Abstract
Restless legs syndrome is a neurological disorder characterized by an urgency to move the legs during periods of rest. Data from a variety of sources provide a compelling argument that the amount of iron in the brain is lower in individuals with restless legs syndrome compared with neurologically normal individuals. Moreover, a significant percentage of patients with restless legs syndrome are responsive to intravenous iron therapy. The mechanism underlying the decreased iron concentrations in restless legs syndrome brains is unknown. We hypothesize that the source of the brain iron deficit is at the blood–brain interface. Thus we analysed the expression of iron management proteins in the epithelial cells of the choroid plexus and the brain microvasculature in post-mortem tissues. The choroid plexus, obtained at autopsy, from 18 neurologically normal controls and 14 individuals who had primary restless legs syndrome was subjected to histochemical staining for iron and immunostaining for iron management proteins. Iron and heavy chain ferritin staining was reduced in the epithelial cells of choroid plexus in restless legs syndrome. Divalent metal transporter, ferroportin, transferrin and its receptor were upregulated in the choroid plexus in restless legs syndrome. Microvessels were isolated from the motor cortex of 11 restless legs syndrome and 14 control brains obtained at autopsy and quantitative immunoblot analyses was performed. Expression of heavy chain ferritin, transferrin and its receptor in the microvessels from restless legs syndrome was significantly decreased compared with the controls but divalent metal protein 1, ferroportin, prohepcidin, mitochondrial ferritin and light-chain ferritin remained unchanged. The presence of an iron regulatory protein was demonstrated in the brain microvasculature and the activity of this protein is decreased in restless legs syndrome; a finding similar to our earlier report in neuromelanin cells from the substantia nigra of restless legs syndrome brains. This study reveals that there are alterations in the iron management protein profile in restless legs syndrome compared with controls at the site of blood–brain interface suggesting fundamental differences in brain iron acquisition in individuals with restless legs syndrome. Furthermore, the decrease in transferrin receptor expression in the microvasculature in the presence of relative brain iron deficiency reported in restless legs syndrome brains may underlie the problems associated with brain iron acquisition in restless legs syndrome. The consistent finding of loss of iron regulatory protein activity in restless legs syndrome brain tissue further implicates this protein as a factor in the underlying cause of the iron deficiency in the restless legs syndrome brain. The data herein provide evidence for regulation of iron uptake and storage within brain microvessels that challenge the existing paradigm that the blood–brain barrier is merely a transport system.
Keywords: iron deficiency, movement disorders, sleep disorders, blood–brain barrier, choroid plexus
Introduction
Restless legs syndrome (RLS) is a sensory disorder characterized by an irresistible urge to move the legs. The symptoms have a strong circadian component, worsening at night (Trenkwalder et al., 1999). Clinical studies have focused on treatment of the symptoms primarily with dopaminergic agents (Allen, 2004) but data from a number of studies are converging to implicate insufficient brain iron status as a, if not the, key component of the disease aetiology (Allen et al., 2001; Schmidauer et al., 2005; Earley et al., 2006). As early as 1953, iron deficiency was suggested as a contributing factor in RLS (Nordlander, 1953). Since then, a growing body of biological evidence, such as a negative correlation between serum ferritin levels and symptoms, changes in iron management proteins in the CSF (Clardy et al., 2006a), and neuroimaging (Allen et al., 2001; Earley et al., 2006) and ultrasound data (Schmidauer et al., 2005; Godau et al., 2007, 2008, 2009) has strengthened the argument for insufficient brain iron status in RLS (Earley et al., 2000a, 2004; Connor et al., 2003, 2004; Allen, 2004; Wang et al., 2004). In the CSF, ferritin is decreased in patients with RLS relative to controls and transferrin is elevated; again a profile consistent with iron deficiency (Mizuno et al., 2005; Clardy et al., 2006a). In addition, prohepcidin, a prohormone responsible for regulating cellular iron efflux is decreased in CSF from patients with RLS compared with controls (Clardy et al., 2006b), which would be consistent with a loss of brain iron. Moreover, autopsy-based studies have revealed less ferritin for iron storage in neuromelanin cells in patients with RLS compared with controls; this observation is consistent with relative cellular iron deficiency (Connor et al., 2003).
The brain, however, exists behind a barrier and brain iron acquisition is via a transcytotic mechanism through endothelial cells (Fishman et al., 1987; Burdo and Connor, 2003). Therefore, the present set of studies were undertaken to determine if the decrease in brain iron in RLS could be associated with alterations in the mechanism associated with brain iron acquisition. Although, brain iron uptake could not be directly studied in our human population, animal studies have informed us that brain iron uptake is a continuous and dynamic process (Dickinson et al., 1996; Malecki et al., 1999). Although, the existing paradigm holds that brain iron concentrations are static, there is recent evidence that reveals that brain iron concentrations are much more dynamic. For example, the ventral midbrain (including, of relevance to RLS, the substantia nigra) has a diurnal variation and profiles of brain iron management proteins can change in response to brain iron status (Bianco et al., 2009). Thus we hypothesized that there would be differences in iron management protein profiles in the microvasculature and choroid plexus in RLS and control brains.
Materials and methods
Patient characteristics
Choroid plexuses were obtained at autopsy from the brains of 14 patients selected from a collection maintained by the RLS Foundation. The clinical RLS diagnosis was confirmed by a RLS expert board certified in sleep medicine who reviewed a detailed diagnostic questionnaire previously completed by the individuals with RLS. The accepted patients met the diagnostic criteria established by the National Institutes of Health consensus workshop on RLS (Allen et al., 2003), had no evidence for any contributing secondary causes of RLS and expressed the early-onset phenotype of RLS (symptoms starting before age 45) (Allen and Earley, 2000). These criteria were selected to reduce variance of disease expression noting evidence for different pathologies related to age-of-onset phenotype (Earley et al., 2006). No other phenotypic data are available on these patients, including serum ferritin levels. The range of age at time of death of the patients with RLS was 53–91 years and all were female except one male. Of the 14 RLS brains used in this study, seven of them had earlier been shown to have altered iron management proteins in a separate study (Connor et al., 2003). The average post-mortem interval was 17 h in the RLS group (range 4–33 h).
The control group consisted of the choroid plexus from 18 individuals (15 females and three males). All control subjects lacked any significant neurological history at the time of death and ranged in age from 49–89 years. None of these brains had been used in our earlier studies and all were obtained from the Harvard Brain Bank. The average post-mortem interval was 15 h with a range of 5–28 h. All choroid plexus tissues were fixed in a 10% formalin solution.
