynamics
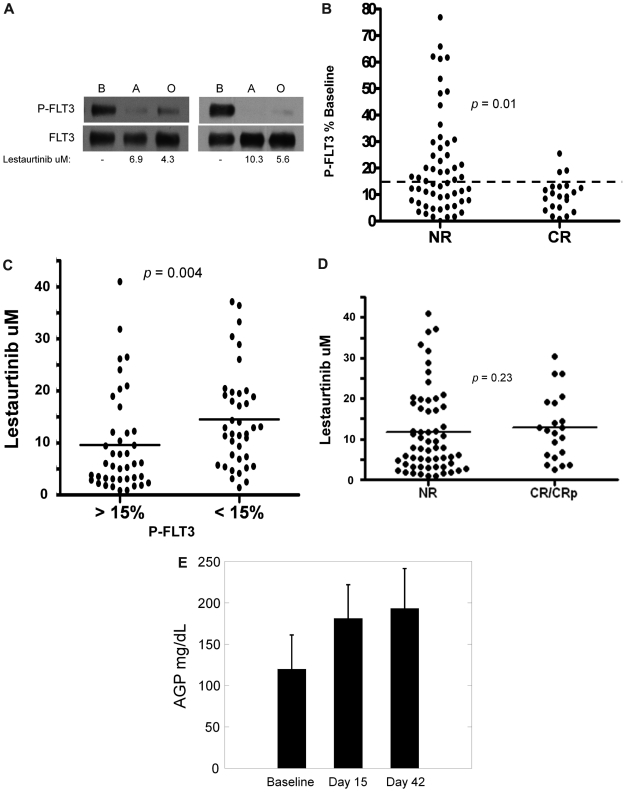
For estimation of FLT3 inhibition in vivo, we performed PIA assays for FLT3 using plasma samples collected at the aplasia and outcome assessments. Of the 107 patients who received lestaurtinib for at least 1 week, 79 had plasma samples from the aplasia assessment time point available for PIA analysis. Based on the data from the monotherapy trial and from preclinical studies, we had estimated that FLT3 needed to be inhibited to less than 15% of its baseline activity to induce a cytotoxic effect and had therefore set this inhibitory level as the goal at the start of the trial.9,17 Figure 4A shows 2 individual examples of the PIA assay. Of the 79 patients analyzed, 46 (58%) achieved FLT3 inhibition to less than 15% of baseline at aplasia assessment, and 21 of these achieved this degree of inhibition at the outcome assessment as well. The 79 patients analyzed had a similar remission rate as the remaining 33 patients whose aplasia samples were not analyzed (21 of 79 vs 8 of 33; P = nonsignificant by Fisher exact test), suggesting that these 79 patients were a fair representation of the lestaurtinib patients. As displayed in Figure 4B (and summarized in Table 4), FLT3 inhibition, as estimated from the PIA assay, was correlated with complete remission (P = .01). In turn, FLT3 inhibition was also correlated with higher plasma lestaurtinib levels (Figure 4C). Interestingly, however, there were several cases in which lestaurtinib levels were relatively high, and yet in vivo FLT3 inhibition was less than expected (Figure 4C). Furthermore, remission rate was not well correlated with drug levels: the mean lestaurtinib level in patients achieving remission was 12.9μM, compared with 11.7μM in the nonresponders (P = .23; Figure 4D).
Figure 4.
Pharmacokinetic and pharmacodynamic correlatives. (A) PIA assay for FLT3 in trial patients. Shown are results from 2 separate patients on the lestaurtinib arm. The upper blots were probed with antiphosphotyrosine. The blots were stripped and reprobed for total FLT3 (lower blots). Shown below the blots are the measured levels of lestaurtinib from plasma obtained at the same time points as that used in the assay. B indicates baseline; A, aplasia; and O, outcome. (B) FLT3 inhibition grouped according to response. Results from individual FLT3 PIA assays from the aplasia assessment are plotted, grouped according to whether or not the patient attained a complete remission. The dashed line indicates the targeted level of 15% of baseline FLT3 activity. (C) Lestaurtinib plasma level grouped according to FLT3 inhibition. Results from individual measurements of plasma lestaurtinib at the aplasia assessment are plotted, grouped according to whether or not the plasma inhibitory activity for FLT3 from the same time point was above (inadequate inhibition) or below (adequate inhibition) the 15% target level. (D) Lestaurtinib plasma level grouped according to response. Results from individual measurements of plasma lestaurtinib at the aplasia assessment are plotted, grouped according to whether or not the patient achieved a complete remission. NR indicates no response. (E) AGP levels. AGP concentrations (milligrams per deciliter of plasma) were determined using an immunodiffusion assay from plasma samples obtained at baseline, the aplasia assessment (day 15), and the outcome assessment (day 42).
