Abstract
Scar tissue at sites of traumatic injury in the adult central nervous system presents a combined physical and molecular impediment to axon regeneration. Of multiple known central nervous system scar associated axon growth inhibitors, semaphorin 3A has been shown to be strongly expressed by invading leptomeningeal fibroblasts. We have previously demonstrated that infusion of the small leucine-rich proteoglycan decorin results in major suppression of several growth inhibitory chondroitin sulphate proteoglycans and growth of adult sensory axons across acute spinal cord injuries. Furthermore, decorin treatment of leptomeningeal fibroblasts significantly increases their ability to support neurite growth of co-cultured adult dorsal root ganglion neurons. In the present study we show that decorin has the ability to suppress semaphorin 3A expression within adult rat cerebral cortex scar tissue and in primary leptomeningeal fibroblasts in vitro. Infusion of decorin core protein for eight days resulted in a significant reduction of semaphorin 3A messenger RNA expression within injury sites compared with saline-treated control animals. Both in situ hybridization and immunostaining confirmed that semaphorin 3A messenger RNA expression and protein levels are significantly reduced in decorin-treated animals. Similarly, decorin treatment decreased the expression of semaphorin 3A messenger RNA in cultured rat leptomeningeal fibroblasts compared with untreated cells. Mechanistic studies revealed that decorin-mediated suppression of semaphorin 3A critically depends on erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 function. Collectively, our studies show that in addition to suppressing the levels of inhibitory chondroitin sulphate proteoglycans, decorin has the ability to suppress semaphorin 3A in the injured central nervous system. Our findings provide further evidence for the use of decorin as a potential therapy for promoting axonal growth and repair in the injured adult mammalian brain and spinal cord.
Keywords: decorin, scar, semaphorin, ErbB4, STAT3
Introduction
The semaphorins are a large family of secreted and membrane-bound glycoproteins that function in axon guidance, fasciculation and synapse formation in the developing and adult nervous system (Pasterkamp and Giger, 2009). Class 3 secreted semaphorins are expressed in the cerebral cortex, where they have been implicated in the patterning of cortical efferent projections (Bagnard et al., 1998; Polleux et al., 1998, 2000), tangential migration of cortical interneurons (Marin et al., 2001; Tamamaki et al., 2001, 2003), control of cortical axonal and dendritic branching (Sasaki et al., 2002; Dent et al., 2004; Fenstermaker et al., 2004; Morita et al., 2006) and dendritic spine morphology and maturation (Yamashita et al., 2007; Tran et al., 2009). Class 3 secreted semaphorins have also been shown to play a role in the regulation of synapse formation and synaptic function of cortical and hippocampal neurons (Sahay et al., 2005; Bouzioukh et al., 2006; Tran et al., 2009). Neuropilin-1 and -2 are high-affinity receptors for class 3 secreted semaphorins (Feiner et al., 1997; Kolodkin et al., 1997; Giger et al., 1998b) and function as the ligand-binding component of a heteromeric receptor complex that also includes members of the Plexin family (Takahashi et al., 1999; Tamagnone et al., 1999). Semaphorin 3E is an exception as it was found to directly bind to PlexinD independent of neuropilins (Gu et al., 2005).
Semaphorin 3A (Sema3A) is the best-characterized member of the secreted class 3 semaphorins. It is expressed during development and in adulthood (Giger et al., 1996, 1998a) and functions as a potent chemorepellent for select neuronal populations in the central and peripheral nervous systems (Messersmith et al., 1995; Puschel et al., 1995; Shepherd et al., 1996). In the adult nervous system, changes in Sema3A expression have been linked to several neurological disorders including stroke (Fujita et al., 2001; Beck et al., 2002; Hou et al., 2008), Alzheimer’s disease (Good et al., 2004), amyotrophic lateral sclerosis (De et al., 2006), multiple sclerosis (Williams et al., 2007) and schizophrenia (Eastwood et al., 2003; Eastwood, 2004). After traumatic injury to the adult CNS, the formation of scar tissue rich in axon growth inhibitory chondroitin sulphate proteoglycans (CSPGs) has been shown to present a major impediment to axon regeneration (Davies et al., 1997, 1999). Importantly, in addition to inhibitory CSPGs, Sema3A has been shown to be upregulated at sites of traumatic CNS injury where it is also thought to act as an inhibitor of axonal regeneration by promoting growth cone collapse (Pasterkamp et al., 1999, 2001; De et al., 2002; Lindholm et al., 2004; Mire et al., 2008). Decreased Sema3A expression correlates with enhanced regeneration in an in vitro organotypic model of CNS injury (Montolio et al., 2009) and suppression of Sema3A-mediated signalling via inhibition of neuropilin-1 with SM-216289 leads to enhanced growth of injured serotonergic axons past the injury site in the rat spinal cord (Kaneko et al., 2006). Collectively, these studies support the idea that Sema3A associated with scar tissue contributes to the growth inhibitory nature of injured adult CNS tissue.
Enhanced Sema3A expression by invading leptomeningeal fibroblasts has been proposed as the major source of Sema3A within scar tissue at sites of CNS injury (Pasterkamp et al., 1999, 2001; De et al., 2002). Increases in neurite extension for dorsal root ganglion neurons co-cultured with meningeal cells treated with Sema3A-blocking antibodies or meningeal cultures derived from Sema3A-deficient mice provide further evidence that Sema3A is a major meningeal cell-derived factor responsible for growth cone collapse and inhibition of neurite growth (Niclou et al., 2003). These findings support the hypothesis that Sema3A upregulation by invading fibroblasts contributes to the axon growth inhibitory environment presented by scar tissue at sites of CNS trauma and that the targeting of Sema3A expression by these cells represents a potential therapy for promoting axon regeneration and functional recovery. At present, however, very little is known about which factors regulate Sema3A expression at sites of CNS injury.
Decorin is a small leucine rich proteoglycan expressed in many tissue types (Stichel et al., 1995; Hocking et al., 1998) that has been shown to suppress fibrotic scarring (Border et al., 1992; Isaka et al., 1996; Fukushima et al., 2001; Kolb et al., 2001a, b; Grisanti et al., 2005; Huijun et al., 2005) in several tissue disorders including brain and spinal cord injury (Logan et al., 1999; Davies et al., 2004). Decorin is capable of inhibiting transforming growth factor-β activity (Yamaguchi et al., 1990) and modulating the activity of downstream signalling pathways linked to several erythroblastic leukaemia viral oncogene homologue (ErbB) receptor family members (Csordas et al., 2000; Seidler et al., 2006). We have previously shown that infusion of human recombinant decorin core protein into acute spinal cord injury in adult rats can suppress the levels of multiple axon growth inhibitory CSPGs and render injury sites permissive for axon growth (Davies et al., 2004). Pre-treatment of adult leptomeningeal fibroblasts with decorin promoted a more than 3-fold increase in neurite length by co-cultured adult dorsal root ganglion neurons (Davies et al., 2004). The ability of decorin to promote axon growth across spinal cord injuries and on leptomeningeal fibroblasts in vitro suggests that decorin may also have the ability to suppress the expression of Sema3A within CNS injuries. In the present study we tested this hypothesis and show that decorin treatment reduces Sema3A expression by leptomeningeal fibroblasts in vitro and suppresses scar-associated Sema3A messenger RNA and protein within cerebral cortex injuries. We also demonstrate that decorin-mediated suppression of Sema3A is dependent on ErbB4 receptor activity and provide evidence of a novel role for signal transducer and activator of transcription 3 (STAT3) signalling in regulating Sema3A expression within CNS scar tissue.
Materials and methods
Animal experiments
All animal care and experiments were carried out according to the guidelines of the National Institutes of Health as well as policies set by our Institutional Animal Care and Use Committee (IACUC). Adult female Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) weighing 200–250 g were anaesthetized with an intraperitoneal injection of an anaesthetic cocktail containing ketamine (80 mg/kg) and xylazine (12 mg/kg). Cerebral cortex injuries were achieved by the surgical insertion of a low profile metal cannula (cannula tip 2.0 mm below pedestal; Plastics One Inc., Roanoke, VA, USA). A small hole was drilled into the skull of each rat at the stereotaxic coordinates AP −3.0 mm and ML +2.0 mm relative to bregma. The dura was carefully pierced and the cannula was inserted into the cerebral cortex until the base of the cannula pedestal was set flat on the skull surface. Cannulae were anchored to the superior end of the skull with Cerebond Skull Fixture Adhesive (Plastics One Inc.). Cannulae were connected via vinyl tubing to Alzet mini-osmotic pumps (mean pumping rate: 1.0 μl/h, Durect Corp., Cupertino, CA, USA) pre-loaded with either isotonic saline or 2.5 mg/ml human recombinant decorin (glycosaminoglycan-free form, Gala Biotech, Middleton, WI, USA) in saline. Following subcutaneous implantation of Alzet pumps, animals were sutured and allowed to recover from anaesthesia.
Tissue processing
Eight days following surgery, animals were euthanized with an overdose of ketamine and xylazine and brains from saline- and decorin-treated animals were collected for total RNA extraction, in situ hybridization or immunohistochemistry. For RNA extraction, brains were quickly removed and placed into RNAlater (Applied Biosystems/Ambion, Austin, TX, USA) for further dissection to isolate cortical lesions. For in situ hybridization, brains were quickly removed and flash frozen in dry-ice cooled isopentane, cryosectioned in the coronal plane at 25 µm per section and mounted on superfrost plus microscope slides. For immunohistochemistry, animals were transcardially perfused with 100 ml of phosphate-buffered saline followed by 400 ml of 4% paraforaldehyde in phosphate-buffered water. Perfused brains were removed and placed into 15% sucrose in phosphate-buffered saline for 24 h and then 30% sucrose for 24 h for cryopreservation. Cryopreserved brains were frozen on dry ice and cryosectioned in the coronal plane at 20 µm per section. Brain sections were mounted onto gelatin-coated microscope slides.
Leptomeningeal fibroblast cultures
Adult rat spinal leptomeningeal fibroblasts were isolated from spinal cord following the method previously described (Davies et al., 2004). Meningeal fibroblasts were also isolated from the dura mater and arachnoid overlying the cerebral cortex (hereafter referred to as brain meningeal fibroblasts). Adult rats were euthanized and after removal of the skull, dura mater and arachnoid overlying the cerebral cortex were collected into serum-free Dulbecco’s modified Eagle’s medium. The spinal column was removed and submerged into sterile calcium–magnesium free Hank’s balanced salt solution (Gibco/Invitrogen, San Diego, CA, USA) at 4°C. All further dissections were conducted at 4°C. Spinous processes were removed and the dura mater and arachnoid were collected from the dorsal surface of the spinal cord, with care taken to specifically exclude peripheral nerve tissue. The brain and spinal meninges were cut into 1 mm2 pieces and transferred to a tube containing dispase I (2.5 U/ml, Roche, Germany) and collagenase II (200 U/ml, Worthington, Lakewood, NJ, USA) in 1 ml of Hank’s balanced salt solution. Tissue was incubated in enzyme for 80 min at 37°C in 5% CO2 atmosphere. Dulbecco’s modified Eagle’s medium/10% foetal bovine serum (Gibco/Invitrogen) was added to stop the enzyme reaction. DNaseI (5 μg/ml) was added to the suspension and the tissue was gently triturated. The suspension was centrifuged at room temperature for 5 min at 1500 rpm using a Microfuge 18 centrifuge (Beckman-Coulter, Fullerton, CA, USA). The supernatant was removed and the cells resuspended in Dulbecco’s modified Eagle’s medium/5% foetal bovine serum and plated in poly-l-lysine coated T-25 vented flasks.