Histochemical staining for iron
Perl’s staining for iron was performed using a protocol described earlier (McManus, 1960). The reaction product was intensified using 0.05% 3,3-diaminobenzidine tetrahydrochloride solution in 0.1 M phosphate buffer (pH 7.4) for 15 min (Nguyen-Legros et al., 1980).
Immunohistochemical staining for iron-related proteins
Immunochemistry was performed on 15 µm thick paraffin sections of choroid plexus. The sections were first deparaffinized with a xylene and ethanol series. Antigen retrieval was carried out by boiling the tissues in citrate buffer for 10 min. Non-specific tissue peroxidase activity was suppressed by incubating the sections in a solution of methanol plus hydrogen peroxide (10:1), followed by incubation in 10% normal serum for 1 h. The tissue was then incubated overnight with antibodies to divalent metal transporter (DMT) 1 (QBC, Hopkinton, MA, USA; 1:1000), ferroportin (Alpha diagnostics, 1:200), heavy chain ferritin, light chain ferritin and mitochondrial ferritin (a gift from Dr Arosio; 1:1000, 1:500 and 1:200), transferrin (BMC, Indianapolis, IN, USA; 1:2000) and transferrin receptor (Zymed, 1:500). The sections were then washed in three changes of phosphate buffered saline and incubated for 1 h at room temperature in a 1:200 dilution of biotinylated IgG secondary antibodies (Vectastain Elite ABC Kit). This was followed by three buffer rinses to remove unbound secondary antibody. The sections were then incubated for 1 h at room temperature with an avidin-biotinylated horseradish peroxidase complex. The reaction was visualized by treatment for 10 min in 0.05% 3,3-diaminobenzidine tetrahydrochloride solution containing 0.05% hydrogen peroxide and 0.2% nickel chloride. The colour reaction was stopped with several washes of Tris buffer. Control sections were incubated with phosphate buffered saline instead of primary antibody; these sections were devoid of immunostaining.
Two investigators who were blinded to the patient’s characteristics examined the slides of iron and immunocytochemical staining independently. Each section was rated as a score from 0 to ++++ based on the staining intensity of the epithelial cells. A score of 0 represents no staining and a score of ++++ represents where the cytoplasm of all cells is completely filled with a robust immunoreaction product. A score of + was given if the staining was light and present in only some of the epithelial cells. A score of ++ was given if the staining product was evenly present in all of the epithelial cells but the intensity was light. The score of +++ was assigned to sections with even staining but the reaction product could not be considered robust. There is no significant difference between the scores of the two investigators according to the Mann–Whitney U-test.
Isolation of microvessels from frozen cortical tissues
Post-mortem cortical tissues were obtained from the brains of 11 patients with RLS selected from a collection from Harvard Brain Bank maintained by the RLS Foundation. Patient characteristics were the same as those described above. All subjects were female. Cortical tissues were also obtained from 14 control females. The control subjects had no significant neurological history at the time of death. Their age ranged from 58–87 years. All the cortical tissues obtained from Harvard Brain Bank were frozen at −70°C.
Microvessels were isolated from frozen brain tissue according to Pardridge et al. (1987) with minor modifications and Buffers A and B required for the process were prepared as described in Pardridge et al. (1987). Briefly, the brain tissues were thawed on ice and their weights were noted. The brain tissues were then rinsed in Buffer B, minced and homogenized with 13 up and down strokes in Buffer A with a dounce homogenizer. Then to the homogenate an equal volume of 26% dextran (to yield 13% dextran) was added, mixed thoroughly and centrifuged at 5800g for 10 min at 4°C. The supernatant was removed and Buffer A (2 ml) was added to the pellet and sieved through a 180 µm pore size mesh placed on a beaker. The filtrate was collected and sieved through a 53 µm pore size mesh. The microvessels on the mesh were collected by washing the mesh in a petri dish with Buffer A and then spun at 50g for 5 min at 4°C. The supernatant was removed and microvessels pellet was washed twice with Buffer B. Microvessels were then passed through a percoll density gradient ranging from 10–50%, under centrifugation for 1 h at 4°C at 27 000g. Microvessels collected from the interphase were then washed twice with Buffer B and the purity of the microvessel preparations routinely monitored by phase-contrast microscopy. Finally, microvessels were stored in Buffer B (with protease inhibitor cocktail) in two aliquots at −70°C.
Protein isolation and immunoblots
The brain microvessels isolated from RLS and control brains, were incubated in Stuart’s protein extraction buffer for 30 min on ice. The Stuart’s extraction buffer consisted of 100 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholic acid, 0.2% sodium dodecyl sulphate, 2 mM EDTA, 10 mM Hepes (pH 7.5) and 1 mM sodium orthovanadate. A microtip (Branson Sonifier 250, Branson Inc., Danbury, CT, USA) on pulse mode was used to produce homogenates. These were centrifuged at 4°C and 4000g for 5 min, sonicated for 30 s and then centrifuged at 4000g for 5 min at 4°C. The protein levels in the microvessel lysates were determined using the Pierce Micro BCA™ assay.
Immunoblot analysis was performed using the Schleicher and Schuell® Minifold® II apparatus (Keene, NH, USA) with a nitrocellulose membrane as described previously (Connor et al., 2004). The antibodies were constituted in 5% non-fat dried milk in Tris buffered saline–Tween. Data from all the blots were normalized using the pooled microvessel lysate on each blot. Each sample was applied at a concentration of 1 µg/500 µl/well. Antibodies used for these assays were H-ferritin monoclonal from Dr P. Arosio, l-ferritin from Santacruz Biotechnology, CA, USA, Tf from EY laboratories Inc., CA, USA, transferrin receptor monoclonal from Zymed, CA, USA, ferroportin, prohepcidin from ADI, TX and DMT1 polyclonal from Dr David Haile. To quantitate the protein, blots were scanned and analysed in the Fuji Film System.
