Abstract
The effects of testosterone on early atherogenesis and the role of aromatase, an enzyme that converts testosterone to estrogens, were assessed in low density lipoprotein receptor-deficient male mice fed a Western diet. Castration of male mice increased the extent of fatty streak lesion formation in the aortic origin compared with testes-intact animals. Administration of anastrazole, a selective aromatase inhibitor, to testes-intact males increased lesion formation to the same extent as that observed with orchidectomized animals. Testosterone supplementation of orchidectomized animals reduced lesion formation when compared with orchidectomized animals receiving the placebo. This attenuating effect of testosterone was not observed when the animals were treated simultaneously with the aromatase inhibitor. The beneficial effects of testosterone on early atherogenesis were not explained by changes in lipid levels. Estradiol administration to orchidectomized males attenuated lesion formation to the same extent as testosterone administration. Aromatase was expressed in the aorta of these animals as assessed by reverse transcription–PCR and immunohistochemistry. These results indicate that testosterone attenuates early atherogenesis most likely by being converted to estrogens by the enzyme aromatase expressed in the vessel wall.
Keywords: atherosclerosis, androgens, anastrazole, mice, estrogen
Men have a greater risk of coronary artery disease (CAD) than women in the reproductive years, and this gender difference diminishes after cessation of estrogen production after menopause (1). On this basis, it has been suggested that this gender difference is caused by an attenuating effect of estrogen on atherogenesis (2, 3). It has been suggested also that this gender difference may be caused in part by a proatherogenic action of androgens (4). Androgen administration has been associated with an increase in total cholesterol and low density lipoprotein (LDL), and both total cholesterol and LDL levels are correlated positively with CAD (5). Testosterone (T) administration has also been associated with an increased extent of atherosclerosis in primates (6) and rabbits (7) fed a high-cholesterol diet. However, recent studies in humans challenge the view that androgens indeed are associated with an increased risk of atherosclerosis (8–10). These differences may in part be because of the type of androgens administered and the route of administration. More recently, the role of T in atherogenesis has assumed great importance, because T administration has been shown to improve libido in postmenopausal women (11), a population that is more prone to clinically significant atherosclerosis. It is essential therefore to reassess the role, if any, of T in modulating the atherogenic process and the potential mechanisms involved.
We have speculated previously (12) that T may attenuate early atherogenesis at least in part by being converted to estradiol by the enzyme aromatase, which also is expressed in endothelial cells (13, 14). The present work was undertaken to test this hypothesis.
Materials and Methods
Chemicals.
[3H]estradiol and [3H]T were purchased from New England Nuclear. T, 17β-estradiol (E2), and placebo pellets were obtained from Innovative Research of America. Anastrazole (A) was supplied as a gift from Zeneca Pharmaceuticals (Cheshire, U.K.). Polyclonal mouse aromatase antibody was supplied kindly by JB Hutchison (Cambridge, U.K.). PST 1 restriction enzyme was obtained from Promega.
Animals and Procedures.
Sexually mature C57BL/6 LDL-receptor knockout (LDLR−/−) male mice were purchased from The Jackson Laboratory. All mice were maintained initially on a regular chow diet. At 8 weeks of age, animals either underwent orchidectomy (Orch) or sham operation according to the following protocol. After anesthesia with ketamine (100 mg/kg i.m.) and acetylpromazine (2.5 mg/kg, i.m.), the scrotum was prepped and draped in a sterile fashion. After incising the scrotum in the midline, the blood vessels supplying the testes were isolated and ligated and the testes were removed through the incision. The incision was closed with absorbable suture. The sham operation consisted of incising the scrotum and closing with suture as above. A skin incision was then made in the nape of the neck, and a T (12.5 mg, 90-day release), 17β-E2 (1.1 mg, 90-day release), or placebo (P) pellet was placed s.c. The incision then was closed with delayed absorbable suture. After 1 week of recovery, animals then were placed on a 42% fat, 0.15% cholesterol “Western” diet (diet number TD88137, Harlan Teklad, Madison, WI) for 8 weeks. Some animals received the aromatase inhibitor, A (10 mg/kg/day), in the drinking water during the entire 8-week feeding period. A is a potent and selective nonsteroidal aromatase inhibitor that at a dose of 1 mg/day in humans inhibited aromatase activity by a mean of 96.7% and suppressed E2 levels by 83.5% (15, 16). The dose for our study was chosen on the basis of the work of other investigators who administered 3 mg/kg/day A s.c. to nude mice and found this dose to be effective in inhibiting growth of breast cancer tumors in vivo (17). Because we elected to administer the A orally, rather than s.c., on a daily basis by adding it to the drinking water, and to ensure adequate inhibition of the aromatase enzyme, we chose to use a higher dose of A. All procedures were in accordance with current National Institutes of Health guidelines and were approved by the University of California Los Angeles Animal Research Committee.