Treatment of spinal leptomeningeal fibroblasts with various inhibitors and decorin
Leptomeningeal fibroblasts were grown until they reached 70–80% confluence except for small interfering RNA experiments described below, in which cells were grown to ∼50% confluence. Leptomeningeal fibroblasts were treated under serum-free conditions for 1 h with the following inhibitors: 10 µM PD158780 (pan-specific inhibitor of ErbB tyrosine kinase activity; Calbiochem, San Diego, CA, USA), 10 μM AG1478 (inhibitor of ErbB1 tyrosine kinase activity; Calbiochem, San Diego, CA, USA), 40 μg/ml Ab-5 (ErbB3 blocking monoclonal antibody; Lab Vision, Fremont, CA, USA), 40 μg/ml Ab-3 (ErbB4 blocking monoclonal antibody; Lab Vision, Fremont, CA, USA), 1 µM L-685458 (γ-secretase inhibitor) or 1 μM cucurbitacin (STAT3 inhibitor). Following inhibitor incubations, fibroblasts were treated with decorin (20 μg/ml) for 48 h.
For ErbB4 small interfering RNA experiments, small interfering RNA duplexes were purchased from Applied Biosystems/Ambion (Austin, TX, USA). The rat ErbB4 target sequences were AAATTTAGTATTTCGGTCAGG and AACATAGTATTTGCGCAAGGC. As a negative control, a Silencer Negative Control #1 small interfering RNA (Applied Biosystems/Ambion) was used. All small interfering RNA duplexes were transfected at 100 nM concentrations in Oligofectamine (Invitrogen). Briefly, spinal leptomeningeal fibroblasts were grown until they reached 50% confluence at which point the media was changed to Optimem I (Invitrogen). The small interfering RNA duplexes and Oligofectamine were diluted in Optimem I and incubated at room temperature for 30 min. The mixture was then added to the cell cultures and incubated at 37°C in 5% CO2 atmosphere for 24 h. Quantitative polymerase chain reaction (PCR) analysis of ErbB4 small interfering RNA-treated fibroblasts verified a significant knockdown in the expression of ErbB4 messenger RNA compared with negative control cultures treated with Silencer Negative Control #1 small interfering RNA (Supplementary Fig. 1). No change in ErbB4 messenger RNA expression levels was detected between Silencer Negative Control small interfering RNA-treated and untreated cultures (data not shown). Following small interfering RNA knockdown of ErbB4 expression, leptomeningeal fibroblasts were then treated with decorin (20 µg/ml) for 48 h under serum-free conditions.
Total RNA isolation
Total RNA was isolated using Trizol reagent according to the manufacturer’s instructions. Briefly, lesioned brain tissue and meningeal fibroblasts were extracted and suspended in 1 ml of Trizol and incubated at room temperature for 5 min. Chloroform (0.2 ml) was added and the solution mixed by vigorous vortex for 30 s. Samples were centrifuged in a 5417C Eppendorf centrifuge (Westbury, NY, USA) at 14 000 rpm for 20 min at 4°C, the aqueous phase was transferred to a clean tube and an equal volume of isopropanol was added. Samples were centrifuged again at 14 000 rpm for 30 min at 4°C and the resulting pellet resuspended in diethylpyrocarbonate-treated water. Total RNA was quantified using a spectrophotometer DU series 500 (Beckman, Fullerton, CA USA).
Complementary DNA synthesis
Complementary DNA was synthesized from 1 μg total RNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The complementary DNA synthesis reaction was performed in a total volume of 100 μl containing 1 × reverse transcriptase buffer, 1 × deoxynucleotide triphosphates, 1 × random primers and 250 U of multiscribe reverse transcriptase (thermal conditions: 25°C for 10 min and 37°C for 120 min) in a PTC-200 Peltier Thermal Cycler (MJ Research/Bio-Rad Laboratories, Watertown, MA and MJ Research, Reno, NV, USA).
Quantitative real-time polymerase chain reaction
Rat messenger RNA quantification was performed using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The primers and probe were selected from the TaqMan Gene Expression Assay (Applied Biosystems). The assay ID numbers were Rn00436469_m1 for rat Sema3A, Rn00580398_m1 for rat ErbB1, Rn00566561_m1 for rat ErbB2, Rn00568107_m1 for rat ErbB3, Rn00572447_m1 for rat ErbB4, Rn00585674_s1 for rat suppressor of cytokine signalling 3 (SOCS3), Rn00588164_m1 for rat Src homology phosphatase 1 (SHP1) and Rn00582371_m1 for rat protein inhibitor of activated STAT3 (PIAS3). These primers and probes span different exons of the rat genes thus excluding the possibility of genomic DNA amplification. The quantitative PCR reaction was performed in a total reaction volume of 20 μl containing 1 × TaqMan Universal PCR Master Mix and 1 × TaqMan Gene Expression Assay Mix (thermal cycling conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 15 s and 60°C for 1 min). Eukaryotic 18S ribosomal RNA endogenous control (VIC/MGB probe) and rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control (VIC/MGB probe) (Applied Biosystems) were included on the same plate as that of the sample complementary DNA as an internal control. Each reaction plate contained non-template controls that did not show any amplification and serial dilutions of complementary DNA were used to estimate the PCR efficiencies of the different genes. The specificity of the reaction was verified by running the PCR products on an agarose gel (2%) and confirming the expected DNA band size. No other bands were seen on the gel. All quantitative PCR reactions were run in triplicate and at least three fully independent experiments were conducted. The threshold cycle (Ct) is the cycle number at which the fluorescence passes a set threshold within the linear range of the all amplification curves. Quantitative analysis was conducted with RQ study software (Version 1.2.2, Applied Biosystems) that uses the comparative method (2ΔΔCt) of relative quantification (Livak and Schmittgen, 2001). We validated the 2ΔΔCt method, comparing the amplification efficiencies of complementary DNA dilutions of the target gene and internal control (18S, GAPDH). Statistical analyses were made using the randomization test of the REST 2005 software (Corbett Research, Technical University Munich).
In situ hybridization
Riboprobes identical (sense) and complementary (anti-sense) to the coding regions of Sema3A were obtained by in vitro transcription of cloned rat complementary DNAs as described (Giger et al., 1996). Briefly, a 20 µl reaction containing 1 µg linearized template complementary DNA, 40 mM Tris–HCl pH 8.0, 6 mM MgCl2, 10 mM dithiothreitol, 2 mM spermidin, 10 mM NaCl, 2 µl 10 × digoxigenin RNA labelling mixture (Boehringer Mannheim/Roche Applied Science, Germany), 10 U RNase inhibitor and either 20 U T3 (sense) or T7 (anti-sense) RNA polymerases. The mixture was incubated for 2 h at 37°C before transcription was terminated by the addition of 20 U of RNase-free DNaseI and further incubation for 15 min. To improve tissue penetration, complementary RNA probes were partially hydrolysed for 1 h at 60°C in 10 mM dithiothreitol, 200 mM NaHCO3/Na2CO3, pH 11, to an average length of 100–250 nucleotides. Hydrolysis was neutralized with 100 mM acetic acid and the complementary RNA probes were precipitated with 1/10 volume of 4 M LiCl and 2.5 volumes of ethanol at −80°C. For in situ hybridization, precipitated complementary RNA fragments were recovered in 20 µl diethylpyrocarbonate-treated water.
To detect Sema3A transcripts in brain tissue sections in situ, brain sections from animals that were either saline or decorin-treated were processed as described previously (Giger et al., 1996). Briefly, sections were air dried at room temperature and fixed for 20 min in freshly prepared 4% paraformaldehyde in phosphate-buffered saline. Sections were then rinsed three times for 5 min in phosphate-buffered saline, and rinsed once briefly in diethylpyrocarbonate-treated water. Sections were acetylated by treating with 0.25% acetic anhydride in 1% triethanolamine for 10 min, and then washed two times in phosphate-buffered saline. Sections were rinsed in 2 × standard saline citrate for 5 min and prehybridized for 6 h at room temperature with a hybridization buffer containing 50% formamide, 5 × Denhardt’s solution, 5 × standard saline citrate, 250 µg/ml bakers yeast transfer RNA and 500 µg/ml sheared and heat-denatured herring sperm DNA. Following prehybridization, 100 µl of hybribization mixture containing 200 ng/ml Sema3A anti-sense Digoxygenin-labelled complementary RNA probe was applied to each slide, sealed with a coverslip and hybridized overnight at 55°C. Specificity control slides were included that were subjected to the entire in situ hybridization protocol described but were hybridized with the corresponding sense complementary RNA probe generated or with no probe added.
Following overnight hybridization, sections were washed under high stringency: 5 min wash at 55°C in 5 × standard saline citrate, 5 min wash at 55°C in 2 × standard saline citrate, 30 min wash at 55°C in 50% formamide in 0.2 × standard saline citrate and 5 min wash at room temperature in 0.2 × standard saline citrate. Sections were then rinsed in Tris-buffered saline (100 mM Tris–HCl, pH 7.5, 150 mM NaCl) at room temperature for 5 min and blocked for 1 h in a 1% solution of blocking reagent (Boehringer Mannheim/Roche Applied Science) in Tris-buffered saline. Digoxygenin-labelled RNA hybrids were detected by incubating sections for 1 h with an anti-digoxygenin Fab fragment conjugated to alkaline phosphatase (Boehringer Mannheim/Roche Applied Science) diluted to 1:2500 in Tris-buffered saline. Sections were then washed twice for 15 min in Tris-buffered saline. To block endogenous phosphatase activity, 0.24 mg/ml levamisole was added to the colour reagents, 300 µg/ml nitrobluetetrazolium chloride and 170 µg/ml 5-bromo-4-chloro-3-indolylphosphate in 100 mM NaCl, 5 mM MgCl2 and 100 mM Tris–HCl, pH 9.5. The colour reagent mixture was added atop sections and incubated overnight at room temperature. Sections were rinsed extensively in 10 mM Tris–HCl, pH 8.0 containing 5 mM ethylenediaminetetraacetic acid and then dehydrated in a graded series of ethanol, cleared in xylene and coverslipped with Histomount (National Diagnostics, Atlanta, GA, USA).