Real-time polymerase chain reaction
Total RNA was isolated from human microvessels isolated from fresh brain cortical tissues using TRIzol® reagent (Invitrogen, CA, USA). Complementary DNA template was generated from 200 ng RNA using the Omniscript® Reverse Transcription kit (Qiagen, CA, USA) according to the manufacturer’s protocol. A sample of complementary DNA template was then diluted (1:2 ratio) and TaqMan® Universal polymerase chain reaction master mix (Applied Biosystems, CA, USA) was added along with a set of forward and reverse primers for transferring transferrin receptor, Ferroportin or iron regulatory protein 1 and 18S (as internal control) (TaqMan Primers, Applied Biosystems, CA, USA) as per the manufacturer’s instructions. The reaction plate was placed in Applied Biosystems 7300 Real Time Polymerase Chain Reaction System and programmed for the real-time polymerase chain reaction. Data were analysed using sequence detection system 1.9.1 software.
Total iron regulatory protein binding activity assay
We determined the total iron regulatory protein activity in the microvessel lysates using an electrophoretic mobility shift assay. A synthetic RNA transcript containing the iron regulatory element was generated from the oligonucleotide template 5′-ttatgctgagtgatatccctctcctaggacgaagttgtcacgaacctgcctagg-3′ with T7 polymerase in the presence of [32P] cytidine triphosphate. Binding reactions were carried out as described previously (Leibold and Munro, 1988) with minor modifications. Briefly, 2.5 µg of protein was incubated with the synthetic radiolabelled iron responsive element probe after treating with 2% β-mercaptoethanol. After a 30 min incubation of the sample with the radiolabelled probe, the RNA–protein complexes were separated on a 4% native polyacrylamide gel and visualized in the FLA 7000 Fuji phosphoimaging system. The image obtained was then analysed with Multigauge software.
Statistical analysis
The scores on Perl staining and immunohistochemistry for iron-related proteins in the choroid plexus were analysed with non-parametric Mann–Whitney U-test. The data on the iron protein profile in the brain microvasculature were analysed using unpaired t-tests with Graph Pad Prism software.
Results
Choroid plexus: Perl staining for iron
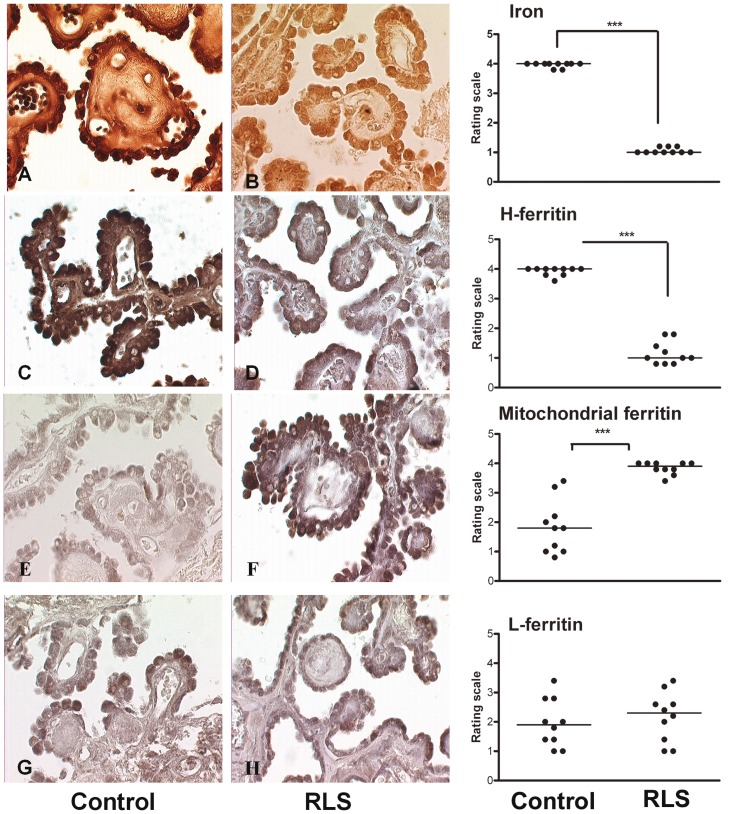
In control sections of choroid plexus (Fig. 1A), the majority of iron was present in the epithelial cells. In the sections from individuals with RLS (Fig. 1B), iron was evenly distributed in the epithelial cells but the staining intensity was dramatically decreased compared with control.
Figure 1.
Representative micrographs from the human choroids plexus comparing control tissues (left) to restless leg syndrome tissues (centre). The right panel presents the graph and statistical significance of the differences in staining between control and RLS tissues. Micrographs (A and B) represent iron staining in the choroid plexus of control and RLS. The iron product is brown in this diaminobenzidene enhanced Perl’s staining. In the control tissue (A), the brown iron product is clearly present in the epithelial cells of the choroid plexus. The iron product is also present in the stroma, but it is much less than that in the epithelial cells. In the RLS tissue (B), iron is evenly distributed in both epithelial and stroma. The staining intensity in the epithelial cells is much lighter compared with the control tissue (***P < 0.001). Micrographs (C and D) represent heavy chain ferritin staining in the choroid plexus of control and RLS. The immunoproduct is grey to black. In the control section (C), the black immunoreaction product is located mainly in the cytoplasm of the epithelial cells but staining is also visible in the stroma. In the RLS section (D), there is significantly less (***P < 0.001) heavy chain ferritin in the cytoplasm of the epithelial cells, as well as the stroma. The immunostaining pattern and intensity for light chain ferritin were not different between control (G) and RLS sections (H) in either epithelial cells or stroma. Mitochondrial ferritin staining in the control tissue (E) is evenly distributed in both epithelial cells and stroma. In the RLS tissue (F), there is a dramatic increase in mitochondrial ferritin (***P < 0.001) in epithelial cells of RLS compared with the control sections. H-ferritin = heavy-chain ferritin; L-ferritin = light-chain ferritin.
Immunohistochemical staining for iron-related proteins
Heavy-chain ferritin (Fig. 1C and D), mitochondrial ferritin (Fig. 1E and F) and light-chain ferritin (Fig. 1G and H), were all detected in the choroid plexus epithelial cells in both control and subjects with RLS. Heavy–chain ferritin immunostaining was less intense in the RLS tissue compared with control, whereas mitochondrial ferritin immunostaining was greater in RLS than control. No detectable differences in immunostaining were apparent for light-chain ferritin between the two groups.
