Abstract
Ethyl 2-((2,3-bis(nitrooxy)propyl)disulfanyl)benzoate (GT-094) is a novel NO chimera containing an NSAID and NO moieties and also a disulfide pharmacophore that in itself exhibits cancer chemopreventive activity. In this study, the effects and mechanism of action of GT-094 were investigated in RKO and SW480 colon cancer cells. GT-094 inhibited cell proliferation and induced apoptosis in both cell lines and this was accompanied by decreased mitochondrial membrane potential (MMP) and induction of reactive oxygen species (ROS), and these responses were reversed after cotreatment with the antioxidant glutathione. GT-094 also downregulated genes associated with cell growth [cyclin D1, hepatocyte growth factor receptor (c-Met), epidermal growth factor receptor (EGFR)], survival (bcl-2, survivin), and angiogenesis [vascular endothelial growth factor (VEGF) and its receptors (VEGFR1 and VEGFR2)]. Results of previous RNA interference studies in this laboratory has shown that these genes are regulated, in part, by specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 that are overexpressed in colon and other cancer cell lines and not surprisingly, GT-094 also decreased Sp1, Sp3 and Sp4 in colon cancer cells. GT-094-mediated repression of Sp and Sp-regulated gene products was due to downregulation of microRNA-27a (miR-27a) and induction of ZBTB10, an Sp repressor that is regulated by miR-27a in colon cancer cells. Moreover, the effects of GT-094 on Sp1, Sp3, Sp4, miR-27a and ZBTB10 were also inhibited by glutathione suggesting that the anticancer activity of GT-094 in colon cancer cells is due, in part, to activation of an ROS-miR-27a:ZBTB10-Sp transcription factor pathway.
Keywords: GT-094, NO-NSAID, Sp proteins, colon cancer, miR-27a:ZBTB10
INTRODUCTION
Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) are invaluable for treatment of fever, pain, arthritis, other inflammatory diseases, and cancer, and applications for these compounds continues to increase (1–4). Several epidemiology studies have reported decreased incidence of multiple cancers associated with NSAIDs (primarily aspirin) intake, and these drugs have been extensively investigated for their chemopreventive and chemotherapeutic activities (3–5). A recent study reported that aspirin use among women with breast cancer decreased the subsequent incidence of metastasis and cancer-related deaths (5). Aspirin and other NSAIDs play a role in colon cancer prevention and therapy (6–14), and specific NSAIDs and other cyclooxygenase (COX) inhibitors also decrease the risk of colon cancer among high risk individuals (12–14).
Nitric oxide (NO) plays an important role in the suppression of GI-induced inflammation and toxicity, and this has led to development of nitro-NSAIDs (NON-SAIDs) which combine the anti-inflammatory activities of NSAIDs with the NO-dependent protection from NSAID-induced GI toxicity. NO-aspirin and other NO-NSAIDs exhibit anticancer activity in a wide range of cancer cell lines and in vivo models (15–27), and these compounds are invariably more potent than their corresponding NSAID analogs. For example, the NO-NSAID analog 2-(acetyloxybenzoic acid 4-nitrooxymethyl)-phenyl ester (NO-ASA) is 700 times more potent than aspirin as an inhibitor of pancreatic cancer cell growth which is due to inhibition of cell proliferation and induction of apoptosis by both compounds (19). The mechanism of action of NO-NSAIDs as cancer chemotherapeutic agents is unclear; however, these compounds clearly inhibit cancer and tumor cell growth, induce apoptosis, and exhibit antiangiogenic and antimetastatic activity.
Ethyl 2-((2,3-bis(nitrooxy)propyl)disulfanyl)benzoate (GT-094) (25, 26) is a novel NO chimera containing an NSAID and NO moieties and also a disulfide pharmacophore that in itself exhibits cancer chemopreventive activity (28). GT-094 significantly decreases aberrant crypt foci, proliferation and inducible NO synthase (iNOS) levels in the azoxymethane-induced rat colon cancer (25) and decreases proliferation and arrests Caco-2 colon cancer cells in G2/M phase of the cell cycle (25, 26).
In this study, we investigated the mechanism of action of NO-NSAIDs using GT-094 as a model in RKO and SW480 colon cancer cells. IGT-094 inhibited colon cancer cell proliferation and induced apoptosis, and this was accompanied by downregulation of genes associated with cell growth [cyclin D1, hepatocyte growth factor receptor (c-Met), epidermal growth factor receptor (EGFR)], survival (bcl-2, survivin), and angiogenesis [vascular endothelial growth factor (VEGF) and its receptors (VEGFR1 and VEGFR2)]. Previous RNA interference studies in this laboratory has shown that all of these genes are regulated, in part, by specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 that are overexpressed in colon and other cancer cell lines (29–37). GT-094 also decreased Sp1, Sp3 and Sp4 in colon cancer cells and this was dependent on a decrease in mitochondrial membrane potential (MMP) and induction of reactive oxygen species (ROS). ROS-mediated repression of Sp and Sp-dependent genes involves downregulation of microRNA-27a (miR-27a) and induction of ZBTB10, an Sp repressor, and comparable results have also been observed for synthetic triterpenoid anticancer drugs in pancreatic and colon cancer cells (35, 38).