Immunohistochemistry
Brain sections were first cleared of paraformaldyde by rinsing slides twice in phosphate-buffered saline for 10 min. Sections were then treated with 10% goat serum in phosphate-buffered saline containing 0.1% Triton X-100 for 30 min at room temperature to block non-specific binding throughout the staining protocol. Rabbit anti-Sema3A (IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-fibronectin (IgM, Sigma, St. Louis, MO, USA) antibodies were then added to sections at 1:100 and 1:500 dilutions, respectively, in 10% goat serum in phosphate-buffered saline containing 0.1% Triton X-100. Sections were incubated with primary antibodies for 16 h at room temperature. For analysis of potential Sema3A immunoreactivity associated with astrocytes and neurons, alternate tissue sections were incubated with rabbit anti-Sema3A antibody as described above followed by either mouse anti-glial fibrillary acidic protein (IgG, Sigma, St. Louis, MO, USA) for astrocytes and mouse anti-NeuN (IgG, Millipore, Temecula, CA, USA) for neurons at dilutions of 1:600 for 4 h at room temperature. Following primary incubation, sections were washed twice in phosphate-buffered saline for 5 min each followed by three washes in phosphate-buffered saline for 10 min each. Primary antibody detection for Sema3A was carried out using a 1:600 dilution of biotinylated goat anti-rabbit IgG in 10% goat serum in phosphate-buffered saline containing 0.1% Triton X-100 for 45 min at room temperature. Following phosphate-buffered saline washes, separate sets of sections were incubated with a 1:2000 dilution of Alexa-488 labelled streptavidin (for Sema3A) and 1:700 dilutions for Alexa-594 goat anti-mouse IgM (for fibronectin), or Alexa-594 goat anti-mouse IgG (for GFAP or NeuN) in 10% goat serum in phosphate-buffered saline containing 0.1% Triton X-100 for 30 min at room temperature. Final washes were carried out and slides were mounted using Fluoromount G (Invitrogen). Stained sections were viewed and images acquired on a Zeiss Observer Z1 microscope (Carl Zeiss Microimaging Inc., Thornwood, NY, USA) and managed using AxioVision software.
Results
Decorin suppresses Sema3A expression in cerebral cortex scar tissue
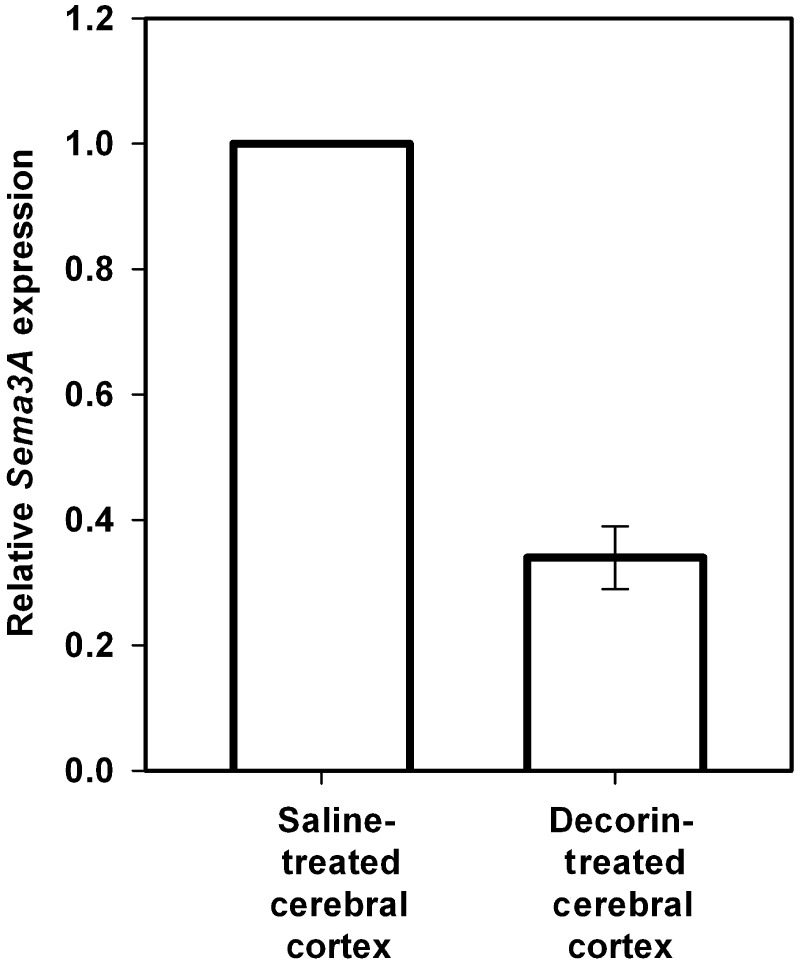
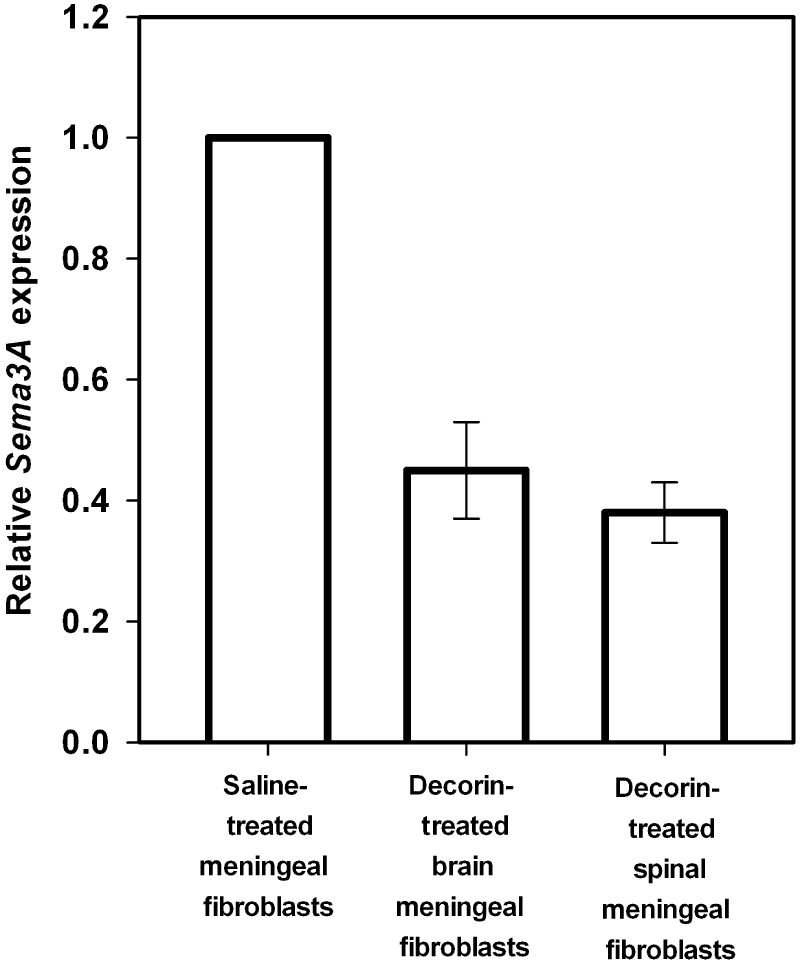
The potential of decorin to regulate Sema3A expression was first tested in cerebral cortex stab injuries resulting from the surgical penetration of a 2.0 mm drug delivery cannula into the primary motor cortex of adult rats. In separate sets of adult rats, human recombinant decorin core protein or saline vehicle alone were infused via osmotic mini-pumps for 8 days, at which time lesion cores were isolated from saline- and decorin-treated injuries for quantitative PCR analysis of Sema3A messenger RNA expression. In accordance with previous data showing high levels of Sema3A expression in the injured adult CNS (Pasterkamp et al., 1999, 2001; De et al., 2002). Quantitative PCR analysis of tissue from control saline-treated injury sites showed significant levels of Sema3A expression (mean Ct value = 24). Decorin treatment of brain injuries however promoted a 66.0% [standard deviation (SD) ± 5.0%] reduction in Sema3A compared with saline-treated injuries (Fig. 1).
Figure 1.
Decorin suppresses Sema3A messenger RNA expression in adult cerebral cortex injuries. Graphs show results of quantitative PCR analysis of Sema3A messenger RNA expression of tissue samples from cortical injuries infused with either human recombinant decorin (2.5 mg/ml) or saline vehicle using Alzet mini-osmotic pumps for 8 days at a flow rate of 1 µl/h. Quantitative analysis was conducted using the comparative (2ΔΔCt) method with samples from saline-treated tissues serving as reference calibrators. Data are represented as relative quantitation of Sema3A expression compared with saline-treated control samples that were given a reference value of 1. Analysis revealed a robust 66.0% (±5.0) reduction in Sema3A expression in decorin-treated injuries compared with saline-treated controls. All quantitative PCR reactions were run in triplicate and at least three fully independent experiments were conducted. Statistical analyses were made using the randomization test of the REST 2005 software. Error bars show standard deviations.
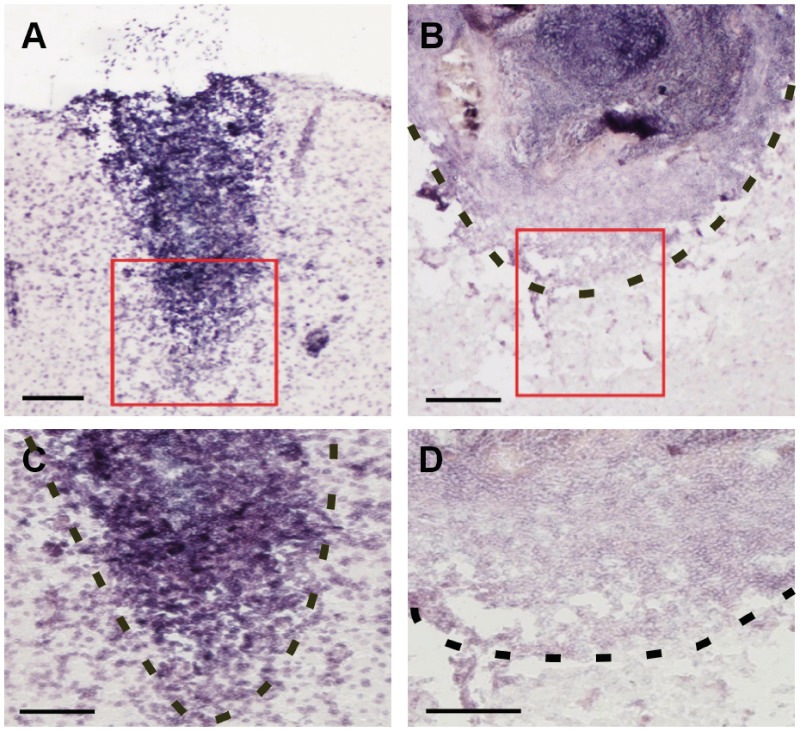
To evaluate the effects of decorin on the distribution of Sema3A expression within cerebral cortex injury sites, in situ hybridization was carried out to detect Sema3A transcript in brain sections from saline- and decorin-treated injuries. The localization pattern of Sema3A expression in saline-treated cortical injuries (Fig. 2A–C) closely resembled that previously reported by other investigators for cerebral cortex stab injuries (Pasterkamp et al., 1999, 2001; De et al., 2002), with abundant levels of Sema3A expression found throughout the central cores and margins of fibrotic scar tissue. Decorin-treated injury sites, however, showed a robust reduction of Sema3A expression within injury centres and margins (Fig. 2B–D).
Figure 2.