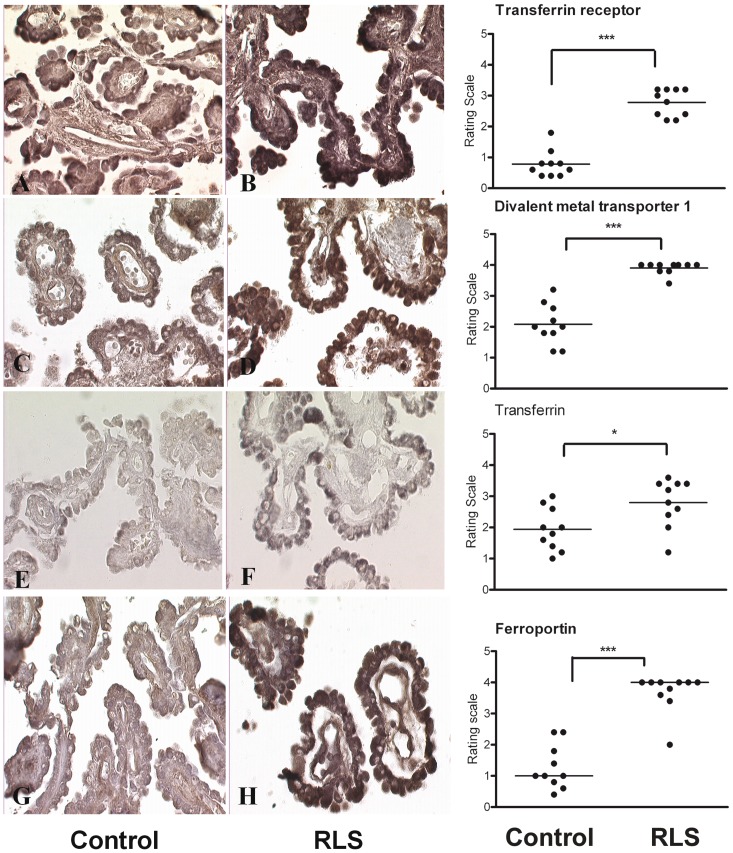
Both transferrin and transferrin receptor expression were found in the epithelial cells of the choroid plexus. Transferrin receptor expression appeared consistently upregulated in the epithelial cells of choroid plexus in RLS (Fig. 2B) compared with the control sections (Fig. 2A). A consistent pattern of increased staining was also apparent for transferrin in RLS (Fig. 2C) compared with the control sections (Fig. 2D).
Figure 2.
Representative micrographs from the human choroid plexus comparing control (left) to RLS tissues (centre). The right panel presents the graph and statistical significance of the differences in staining between control and RLS tissues. (A and B) The immunoreaction product for transferrin receptor is present in the cytoplasm of the epithelial cells in controls (A) and RLS choroid plexus (B). The immunoreaction product is considerably stronger in the RLS tissue (B) than that in the control (A). (C and D) DMT1 is present in the epithelial cells of the choroid plexus. In the RLS choroid plexus (C), DMT 1 was significantly more (***P < 0.001) than that in the control (D) epithelial cells. (E and F) Transferrin staining was present in both the control tissue (E) and the RLS tissue (F) in the epithelial cells. There was an increase in the staining intensity in RLS (*P < 0.05) compared with control sections. Ferroportin staining was significantly increased in the choroid plexus of RLS (G) (***P < 0.001) compared with the control sections (H). H-ferritin = heavy-chain ferritin; L-ferritin = light-chain ferritin.
DMT1 was expressed in the epithelial cells of the choroid plexus (Fig. 2E and F). In control sections, DMT1 immunoproduct could be detected but the intensity of the reaction was consistently higher in RLS tissue.
Ferroportin was detected in choroid plexus but was difficult to detect in a number of the control samples (Fig. 2G), whereas in RLS brains, the expression of ferroportin was consistently strong (Fig. 2H).
Brain microvasculature
Immunoblot analyses for iron-related proteins
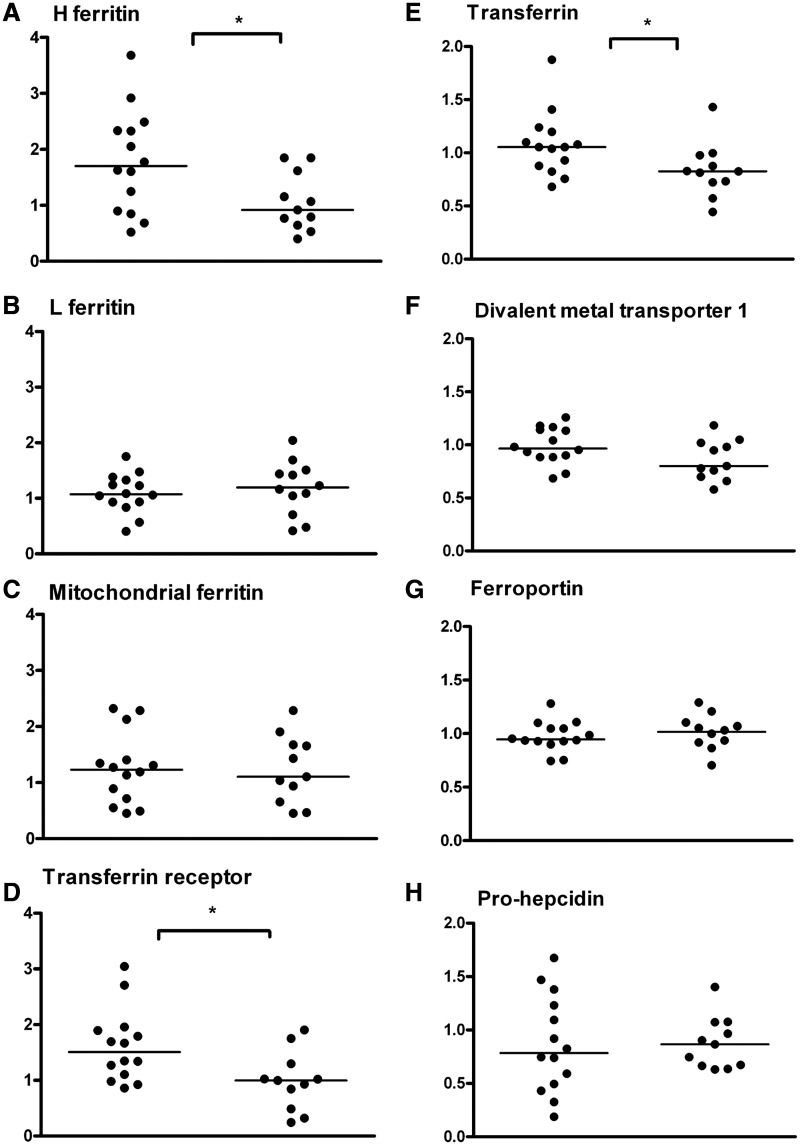
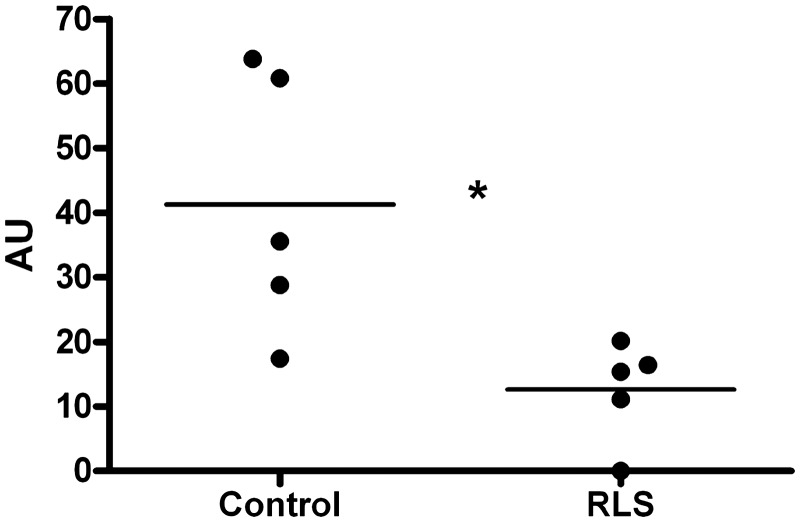
The iron-management protein levels were determined in the microvasculature of RLS and control autopsy brain tissues. The two subunits of ferritin were analysed individually. The expression of heavy-chain ferritin was significantly (P < 0.05) decreased in the RLS microvasculature compared with the control (Fig. 3A), while the expression of light chain ferritin was not different between the groups (Fig. 3B). There was no difference in the expression of mitochondrial ferritin (Fig. 3C) between RLS and controls. The expression of transferrin receptor was decreased significantly in RLS (P < 0.05) compared with the controls (Fig. 3D). Transferrin levels were also significantly (P < 0.05) decreased in RLS (Fig. 3E). The levels of DMT1 (Fig. 3F), the iron transport protein ferroportin (Fig. 3G) and the iron regulatory hormone prohepcidin (Fig. 3H) were all similar for RLS and controls.
Figure 3.