MATERIALS AND METHODS
Cell lines, reagents and antibodies
RKO and SW480 human colon carcinoma cell lines were obtained from American Type Culture Collection (Manassas, VA). RKO and SW480 cells were maintained in Dulbecco's modified/Ham's F-12 (Sigma-Aldrich, St. Louis, MO) with phenol red supplemented with 0.22% sodium bicarbonate, 5% fetal bovine serum, and 10 ml/L 100× antibiotic antimycotic solution (Sigma). Cells were grown in 150 cm2 culture plates in an air/CO2 (95:5) atmosphere at 37°C and passaged approximately every 3–5 days. GT-094 was synthesized in the laboratory of Dr. Gregory R. Thatcher (University of Illinois, Chicago). All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), except cleaved poly (ADP) ribose polymerase (PARP) and c-Met (Cell Signaling Technology, Danvers, MA), Sp1 and VEGFR2 (Millipore, Temecula, CA), survivin (R&D Systems, Minneapolis, MN), VEGFR1 (Abcam Inc. Cambridge, MA), and β-actin antibodies (Sigma-Aldrich). Glutathione, 98% (γ-glu-cys-gly, GSH) and carbonyl cyanide 3-chlorophenylhydrazone (CCCP) were purchased from Sigma-Aldrich. Dithiothretol (DTT, 98%) was obtained from Boehringer Mannheim Corp, (Indianapolis, IN). CM-H2DCFDA was purchased from Invitrogen. MG132 and lactacystin are the proteasomal inhibitors purchased from Calbiochem (Sandiago, CA) and Sigma Chemicals Co. (St Louis, MO), respectively.
Cell proliferation assays
RKO and SW480 cancer cell lines were plated (3 × 104 per well) using DMEM:Ham's F-12 medium containing 2.5% charcoal stripped fetal bovine serum (FBS) in 12-well plates and left to attach for 24 h. Cells were then treated with either vehicle or the indicated concentrations of GT-094. After 24, 48 and 72 h of treatment, cells were counted using a Coulter Z1 particle counter (39). Each experiment was carried out in triplicate and results are expressed as means ± SE for each determination.
Western blots
RKO and SW480 cancer cells were seeded in DMEM:Ham's F-12 medium containing 2.5% charcoal-stripped FBS and, after 24 h, cells were treated with either vehicle (DMSO) or the indicated compounds. Cells were collected using high-salt buffer (50 mM HEPES, 0.5 mol/L NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, and 1% Triton-X-100) and 10 ml/L Protease Inhibitor Cocktail (Sigma-Aldrich). Protein lysates were incubated for 3 min at 100°C before electrophoresis and then separated on 10% SDS-polyacrylamide gel electrophoresis 120 V for 3 to 4 h. Proteins were transferred onto polyvinylidene difluoride membranes by wet electroblotting in a buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol for 1.5 h at 180 mÅ. Membranes were blocked for 30 min with 5% TBST-Blotto (10 mM Tris-HCl, 150 mM NaCl, pH 8.0, 0.05% Triton X-100, and 5% nonfat dry milk) and incubated in fresh 5% TBST-Blotto with 1:500 primary antibody overnight with gentle shaking at 4°C. After washing with TBST for 10 min, the polyvinylidene difluoride membrane was incubated with secondary antibody (1:5000) in 5% TBST-Blotto for 2 h by gentle shaking. The membrane was washed with TBST for 10 min, incubated with 6 ml of chemiluminescence substrate for 1 min, and exposed to Kodak image station 4000 mm Pro (Carestream Health, Rochester, NY).
ROS estimation
Cellular ROS levels were evaluated with the cell permeant probe CM-H2DCFDA (5-(and-6)-chloromethyl-2',7' dichlorodihydrofluorescein diacetate acetyl ester). CM-H2DCFDA is nonfluorescent until removal of the acetate groups by intercellular esterases and oxidation occurs within the cell. Following treatment of the cells for 12 h, 48-well cell culture plates were loaded with 10 μM CM-H2DCFDA for 30 min, washed once with serum-free medium, and analyzed for ROS levels using Bio Tek Synergy 4 plate reader (Bio Tek Instruments, Inc., Winooski, VT) set at 480 nm and 525 nm excitation and emission wavelength, respectively. Cells were then washed twice with PBS and fixed with methanol for 3 min at room temperature. Methanol was then completely removed and 1 mg/ml Janus green was added to the cultures for 3 min. Following removal of Janus green, cultures were washed twice with PBS and 100 μl of 50% methanol was added to each well. Cell counts were then determined with the plate reader set to an absorbance of 654 nm, and ROS intensities were corrected accordingly. Three experiments were performed and analyzed on different days using 8 wells per treatment group, and results are expressed as means ± SE for each determination.
Terminal deoxyribonucleotide transferase-mediated nick-end labeling (TUNEL) assay
RKO and SW480 cells (10 × 104) were seeded in two-chambered glass slides and left overnight to attach. After treatment with indicated compounds for 12 h, the in situ cell death detection POD kit was used for the terminal deoxyribonucleotide transferase-mediated nick-end labeling (TUNEL) assay according to the instructions in the protocol manual for fixed cells. The percentage of apoptotic cells was calculated by counting the stained cells in eight fields, each containing 50 cells. The total number of apoptotic cells was plotted as a percentage in both cell lines.
Measurement of MMP
MMP was measured with the Mitochondrial Membrane Potential Detection Kit (Stratagene, Cedar Creek, TX) according to manufacturer's protocol using JC-1 dye. RKO and SW480 colon cancer cells were plated on two-well Lab-Tex Coverglass slides (NUNC A/S, Roskilde, Denmark) and, after 24 h, cells were treated with DMSO, CCCP (25 μmol/L), GT-094 (50 μmol/L) alone or with GSH (5 mmol/L) for 12 h. Cells were then incubated with 1× JC-1 dye at 37°C for 15 min and washed twice with assay buffer according to manufacturer's protocol, and then cells were subjected to microscopic analysis using Zeiss Stallion Dual Detector Imaging System (Carl Zeiss Microimaging Inc., Thornwood, NY). J-aggregates are detected as red fluorescence, and J-monomers are detected as green fluorescence. The ratio of red fluorescence to green fluorescence was measured using ImageJ Software. Cells were examined in more than 10 fields per slide on multiple slides. Data represent the average of all the fields and results are expressed as means ± SE for each determination.