Decorin decreases scar-associated Sema3A messenger RNA in the injured adult cerebral cortex. (A) Saline-treated cerebral cortex injuries show robust detection of Sema3A messenger RNA throughout the injury centre as well as at injury margins (C). (B) Decorin-treated injuries display suppression of Sema3A messenger RNA levels throughout injury centres and at injury margins (D). Red squares in A and B delineate injury regions that are shown at higher magnification in C and D, respectively. Dotted lines in B, C and D indicate injury margins where fibrotic scar tissue interfaces with cerebral cortex grey matter. Scale bars: A = 200 µm; B and C = 100 µm; D = 50 µm.
Decorin treatment decreases CNS scar-associated semaphorin 3A protein
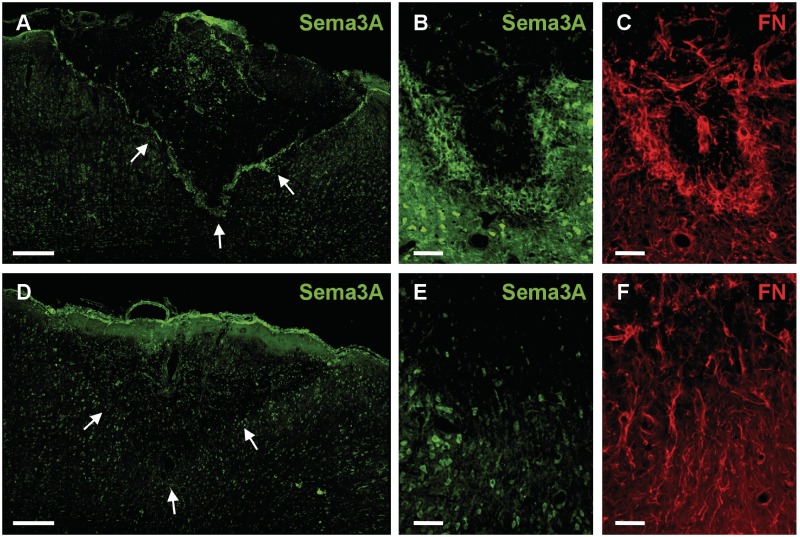
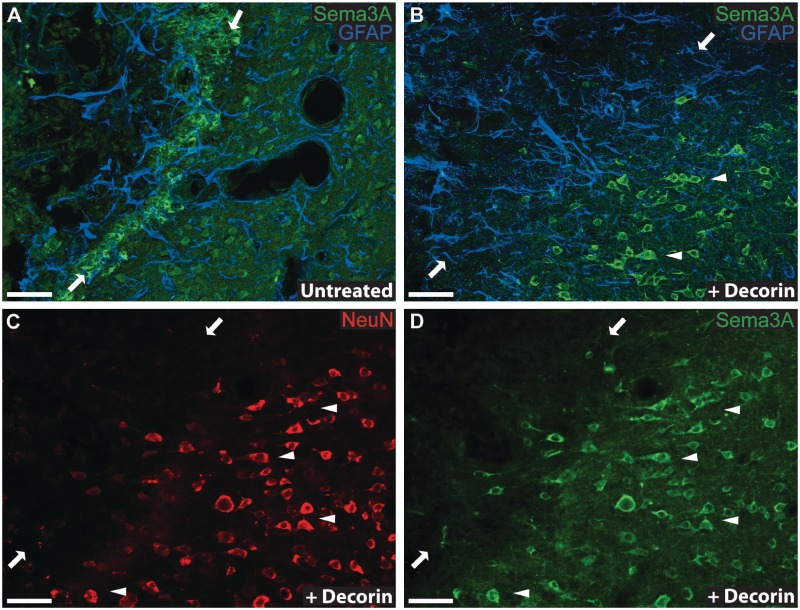
To verify that decorin suppression of Sema3A messenger RNA translated to equally robust reductions in Sema3A protein within cerebral cortex injuries, immunohistochemistry for Sema3A protein was conducted for saline- and decorin-treated brain injuries. In accordance with the patterns of Sema3A messenger RNA expression observed within scar tissue, the highest levels of Sema3A protein were found within the centre and at the margins of control saline-treated injuries (Fig. 3A). Notably intense staining for Sema3A was observed to co-localize with fibronectin positive fibroblasts at injury margins (Fig. 3B and C), thus supporting previous reports that infiltrating leptomeningeal fibroblasts are responsible for high levels of Sema3A expression within CNS injuries (Pasterkamp et al., 1999, 2001; De et al., 2002). Consistent with previous studies showing Sema3A expression by cerebral cortex neurons (Pasterkamp et al., 1999), Sema3A immunoreactivity was observed for neurons within uninjured cerebral cortex grey matter (Supplementary Fig. 2) and grey matter adjacent to saline-treated injury sites (Figs 3A and B, and 4A). However, minimal Sema3A immunoreactivity was observed associated with glial fibrillary acidic protein positive astrocyte cell bodies and processes at injury margins or within adjacent grey matter of saline- (Fig. 4A) or decorin-treated (Fig. 4B) injury sites.
Figure 3.
Decorin suppresses scar-associated semaphorin 3A immunoreactivity in adult cerebral cortex scar tissue. (A) Saline-treated injuries show robust immunoreactivity for Sema3A within fibrotic injury centres and predominantly along ventral injury margins. (B and C) A higher power image of the margin of a saline-treated cerebral cortex injury showing robust immunostaining for Sema3A (B) that co-localizes with fibronectin (FN) (C). (D) Decorin-treated injuries display diminished Sema3A immunoreactivity throughout the injury centre and at ventral injury margins. High-power images showing reduced immunoreactivity for Sema3A (E) and fibronectin (F) at the injury margins of decorin-treated injury sites. Arrows in panels A and C delineate the ventral injury margins. Scale bars: A and D = 200 µm; B, C, E and F = 50 µm.
Figure 4.
Decorin suppresses scar-associated semaphorin 3A immunoreactivity at injury margins. (A) Control saline-treated injuries show a band of robust Sema3A immunoreactivity along the injury margins of cerebral cortex scar tissue that is absent in (B) decorin-treated injuries (opposed arrows indicate the alignment of injury margins). Note the lack of co-localization of Sema3A (green) and glial fibrillary acidic protein (GFAP) immunoreactivity (blue), indicating that GFAP+ astrocytes at injury margins display little if any immunoreactivity for Sema3A in both control saline (A) and decorin-treated (B) injuries. Robust Sema3A immunoreactivity is however displayed by neurons within cerebral cortex grey matter immediately adjacent to both (A) control saline and (B) decorin-treated injuries (groups of Sema3A+ neurons are identified by arrowheads). Immunological co-staining for NeuN (C) and Sema3A (D) in decorin-treated injuries confirms that Sema3A (green) positive cells within grey matter adjacent to injury margins (indicated by opposing arrows) are NeuN+ neurons (red). Arrowheads indicate representative groups of neurons co-stained for NeuN (C) and Sema3A (D). Decorin infusion therefore resulted in a specific reduction in Sema3A immunoreactivity within cerebral cortex scar tissue but did not suppress neuronal Sema3A immunoreactivity within adjacent grey matter. Scale bars = 50 μm.
Immunoreactivity for Sema3A protein was greatly reduced within the fibrotic injury centres and at the lateral/ventral margins of decorin-treated cerebral cortex stab injuries (Figs 3D and B, and 4D). Sema3A staining was, however, still evident at the dorsal surface of decorin-treated injury sites and was most likely due to the more ventral placement of cannulae tips within the central cores of injury sites, resulting in infusion of decorin primarily to central and ventral regions of the injury site, i.e. well below and away from the dorsal surface. High-power imaging showed that decorin infusion had suppressed immunoreactivity for both Sema3A and fibronectin at injury margins (Fig. 3E and F), however no reduction in Sema3A immunoreactivity was observed for NeuN positive neurons within grey matter immediately adjacent to injury margins (Figs 3E and 4B–D).
Decorin reduces Sema3A messenger RNA expression by leptomeningeal fibroblasts
In light of the results of our current study and previous investigations indicating leptomeningeal fibroblasts to be the main source of Sema3A expression in adult CNS injuries, we tested whether decorin regulates Sema3A expression in cultured leptomeningeal fibroblasts isolated from adult rat brain and spinal meninges. Fibroblasts were plated in serum-free media either untreated or treated with decorin and cultured for 48 h. Cells were then extracted and Sema3A messenger RNA expression levels quantified by quantitative PCR. Quantitative PCR analysis confirmed the expression of Sema3A messenger RNA by brain and spinal leptomeningeal fibroblasts, with a measured Ct value of 29 recorded for both cell types. Treatment of spinal and brain meningeal fibroblasts with decorin significantly reduced the expression level of Sema3A to ∼40 and 48%, respectively, of that measured for untreated cells (Fig. 5). Thus, decorin treatment of fibroblasts isolated from both brain and spinal meninges resulted in equally robust suppression of Sema3A expression, a finding that correlates with the robust increases in axon growth observed in cultures of spinal cord leptomeningeal fibroblasts treated with decorin (Davies et al., 2004).
Figure 5.
Decorin reduces Sema3A expression by cultured leptomeningeal fibroblasts isolated from both brain and spinal cord meninges. The graph shows a significant reduction in the expression of Sema3A after decorin treatment of both spinal and brain leptomeningeal meningeal fibroblasts compared with saline-treated fibroblasts. Data are represented as relative quantitation of Sema3A expression compared with saline-treated control samples that were given a reference value of 1. Error bars = standard deviations. Decorin treatment resulted in reductions of 62.0% (±5.0) and 55.0% (±8.0) in Sema3A expression, respectively, compared with untreated cells.
Decorin suppression of Sema3A expressed by leptomeningeal fibroblasts is erythroblastic leukaemia viral oncogene homologue B4-dependent
We next investigated the mechanism through which decorin suppresses Sema3A expression in leptomeningeal fibroblasts. The ErbB family of receptor tyrosine kinases includes epidermal growth factor receptor (EGFR), ErbB1, ErbB2, ErbB3 and ErbB4, and has been linked to several biological functions regulated by decorin (Moscatello et al., 1998; Patel et al., 1998; Iozzo et al., 1999; Santra et al., 2000; Zafiropoulos and Tzanakakis, 2008). We therefore hypothesized that one or more ErbB receptor(s) might play a role in the ability of decorin to suppress Sema3A expression by leptomeningeal fibroblasts. Quantitative PCR analysis was first carried out to confirm that ErbB receptor family members are expressed by meningeal fibroblasts in culture. Our analysis confirmed that spinal leptomeningeal fibroblasts expressed all four known ErbB tyrosine kinase receptors, with expression levels of EGFR being similar to ErbB4 (Ct values = 30), but 5-fold lower than ErbB2 (Ct = 28) and 10-fold lower than ErbB3 (Ct = 27).