Table 4.
Results of PIA analysis and primary outcome
| Phosphorylated FLT3 | No. of patients | No. (%) with CR/CRp |
|---|---|---|
| Patients with samples available for analysis | 79 | 21 (26.6) |
| Inhibited at aplasia assessment | 46 | 18 (39.1) |
| Not inhibited at aplasia assessment | 33 | 3 (9.1) |
| Inhibited both at aplasia and outcome assessments | 21 | 12 (57.1) |
The difference in the CR/CRp rate between those who were inhibited at the aplasia assessment and those who were not was highly significant (P = .004; Fisher exact test).
Failure to achieve FLT3 inhibition to less than 15% of baseline (in the 79 patients analyzed for this parameter) was associated with a lower remission rate compared with patients on the control arm (9.1% vs 21%; P = .20, 2-sided Fisher exact test). Furthermore, patients who received lestaurtinib but failed to achieve target inhibition had median survival of 92 days compared with 160 days for those receiving lestaurtinib who did achieve this degree of inhibition (P = .02). Thus, being treated with lestaurtinib and not achieving in vivo FLT3 inhibition was associated with worse overall clinical outcomes.
Clinical response in the patients randomized to lestaurtinib was correlated with in vivo FLT3 inhibition, and yet a significant number of patients failed to achieve adequate FLT3 in inhibition sustained over the course of treatment. Certainly, one explanation for this would simply be the decrease in plasma lestaurtinib levels from the aplasia to the outcome time points (Figure 2). As illustrated in Figure 4C, levels of lestaurtinib correlated with in vivo FLT3 inhibition. This might imply that only sustained FLT3 inhibition (ie, over the entire 42 days) can result in clinical benefit. However, another possible explanation for the lower-than-expected FLT3 inhibition overall was that free, biologically active drug levels were still inadequate. In human plasma, lestaurtinib binds with high affinity to AGP, levels of which can increase in response to a variety of stimuli.25 Higher levels of AGP in plasma translate to lower net levels of free lestaurtinib. As shown in Figure 4E, AGP levels rose from baseline by day 15 and remained elevated by day 42. Given the drop in total plasma drug levels on day 42 (Figure 2), this would be expected to result in an overall decrease in free lestaurtinib on day 42 compared with day 15.
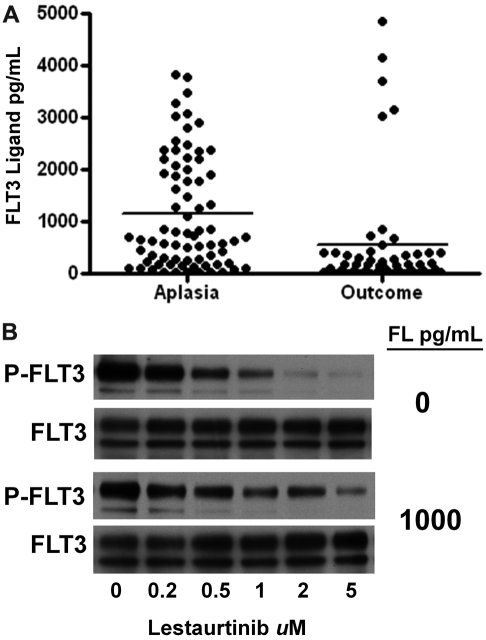
In addition to AGP levels, FL levels in plasma after chemotherapy may also have resulted in less effective FLT3 inhibition. We have recently shown that FL can interfere with the efficacy of FLT3 inhibitors.26 FL concentrations in the plasma of the lestaurtinib arm patients were uniformly less than 20 pg/mL at baseline. However, as shown in Figure 5A, at the time of the aplasia assessment, FL levels were markedly elevated from baseline in all cases analyzed. FL levels rose to greater than 1000 pg/mL in many patients, and in some cases remained elevated at the outcome assessment. Shown in Figure 5B is an experiment demonstrating the blunted inhibitory activity of lestaurtinib in the presence of FL.
Figure 5.