Quantitative real-time PCR analysis of mRNAs and miRNAs
miRNA was extracted using the mirvaRNA extraction kit (Applied Biosystems). Quantification of miRNA (RNU6B, miRNA-27a) was performed using the Taqman miRNA kit (Applied Biosystems) according to the manufacturer's protocol with real-time PCR. U6 small nuclear RNA was used as a control to determine relative miRNA expression. Total RNA was isolated using the RNeasy Protect Mini kit (Qiagen) according to the manufacturer's protocol. RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's protocol. PCR was carried out with the SYBR Green PCR Master Mix from PE Applied Biosystems on an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems) using 0.5 μmol/L of each primer and 2 μl cDNA template in each 25 μl reaction. TATA binding protein (TBP) was used as an exogenous control to compare the relative amount of target gene in different samples. The PCR profile was as follows: one cycle of 95°C for 10 min, then 40 cycles of 95°C for 15 s, and 60°C for 1 min. The comparative CT method was used for relative quantitation of samples, and results are expressed as means ± SE for at least 3 separate determinations. Primers were purchased from Integrated DNA Technologies. The following primers were used.
TBP (F): 5′-TGCACAGGAGCCAAGAGTGAA-3′
TBP (R): 5′-CACATCACAGCTCCCCACCA-3′
ZBTB10 (F): 5′-GCTGGATAGTAGTTATGTTGC-3′
ZBTB10 (R): 5′-CTGAGTGGTTTGATGGACAGA-3′
Statistical analysis
Statistical significance of differences was determined by analysis of variance and student t-test, and the levels of probability were noted. All statistical tests were two-sided. IC50 values were calculated using non-linear regression analysis and expressed in μM, at 95% confidence intervals.
RESULTS
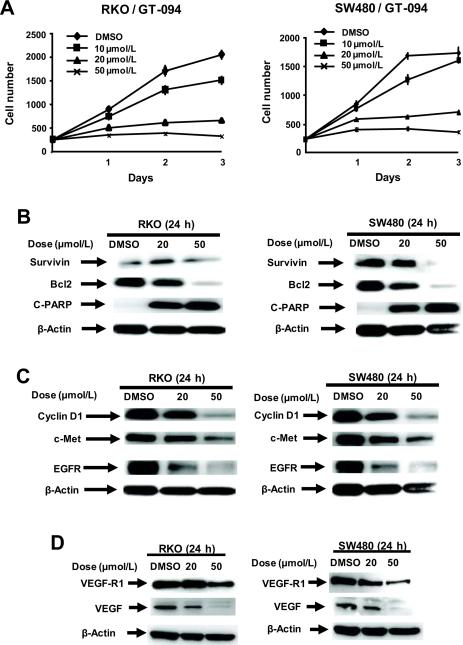
GT-094 is a model nitro-NSAID analog (NO-chimera) that contains both the aliphatic nitro group and a disulfide linker, and this compound exhibits cancer chemoprevention activity in an in vivo model for colon cancer. In this study, we initially investigated the concentration and time-dependent effects of GT-094 on proliferation of RKO and SW480 colon cancer cells (Fig. 1A). Significant growth inhibition was observed in both cell lines and the 24-h IC50 values for growth inhibition were 36 and 44 μmol/L in RKO and SW480 cells, respectively. In addition, the proapoptotic effects were examined in RKO and SW480 cells, and GT-094 induced caspase-dependent PARP cleavage in both cell lines (Fig. 1B). GT-094 decreased expression of pro-survival survivin and bcl-2 proteins, and this was consistent with the observed caspase-dependent PARP cleavage. GT-094 inhibited cell proliferation and induced apoptosis in RKO and SW480 cells and therefore, the effects of GT-094 on gene products associated with cell proliferation and apoptosis were also investigated. Results in Figure 1C show that GT-094 decreased expression of several proteins involved in cell proliferation and these included cyclin D1, c-Met and EGFR in both RKO and SW480 cells. In addition, we observed that GT-094 decreased expression of the angiogenic growth factor VEGF and its receptor VEGFR1 in RKO and SW480 cells (Fig. 1D).
Figure 1.
GT-094 inhibits growth and induces apoptosis in colon cancer cells. (A) Inhibition of RKO and SW480 cell growth. Cells were treated with different concentrations of GT-094, and cell numbers were determined on days 1, 2 and 3 as described in the Materials and Methods. Western blot analysis of Sp-regulated survival (B), proliferative (C), and angiogenic (D) gene products in RKO and SW480 cells treated with GT-094. Cells were treated with GT-094 (20 and 50 μmol/L) for 24 h, and whole cell lysates were analyzed by western blots as described in the Materials and Methods.