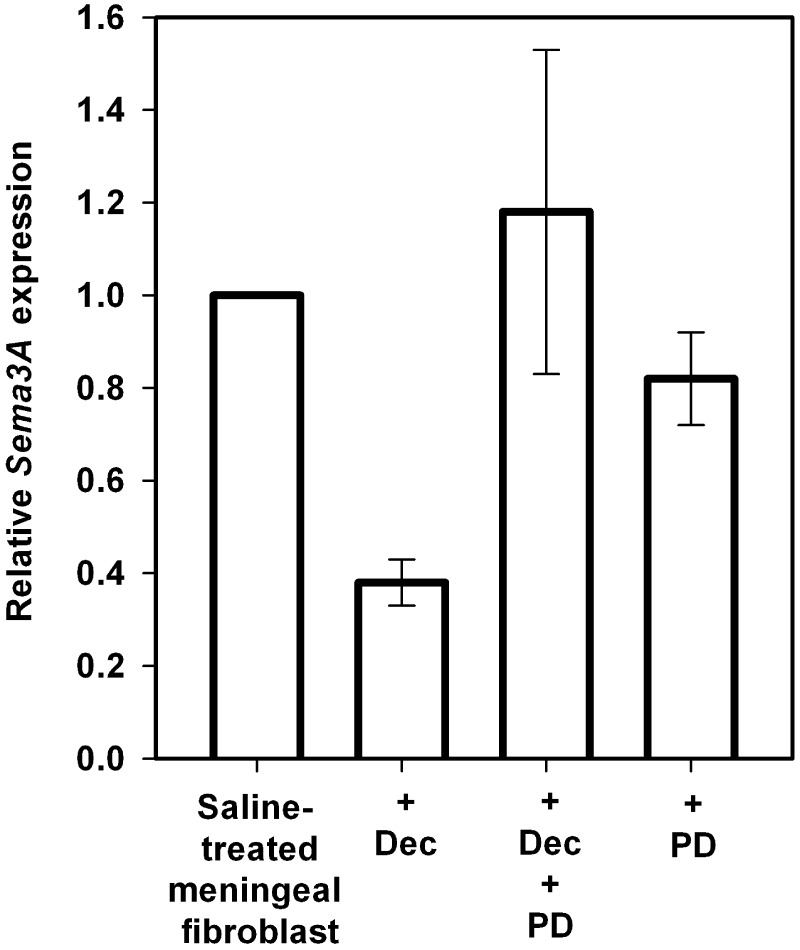
To establish a role for ErbB receptor activity in decorin-mediated suppression of Sema3A, we first tested the ability of PD158780, a well-known pan-specific inhibitor of ErbB tyrosine kinase activity (Rewcastle et al., 1998), to block decorin-mediated suppression of Sema3A expression by leptomeningeal fibroblasts. Treatment with PD158780 alone in control spinal fibroblast cultures showed no effect on the basal levels of Sema3A expression (Fig. 6), however, PD158780 treatment completely blocked the decorin-mediated reduction in Sema3A expression (Fig. 6). This result confirmed that decorin suppresses Sema3A expression via an ErbB-dependent mechanism in cultured leptomeningeal fibroblasts. We then tested AG1478, a well-characterized inhibitor of EGFR (ErbB1) tyrosine kinase activity (Levitzki and Gazit, 1995). Treatment of leptomeningeal fibroblasts with AG1478 and decorin did not block the ability of decorin to suppress Sema3A (Fig. 7). This finding demonstrates that decorin suppression of Sema3A production by leptomeningeal fibroblasts is not mediated by decorin signalling through EGFR tyrosine kinase activity.
Figure 6.
Decorin inhibits Sema3A expression by leptomeningeal fibroblasts via an ErbB-dependent mechanism. Graph shows relative Sema3A levels expressed by spinal leptomeningeal fibroblasts treated with saline, decorin alone (Dec), decorin in combination with PD158780 (PD) and PD158780 alone. Data are represented as relative quantitation of Sema3A expression compared with saline-treated control samples that were given a reference value of 1. Error bars = standard deviations. Co-treatment of fibroblasts with decorin and PD158780 completely blocked decorin-mediated suppression of Sema3A, confirming an ErbB-dependent mechanism in the ability of decorin to regulate Sema3A expression. Treatment of fibroblasts with PD158780 alone resulted in no significant difference in Sema3A expression compared with saline-treated fibroblasts.
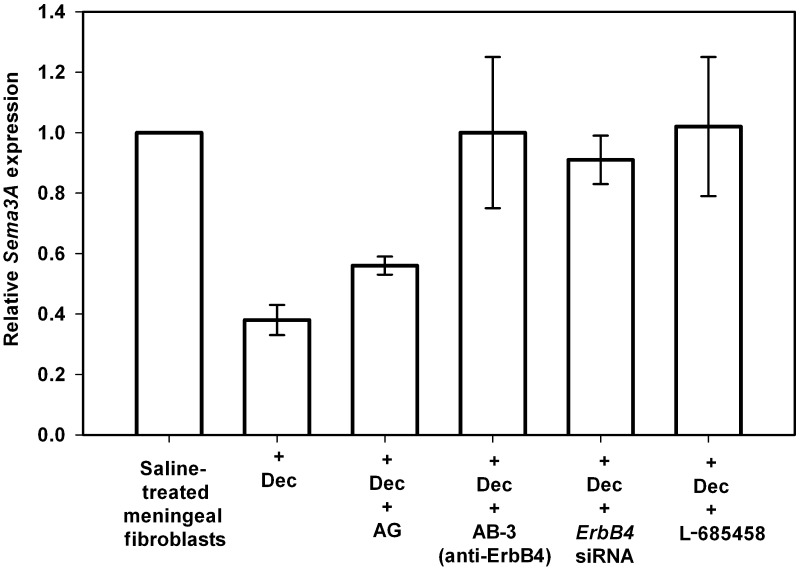
Figure 7.
Decorin suppresses Sema3A expression by leptomeningeal fibroblasts through a mechanism that involves ErbB4 receptor signalling. Spinal leptomeningeal fibroblasts were treated with either saline, decorin alone (Dec), decorin + AG1478 (AG), decorin + anti-ErbB4 Ab-3 antibody, decorin + ErbB4 small interfering RNA (siRNA) or decorin + L-685458. Data are represented as relative quantitation of Sema3A expression compared with saline-treated control samples that were given a reference value of 1. Error bars = standard deviations. Inhibition of ErbB4 activity using either Ab-3 or small interfering RNA completely reversed the decorin-mediated suppression of Sema3A synthesis by leptomeningeal fibroblasts. Additionally, decorin suppression of Sema3A was also completely blocked by co-treatment with L-685458, a γ-secretase inhibitor of the ErbB4 signalling pathway.
In addition to EGFR, decorin has also been shown to bind to both ErbB4 and ErbB2 receptors but not the ErbB3 receptor (Santra et al., 2000). Importantly, at present it is generally accepted that ErbB2 only acts via dimerization with other ErbB receptors and that ErbB2 activity is suppressed via decorin interaction with ErbB4, as demonstrated in breast carcinoma cells (Santra et al., 2000). To test the role of the ErbB4 receptor in decorin-mediated suppression of Sema3A in fibrotic CNS scar tissue, leptomeningeal fibroblast cultures were treated with either a specific ErbB4 blocking antibody (AB-3) or an ErbB4 small interfering RNA mixture. Co-treatment with AB-3 completely blocked decorin-mediated suppression of Sema3A by spinal leptomeningeal fibroblasts (Fig. 7). This was in contrast to results observed using an ErbB3-specific blocking antibody (AB-5) that had no effect on decorin-mediated suppression of Sema3A (data not shown). Similarly, knockdown of ErbB4 using small interfering RNA also completely blocked suppression of Sema3A by decorin (Fig. 7), further providing evidence that in leptomeningeal fibroblasts, decorin suppresses expression of Sema3A in an ErbB4-dependent manner.
Activation of the ErbB4 receptor has been shown to induce its cleavage by TNF-α converting enzyme (tumor necrosis-alpha converting enzyme) (Rio et al., 2000; Carpenter, 2003a; Thiel and Carpenter, 2006) and further processing by γ-secretase (Ni et al., 2001, 2003) to allow release and translocation of the intracellular domain of the ErbB4 receptor (E4ICD) from the cell membrane to the nucleus (Zhou and Carpenter, 2002; Carpenter, 2003a, b). Notably, of the ErbB family of receptor tyrosine kinases, only ErbB4 has been shown to signal via a γ-secretase-dependent mechanism (Ni et al., 2001, 2003; Carpenter, 2003a). Treatment of control fibroblast cultures with L-685458, a potent γ-secretase inhibitor (Shearman et al., 2000) did not have any effect on the basal expression levels of Sema3A (data not shown). Treatment of adult rat leptomeningeal fibroblasts with L-685458 and decorin, however, completely blocked decorin-mediated suppression of Sema3A (Fig. 7). This result further supports our finding that decorin suppresses Sema3A by leptomeningeal fibroblasts through a signalling mechanism requiring ErbB4 receptor activity.
Regulation of semaphorin 3A expression via the signal transducer and activator of transcription 3 signalling pathway
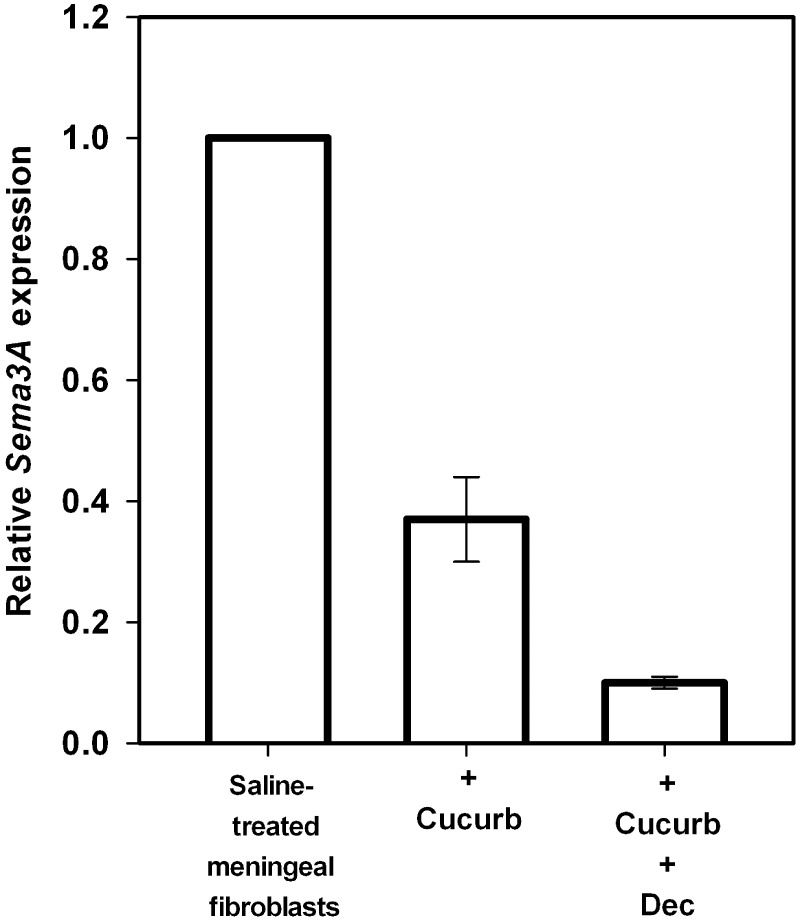
ErbB4 activation has been shown to induce the tyrosine phosphorylation and activation of STAT3 (Puricelli et al., 2002). Sequence analysis of the promoters of the human, mouse and rat Sema3A gene has revealed strong consensus binding motifs for STAT3. We therefore tested and found that treatment of leptomeningeal fibroblasts with cucurbitacin, a specific inhibitor of STAT3 (Blaskovich et al., 2003), decreased the expression of Sema3A to 37% (SD ± 7.0%) of that observed for untreated cultures (Fig. 8), a degree of Sema3A suppression that was strikingly similar to that achieved by decorin treatment alone. Moreover, co-treatment of cultures with decorin and cucurbitacin had a robust synergistic effect leading to a 90% (SD ± 1.0%) reduction in the expression of Sema3A compared with that observed for untreated leptomeningeal fibroblasts (Fig. 8). This suggests that decorin suppresses Sema3A expression by inhibition of STAT3 activity, a mechanism that is complementary to that employed by cucurbitacin.