FLT3 ligand levels. (A) FL levels were determined with an enzyme-linked immunosorbent assay using plasma samples obtained at the aplasia and outcome assessments. The individual results are plotted, with solid lines indicating the mean level for each group. (B) The effect of FL on the FLT3 inhibitory activity of lestaurtinib. TF/ITD cells, expressing ITD-mutated FLT3, were incubated in plasma containing increasing concentrations of lestaurtinib in the presence or absence of 1000 pg/mL FL. After immunoprecipitation for FLT3 and electrophoresis, the blots were probed with antiphosphotyrosine (top blot), and then stripped and reprobed for total FLT3 (bottom blot).
Discussion
The clinical data from the Cephalon 204 trial indicate that lestaurtinib administered after salvage chemotherapy provides no benefit to adult AML patients with FLT3 mutations in first relapse. By intention-to-treat analysis, there was no statistically significant improvement in remission rate or OS for patients receiving lestaurtinib after salvage chemotherapy. There was also no difference in remission rate or survival evident in the analysis of the predefined subgroups, which included patients more than 50 and less than 50 years of age, and in patients with first remission duration of 1 to 6 months and more than 6 months. One very encouraging aspect of our results, however, was that in vivo FLT3 inhibition correlated very highly with remission rate, suggesting that FLT3 inhibition as a therapeutic modality still is very promising. AML in first relapse has an extraordinarily poor prognosis, and such patients are, therefore, very difficult to study. Ongoing studies of chemotherapy combined with FLT3 inhibitors in newly diagnosed FLT3 mutant AML may offer a better approach to establishing any benefit of FLT3 inhibition.27,28
The Cephalon 204 trial was not powered to detect a significant difference in younger AML patients treated with lestaurtinib, but there was a suggestion that patients younger than 50 years may have benefited from the drug (eg, Table 2). Currently, there are 2 randomized trials of lestaurtinib in younger AML patients with FLT3 mutations: the Medical Research Council AML17 trial and the Children's Oncology Group trial.28 In addition, midostaurin (another indolocarbazole FLT3 inhibitor) is being evaluated in younger patients in a randomized setting.27 Whether or not meaningful FLT3 inhibition can be achieved with the agents in these settings remains unclear.
Why did lestaurtinib fail to provide a benefit to patients on the Cephalon 204 trial? Certainly, the population being studied represents a significant challenge. Numerous resistance mechanisms are probably already present in the leukemia cells of these patients (activation of alternate pathways, resistance mutations, FLT3 up-regulation) that we would not have detected with our correlative assays.29–31 However, our correlative studies do suggest some explanations. Steady-state plasma levels of lestaurtinib varied from < 1μM to > 40μM, a remarkably large variation that was not observed in monotherapy trials. High levels of drug seemed to predict toxicity (presumably from off-target effects), and yet they did not predict in vivo FLT3 inhibition. In vivo FLT3 inhibition (even at just one time point) was correlated with an increased remission rate but was observed in only a little more than one-half of the patients. Certainly, the decreasing levels of plasma lestaurtinib over the course of treatment could have contributed to the lack of FLT3 inhibition. However, although lestaurtinib levels in general correlated with in vivo FLT3 inhibition, several patients had high plasma levels of drug without achieving FLT3 inhibition. This lack of correlation between high lestaurtinib levels and in vivo FLT3 inhibition may in turn be attributable to elevated AGP and FL levels after chemotherapy, resulting in low free lestaurtinib levels. It seems probable, therefore, that some patients in this trial benefited from lestaurtinib, whereas an equal number were harmed. A high plasma level of lestaurtinib might have caused toxicity but did not guarantee FLT3 inhibition. The conclusion that can be drawn, therefore, is that administration of an FLT3 inhibitor after chemotherapy might have some benefit for adult patients with relapsed FLT3 mutated AML but that lestaurtinib's complex pharmacokinetics and off-target effects limit its utility in this regard.
What are the implications of these findings for ongoing and future trials of FLT3 inhibitors? The rise in FL after intensive chemotherapy that we observed in this trial is potentially concerning. Elevations in plasma FL could theoretically affect all known small molecule FLT3 inhibitors. This is a phenomenon that is being actively investigated,26 and, if established to be real, could represent an important obstacle in moving from a monotherapy trial to a chemotherapy combination trial. The dose of a drug required to inhibit FLT3 when it is administered in the context of chemotherapy may be completely different from the dose found to inhibit the target in a monotherapy trial.
In conclusion, the addition of lestaurtinib to a salvage chemotherapy regimen conferred no additional benefit for patients with relapsed FLT3-mutated AML. Although the failure to improve survival with lestaurtinib is discouraging, our correlative data suggest that FLT3 inhibition using an inhibitor with better pharmacokinetics may ultimately prove effective.