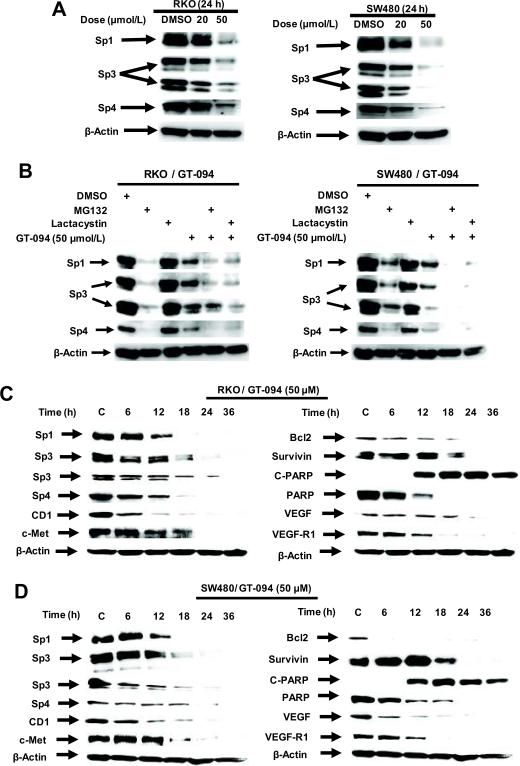
Previous studies with agents such as curcumin, betulinic acid, synthetic triterpenoid anticancer drugs, and the NSAID tolfenamic acid show that these compounds decrease expression of bcl-2, survivin, c-Met, EGFR, cyclin D1, VEGF and VEGFR1 protein in several cancer cell lines, including colon cancer cells, and RNA interference studies confirmed that these genes are regulated by Sp1, Sp3 and Sp4 transcription factors which are overexpressed in cancer cells (29, 34–38). Treatment of RKO and SW480 cancer cells with 20 and 50 μmol/L GT-094 decreased expression of Sp1, Sp3 and Sp4 proteins (Fig. 2A) and these results were consistent with decreased expression of Sp-regulated proteins illustrated in Figure 1. However, in contrast to the effects of betulinic acid and tolfenamic acid in prostate or pancreatic cancer cells, the effects of GT-094 on downregulation of Sp1, Sp3 and Sp4 proteins were not reversed by the proteasome inhibitors MG132 or lactacystin (Fig. 2B). MG132 alone decreased expression of Sp1, Sp3 and Sp4 in RKO and SW480 cells, whereas lactacystin alone slightly decreased Sp protein levels only in SW480 cells; however, a combination of lactacystin plus GT-094 did not reverse Sp downregulation but appeared to enhance this response. The time-dependent effects of GT-094 on Sp1, Sp3, Sp3 and Sp4 protein expression and levels of Sp-regulated genes were also determined in RKO (Fig. 2C) and SW480 (Fig. 2D) cells. In RKO cells, Sp1, Sp3 and Sp4 proteins decreased between 6–12 h after treatment and after 12 h, there was almost complete loss of these transcription factors. The time-dependent decrease in expression of bcl-2, survivin, c-MET, and VEGFR-R1 and induction of cleaved PARP followed a pattern similar to that observed for Sp proteins. However, cyclin D1 was significantly decreased within 6 h, whereas decreased VEGF expression was delayed (> 18 h), suggesting that other GT-094-mediated pathways may also be involved. In SW480 cells, the time-dependent pattern of GT-094-induced downregulation of Sp1, Sp3, Sp4 and Sp-regulated gene products was similar with decreased expression observed within 6–12 h and highly significant downregulation after 18 h. The major exceptions were the rapidly decreased responses (within 6 h) for bcl-2, VEGF and the low molecular weight Sp3 protein.
Figure 2.
GT-094-mediated effects on Sp protein and Sp-regulated gene products. (A) GT-094 decreases Sp1, Sp3 and Sp4 protein expression in colon cancer cells. RKO and SW480 cells were treated with 20 or 50 μmol/L GT-094 for 24 h, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. (B) Effects of proteasome inhibitors on Sp downregulation. Cells were treated with 50 μmol/L GT-094, MG132 or lactacystin alone or in combination, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. Results in Figure 2 are typical of replicate (at least 2) experiments. Time-course effects of GT-094 (50 μM) in RKO (C) and SW480 (D) cells. Cells were treated with DMSO (0 time) or GT-094 for 6, 12, 18, 24 and 36 h, and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods.
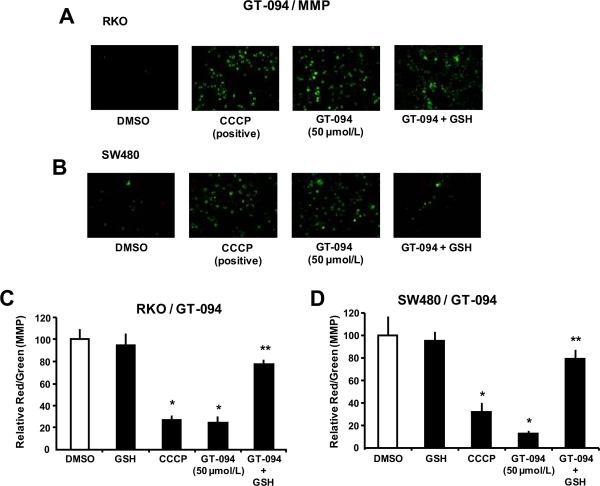
Ongoing studies with arsenic trioxide and other pro-oxidants that also decrease expression of Sp transcription factors suggest that mitochondria are the initial targets for these agents (40, 41). Figure 3A illustrates the effects of 50 μmol/L GT-094 and the mitochondriotoxic carbonyl cyanide 3-chlorphenyl hydrazone (CCCP) (24 μmol/L) which depolarizes mitochondria and decreases MMP. In RKO cells treated with the fluorescent dye JC-1 and DMSO or GSH, JC-1 formed a red fluorescent aggregate which is characteristic of high MMP. However, after treatment with GT-094 or CCCP, there was a decrease in red and an increase in green fluorescence of JC-1 which is typical of low MMP, and this was accompanied by a decreased red/green fluorescence ratio. However, cotreatment of RKO cells with GT-094 and the cellular antioxidant GSH (5 mmol/L) reversed the effects of GT-094 and restored MMP. Similar responses were observed in SW480 cells (Fig. 3B) and the results were quantitated in both cell lines (Figs. 3C and 3D).
Figure 3.