Figure 8.
Sema3A expression by leptomeningeal fibroblasts in culture is regulated by the transcription factor STAT3. The graph shows relative Sema3A levels expressed by spinal leptomeningeal fibroblasts treated with saline, cucurbitacin I (Cucurb), an inhibitor of STAT3 signalling and combined cucurbitacin I + decorin (Dec). Data is represented as relative quantitation of Sema3A expression compared with saline-treated control samples that were given a reference value of 1. Error bars = standard deviations. Analysis of leptomeningeal fibroblasts treated with cucurbitacin I alone revealed a robust 63.0% (±7.0) reduction in Sema3A expression compared with saline-treated control samples. Co-treatment of fibroblasts with decorin and cucurbitacin I results in a synergistic 90% (±1.0) reduction in the expression of Sema3A compared with that observed for saline-treated control leptomeningeal fibroblasts.
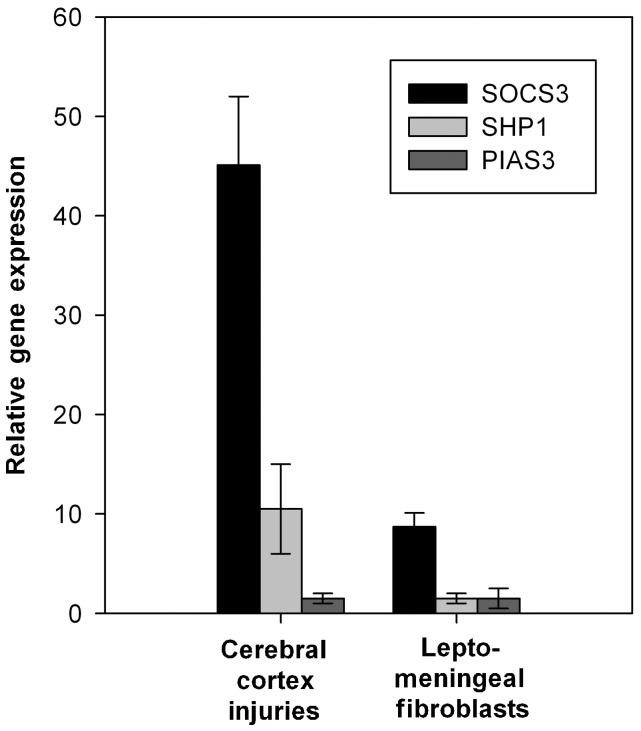
We therefore investigated the ability of decorin to upregulate the expression of three well-established STAT3 inhibitors, SOCS3, SHP1 and PIAS3 (Rakesh and Agrawal, 2005). Quantitative PCR analysis of decorin-infused adult rat cerebral cortex injuries revealed a robust 45.1-fold (SD ± 6.9) increase in the expression of SOCS3 messenger RNA and a 10.5-fold (SD ± 4.5) increase in the expression of SHP1 compared with saline-treated control cerebral cortex injuries (Fig. 9). There was no change in the expression of PIAS3. In vitro decorin increased the expression of SOCS3 messenger RNA by 8.7-fold (SD ± 1.4) in adult rat leptomeningeal fibroblast cultures as assessed by quantitative PCR, but did not increase SHP1 or PIAS3 expression (Fig. 9).
Figure 9.
Decorin induces the expression of STAT3-inhibitors SOCS3 and SHP1 in cerebral cortex injuries and SOCS3 by cultured leptomeningeal fibroblasts. The graph shows the expression levels of SOCS3, SHP1 and PIAS3 in decorin-treated cerebral cortex injuries and cultured leptomeningeal fibroblasts compared with saline-treated samples. Data is represented as relative quantitation of SOCS3, SHP1 and PIAS3 gene expression compared with saline-treated control samples that were given a reference value of 1. Error bars = standard deviations. Decorin treatment of cerebral cortex injuries induced a robust 45.1-fold (±6.9) increase in the expression of SOCS3 messenger RNA and a 10.5-fold (±4.5) increase in the expression of SHP1 compared with saline-treated control cerebral cortex injuries. Decorin did not induce the expression of PIAS in cerebral cortex injuries. In cultured leptomeningeal fibroblasts, decorin increased the expression of SOCS3 messenger RNA by 8.7-fold (±1.4) but did not alter expression levels of SHP1 or PIAS compared with untreated control cultures.
Discussion
Our present study demonstrates for the first time that infusion of decorin core protein robustly suppresses messenger RNA and protein levels of the axon growth inhibitory molecule Sema3A within a CNS injury. Taken together, our immunohistological and in situ analyses of untreated control and decorin-treated injury sites revealed a specific reduction in Sema3A protein and messenger RNA by invading leptomeningeal fibroblasts within fibrotic scar tissue at the centres and margins of cerebral cortex injury sites. The ability of decorin core protein to robustly suppress Sema3A messenger RNA expression by leptomeningeal fibroblasts was confirmed by quantitative PCR analysis of decorin-treated cultures of both brain and spinal cord leptomeningeal fibroblasts. Further quantitative PCR analysis of cultured leptomeningeal fibroblasts treated with a series of ErbB receptor specific inhibitors demonstrated that decorin-mediated suppression of Sema3A is dependent on ErbB4 receptor activity. We also showed a novel role for STAT3 signalling in regulating Sema3A expression through treatment of leptomeningeal fibroblasts with cucurbitacin, a STAT3 specific inhibitor. Lastly, we demonstrated a novel function for decorin to induce the expression of two endogenous STAT3 inhibitors, SOCS3 and SHP1, in injured cerebral cortex in vivo and SOCS3 in cultured fibroblasts in vitro. These studies provide a potential mechanism by which decorin suppresses Sema3A within injured adult CNS tissue.
Decorin, a potent inhibitor of CNS scar formation
Decorin has been shown to suppress fibrotic scarring following injury to many types of tissues, including muscle (Fukushima et al., 2001), kidney (Border et al., 1992; Isaka et al., 1996; Huijun et al., 2005), eye (Grisanti et al., 2005) and lung (Kolb et al., 2001a, b). In the injured adult CNS, Logan et al. (1999) first demonstrated that infusion of decorin core protein attenuated laminin and fibronectin deposition within cerebral cortex stab injuries. In addition, our laboratory demonstrated that decorin infusion robustly suppresses astrocyte reactivity and levels of multiple axon growth-inhibitory CSPGs, namely neurocan, brevican, NG2 and phosphacan, at sites of spinal cord injury, effects that correlated with enhanced axon growth across decorin-treated spinal cord injuries (Davies et al., 2004). Here we expand on these studies and now report that in addition to axon growth inhibitory CSPGs, decorin also has the ability to suppress Sema3A expression, another important CNS scar-associated inhibitor of axonal growth.
Taken together, the results of our in vitro and in vivo studies indicate that decorin treatment of cerebral cortex injuries results in a specific reduction in Sema3A expression by invading leptomeningeal fibroblasts. That fibronectin immunoreactivity was also reduced within decorin-treated injury sites raises the possibility that, in addition to directly suppressing fibronectin and Sema3A synthesis by invading fibroblasts, a decorin-mediated suppression of the numbers of invading fibroblasts may also contribute to the robust suppression of Sema3A expression within decorin-treated injuries. That decorin infusion did not suppress Sema3A immunoreactivity associated with NeuN+ neurons within adjacent grey matter suggests that either decorin-independent mechanisms are involved in the synthesis of Sema3A in adult rat cerebral cortex neurons, or that decorin infusion cannot promote the removal of Sema3A once it is established within the perineuronal matrix. Future investigations will determine the ability of decorin to regulate the synthesis of Sema3A by neurons.
Erythroblastic leukaemia viral oncogene homologue B4 receptor and decorin suppression of semaphorin 3A
Having shown suppression of Sema3A synthesis by leptomeningeal fibroblasts in cerebral cortex injuries and in vitro, we next focused on the signalling mechanism through which decorin suppresses Sema3A synthesis at sites of CNS injury. Several studies have identified multiple cell-surface receptors that interact with decorin, including ErbB receptors (Iozzo et al., 1999; Csordas et al., 2000; Santra et al., 2000), insulin-like growth factor receptor (Schaefer et al., 2007), lipoprotein-receptor related protein (Brandan et al., 2006; Cabello-Verrugio and Brandan, 2007) and Met receptor (Goldoni et al., 2009). As initial quantitative PCR analysis demonstrated that all four ErbB family members are expressed by leptomeningeal fibroblasts in culture, we therefore focused our attention on the potential role of ErbB receptors in decorin-mediated suppression of Sema3A.
That treatment of cells with PD15878, a pan-specific inhibitor of general ErbB tyrosine kinase activity, completely abolished the ability of decorin to inhibit Sema3A expression, confirmed that activity of an ErbB family receptor is required for decorin-mediated suppression of Sema3A. Further testing with ErbB receptor blocking antibodies revealed that only the AB-3 antibody specific to ErbB4 inhibited decorin-mediated suppression of Sema3A, thus demonstrating a specific requirement for ErbB4 activity. This result was confirmed using small interfering RNA knockdown of ErbB4 and represents the first demonstration of ErbB4 activity-dependant regulation of Sema3A expression. Importantly, however, our finding that treatment of fibroblasts with PD15878 alone did not change Sema3A gene expression indicates that a signalling mechanism independent of ErbB receptor activity is responsible for maintaining the constitutive expression of Sema3A by leptomeningeal fibroblasts in culture. Taken together, these findings support the hypothesis that decorin suppresses Sema3A levels via ErbB4 receptor-mediated inhibition of Sema3A synthesis, rather than by binding to and blocking cell surface receptor activity required to promote Sema3A synthesis (see summary schematic Fig. 10).
Figure 10.