Quantitative immunoblot assays were performed to determine the relative amounts of the iron-transport proteins in the microvessels isolated from RLS (n = 11) and control (n = 14) brain autopsies. The horizontal line in the graph represents the median mean value for each group. Statistical analysis of the data indicates a significant difference between the means of the two groups (control and RLS) for heavy chain ferritin (A), transferrin receptor (D) and transferrin (E). These three proteins were significantly decreased in the microvasculature of RLS brains compared with the controls (*P < 0.05). The relative amounts of light chain ferritin (B), mitochondrial ferritin (C), DMT1 (F), ferroportin (G) and prohepcidin (H) were similar between the RLS and control groups. H-ferritin = heavy-chain ferritin; L-ferritin = light-chain ferritin.
Polymerase chain reaction analysis in human brain microvasculature
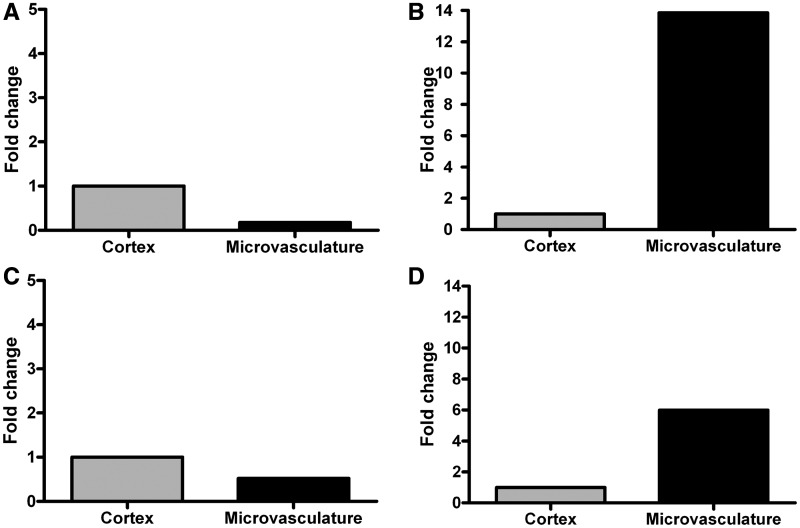
The presence of ferroportin has not been previously reported in human brain microvasculature. The presence of this protein represents a previously unreported mechanism for iron to be released from the blood–brain-barrier therefore real-time polymerase chain reaction analysis was performed on RNA isolated from fresh autopsy human brain and microvessels of human brain for the messenger RNA to further validate the presence of the protein (Fig. 4A). The messenger RNA for transferrin receptor was used as a positive control (Fig. 4B). The existing paradigm for iron transport states that transferrin is taken up by the endothelial cells and transports iron from the luminal to the abluminal membrane. We, however, determined that messenger RNA for transferrin could be detected in human brain microvasculature (Fig. 4C). It was not possible to isolate the RNA from the frozen brains so no comparison of RLS and control was possible. In each of the messenger RNA assays, the amount in the microvasculature was compared with an isolate from the cortex. The cortical tissue used served as a positive control as well as a control for relative amounts of expression.
Figure 4.
Real-time polymerase analysis of RNA extracted from fresh autopsy human brain microvessels and the cortical tissue revealed the presence of message for ferroportin (A), transferrin (B), transferrin receptor (C) and iron regulatory protein 1 (D). The cortical tissue is used as a positive control and to demonstrate the relative amounts of messenger RNA in a cortical sample versus the microvasculature.
Total iron regulatory protein binding activity in restless legs syndrome and control brain microvasculature
The regulation of transferrin receptors, DMT1 and ferritin is at the post-transcriptional level by iron regulatory proteins. Given the current paradigm that iron is simply transcytosed across the blood–brain barrier, no regulatory mechanism has been put forward for transferrin receptor expression, an obvious key component of how iron acquisition by the brain could be regulated. Furthermore, we have identified previously the iron regulatory proteins as the potential site for misregulation of transferring receptor expression in RLS (Connor et al., 2004). Therefore, we performed binding assays for iron regulatory proteins in the microvasculature. Iron regulatory protein activity was detected in the microvasculature. Moreover, the activity was significantly lower in RLS (P < 0.05) compared with control (Fig. 5). Because of the novelty and significance of the presence of iron regulatory proteins in the brain microvasculature, we confirmed the presence of iron regulatory protein 1 messenger RNA (Fig. 4D).