GT-094 decreases MMP in colon cancer cells. Effects of GT-094 in RKO (A) and SW480 (B) cells. Cells were treated with 50 μmol/L GT-094, 25 μmol/L CCCP, and GT-094 plus GST for 12 h and analyzed for changes in MMP as outlined in the Materials and Methods. Quantitative changes in MMP in RKO (C) and SW480 (D) cells. Changes in MMP were determined as described in the Materials and Methods. Significant (p<0.05) decreases by GT-094 or CCCP (*) and reversal of these effects by GSH (**) are indicated.
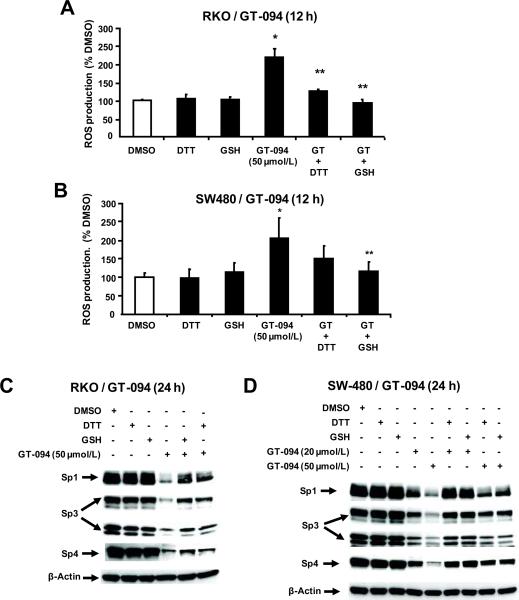
Drug-induced opening of the mitochondrial pore is associated with induction of ROS, and Figure 4A shows that treatment of RKO cells with 50 μM GT-094 significantly induced ROS levels as determined by hydrolysis of the cell permeant probe to give a fluorescent product. In addition, cotreatment of these cells with thiol antioxidants (DTT or GSH) significantly decreased induction of ROS by GT-094. In parallel experiments in SW480 cells, we also observed that GT-094 induced ROS and this response was also attenuated after cotreatment with the thiol antioxidants (Fig. 4B). The role of ROS in mediating GT-094-dependent downregulation of Sp1, Sp3 and Sp4 proteins was investigated in RKO and SW480 cells. Treatment of RKO (Fig. 4C) and SW480 (Fig. 4D) cells with GT-094 decreased expression of Sp1, Sp3 and Sp4 proteins in both cell lines and this response was attenuated in cells cotreated with GT-094 plus the thiol antioxidants. These results demonstrate that GT-094-mediated downregulation of Sp1, Sp3 and Sp4 proteins in colon cancer cells is dependent on decreased MMP and induction of ROS, and we have observed similar effects for arsenic trioxide and curcumin in bladder and pancreatic cancer cells, respectively (38, 40).
Figure 4.
Role of ROS in GT-094-mediated downregulation of Sp1, Sp3 and Sp4 proteins. Induction of ROS in RKO (A) and SW480 (B) cells. Cells were treated with 50 μmol/L GT-094, 1 mmol/L DTT, and 5 mmol/L GSH alone or in combination for 12 h, and ROS was determined as described in the Materials and Methods. Significant (p<0.05) induction by GT-094 (*) and inhibition of this response by GSH or DTT (**) are indicated. Effects of GSH or DTT on GT-094-mediaited Sp downregulation in RKO (C) and SW480 (D) cells. Cells were treated with different concentrations as indicated for 24 h, and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods. Results are typical of replicate (2) experiments.
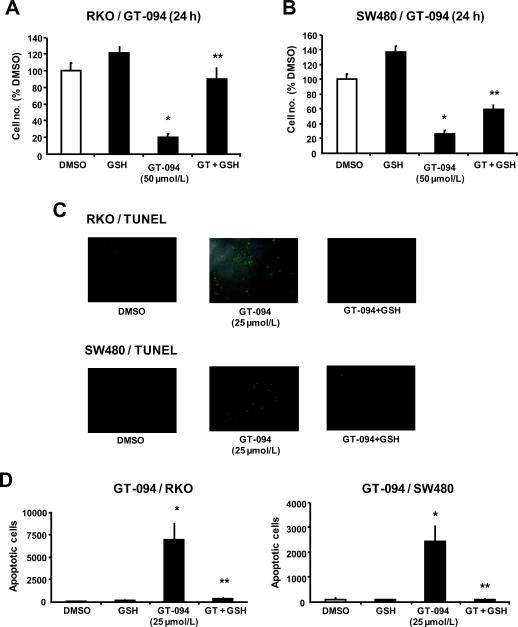
Since GT-094-induced mitochondrial disruption and induction of ROS mediates downregulation of Sp1, Sp3 and Sp4, the role of this pathway on inhibition of colon cancer cell growth and induction of apoptosis was investigated. Treatment of RKO and SW480 cells with 50 μM GT-094 for 24 h significantly inhibited proliferation of both cell lines; however, in cells cotreated with GT-094 and the antioxidant GSH, the growth inhibitory effects of the nitro-NSAID was attenuated (Figs. 5A and 5B). The results also show that the role of ROS-induced growth inhibition was more dominant in RKO than in SW480 cells, suggesting that other ROS-independent pathways are involved in the inhibitory effects of GT-094 in SW480 cells. Figures 5C and 5D show that GT-094 enhanced TUNEL staining, indicative of apoptosis in RKO and SW480 cells, and this effect was inhibited in cells cotreated with GT-094 plus GSH. These results confirm that GT-094-dependent activation of ROS plays an important role in the observed downregulation of Sp1, Sp3 and Sp4 transcription factors, decreased cell proliferation, and induction of apoptosis in colon cancer cells.
Figure 5.