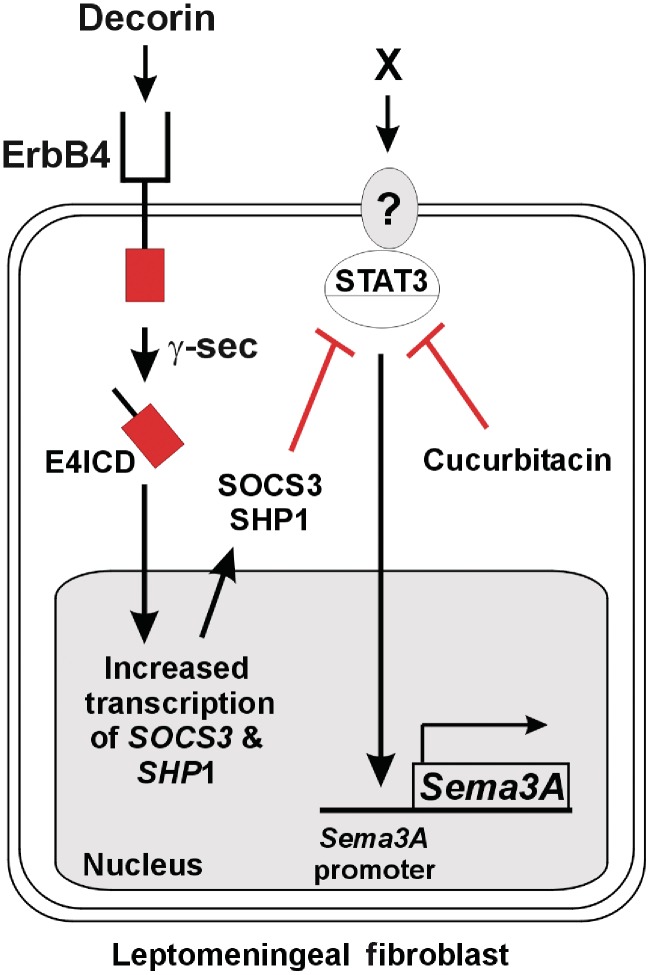
Potential signalling pathways by which decorin suppresses Sema3A expression via regulation of ErbB4, SOCS3 and STAT3 activity. Decorin activation of ErbB4 receptor induces a γ-secretase (γ-sec) dependent release of the ErbB4 receptor E4ICD intracellular signalling domain. Translocation of E4ICD to the nucleus induces the expression of the STAT3 inhibitors SOCS3 and SHP1. SOCS3 and SHP1 inhibit STAT3-mediated induction of Sema3A expression by CNS scar-associated leptomeningeal fibroblasts. The robust synergistic reduction in Sema3A expression observed after decorin/cucurbitacin co-treatment of leptomeningeal fibroblasts predicts that induction of Sema3A is promoted by an as yet unknown ligand ‘X’/receptor signalling event upstream of STAT3.
ErbB receptors function through activation of several signalling pathways, including those that involve extracellular signal-regulated kinase 1/2 (ERK 1/2), mitogen-activated protein kinase (MAPK), phosphatidylinosithol-3-kinase (PI3K) and STATs (Linggi and Carpenter, 2006; Warren and Landgraf, 2006). Upon ligand binding, all ErbB family members undergo activation via auto-phosphorylation followed by the formation of either ErbB homo- or hetero-dimers (Linggi and Carpenter, 2006; Warren and Landgraf, 2006). In addition to activating signalling pathways via phosphorylation of downstream effectors, the ErbB4 receptor can also signal through a mechanism unique among the ErbB receptor family where it undergoes proteolysis of its extracellular domain by TNF-α converting enzyme and its intracellular signalling domain by γ-secretase. Following proteolysis, the ErbB4 intracellular domain, E4ICD, is translocated to the nucleus where it induces gene transcription. Our finding that the γ-secretase specific inhibitor L-685458 (Shearman et al., 2000) completely inhibited the ability of decorin to suppress Sema3A expression by leptomeningeal fibroblasts in culture indicates a requirement for activity of the ErbB4/E4ICD signalling pathway for decorin-mediated down-regulation of Sema3A expression (Fig. 10).
Signal transducer and activator of transcription 3 activity and semaphorin 3A expression
At present, the ligands and receptors promoting the expression of Sema3A by leptomeningeal fibroblasts in either CNS injuries or in culture are currently unknown. In considering the potential downstream signalling pathways that may induce Sema3A in scar tissue, we focused on the transcription factor STAT3, as it has been shown to actively promote scar formation in a variety of tissue types (Lim et al., 2006; Ogata et al., 2006; Arakawa et al., 2008; Herrmann et al., 2008; Berthier et al., 2009). Activation of STAT3 has been shown in several CNS disorders, including excitotoxic injury to the sensorimotor cortex (Acarin et al., 2000), amyotrophic lateral sclerosis (Shibata et al., 2009) and spinal cord injury (Yamauchi et al., 2006; Herrmann et al., 2008; Su et al., 2010) where its activity is associated with inflammation, microglia activation and reactive astrogliosis. Su et al. (2010) recently demonstrated beneficial effects of blocking STAT3 signalling following spinal cord injury, including decreased glial scarring and improved behavioural recovery. We therefore considered the possibility that decorin may inhibit Sema3A expression following CNS injury by suppressing STAT3 signalling in leptomeningeal fibroblasts.
Sequence analyses of the human, rat and murine Sema3A promoters demonstrated that each gene contained consensus DNA binding motifs for STAT3. To examine a possible role for STAT3-mediated regulation of the Sema3A promoter in leptomeningeal fibroblasts, we utilized cucurbitacin I, a selective inhibitor of the Janus kinase/STAT3 signalling pathway. Although the exact mechanism of cucurbitacin I inhibition on STAT3 signalling is presently unknown, treatment of various cell types with cucurbitacin I has been shown to result in diminished activated phosphotyrosine STAT3 without changes in the total protein levels of STAT3 (Blaskovich et al., 2003). Here, we were able to demonstrate that cucurbitacin I treatment of leptomeningeal fibroblasts suppressed Sema3A transcription to a level similar to that achieved with decorin treatment alone. As such, we identify STAT3 as a transcription factor regulating the induction of Sema3A expression in leptomeningeal fibroblasts following CNS injury. The synergistic reduction in Sema3A expression we observed with combined decorin/cucurbitacin I treatment of leptomeningeal fibroblasts indicated that decorin may suppress Sema3A via a mechanism complementary to that used by cucurbitacin I and raises the possibility that decorin/ErbB4 signalling also targets STAT3 activity (Fig. 10).
Decorin upregulation of signal transducer and activator of transcription 3 inhibitors suppressor of cytokine signalling 3 and Src homology phosphatase 1
STAT3 activation has been shown to be under the tight control of three major inhibitors of STAT3-mediated signal transduction pathways: SOCS3, SHP1 and PIAS (for review see Wormald and Hilton, 2004). SOCS3 can suppress STAT3 signalling through inhibition of Janus kinases, competition with STATs for binding sites on receptors or by mediation of proteosomal degradation (Krebs and Hilton, 2000; Kamizono et al., 2001; Kile et al., 2002). SHP1 is a phosphatase that has been shown to negatively regulate receptor tyrosine kinases and Janus kinase 2 (David et al., 1995; Klingmuller et al., 1995). PIAS suppresses STAT3 signalling by blocking STAT3 binding to promoter DNA (Wormald and Hilton, 2004). We tested the hypothesis that decorin/ErbB4-mediated signalling leads to enhanced transcription of one or more of these known STAT3 signalling inhibitors. Our results demonstrate that decorin robustly upregulated the expression of SOCS3 and SHP1 in cerebral cortex injuries and SOCS3 in cultured leptomeningeal fibroblasts. Interestingly, adenoviral vector-mediated expression of SOCS3 has been shown to reduce fibrotic scarring in a rodent model of diabetic nephropathy (Ortiz-Munoz et al., 2010). Importantly in the context of the present study, the ability of decorin to upregulate known STAT3 inhibitors provides a putative signalling mechanism by which decorin may suppress STAT3-mediated induction of Sema3A expression by CNS scar associated leptomeningeal fibroblasts (Fig. 10).
In conclusion, these findings represent, to the best of our knowledge, the first demonstration of a naturally occurring molecule that can regulate Sema3A expression within CNS scar tissue. As such, decorin treatment of CNS injuries represents an complementary approach to other strategies designed to target the axon growth inhibitory effects of Sema3A by suppressing Sema3A signalling through receptors such as neuronal neuropilin-1 (Williams et al., 2005; Kaneko et al., 2006; Mire et al., 2008; Montolio et al., 2009). The ability of decorin to downregulate the de novo expression of Sema3A, multiple inhibitory CSPGs and also desensitize neurons to the inhibitory effects of CSPGs and myelin associated inhibitors (Minor et al., 2008), makes decorin a promising therapeutic agent for promoting axon regeneration in the injured adult mammalian CNS.
Funding
Supported by NIH 7NS047333, the David Van Wagener Spinal Cord Fund and private donations by the international spinal cord injury community.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Gala Biotech (Middleton, WI) for supplying recombinant human decorin core protein.