Acknowledgments
This work was supported the National Cancer Institute (NCI; Leukemia SPORE P50 CA100632-06, R01 CA128864) and the American Society of Clinical Oncology (M.L.). M.L. is a Clinical Scholar of the Leukemia & Lymphoma Society.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.L. was the lead investigator, designed the study, designed and performed correlative studies, and wrote the manuscript; F.R., E.S.W., M.R.B., and B.D.S. served on the publication committee and enrolled patients on the protocol; D.S. designed the study and correlative studies; H.M.K. designed the study; L.T. and D.M.B.-K. are employees of Cephalon, Inc whose product, lestaurtinib, was investigated in this study; A.P., S.C., R.K.S., M.B., L.D.C., M.S.T., G.M., H.E., L.A.G., A.A.L., S.A., I.D.L., A.N., R.S., K.Y., A.A., D.D., W.W.-J., G.J., M.R.L., S.P., and M.S. enrolled patients on the protocol; and all authors reviewed the clinical data and helped edit the manuscript.
Conflict-of-interest disclosure: M.L. is on the Clinical Advisory Board of Cephalon Inc. H.E. is a promotional speaker for Cephalon, Inc. F.R. has received research funding and is on the Clinical Advisory Board of Cephalon Inc. A.A. and H.M.K. have received research funding from Cephalon Inc. D.D. has received research funding and is on the Clinical Advisory Board and Speaker's Bureau of Cephalon Inc. B.D.S. is on the Clinical Advisory Board of Cephalon Inc. L.T. and D.M.B.-K. are employees of Cephalon Inc, whose product, lestaurtinib, was investigated in this study. The remaining authors declare no competing financial interests.
Correspondence: Mark Levis, Kimmel Cancer Center at Johns Hopkins, 1650 Orleans St, Rm 243, Baltimore, MD 21231; e-mail: levisma@jhmi.edu.
References
- 1.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918. [PubMed] [Google Scholar]
- 2.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 3.Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93(9):3074–3080. [PubMed] [Google Scholar]
- 4.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 5.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 7.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 8.Levis M, Small D. FLT3 tyrosine kinase inhibitors. Int J Hematol. 2005;82(2):100–107. doi: 10.1532/IJH97.05079. [DOI] [PubMed] [Google Scholar]
- 9.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 10.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 11.O'Farrell AM, Foran JM, Fiedler W, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9(15):5465–5476. [PubMed] [Google Scholar]
- 12.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 13.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113(17):3938–3946. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113(26):6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Paietta E, Racevskis J, Wiernik PH. Complete resolution of leukemia cutis with sorafenib in an acute myeloid leukemia patient with FLT3-ITD mutation. Am J Hematol. 2009;84(10):701–702. doi: 10.1002/ajh.21511. [DOI] [PubMed] [Google Scholar]
- 16.Crump M, Hedley D, Kamel-Reid S, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk Lymphoma. 2010;51(2):252–260. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- 17.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 18.George DJ, Dionne CA, Jani J, et al. Sustained in vivo regression of Dunning H rat prostate cancers treated with combinations of androgen ablation and Trk tyrosine kinase inhibitors, CEP-751 (KT-6587) or CEP-701 (KT-5555). Cancer Res. 1999;59(10):2395–2401. [PubMed] [Google Scholar]
- 19.Marshall JL, Kindler H, Deeken J, et al. Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs. 2005;23(1):31–37. doi: 10.1023/B:DRUG.0000047103.64335.b0. [DOI] [PubMed] [Google Scholar]
- 20.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 21.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104(4):1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 22.Murphy KM, Levis M, Hafez MJ, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003;5(2):96–102. doi: 10.1016/S1525-1578(10)60458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fournier T, Medjoubi NN, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482(1):157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Knapper S, White P, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286–3293. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone RM, Fischer T, Paquette R, et al. Phase IB study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (DA) induction and high-dose cytarabine consolidation in newly diagnosed patients with AML [abstract]. Blood. 2005;106:121a. [Google Scholar]
- 28.Knapper S, Burnett A, Hills RK, Small D, Levis M. Lestaurtinib FLT3 inhibitory activity is modulated by concomitant azole therapy and may influence relapse risk [abstract]. Blood. 2009;114:326a. [Google Scholar]
- 29.Piloto O, Wright M, Brown P, Kim KT, Levis M, Small D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007;109(4):1643–1652. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107(1):293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 31.Ozeki K, Kiyoi H, Hirose Y, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103(5):1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]