GSH blocks GT-094-induced growth inhibition and apoptosis. Inhibition of RKO (A) and SW480 (B) cell growth by GT-094 blocked by GSH. Cells were treated with 50 μmol/L GT-094 or 5 mmol/L GSH alone or in combination for 24 h, and cell numbers were determined as described in the Materials and Methods. Significant (p<0.05) inhibition by GT-094 (*) or reversal of this effect by GSH (**) are indicated. Induction of apoptosis by GT-094 ± GSH in RKO (C) and SW480 (D) cells. Cells were treated with GT-094 ± GSH for 24 h, and apoptosis was determined by TUNEL staining as described in the Materials and Methods. Significant (p<0.05) induction of apoptosis by GT-094 (*) and inhibition after cotreatment with GSH (**) are indicated.
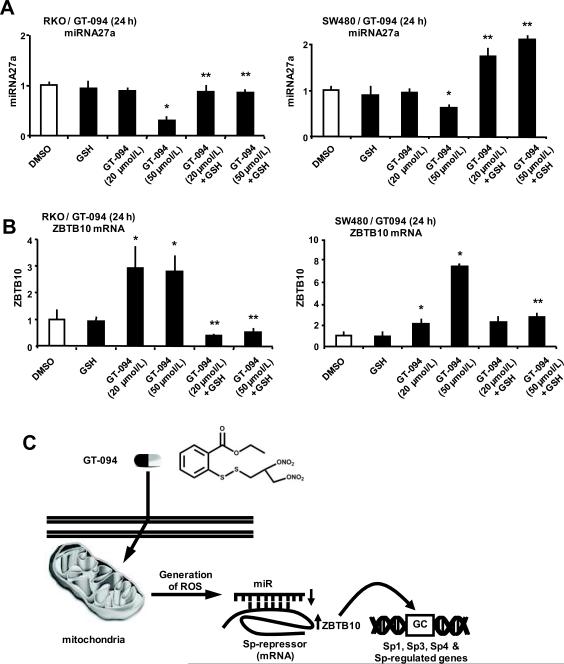
A recent report shows that downregulation of Sp transcription by methyl 2-cyano-3,11-dioxo-18β-oleana-1,12-dien-30-oate (CDODA-Me) is due to disruption of miR-27a:ZBTB10 interactions in colon cancer cells, resulting in decreased expression of miR-27a and induction of ZBTB10, an Sp repressor (35). Treatment of RKO and SW480 cells with GT-094 decreased miR-27a expression in both cell lines (Fig. 6A) and cotreatment with GSH reversed this effect. In parallel experiments, we observed that GT-094 induced ZBTB10 mRNA levels in RKO and SW480 cells (Fig. 6B) cells and this response was also attenuated after cotreatment with GSH. Downregulation of miR-27a was only observed using 50 μM GT-094, whereas 20 and 50 μM GT-094 induced ZBTB10 and this was particularly evident in RKO cells, suggesting that other microRNAs or other factors that play a role in regulating ZBTB10 expression may be affected by GT-094. The time-dependent effects of GT-094 (50 μM) on miR-27a and ZBTB4 were also investigated (Supplement Fig. 1). GT-094 decreased miR-27a expression in both cell lines within 6 h, whereas ZBTB10 was induced after 6–12 and 12–18 h in RKO and SW480 cells, respectively, indicating some cell context-dependent differences.
Figure 6.
GT-094 modulates miR-27a and ZBTB10 expression. (A) GT-094 decreases miR-27a expression in RKO and SW480 cells. Cells were treated with 20 or 50 μmol/L GT-094 and 5 mmol/L GSH alone or in combination for 24 h, and miR-27a was determined by real time PCR as outlined in the Materials and Methods. (B) GT-094 induces ZBTB10 in RKO and SW480 cells. Cells were treated with 20 or 50 μmol/L GT-094 and 5 mmol/L GSH alone or in combination for 24 h, and ZBTB10 mRNA levels were determined by real time PCR as outlined in the Materials and Methods. Significant (p<0.05) inhibition of miR-27a and induction of ZBTB10 (*) and inhibition of these responses by GSH (**) are indicated. (C) Proposed molecular mechanism for GT-094-induced ROS and ROS-dependent disruption of the miR-27a:ZBTB10-Sp/Sp-regulated gene axis.
DISCUSSION
Nitro-NSAIDs are a new class of NSAIDs that have been developed to provide the prototypical anti-inflammatory and analgesic effects of NSAIDs but have minimal toxic gastrointestinal side-effects observed for aspirin and related drugs. The NO moiety enhances gastric mucosal repair and ulcer healing and ameliorates NSAID-induced toxicity. NO-NSAIDs have also been investigated for their anticancer activities and the results of in vitro cell culture and in vivo animal studies have been promising (17–26). These compounds typically inhibit cell growth and induce apoptosis in various cancer cell lines and this includes inhibition of β-catenin/Tcf complexes and downregulation of cyclin D1 in colon cancer cells (17). Nitro-aspirin derivatives inhibit growth of tumors in mouse xenograft experiments using HT-29 colon cancer cells and this is accompanied by decreased vascularity in the tumors and downregulation of VEGF (27). GT-094 also inhibits cancer cell and tumor growth and, in both Caco-2 and HT-29 colon cancer cells, there was no evidence for induction of apoptosis (25, 26).