Glossary
Abbreviations
- CSPGs
chondroitin sulphate proteoglycans
- EGFR
epidermal growth factor receptor
- ErbB
erythroblastic leukaemia viral oncogene homologue
- PCR
polymerase chain reaction
- PIAS3
protein inhibitor of activated STAT3
- Sema3A
semaphorin 3A
- SHP1
Src homology phosphatase 1
- SOCS3
suppressor of cytokine signalling 3
- STAT3
signal transducer and activator of transcription 3
- TACE
tumor necrosis-alpha converting enzyme
References
- Acarin L, Gonzalez B, Castellano B. STAT3 and NFkappaB activation precedes glial reactivity in the excitotoxically injured young cortex but not in the corresponding distal thalamic nuclei. J Neuropathol Exp Neurol. 2000;59:151–63. doi: 10.1093/jnen/59.2.151. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Masaki T, Hirai T, Doi S, Kuratsune M, Arihiro K, et al. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol Dial Transplant. 2008;23:3418–26. doi: 10.1093/ndt/gfn314. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–53. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Puschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol. 2002;61:339–50. doi: 10.1093/jnen/61.4.339. [DOI] [PubMed] [Google Scholar]
- Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58:469–77. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–4. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Bouzioukh F, Daoudal G, Falk J, Debanne D, Rougon G, Castellani V. Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur J Neurosci. 2006;23:2247–54. doi: 10.1111/j.1460-9568.2006.04783.x. [DOI] [PubMed] [Google Scholar]
- Brandan E, Retamal C, Cabello-Verrugio C, Marzolo MP. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J Biol Chem. 2006;281:31562–71. doi: 10.1074/jbc.M602919200. [DOI] [PubMed] [Google Scholar]
- Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem. 2007;282:18842–50. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res. 2003a;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr Opin Cell Biol. 2003b;15:143–8. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275:32879–87. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- David M, Chen HE, Goelz S, Larner AC, Neel BG. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995;15:7050–8. doi: 10.1128/mcb.15.12.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226–42. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–3. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–22. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De WF, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, et al. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- De WF, Vo T, Stam FJ, Wisman LA, Bar PR, Niclou SP, et al. The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol Cell Neurosci. 2006;32:102–17. doi: 10.1016/j.mcn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–12. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL. The synaptic pathology of schizophrenia: is aberrant neurodevelopment and plasticity to blame? Int Rev Neurobiol. 2004;59:47–72. doi: 10.1016/S0074-7742(04)59003-7. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–55. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19:539–45. doi: 10.1016/s0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Fenstermaker V, Chen Y, Ghosh A, Yuste R. Regulation of dendritic length and branching by semaphorin 3A. J Neurobiol. 2004;58:403–12. doi: 10.1002/neu.10304. [DOI] [PubMed] [Google Scholar]
- Fujita H, Zhang B, Sato K, Tanaka J, Sakanaka M. Expressions of neuropilin-1, neuropilin-2 and semaphorin 3A mRNA in the rat brain after middle cerebral artery occlusion. Brain Res. 2001;914:1–14. doi: 10.1016/s0006-8993(01)02765-2. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Pasterkamp RJ, Heijnen S, Holtmaat AJ, Verhaagen J. Anatomical distribution of the chemorepellent semaphorin III/collapsin-1 in the adult rat and human brain: predominant expression in structures of the olfactory-hippocampal pathway and the motor system. J Neurosci Res. 1998a;52:27–42. doi: 10.1002/(SICI)1097-4547(19980401)52:1<27::AID-JNR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998b;21:1079–92. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Wolfer DP, De Wit GM, Verhaagen J. Anatomy of rat semaphorin III/collapsin-1 mRNA expression and relationship to developing nerve tracts during neuroembryogenesis. J Comp Neurol. 1996;375:378–92. doi: 10.1002/(SICI)1096-9861(19961118)375:3<378::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, et al. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–54. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good PF, Alapat D, Hsu A, Chu C, Perl D, Wen X, et al. A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer's disease. J Neurochem. 2004;91:716–36. doi: 10.1111/j.1471-4159.2004.02766.x. [DOI] [PubMed] [Google Scholar]
- Grisanti S, Szurman P, Warga M, Kaczmarek R, Ziemssen F, Tatar O, et al. Decorin modulates wound healing in experimental glaucoma filtration surgery: a pilot study. Invest Ophthalmol Vis Sci. 2005;46:191–6. doi: 10.1167/iovs.04-0902. [DOI] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–8. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- Hou ST, Keklikian A, Slinn J, O'Hare M, Jiang SX, Aylsworth A. Sustained up-regulation of semaphorin 3A, Neuropilin1, and doublecortin expression in ischemic mouse brain during long-term recovery. Biochem Biophys Res Commun. 2008;367:109–15. doi: 10.1016/j.bbrc.2007.12.103. [DOI] [PubMed] [Google Scholar]
- Huijun W, Long C, Zhigang Z, Feng J, Muyi G. Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis. Exp Mol Pathol. 2005;78:17–24. doi: 10.1016/j.yexmp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–92. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–23. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–8. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–9. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Kile BT, Schulman BA, Alexander WS, Nicola NA, Martin HM, Hilton DJ. The SOCS box: a tale of destruction and degradation. Trends Biochem Sci. 2002;27:235–41. doi: 10.1016/s0968-0004(02)02085-6. [DOI] [PubMed] [Google Scholar]
- Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–38. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Galt T, Sime PJ, Xing Z, Schmidt M, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med. 2001a;163:770–7. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2001b;280:L1327–34. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–62. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113(Pt 16):2813–9. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–8. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Lim CP, Phan TT, Lim IJ, Cao X. Stat3 contributes to keloid pathogenesis via promoting collagen production, cell proliferation and migration. Oncogene. 2006;25:5416–25. doi: 10.1038/sj.onc.1209531. [DOI] [PubMed] [Google Scholar]
- Lindholm T, Skold MK, Suneson A, Carlstedt T, Cullheim S, Risling M. Semaphorin and neuropilin expression in motoneurons after intraspinal motoneuron axotomy. Neuroreport. 2004;15:649–54. doi: 10.1097/00001756-200403220-00015. [DOI] [PubMed] [Google Scholar]
- Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–56. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Logan A, Baird A, Berry M. Decorin attenuates gliotic scar formation in the rat cerebral hemisphere. Exp Neurol. 1999;159:504–10. doi: 10.1006/exnr.1999.7180. [DOI] [PubMed] [Google Scholar]
- Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–5. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–59. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Minor K, Tang X, Kahrilas G, Archibald SJ, Davies JE, Davies SJ. Decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons. Neurobiol Dis. 2008;32:88–95. doi: 10.1016/j.nbd.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Mire E, Thomasset N, Jakeman LB, Rougon G. Modulating Sema3A signal with a L1 mimetic peptide is not sufficient to promote motor recovery and axon regeneration after spinal cord injury. Mol Cell Neurosci. 2008;37:222–35. doi: 10.1016/j.mcn.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montolio M, Messeguer J, Masip I, Guijarro P, Gavin R, Antonio Del RJ, et al. A semaphorin 3A inhibitor blocks axonal chemorepulsion and enhances axon regeneration. Chem Biol. 2009;16:691–701. doi: 10.1016/j.chembiol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, et al. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci. 2006;26:2971–80. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–12. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. Gamma-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Ni CY, Yuan H, Carpenter G. Role of the ErbB-4 carboxyl terminus in gamma-secretase cleavage. J Biol Chem. 2003;278:4561–5. doi: 10.1074/jbc.M210504200. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Franssen EH, Ehlert EM, Taniguchi M, Verhaagen J. Meningeal cell-derived semaphorin 3A inhibits neurite outgrowth. Mol Cell Neurosci. 2003;24:902–12. doi: 10.1016/s1044-7431(03)00243-4. [DOI] [PubMed] [Google Scholar]
- Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, et al. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520–30. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]
- Ortiz-Munoz G, Lopez-Parra V, Lopez-Franco O, Fernandez-Vizarra P, Mallavia B, Flores C, et al. Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol. 2010;21:763–72. doi: 10.1681/ASN.2009060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Anderson PN, Verhaagen J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur J Neurosci. 2001;13:457–71. doi: 10.1046/j.0953-816x.2000.01398.x. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–74. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, de WJ, De WF, et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–66. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- Patel S, Santra M, McQuillan DJ, Iozzo RV, Thomas AP. Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 carcinoma cells. J Biol Chem. 1998;273:3121–4. doi: 10.1074/jbc.273.6.3121. [DOI] [PubMed] [Google Scholar]
- Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science. 1998;282:1904–6. doi: 10.1126/science.282.5395.1904. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–73. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Puricelli L, Proietti CJ, Labriola L, Salatino M, Balana ME, Aguirre GJ, et al. Heregulin inhibits proliferation via ERKs and phosphatidyl-inositol 3-kinase activation but regulates urokinase plasminogen activator independently of these pathways in metastatic mammary tumor cells. Int J Cancer. 2002;100:642–53. doi: 10.1002/ijc.10533. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–8. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Rakesh K, Agrawal DK. Controlling cytokine signaling by constitutive inhibitors. Biochem Pharmacol. 2005;70:649–57. doi: 10.1016/j.bcp.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Rewcastle GW, Murray DK, Elliott WL, Fry DW, Howard CT, Nelson JM, et al. Tyrosine kinase inhibitors. 14. Structure-activity relationships for methylamino-substituted derivatives of 4-[(3-bromophenyl)amino]-6-(methylamino)-pyrido[3,4-d]pyrimidine (PD 158780), a potent and specific inhibitor of the tyrosine kinase activity of receptors for the EGF family of growth factors. J Med Chem. 1998;41:742–51. doi: 10.1021/jm970641d. [DOI] [PubMed] [Google Scholar]
- Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275:10379–87. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, Ginty DD, et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25:3613–20. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153–61. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002;35:907–20. doi: 10.1016/s0896-6273(02)00857-7. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, et al. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. Am J Pathol. 2007;170:301–15. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–18. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, et al. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry. 2000;39:8698–704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- Shepherd I, Luo Y, Raper JA, Chang S. The distribution of collapsin-1 mRNA in the developing chick nervous system. Dev Biol. 1996;173:185–99. doi: 10.1006/dbio.1996.0016. [DOI] [PubMed] [Google Scholar]
- Shibata N, Kakita A, Takahashi H, Ihara Y, Nobukuni K, Fujimura H, et al. Activation of signal transducer and activator of transcription-3 in the spinal cord of sporadic amyotrophic lateral sclerosis patients. Neurodegener Dis. 2009;6:118–26. doi: 10.1159/000213762. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Kappler J, Junghans U, Koops A, Kresse H, Muller HW. Differential expression of the small chondroitin/dermatan sulfate proteoglycans decorin and biglycan after injury of the adult rat brain. Brain Res. 1995;704:263–74. doi: 10.1016/0006-8993(95)01131-5. [DOI] [PubMed] [Google Scholar]
- Su Z, Yuan Y, Cao L, Zhu Y, Gao L, Qiu Y, et al. Triptolide promotes spinal cord repair by inhibiting astrogliosis and inflammation. Glia. 2010;58:901–15. doi: 10.1002/glia.20972. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori K, Nojyo Y, Kaneko T, Takauji R. Evidence that Sema3A and Sema3F regulate the migration of GABAergic neurons in the developing neocortex. J Comp Neurol. 2003;455:238–48. doi: 10.1002/cne.10476. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Kaneko T. Cell migration from the corticostriatal angle to the basal telencephalon in rat embryos. Neuroreport. 2001;12:775–80. doi: 10.1097/00001756-200103260-00032. [DOI] [PubMed] [Google Scholar]
- Thiel KW, Carpenter G. ErbB-4 and TNF-alpha converting enzyme localization to membrane microdomains. Biochem Biophys Res Commun. 2006;350:629–33. doi: 10.1016/j.bbrc.2006.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–9. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Landgraf R. Signaling through ERBB receptors: multiple layers of diversity and control. Cell Signal. 2006;18:923–33. doi: 10.1016/j.cellsig.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Williams A, Piaton G, Aigrot MS, Belhadi A, Theaudin M, Petermann F, et al. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain. 2007;130:2554–65. doi: 10.1093/brain/awm202. [DOI] [PubMed] [Google Scholar]
- Williams G, Eickholt BJ, Maison P, Prinjha R, Walsh FS, Doherty P. A complementary peptide approach applied to the design of novel semaphorin/neuropilin antagonists. J Neurochem. 2005;92:1180–90. doi: 10.1111/j.1471-4159.2004.02950.x. [DOI] [PubMed] [Google Scholar]
- Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279:821–4. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Osuka K, Takayasu M, Usuda N, Nakazawa A, Nakahara N, et al. Activation of JAK/STAT signalling in neurons following spinal cord injury in mice. J Neurochem. 2006;96:1060–70. doi: 10.1111/j.1471-4159.2005.03559.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–4. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Morita A, Uchida Y, Nakamura F, Usui H, Ohshima T, et al. Regulation of spine development by semaphorin3A through cyclin-dependent kinase 5 phosphorylation of collapsin response mediator protein 1. J Neurosci. 2007;27:12546–54. doi: 10.1523/JNEUROSCI.3463-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafiropoulos A, Tzanakakis GN. Decorin-mediated effects in cancer cell biology. Connect Tissue Res. 2008;49:244–8. doi: 10.1080/03008200802147746. [DOI] [PubMed] [Google Scholar]
- Zhou W, Carpenter G. ErbB-4: a receptor tyrosine kinase. Inflamm Res. 2002;51:91–101. doi: 10.1007/BF02684009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.