Groups.
Animals were divided into the following groups: (i) testes intact (TI; sham operated) with P pellet (n = 9); (ii) Orch with P pellet (n = 11); (iii) Orch with T pellet (n = 8); (iv) TI (sham operated) with P pellet and administered A (n = 11); (v) Orch with T pellet and administered A (n = 10); and (vi) Orch with 17β-E2 pellet (n = 6). Then 1 week after surgery, all animals were placed on the Western diet for 8 weeks. At the conclusion of the feeding period, blood was collected for lipid analysis and hormone assays, and the left ventricle and proximal aorta were prepared for analysis of fatty streak lesion formation.
Tissue Preparation and Lesion Analysis.
The methods that were used for the quantitation of atheromatous lesions at the aortic valve were analyzed as reported (18). Briefly, at sacrifice the upper portion of the heart and proximal aorta were obtained, embedded in OCT compound, and stored at −70°C. Serial 10-μm-thick cryosections from the middle portion of the left ventricle to the aortic arch were collected for a distance of 400 μm and mounted on polyD-lysine coated slides. These sections were stained with Oil red O and hematoxylin. The lipid-staining areas of 25 sections were counted in a blinded fashion by light microscopy. Then the mean value of lipid-staining areas of aortic wall per section was calculated. The aortic origin in the vicinity of the aortic valve was chosen as the site for assessment of lesion formation, because it is one of the areas in which lesions can be detected first, and also it allows for a standardized comparison between groups. Furthermore, lesions in this area have been shown to correlate with later development of more advanced atherosclerotic lesions in the entire aorta (19).
Reverse Transcription (RT)–PCR For Aromatase.
The RT-PCR technique was used to assess the presence of aromatase in aortas obtained from male LDLR−/− mice. Male mouse brain was used as a positive control. Aortas were pooled from six mice. RNeasy mini kit from Qiagen (Chatsworth, CA) was used for homogenization and extraction of total cellular RNA. RT was performed by using 3 μg of total RNA per sample, 50 units of Moloney murine leukemia virus, and 5 μm of oligo dT reverse transcriptase and running the reaction at 42°C for 25 min. The resulting cDNA samples were PCR amplified by using the GENE AMP RNA PCR kit using 50 ng each of sense and antisense primers (Perkin–Elmer) in a 100-μl reaction mixture. The final reaction mixture was heat denatured at 94°C for 5 min and then subjected to touch-down PCR with amplification for 10 cycles (94°C for 35 sec, 62°C for 45 sec, −0.5°C per cycle, 72°C for 2 min), subsequent amplification for 25 cycles (94°C for 35 sec, 57°C for 45 sec, 72°C for 2 min), and one cycle at 72°C for 7 min. The PCR was carried out in the MJ Research (Cambridge, MA) PTC-200 thermal cycler.
The primers used for P450 aromatase PCR were based on the published mouse cDNA sequence (ref. 20; GenBank accession no. D00659): forward primer, 5′-GGA TTG GAA GTG CCT GCA ACT ACT-3′; and reverse primer, 5′-GAG CAT GTT AGA GGT GTC CAG CAT-3′. The expected product size is 400 bp. The resulting PCR products (30 μl) were analyzed on a 1.5% agarose gel by using appropriate molecular-weight markers to verify the required size of the final PCR product and then visualized by ethidium bromide staining. The identity of the PCR product was confirmed by appropriate restriction digestion.
Immunohistochemical Analysis of Aromatase.