Figure 5.
Total iron regulatory protein binding activity in the RLS and control brain microvasculature was analysed using a gel shift assay on 4% native polyacrylamide gel. This assay showed significant decrease (*P < 0.05) in the total binding activity in RLS microvasculature compared with the controls.
Discussion
The results of this study demonstrate that the patterns of protein expression for iron management proteins in the choroid plexus and brain microvasculature are different in individuals with RLS compared with controls. The presence of iron regulatory protein activity in the microvasculature also demonstrates that there is regulation of transferrin receptor, ferritin and DMT1 expression at the post-transcriptional level because these proteins all contain iron responsive elements in their messenger RNA. The binding of the iron regulatory protein to the iron responsive element is responsive to the intracellular iron status (Pantopoulos, 2004), which means that the expression of transferrin receptors, ferritin and DMT1 in the brain endothelial cells is controlled by the iron status of these cells. Thus, in addition to the relevance of our findings for RLS in this study, the presence of iron regulatory protein binding activity in brain microvasculature is a significant discovery for brain iron uptake at the blood–brain barrier because it indicates that the control of the transferrin receptors and hence ultimately the amount of iron that can enter the brain via transferrin is regulated by the endothelial cells forming the blood–brain-barrier. It follows then that there must be a source of iron for the endothelial cells themselves and iron is not simply transcytosed across the blood–brain-barrier as the current paradigm states, but rather iron must also be released into the endothelial cell; a concept we have presented earlier (Connor et al., 2001). The presence of DMT1 in the endothelial cells is one method for iron release within the endothelial cells because the role of this protein is to export iron from the endosome to the cytosol (Gunshin et al., 1997). We have also reported DMT1 in rat brain microvasculature (Burdo et al., 2001) although its presence is not uniformly accepted (Moos et al., 2006). The expression of ferritin in both the choroid plexus and microvasculature reveals the capacity of endothelial cells to store iron and thus further dispels the current concept that the blood–brain barrier is a simple conduit for iron transport. There is less heavy chain ferritin in the microvasculature from the RLS brains compared with control indicating that there is less iron storage in these cells in RLS compared with control. The relative decrease in iron storage in the RLS tissue compared with control is consistent with our earlier report that neuromelanin cells in RLS contain less iron than normal (Connor et al., 2004).
Mechanisms of iron release
Unlike the neuromelanin cells, however, the function of the choroid plexus epithelial cells and endothelial cells of the microvasculature is to release iron into the brain. Release of iron from the choroid plexus and microvasculature is thought to be via transferrin, secreted from the choroid plexus or transcytosed from blood in the endothelial cells. The amount of transferrin in the choroid plexus did not appear different in RLS and controls, but was decreased in RLS microvasculature. The decrease in transferrin in the microvasculature could be associated with more rapid transcytosis or less transcytosis, but we have shown transferrin can be synthesized in the endothelial cells in this study and its synthesis in the choroid plexus has been demonstrated earlier (Connor et al., 2001). The regulation of transferrin synthesis and secretion from any cell type is not known at this time, but the presence of transferrin in the CSF is elevated in RLS (Earley et al., 2000b) suggesting increased secretion by the choroid plexus. Secretion of transferrin from the cells of the blood–brain barrier has not been demonstrated. Thus the decrease in transferrin in endothelial cells, particularly coupled with the decrease in ferritin and transferrin receptors in the brain microvasculature, suggests there is less iron moving across the blood–brain barrier in RLS. This idea would be consistent with the decrease in serum ferritin, suggesting lower systemic iron, which is a consistent finding in RLS (O'Keeffe et al., 1994; Sun et al., 1998; Trenkwalder et al., 2008).
Another mechanism for iron release from cells, although previously not considered as part of the paradigm in iron transport at the blood–brain barrier, is ferroportin. This protein resides on cell membranes and functions to export iron (Thomas and Oates, 2004). Ferroportin expression in the brain microvasculature was not different between the two groups and was increased in the choroid plexus in RLS. The data suggest greater release of iron from the choroid plexus cells via ferroportin, an observation consistent with the decreased hepcidin measured in the CSF of patients with RLS (Clardy et al., 2006b). The extent to which ferroportin plays a role in iron movement across the blood–brain barrier has not yet been investigated, but non-transferrin associated iron can be released from iron in a model of the blood–brain barrier (Burdo et al., 2003). The lack of a change in ferroportin expression in the microvasculature suggests the non-transferrin mediated iron release in RLS at the blood–brain barrier could be normal. Based on the decrease in heavy chain ferritin and transferrin receptors; however, the indication would be that there is less iron available for release via ferroportin.
Mechanisms of iron uptake
The mechanism for iron uptake into both the endothelial cells of the brain microvasculature and choroid plexus is thought to be primarily via the interaction of transferrin with its receptor (Fishman et al., 1987; Descamps et al., 1996) although non-transferrin mediated uptake has been shown for both the choroid plexus and brain microvasculature (Fishman et al., 1987; Moos and Morgan, 1998; Deane et al., 2004). The expression of transferrin receptors in the choroid plexus is increased in RLS but decreased in RLS in the microvasculature. In both tissues, as mentioned earlier, ferritin is decreased. Thus, the increase in transferrin receptors in the choroid plexus in RLS is consistent with the interpretation of decreased intracellular iron, whereas the decrease in the microvasculature is not. The decrease in transferrin receptor expression in the microvasculature coupled with a relative decrease in ferritin is consistent, however, with our earlier report in neuromelanin cells in RLS (Connor et al., 2004). The regulation of ferritin and transferrin receptor expression is through the iron regulatory protein that interacts with their messenger RNA (Kim et al., 1995). We have demonstrated herein the presence of iron regulatory protein binding activity in the blood–brain barrier. Moreover, we found that iron regulatory protein activity is decreased in the RLS microvasculature compared with control. The function of the iron regulatory proteins is to stabilize transferrin receptor messenger RNA to increase translation of protein when intracellular iron status is low. Because ferritin expression in the microvasculature in RLS is low, indicating that there is low intracellular iron, the decrease in iron regulatory protein binding activity in RLS would be surprising if not consistent with our earlier report in neuromelanin cells (Connor et al., 2004). The consistent decrease in iron regulatory protein activity in RLS could be due to a number of reasons that are beyond the scope of the current study.