Studies in this laboratory have shown that COX-2 inhibitors such as celecoxib decreased colon cancer cell growth and the NSAID tolfenamic acid decreased pancreatic cancer cell growth, and these effects were due, in part, to downregulation of Sp1, Sp3 and Sp4 transcription factors (30, 34). GT-094 inhibited growth of RKO and SW480 colon cancer cells (Fig. 1A), induced PARP cleavage (Fig. 1B), and TUNEL staining (Fig. 5C) consistent with induction of apoptosis. Moreover, GT-094 also decreased expression of the antiapoptotic survivin and bcl-2 proteins and this was consistent with induction of apoptosis. GT-094 decreased expression of other proteins that play a role in cell growth (cyclin D1, c-Met and EGFR) and angiogenesis (VEGF and VEGFR1) (Figs. 1C and 1D) and this was consistent with previous studies on NONSAIDs which report downregulation of cyclin D1 and VEGF (24, 27). Previous studies in this laboratory have demonstrated by RNA interference that knockdown of Sp1, Sp3 and Sp4 individually or in combination decreases expression of the prosurvival, growth promoting, and angiogenic proteins (29, 31, 32, 37–41). Results shown in Figure 1 suggest that an underlying mechanism for these responses in RKO and SW480 cells may be related to downregulation of Sp1, Sp3 and Sp4 proteins and this was observed in these cells treated with GT-094 (Fig. 2A). The time-dependent decrease in expression of Sp1, Sp3, Sp4 and Sp-regulated genes was also determined in RKO and SW480 cells treated with GT-094 (Figs. 2C and 2D). Although the rate of decrease in expression of these proteins was similar in each of the cell lines, there were also notable exceptions, particularly for some putative Sp-regulated genes which have previously been characterized by Sp1, Sp3 and Sp4 knockdown in RNA interference studies (29, 31, 32, 37–41). For example, expression of cyclin D1 (RKO cells) and bcl-2 (SW480 cells) was rapidly decreased (< 6 h) prior to Sp transcription factors or most other Sp-regulated genes, suggesting that GT-094 also induced other pathways that repress genes independent of Sp downregulation.
At least two different pathways have been linked to drug-dependent downregulation of Sp transcription factors, namely via activation of proteasomes (29, 34, 36) or through downregulation of miR-27a and the subsequent induction of the Sp repressor ZBTB10 (35, 36). GT-094-dependent downregulation of Sp1, Sp3 and Sp4 was not reversed by proteasome inhibitors (Fig. 2B), whereas the NSAID tolfenamic acid and the triterpenoid betulinic acid induce proteasome-dependent degradation of Sp transcription factors in pancreatic and prostate cancer cells, respectively (34, 36). In contrast, CDODA-Me-dependent downregulation of Sp1, Sp3 and Sp4 in colon cancer cells was proteasome-independent as observed in this study, and the effects of CDODA-Me were due to decreased miR-27a and the subsequent induction of ZBTB10, a miR-27a-regulated mRNA (35). Moreover, in SW480 and RKO cells transfected with antisense-miR-27a or ZBTB10 expression plasmid, there is also a decrease in Sp1, Sp3, Sp4 and Sp-regulated genes (35). This pathway was also relevant for GT-094 which induced ZBTB10 and decreased miR-27a in colon cancer cells (Fig. 6 and Supplemental Figure 1). ZBTB10 is a member of the POK family of transcriptional repressors (42) and was first identified as an inhibitor of gastrin gene expression (43). ZBTB10 binds to the GC-rich gastrin gene promoter and inhibits Sp1-dependent transactivation and presumably inhibits expression of Sp1, Sp3, Sp4 and Sp-regulated genes through similar interactions with their GC-rich promoters.
In ongoing studies with arsenic trioxide (40) and other pro-oxidants including curcumin and CDDO-Me in pancreatic cancer cells (38, 41), we have observed that these compounds decreased MMP and increased ROS and ROS-induced downregulation of Sp and Sp-regulated gene products. Results illustrated in Figures 3 and 4 indicate that GT-094 decreased MMP and induced ROS in RKO and SW480 cells and this was reversed in cells cotreated with the thiol antioxidant GSH. The effects of GT-094 on MMP and ROS were similar to the induction of ROS by NO-ASA in colon cancer cell lines (18). However, induction of ROS by GT-094 in this study was also related to modulation of the miR-27a:ZBTB10-Sp1/Sp3/Sp4 axis since cotreatment with GSH attenuated GT-094-mediated downregulation of miR-27a and induction of ZBTB10 (Fig. 6) decreased expression of Sp1, Sp3 and Sp4 (Figs. 4C and 4D), increased TUNEL staining (Figs. 5C and 5D), and decreased growth (Figs. 5A and 5B). It was also apparent from the cotreatment studies (GT-094 + GSH) that there were cell context-dependent differences in the inhibitory effects of GSH. For example, GSH almost totally reversed GT-094-mediated inhibition of RKO cell proliferation but only partially reversed these effects in SW480 cells (Figs. 5A and 5B), suggesting that other GT-094-induced growth inhibitory responses were important in the latter cell line. In RKO cells, 20 μM GT-094 induced ZBTB10 mRNA but did not downregulate miR-27a (Figs. 6A and 6B), suggesting that other miRs or other factors contributed to expression of this gene and these are currently being investigated.
In summary, this study shows that like CDODA-Me, GT-094 decreases miR-27a and induces ZBTB10 expression in colon cancer cells and the subsequent downregulation of Sp1, Sp3, Sp4 and Sp-regulated proteins contributes to the anticancer activities of this compound. Moreover, GT-094 disruption of mitochondria and induction of ROS are critical elements for the subsequent ROS-dependent downstream disruption of the miR-27a:ZBTB10-Sp transcription factor axis (Fig. 6C). In addition, this study and a previous report in pancreatic cancer cells demonstrates that ROS suppresses miR-27a, and we have also observed similar ROS-dependent effects on the miR-27a promoter. We are currently investigating the specific cis-elements and trans-acting factors responsible for ROS-miR-27a interactions. These results demonstrate a hitherto unknown mechanism of action for GT-094 and other NONSAIDs in cancer cells (data not shown), and current studies are focused on the role of Sp transcription factors as targets for NO-NSAIDs and NSAIDs in cancer cells and tumors.
Supplementary Material
Acknowledgments
FUNDING: This research was supported by the National Institutes of Health [CA136571 (S.S.) and CA102590 (G.R.J.T.)] and Texas AgriLife Research.