The presence of aromatase in the aorta of C57BL/6 LDLR−/− mice was determined by immunohistochemical analysis as described (21). Frozen 10-μm-thick sections of the aorta were fixed with acetone for 20 min and washed with PBS. Fixed sections were incubated with a rabbit polyclonal antibody to mouse aromatase (1:400 dilution) for 1 h at room temperature. After washing, a biotinylated anti-rabbit secondary antibody was applied for 30 min followed by a 30-min incubation with avidin–biotin complex linked to horseradish peroxidase (Vector Laboratories, Portland, ME). Antibody binding was visualized with a peroxidase chromogen AEC kit (Biomeda, Foster City, CA). Control sections were treated identically except that the primary antibody was omitted.
Plasma Lipid Measurements.
Mice were fasted overnight and bled through retro-orbital veins under isofluorane anesthesia. Enzymatic assays for plasma total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides were performed as described by Hedrick et al. (22).
RIA for E2 and T.
Known amounts of [3H]T and [3H]E2 were added to 0.6–0.8 ml of plasma pooled from the animals (5–6) in each group, and the assays were performed as described (23–25).
Statistics.
All values are expressed as mean ± SEM with the exception of the T and E2 levels, which were pooled samples and expressed as mean. ANOVA was used to determine differences between groups in lesions or lipid levels. Differences were considered statistically significant at P < 0.05. The number of animals in each group ranged from 8–11 with the exception of the E2 group, in which there were 6 animals.
Results
Effect of T and Aromatase Inhibitors on Lesion Area.
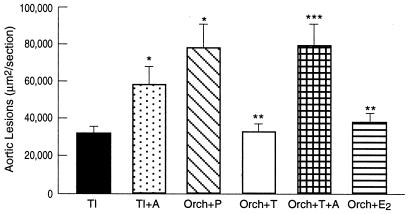
TI LDLR−/− male mice fed a cholesterol-enriched diet developed early fatty lesions that were significantly smaller than those observed in Orch mice given P, suggesting a role of T in attenuating atherogenesis (Fig. 1). When TI males were treated with the aromatase inhibitor, there was an increase in lesion area similar to that observed in Orch animals given P. Supplementation of T to Orch males significantly reduced lesion area when compared with Orch animals receiving P. This reduction with T was not observed when animals were treated simultaneously with the aromatase inhibitor (Fig. 1). No obvious differences in lesion morphology were observed amongst the various groups.
Figure 1.
Atherosclerotic lesions at the aortic root of male C57BL/6J, LDLR−/− mice. The mice were divided into the following groups: (i) TI (n = 11); (ii) TI + A (n = 11); (iii) Orch + P (n = 11); (iv) Orch + T (n = 8); (v) Orch + T + A (n = 10); and (vi) Orch + 17β-E2 (n = 6). Animals were fed a Western diet for 8 weeks. Values are expressed as mean ± SEM. *, P < 0.05 vs. TI, **, P < 0.05 vs. Orch + P, and ***, P < 0.05 vs. Orch + T
Effect of E2 Supplementation on Lesion Area in Orch Males.
E2 supplementation to Orch males significantly decreased lesion area similar to that observed in T-treated animals (Fig. 1).
Circulating Levels of T and E2.
The circulating levels of T and E2 (Table 1) were decreased significantly in Orch animals given P when compared with those that were gonad-intact. On the other hand, in TI animals treated with the aromatase inhibitor, the level of T was similar to that of TI males, whereas the levels of E2 were much lower (Table 1). Supplementation with T to Orch mice increased both T and E2 levels. Orch animals that were supplemented with T and treated simultaneously with the aromatase inhibitor had increased T levels compared with Orch animals but low levels of E2 (Table 1). In Orch animals administered E2, E2 levels were significantly higher than those in Orch animals receiving P (Table 1).
Table 1.
Plasma levels from pooled samples of T and E2 in TI and Orch males given T, E2, P, and/or A
| Treatment | T, pg/ml | E2, pg/ml |
|---|---|---|
| TI | 1,475 | 18 |
| TI + A | 1,762 | 9 |
| Orch + P | 27 | 6 |
| Orch + T | 1,213 | 15 |
| Orch + T + A | 1,350 | 7 |
| Orch + E2 | 18 | 392 |
Lipid Profiles.