The presence of DMT1 in both the choroid plexus and the brain microvasculature indicates that iron is released into these cells from the endosomes (Burdo et al., 2001). The increase in DMT1 in the choroid plexus is consistent with decreased intracellular iron. The decrease in DMT1 expression in the microvasculature, in spite of apparent decrease in iron content, is consistent with the transferrin receptor decrease. Moreover, DMT1 expression can be regulated by the iron regulatory protein. Therefore, the underlying mechanism for apparent discordant response to iron status is consistent with a loss of iron regulatory protein binding activity. A decrease in DMT1 levels was also reported in the neuromelanin cells in RLS (Connor et al., 2004).
Iron storage
Three types of ferritin have been examined in this study, heavy-chain ferritin, light-chain ferritin and mitochondrial ferritin. The three types of ferritins are distributed unevenly in the CNS (Connor et al., 1994; Snyder et al., 2009), and they are functionally distinct, although we do not know the exact role of the mitochondrial ferritin. The level of heavy chain ferritin was reduced, and light-chain ferritin was unchanged in RLS samples. The results are consistent with our finding from CSF (Clardy et al., 2006a) and substantia nigra (Connor et al., 2003, 2004). The levels of mitochondrial ferritin were increased in the choroid plexus of patients with RLS, and this agrees with our observation of increased expression of mitochondrial ferritin in the substantia nigra of subjects with RLS (Snyder et al., 2009). Overexpression of mitochondrial ferritin can decrease cytosolic iron levels (Nie et al., 2005). Therefore, the increase in mitochondrial ferritin is consistent with an increase in transferrin receptors.
Conclusion
This study demonstrates that iron regulatory proteins are present in the brain microvasculature. The presence of iron regulatory proteins indicates that transferrin receptors on endothelial cells are regulated by the cellular iron content. The presence of iron regulatory proteins and DMT1 is irrefutable evidence that iron is released into the endothelial cells indicating the blood–brain barrier is not a simple conduit for iron transport into the brain. The decrease in iron regulatory protein activity in the RLS microvasculature reveals a potential discordance between cellular iron status and expression of transferrin receptors and DMT1, which is what we also reported in our study of the substantia nigra from RLS brains. The study further reveals differences in the profile of iron management proteins between RLS and control individuals at the level of the brain and blood interface suggesting the relatively low brain iron concentrations in RLS may stem from misregulation of iron transport across the blood–brain barrier. We propose that in toto our data suggest that the endothelial cells of the blood–brain barrier serve as an iron reservoir for the brain and that the underlying problem in RLS is the lack of sufficient iron in reserve in the endothelial cells to meet physiological challenges, such as circadian changes in serum iron (Casale et al., 1981; Uchida et al., 1983; Scales et al., 1988) and the increased demands of iron during pregnancy (Ekbom, 1960; Manconi et al., 2004), conditions or times known to increase symptoms of RLS. Perhaps the mechanism underlying the use of intravenous iron supplements in treating symptoms of RLS is the ability of the iron compounds to ‘refill’ the reservoir.
Funding
This work was supported by a Program project grant from the National Institute of Health (1 P01 AG021190) awarded to the group studying pathophysiology of restless legs syndrome (to C.J.E.).
Acknowledgements
The authors are grateful to the restless Leg Syndrome Foundation for access to the restless Legs Syndrome Brain Donation Centre.
Glossary
Abbreviations
- DMT
Divalent metal transporter
- RLS
restless legs syndrome
References
- Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–5. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- Allen RP, Earley CJ. Defining the phenotype of the restless legs syndrome (RLS) using age-of-symptom-onset. Sleep Med. 2000;1:11–19. doi: 10.1016/s1389-9457(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Bianco LE, Unger EL, Earley CJ, Beard JL. Iron deficiency alters the day–night variation in monoamine levels in mice. Chronobiol Int. 2009;26:447–63. doi: 10.1080/07420520902820905. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Antonetti DA, Wolpert EB, Connor JR. Mechanisms and regulation of transferrin and iron transport in a model blood–brain barrier system. Neuroscience. 2003;121:883–90. doi: 10.1016/s0306-4522(03)00590-6. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Connor JR. Brain iron uptake and homeostatic mechanisms: an overview. Biometals. 2003;16:63–75. doi: 10.1023/a:1020718718550. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, et al. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- Casale G, Migliavacca A, Bonora C, Zurita IE, de Nicola P. Circadian rhythm of plasma iron, total iron binding capacity and serum ferritin in arteriosclerotic aged patients. Age Ageing. 1981;10:115–8. doi: 10.1093/ageing/10.2.115. [DOI] [PubMed] [Google Scholar]
- Clardy SL, Earley CJ, Allen RP, Beard JL, Connor JR. Ferritin subunits in CSF are decreased in restless legs syndrome. J Lab Clin Med. 2006a;147:67–73. doi: 10.1016/j.lab.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Clardy SL, Wang X, Boyer PJ, Earley CJ, Allen RP, Connor JR. Is ferroportin–hepcidin signaling altered in restless legs syndrome? J Neurol Sci. 2006b;247:173–9. doi: 10.1016/j.jns.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Connor JR, Boeshore KL, Benkovic SA, Menzies SL. Isoforms of ferritin have a specific cellular distribution in the brain. J Neurosci Res. 1994;37:461–5. doi: 10.1002/jnr.490370405. [DOI] [PubMed] [Google Scholar]
- Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Ondo WG, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–9. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL, Burdo JR, Boyer PJ. Iron and iron management proteins in neurobiology. Pediatr Neurol. 2001;25:118–29. doi: 10.1016/s0887-8994(01)00303-4. [DOI] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Patton SM, Menzies SL, Troncoso JC, Earley CJ, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62:1563–7. doi: 10.1212/01.wnl.0000123251.60485.ac. [DOI] [PubMed] [Google Scholar]