REFERENCES
- 1.Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–50. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–9. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 4.Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- 5.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–72. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–58. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–66. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grau MV, Sandler RS, McKeown-Eyssen G, et al. Nonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: observational follow-up of a randomized study. J Natl Cancer Inst. 2009;101:267–76. doi: 10.1093/jnci/djn484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dube C, Rostom A, Lewin G, et al. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–75. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 10.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 11.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 12.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 13.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 14.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–82. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 15.del Soldato P, Sorrentino R, Pinto A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol Sci. 1999;20:319–23. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- 16.Kashfi K, Rigas B. The mechanism of action of nitric oxide-donating aspirin. Biochem Biophys Res Commun. 2007;358:1096–101. doi: 10.1016/j.bbrc.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Williams JL, Nath N, Chen J, et al. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res. 2003;63:7613–8. [PubMed] [Google Scholar]
- 18.Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci U S A. 2005;102:17207–12. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashfi K, Ryan Y, Qiao LL, et al. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect. J Pharmacol Exp Ther. 2002;303:1273–82. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- 20.Royle JS, Ross JA, Ansell I, Bollina P, Tulloch DN, Habib FK. Nitric oxide donating nonsteroidal anti-inflammatory drugs induce apoptosis in human prostate cancer cell systems and human prostatic stroma via caspase-3. J Urol. 2004;172:338–44. doi: 10.1097/01.ju.0000132367.02834.41. [DOI] [PubMed] [Google Scholar]
- 21.Rao CV, Reddy BS, Steele VE, et al. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: effects on molecular targets. Mol Cancer Ther. 2006;5:1530–8. doi: 10.1158/1535-7163.MCT-06-0061. [DOI] [PubMed] [Google Scholar]
- 22.Williams JL, Kashfi K, Ouyang N, del Soldato P, Kopelovich L, Rigas B. NO-donating aspirin inhibits intestinal carcinogenesis in Min (APC(Min/+)) mice. Biochem Biophys Res Commun. 2004;313:784–8. doi: 10.1016/j.bbrc.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–36. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 24.Bratasz A, Selvendiran K, Wasowicz T, et al. NCX-4040, a nitric oxide-releasing aspirin, sensitizes drug-resistant human ovarian xenograft tumors to cisplatin by depletion of cellular thiols. J Transl Med. 2008;6:9. doi: 10.1186/1479-5876-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagos GK, Carroll RE, Kouznetsova T, et al. Colon cancer chemoprevention by a novel NO chimera that shows anti-inflammatory and antiproliferative activity in vitro and in vivo. Mol Cancer Ther. 2007;6:2230–9. doi: 10.1158/1535-7163.MCT-07-0069. [DOI] [PubMed] [Google Scholar]
- 26.Hagos GK, Abdul-Hay SO, Sohn J, et al. Anti-inflammatory, antiproliferative, and cytoprotective activity of NO chimera nitrates of use in cancer chemoprevention. Mol Pharmacol. 2008;74:1381–91. doi: 10.1124/mol.108.046664. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang N, Williams JL, Rigas B. NO-donating aspirin inhibits angiogenesis by suppressing VEGF expression in HT-29 human colon cancer mouse xenografts. Carcinogenesis. 2008;29:1794–8. doi: 10.1093/carcin/bgn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Druesne N, Pagniez A, Mayeur C, et al. Diallyl disulfide (DADS) increases histone acetylation and p21(waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis. 2004;25:1227–36. doi: 10.1093/carcin/bgh123. [DOI] [PubMed] [Google Scholar]
- 29.Chadalapaka G, Jutooru I, Chintharlapalli S, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–54. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expession in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol. 2005;68:317–29. doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- 31.Abdelrahim M, Smith R, III, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64:6740–9. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 32.Higgins KJ, Abdelrahim M, Liu S, Yoon K, Safe S. Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun. 2006;345:292–301. doi: 10.1016/j.bbrc.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 33.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein (Sp) transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 34.Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–68. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 35.Chintharlapalli S, Papineni S, Abdelrahim M, et al. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate in colon cancer cells. Int J Cancer. 2009;125:1965–74. doi: 10.1002/ijc.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–23. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 37.Chadalapaka G, Jutooru I, Burghardt R, Safe S. Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol Cancer Res. 2010;8:739–50. doi: 10.1158/1541-7786.MCR-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jutooru I, Chadalapaka G, Abdelrahim M, et al. Methyl 2-Cyano-3,12-dioxooleana-1,9-dien-28-oate (CDDO-Me) decreases specificity protein (Sp) transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a. Mol Pharmacol. 2010;78:226–36. doi: 10.1124/mol.110.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdelrahim M, Samudio I, Smith R, Burghardt R, Safe S. Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J Biol Chem. 2002;277:28815–22. doi: 10.1074/jbc.M203828200. [DOI] [PubMed] [Google Scholar]
- 40.Jutooru I, Chadalapaka G, Sreevalsan S, et al. Arsenic trioxide downregulation of specificity protein (Sp) transcription factors in bladder cancer cells is dependent on reactive oxygen species (ROS) Exper Cell Res. 2010;316:2174–88. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFκB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein downregulation. J Biol Chem. 2010;285:25332–44. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costoya JA. Functional analysis of the role of POK transcriptional repressors. Brief Funct Genomic Proteomic. 2007;6:8–18. doi: 10.1093/bfgp/elm002. [DOI] [PubMed] [Google Scholar]
- 43.Tillotson LG. RIN ZF, a novel zinc finger gene, encodes proteins that bind to the CACC element of the gastrin promoter. J Biol Chem. 1999;274:8123–8. doi: 10.1074/jbc.274.12.8123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.