The plasma total cholesterol level was significantly higher in Orch animals receiving P when compared with Orch animals receiving T (Table 2). Total cholesterol levels in animals given the aromatase inhibitor were not significantly different from those in TI animals given vehicle or Orch animals given T and vehicle, despite greater lesion formation in the animals receiving the aromatase inhibitor (Table 2).
Table 2.
Fasting plasma levels of total cholesterol (Chol), HDL-Chol, and triglycerides in TI and Orch males given T, E2, P, and/or A
| Treatment | Chol, mg/dl | HDL-Chol, mg/dl | Triglycerides, mg/dl |
|---|---|---|---|
| TI | 1,448 ± 46 | 98 ± 6 | 297 ± 34 |
| TI + A | 1,276 ± 37 | 117 ± 6 | 267 ± 22 |
| Orch + P | 1,677 ± 125 | 64 ± 4* | 250 ± 26 |
| Orch + T | 1,290 ± 54** | 64 ± 10* | 247 ± 19 |
| Orch + T + A | 1,405 ± 85** | 81 ± 11 | 300 ± 33 |
| Orch + E2 | 1,188 ± 152** | 89 ± 10**,*** | 122 ± 16*,**,*** |
*, P < 0.05 vs. TI; **, P < 0.05 vs. Orch + P; ***, P < 0.05 vs. Orch + T.
In TI mice given the aromatase inhibitor, HDL levels were similar to those in TI animals given vehicle, despite greater lesion formation in the animals receiving the aromatase inhibitor (Table 2). HDL levels were significantly higher in TI animals as compared with Orch mice given P (Table 2). In Orch animals, HDL levels were not significantly different between animals given P, T, or T plus the aromatase inhibitor, despite differences in lesion area between these groups (Table 2). Triglyceride levels were not affected by Orch, supplementation with T, or administration of the aromatase inhibitor in both TI and Orch animals (Table 2). E2 administration to Orch mice decreased total cholesterol, increased HDL levels, and decreased triglycerides compared with Orch animals given P (Table 2).
Aromatase Expression in Aorta.
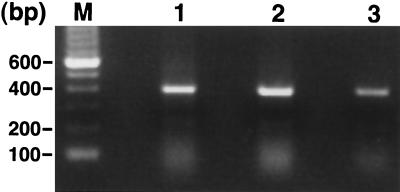
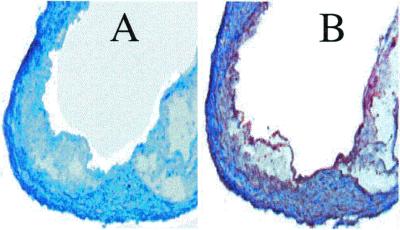
As judged by RT-PCR analysis of RNA extracted from aorta, LDLR−/− male mice expressed aromatase mRNA in the aorta (Fig. 2). Sections of aorta also stained positively with a polyclonal antibody specific for aromatase (Fig. 3). The inner surface of the atherosclerotic lesion was stained abundantly with the aromatase antibody, and some staining in the smooth muscle layer was observed also (Fig. 3B). No staining was observed within the lesion area (Fig. 3B). Control sections in which the primary antibody was omitted failed to show significant staining (Fig. 3A).
Figure 2.
RT-PCR analysis of aromatase mRNA from mouse brain (lane 1), mouse brain (lane 2), and LDLR−/− mouse aorta (lane 3).
Figure 3.
Immunohistochemical analysis of aromatase expression in the vessel wall of LDLR−/− mice. Frozen sections were fixed with acetone, and incubated with a rabbit polyclonal antibody against mouse aromatase. (A) Section on which incubation with polyclonal antibody was omitted. (B) Section incubated with polyclonal antibody. Original magnification was ×40.