- Deane R, Zheng W, Zlokovic BV. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J Neurochem. 2004;88:813–20. doi: 10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps L, Dehouck MP, Torpier G, Cecchelli R. Receptor-mediated transcytosis of transferrin through blood–brain barrier endothelial cells. Am J Physiol. 1996;270:H1149–58. doi: 10.1152/ajpheart.1996.270.4.H1149. [DOI] [PubMed] [Google Scholar]
- Dickinson TK, Devenyi AG, Connor JR. Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. J Lab Clin Med. 1996;128:270–8. doi: 10.1016/s0022-2143(96)90028-1. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. J Neurosci Res. 2000a;62:623–8. doi: 10.1002/1097-4547(20001201)62:5<623::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000b;54:1698–700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–5. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Barker PB, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7:458–61. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Ekbom KA. Restless legs syndrome. Neurology. 1960;10:868–73. doi: 10.1212/wnl.10.9.868. [DOI] [PubMed] [Google Scholar]
- Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE. Receptor-mediated transcytosis of transferrin across the blood–brain barrier. J Neurosci Res. 1987;18:299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- Godau J, Klose U, Di Santo A, Schweitzer K, Berg D. Multiregional brain iron deficiency in restless legs syndrome. Mov Disord. 2008;23:1184–7. doi: 10.1002/mds.22070. [DOI] [PubMed] [Google Scholar]
- Godau J, Manz A, Wevers AK, Gaenslen A, Berg D. Sonographic substantia nigra hypoechogenicity in polyneuropathy and restless legs syndrome. Mov Disord. 2009;24:133–7. doi: 10.1002/mds.22391. [DOI] [PubMed] [Google Scholar]
- Godau J, Schweitzer KJ, Liepelt I, Gerloff C, Berg D. Substantia nigra hypoechogenicity: definition and findings in restless legs syndrome. Mov Disord. 2007;22:187–92. doi: 10.1002/mds.21230. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Kim HY, Klausner RD, Rouault TA. Translational repressor activity is equivalent and is quantitatively predicted by in vitro RNA binding for two iron-responsive element-binding proteins, IRP1 and IRP2. J Biol Chem. 1995;270:4983–6. doi: 10.1074/jbc.270.10.4983. [DOI] [PubMed] [Google Scholar]
- Leibold EA, Munro HN. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci USA. 1988;85:2171–5. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki EA, Cook BM, Devenyi AG, Beard JL, Connor JR. Transferrin is required for normal distribution of 59Fe and 54Mn in mouse brain. J Neurol Sci. 1999;170:112–8. doi: 10.1016/s0022-510x(99)00203-8. [DOI] [PubMed] [Google Scholar]
- Manconi M, Govoni V, De Vito A, Economou NT, Cesnik E, Casetta I, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63:1065–9. doi: 10.1212/01.wnl.0000138427.83574.a6. [DOI] [PubMed] [Google Scholar]
- McManus JF, Mowry RW. Staining methods: histological and histochemical. Medical Division of Harper and Brothers, New York, NY, 1960. [Google Scholar]
- Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14:43–7. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- Moos T, Morgan EH. Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. J Neurosci Res. 1998;54:486–94. doi: 10.1002/(SICI)1097-4547(19981115)54:4<486::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Moos T, Skjoerringe T, Gosk S, Morgan EH. Brain capillary endothelial cells mediate iron transport into the brain by segregating iron from transferrin without the involvement of divalent metal transporter 1. J Neurochem. 2006;98:1946–58. doi: 10.1111/j.1471-4159.2006.04023.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Bizot J, Bolesse M, Pulicani JP. [“Diaminobenzidine black” as a new histochemical demonstration of exogenous iron (author’s translation)] Histochemistry. 1980;66:239–44. doi: 10.1007/BF00495737. [DOI] [PubMed] [Google Scholar]
- Nie G, Sheftel AD, Kim SF, Ponka P. Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood. 2005;105:2161–7. doi: 10.1182/blood-2004-07-2722. [DOI] [PubMed] [Google Scholar]
- Nordlander NB. Therapy in restless legs. Acta Med Scand. 1953;145:453–7. [PubMed] [Google Scholar]
- O'Keeffe ST, Gavin K, Lavan JN. Iron status and restless legs syndrome in the elderly. Age Ageing. 1994;23:200–3. doi: 10.1093/ageing/23.3.200. [DOI] [PubMed] [Google Scholar]
- Pantopoulos K. Iron metabolism and the IRE/IRP regulatory system: an update. Ann NY Acad Sci. 2004;1012:1–13. doi: 10.1196/annals.1306.001. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Yang J, Eisenberg J, Tourtellotte WW. Isolation of intact capillaries and capillary plasma membranes from frozen human brain. J Neurosci Res. 1987;18:352–7. doi: 10.1002/jnr.490180213. [DOI] [PubMed] [Google Scholar]
- Scales WE, Vander AJ, Brown MB, Kluger MJ. Human circadian rhythms in temperature, trace metals, and blood variables. J Appl Physiol. 1988;65:1840–6. doi: 10.1152/jappl.1988.65.4.1840. [DOI] [PubMed] [Google Scholar]
- Schmidauer C, Sojer M, Seppi K, Stockner H, Hogl B, Biedermann B, et al. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol. 2005;58:630–4. doi: 10.1002/ana.20572. [DOI] [PubMed] [Google Scholar]
- Snyder AM, Wang X, Patton SM, Arosio P, Levi S, Earley CJ, et al. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol. 2009;68:1193–9. doi: 10.1097/NEN.0b013e3181bdc44f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep. 1998;21:371–7. [PubMed] [Google Scholar]
- Thomas C, Oates PS. Ferroportin/IREG-1/MTP-1/SLC40A1 modulates the uptake of iron at the apical membrane of enterocytes. Gut. 2004;53:44–9. doi: 10.1136/gut.53.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkwalder C, Hening WA, Walters AS, Campbell SS, Rahman K, Chokroverty S. Circadian rhythm of periodic limb movements and sensory symptoms of restless legs syndrome. Mov Disord. 1999;14:102–10. doi: 10.1002/1531-8257(199901)14:1<102::aid-mds1017>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Hogl B, Benes H, Kohnen R. Augmentation in restless legs syndrome is associated with low ferritin. Sleep Med. 2008;9:572–4. doi: 10.1016/j.sleep.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Uchida T, Akitsuki T, Kimura H, Tanaka T, Matsuda S, Kariyone S. Relationship among plasma iron, plasma iron turnover, and reticuloendothelial iron release. Blood. 1983;61:799–802. [PubMed] [Google Scholar]
- Wang X, Wiesinger J, Beard J, Felt B, Menzies S, Earley C, et al. Thy1 expression in the brain is affected by iron and is decreased in Restless Legs Syndrome. J Neurol Sci. 2004;220:59–66. doi: 10.1016/j.jns.2004.02.004. [DOI] [PubMed] [Google Scholar]