Discussion
The effects of T on early atherogenesis were assessed in LDLR−/− mice that were fed a cholesterol-enriched diet. We elected to evaluate lesion development in the aortic origin in the vicinity of the aortic valve, because this is one of the areas in which lesions can be detected first, and it also provides a standardized site for comparison between different groups (19). Furthermore, lesions in this area have been shown to correlate with later development of more advanced atherosclerotic lesions in the entire aorta (19). We demonstrate that T attenuates early atherogenesis in LDLR−/− mice that are fed a cholesterol-enriched diet, because the extent of lesion formation in Orch animals was greater than that of TI animals or Orch animals supplemented with T. Our results are similar to those reported by other investigators in castrated cholesterol-fed rabbits, where animals given T demonstrated less aortic atherosclerosis than those given P (26). However, these investigators did not elucidate the mechanism(s) by which T attenuated atherogenesis.
We next evaluated whether the antiatherogenic action of T was due to a direct effect of T itself or by its conversion to E2. T is converted by an enzyme complex termed aromatase (27), which has been shown to be present in various tissues including endothelial cells (13, 14) and vascular smooth muscle (27). Local formation of estrogens from circulating androgens via aromatase has been implicated as a regulatory effect of androgens on various functions in the brain (28, 29) and ovary (27). However, the role of aromatase in the vascular wall and in modulating atherogenesis has not been assessed previously.
The observation that in TI animals given the aromatase inhibitor, a greater extent of early lesion formation was seen as compared with TI animals given vehicle suggests that T inhibited atherogenesis by its conversion to E2 rather than by a direct action of its own. This concept was confirmed further by the finding that in Orch mice supplemented with T and simultaneously given the aromatase inhibitor, the extent of lesion formation was increased significantly compared with those administered T alone. This suggestion is supported by the fact that the extent of lesion development appeared to be correlated inversely with circulating E2 levels.
If T indeed did inhibit atherogenesis through conversion to E2, then it would be important to demonstrate a beneficial effect of E2 administration in male animals. We found that E2 supplementation also attenuated early atherogenesis in male mice approximately to the same extent as that after administration of T. However, it may not be appropriate to supplement men with E2 to attenuate atherogenesis, because E2 administration to men led to an increase in cardiovascular events (30). The exact etiology for the high morbidity rate in men is not known, but may be caused by an increase in coagulation factors (31). One advantage of local conversion in the vascular wall of T to E2 is that it would lead to higher local levels but relatively lower circulating levels of E2. This local conversion would thereby limit the exposure of other tissues such as the liver to E2 and thereby reduce its adverse effects. This concept is supported by our observation that aromatase is present in the vascular wall of mice similar to that reported by investigators in other species including humans (13, 14, 27).
The aromatase inhibitor did not accentuate atherogenesis itself as in oophorectomized female LDLR−/− mice, E2 supplementation, when given alone or in combination with the aromatase inhibitor, inhibited atherogenesis to the same extent (L.N., unpublished observations).
The mechanism(s) by which E2 generated by conversion of T led to decreased lesion formation in male mice was not explored in this study, but it seems the effects of T may have been mediated in part via an action on the vascular wall rather than primarily by effects on lipids. Orch did produce adverse effects on lipid profiles, and supplementation with either T or E2 reversed some of these changes. However, administration of T did not improve lipid profiles to the same extent as that observed with exogenous administration of E2. This effect of T on lipids may be because T led to formation of E2 only at selective sites at which aromatase is present, whereas exogenous E2 led to higher circulating levels of E2 and possibly more pronounced effects on the liver, thereby leading to more profound changes in circulating lipoproteins. Regardless of the different effects of T and E2 on lipids, changes in lipids did not explain the changes in lesion area in some groups of animals. For example, the lipid levels did not differ between TI animals given vehicle and those given the aromatase inhibitor, nor did they differ between castrated animals given T and those given T plus the aromatase inhibitor, despite the significant differences in the lesion area between these groups. Therefore, it seems the effects of T after conversion to E2 most likely are mediated, at least in part, by a direct action on the vascular wall, and that effects on lipids are not a prerequisite for the anti-atherogenic action of T. We previously demonstrated decreases in vascular cell adhesion molecule-1 (12) and monocyte chemoattractant protein-1 (32) expression in vivo in hypercholesterolemic animals receiving E2. This finding may indicate a similar direct action of E2 on the vascular wall in attenuating atherogenesis.
Results from our studies may offer a potential explanation as to why, in certain strains of mice, the males are protected from atherosclerosis compared with females (33). In female mice, E2 levels fluctuate in a cyclic fashion, and therefore the animals are not exposed constantly to steady-state levels of E2. On the other hand, males, when compared with females, have much higher steady-state levels of T, which will ensure constant steady levels of E2 in the vascular tissue after conversion of T by aromatase. It would be interesting to assess whether there is increased expression of aromatase in the vascular wall of males in strains of mice in which males develop less atherosclerosis than females. Further studies also need to be carried out to assess whether the beneficial effects of T in attenuating atherogenesis in male mice are applicable also to females, and whether this effect is seen across species and in different vascular beds. It would be interesting also to assess whether the conversion of T to E2 by aromatase in the vascular wall is applicable to all species.
It is quite likely that T may exert cardioprotective effects in humans. Intramuscular administration of T to hypogonadal or elderly men decreased total cholesterol and the atherogenic fraction of LDL cholesterol without significantly altering HDL cholesterol (34). In a more recent study, it was observed that men with coronary artery disease had significantly lower levels of androgens than controls, challenging the preconception that physiologically high levels of androgens in men account for their increased relative risk for coronary artery disease (9, 10). Furthermore, a randomized crossover study of men with coronary artery disease demonstrated that when subjects received T intravenously, fewer ischemic changes were observed than when they received P (8). However, these investigators did not establish the precise mechanism(s) by which this protection by T occurs. On the basis of these studies, as well as the present study, it is essential to assess the role of T in human atherogenesis and the ideal route of administration to observe an atheroprotective action.
Our findings, if confirmed in humans, may have important clinical implications, because they would suggest that T could be administered to both men and postmenopausal women to increase their libido, with the expectation that its administration would not increase and actually may decrease coronary artery disease risk. Similarly, it would be interesting to evaluate whether transfection of the aromatase gene to the vascular graft used for coronary bypass to overexpress aromatase may ensure increased local concentrations of E2 in the vascular graft without significant increases in circulating E2 levels. Such increases in the local concentration of E2 in the bypass graft may delay or prevent the known complication of graft restenosis without untoward effects of increased circulating E2 levels.
Acknowledgments
This work was supported in part by the National Institute of Aging Grant AG-15B57 (to G.C. and L.N.), the National Heart, Lung, and Blood Institute Grants HL-46843 (to G.C.) and HL-30568 (to A.J.L.), and the Laubisch Fund for Cardiovascular Research, University of California Los Angeles. L.N. is a recipient of the Pfizer/Society for Women's Health Research Scholars Grant for Faculty Development in Women's Health.
Abbreviations
- LDL
low density lipoprotein
- T
testosterone
- LDLR−/−
LDL-receptor knockout
- Orch
orchidectomized mouse/mice
- E2
estradiol
- P
placebo
- TI
testes intact
- RT
reverse transcription
- HDL
high density lipoprotein
- A
anastrazole
References
- 1.Kannel W B, Hjortland M C, McNamara P M, Gordon T. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer J M, Colditz G A. Prev Med. 1991;20:47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 3.Green A, Bain C. Ballieres Clin Endocrinol Metab. 1993;7:95–112. doi: 10.1016/s0950-351x(05)80272-1. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan M L, Martinez C M, Gennis P, Gallagher E J. Prog Cardiovasc Dis. 1998;41:1–15. doi: 10.1016/s0033-0620(98)80019-4. [DOI] [PubMed] [Google Scholar]
- 5.Hayward C S, Kelly R P, Collins P. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 6.Adams M R, Williams J K, Kaplan J R. Arterioscler Thromb Vasc Biol. 1995;15:562–570. doi: 10.1161/01.atv.15.5.562. [DOI] [PubMed] [Google Scholar]
- 7.Fischer G M, Bashey R I, Rosenbaum H, Lyttle C R. Exp Mol Pathol. 1985;43:288–296. doi: 10.1016/0014-4800(85)90066-8. [DOI] [PubMed] [Google Scholar]
- 8.Webb C M, Adamson D L, de Zeigler D, Collins P. Am J Cardiol. 1999;83:437–439. doi: 10.1016/s0002-9149(98)00880-7. [DOI] [PubMed] [Google Scholar]
- 9.Phillips G B, Pinkernell B H, Jing T M. Arterioscler Thromb. 1994;14:701–706. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- 10.English K M, Mandour O, Steeds R P, Diver M J, Jones T H, Channer K S. Eur Heart J. 2000;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 11.Shifren J L, Braunstein G D, Simon J A, Casson P R, Buster J E, Redmond G P, Burki R E, Ginsburg E S, Rosen R C, Leiblum S R, et al. N Engl J Med. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 12.Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- 13.Sasano H, Murakami H, Shizawa S, Satomi S, Nagura H, Harada N. Endocr J. 1999;46:233–242. doi: 10.1507/endocrj.46.233. [DOI] [PubMed] [Google Scholar]
- 14.Diano S, Horvath T L, Mor G, Register T, Adams M, Harada N, Naftolin F. Menopause. 1999;6:21–28. [PubMed] [Google Scholar]
- 15.Dowsett M. Ann Oncol. 1997;8:631–632. doi: 10.1023/a:1008282315089. [DOI] [PubMed] [Google Scholar]
- 16.Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, Kormeset P O, Lonning P E. Br J Cancer. 1996;74:1286–1291. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Q, Yue W, Wang J, Liu Y, Long B, Brodie A. Breast Cancer Res Treat. 1998;50:63–71. doi: 10.1023/a:1006004930930. [DOI] [PubMed] [Google Scholar]
- 18.Qiao J H, Xie P Z, Fishbein M C, Kreuzer J, Drake T A, Demer L L, Lusis A J. Arterioscler Thromb. 1994;14:1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- 19.Tangirala R K, Rubin E M, Palinski W. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 20.Terashima M, Toda K, Kawamoto T, Kuribayashi I, Ogawa Y, Maeda T, Shizuta Y. Arch Biochem Biophys. 1991;285:231–237. doi: 10.1016/0003-9861(91)90354-l. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Segura L M, Wozniak A, Azcoitia I, Rodriguez J R, Hutchison R E, Hutchison J B. Neuroscience. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 22.Hedrick C C, Castellani L W, Warden C H, Puppione D L, Lusis A J. J Biol Chem. 1993;268:20676–20682. [PubMed] [Google Scholar]
- 23.DeVane G W, Vzekala N M, Judd H L, Yen S S C. Am J Obstet Gynecol. 1975;121:496–500. doi: 10.1016/0002-9378(75)90081-2. [DOI] [PubMed] [Google Scholar]
- 24.Anderson D C, Hopper B R, Lasley B L, Yen S S C. Steroids. 1976;28:179–196. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 25.Lu K H, Hopper B R, Vargo T M, Yen S S C. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 26.Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Circ Res. 1999;84:813–819. doi: 10.1161/01.res.84.7.813. [DOI] [PubMed] [Google Scholar]
- 27.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Circ Res. 1999;84:1285–1291. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 28.Balthazart J, Foidart A. J Steroid Biochem Mol Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 29.Roselli C E, Abdelgadir S E, Resko J A. Brain Res Bull. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 30.Coronary Drug Project Research Group. J Am Med Assoc. 1970;214:1303–1313. [Google Scholar]
- 31.Nabulsi A A, Folsom A R, White A, Patsch W, Heiss G, Wu K K, Szklo M. N Engl J Med. 1993;328:1069–1075. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
- 32.Pervin S, Singh R, Rosenfeld M E, Navab M, Chaudhuri G, Nathan L. Arterioscler Thromb Vasc Biol. 1998;18:1575–1582. doi: 10.1161/01.atv.18.10.1575. [DOI] [PubMed] [Google Scholar]
- 33.Paigen B, Holmes P A, Mitchell D, Albee D. Atherosclerosis (Shannon, Ireland) 1987;64:215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- 34.Zgliczynski S, Ossowski M, Slowinska-Srzednicka J, Brzezinska A, Zgliczynski W, Soszynski P, Chotkowska E, Srzednicki M, Sadowski Z. Atherosclerosis (Shannon, Ireland) 1996;121:35–43. doi: 10.1016/0021-9150(95)05673-4. [DOI] [PubMed] [Google Scholar]