Abstract
Epilepsy is a prevalent childhood neurological disorder, but there are few prospective quantitative magnetic resonance imaging studies examining patterns of brain development compared to healthy controls. Controlled prospective investigations initiated at or near epilepsy onset would best characterize the nature, timing and course of neuroimaging abnormalities in paediatric epilepsy. In this study, we report the results of a deformation-based morphometry technique to examine baseline and 2-year prospective neurodevelopmental brain changes in children with new and recent onset localization-related epilepsies (n = 24) and idiopathic generalized epilepsies (n = 20) compared to healthy controls (n = 36). Children with epilepsy demonstrated differences from controls in baseline grey and white matter volumes suggesting antecedent anomalies in brain development, as well as abnormal patterns of prospective brain development that involved not only slowed white matter expansion, but also abnormalities of cortical grey matter development involving both greater and lesser volume changes compared to controls. Furthermore, abnormal neurodevelopmental changes extended outside the cortex affecting several subcortical structures including thalamus, cerebellum, brainstem and pons. Finally, there were significant differences between the epilepsy syndromes (localization-related epilepsies and idiopathic generalized epilepsies) with the idiopathic generalized epilepsies group showing a more disrupted pattern of brain structure both at baseline and over the 2-year interval.
Keywords: MRI, prospective neurodevelopment changes, deformation-based morphometry, new and recent onset epilepsy, localization-related epilepsy, idiopathic generalized epilepsy
Introduction
Longitudinal quantitative MRI investigations of healthy children have shown age, gender and region-specific declines in cerebral grey matter volume along with increases in cerebral white matter volume (Giedd et al., 1999; Paus et al., 1999; Gogtay et al., 2004; Sowell et al., 2003, 2004; Lenroot and Giedd, 2006; Toga et al., 2006; Wilke et al., 2006; Shaw et al., 2008; Hua et al., 2009), the majority of these changes occur in the frontal and parietal regions in late childhood and adolescence (Sowell et al., 2003, 2004; Marsh et al., 2008; Paus et al., 2008; Giedd et al., 2009). Against this background of normal neurodevelopmental change, prospective investigations have identified patterns of abnormal development in children and adolescents with a variety of clinical conditions including childhood schizophrenia (Rapoport et al., 1999; Greenstein et al., 2006; Gogtay et al., 2008), autism (Wassink et al., 2007; Gogtay et al., 2008), attention deficit/hyperactivity disorder (Castellanos et al., 2002; Shaw et al., 2006, 2007; van't Ent et al., 2007), very low birth weight (Peterson et al., 2000; Ment et al., 2009), childhood-onset schizophrenia (Gogtay et al., 2008) and bipolar disorder (Gogtay et al., 2007; Lazaro et al., 2009).
Epilepsy is a prevalent childhood neurological disorder (Shinnar and Pellock, 2002), but there are few prospective quantitative MRI studies examining patterns of brain development compared to healthy controls. Cross-sectional studies of children with chronic localization-related epilepsy (LRE) using traditional volumetrics and voxel-based morphometry have revealed abnormalities in the overall cerebrum, cerebellum, frontal and temporal lobes, hippocampus, amygdala, and thalamus (Lawson et al., 1997, 1998, 2000a, b; Cormack et al., 2005; Daley et al., 2006, 2008; Guimaraes et al., 2007). Similar cross-sectional investigations of children, adolescents and young adults with idiopathic generalized epilepsies (IGE) including childhood absence and juvenile myoclonic epilepsy have reported distributed patterns of abnormality predominantly affecting thalamus and frontal lobe (Betting et al., 2006a, b, c; Tae et al., 2006, 2008; Kim et al., 2007; Pardoe et al., 2008; Caplan et al., 2009a, b; de Araújo Filho et al., 2009; Pulsipher et al., 2009). Collectively, these studies clearly indicate a neurodevelopmental contribution to anatomic abnormalities that have been observed in adults with these syndromes of epilepsy (Hermann et al., 2009), but the onset and course of their emergence remains uncertain.
Controlled prospective investigations initiated at or near epilepsy onset would best characterize the nature, timing and course of neuroimaging abnormalities in paediatric epilepsy. In the only longitudinal investigation to date, expansion of cerebral white matter volume was found to be slowed in children with epilepsy over a 2-year interval, especially in frontal regions, with normal appearing reduction in grey matter volumes (Hermann et al., 2010). While clearly indicating abnormalities in brain development in children with uncomplicated epilepsies, the techniques used likely understated the amount of developmental abnormality as only gross total volumes of cortical lobar grey and white matter were examined and no information was provided regarding subcortical structures or cerebellum. In addition to a broader characterization of neural structures, it is certainly possible that a more sophisticated examination of the cortical mantle and associated white matter, as well as subcortical structures and cerebellum using voxel-based approaches might identify localized or lateralized areas of abnormalities not detected in total lobar analyses.
To that end, we report the results from the use of a deformation-based morphometry technique to examine brain neurodevelopment in children with new and recent onset LRE and IGE. Deformation-based morphometry is a relatively new image analysis technique that can be used to identify regional structural differences in the brain either cross-sectionally (i.e. baseline status) or over time (i.e. prospective neurodevelopmental change) (Leow et al., 2006; Studholme et al., 2006) across groups from the gradients of the deformation fields that warp images. Widely used voxel-based morphometry relies on the automated segmentation of images into grey matter, white matter and cerebrospinal fluid density maps and a rough transformation of grey matter density map of a subject’s brain into a target space. This approach can be particularly problematic in longitudinal studies as white matter tissue expansion or shrinkage causes changes in the location of overlying grey matter and subcortical grey matter. For these reasons, voxel-based morphometry is suboptimal for investigating prospective white matter changes or determining subcortical involvement in prospective neurodevelopmental changes. Recent improvements in image alignment allow a much more precise transformation of a subject’s brain into a target space with deformation-based morphometry used to provide more direct quantitative maps of anatomical variation (Studholme et al., 2004). By avoiding the need for image segmentation and using robust registration methods, deformation-based morphometry is a more suitable and sensitive technique for investigating anatomical variation in prospective neurodevelopmental changes in epilepsy.
Our hypotheses were as follows: (i) abnormalities in the rate of white matter expansion would be replicated in the frontal lobes of children with epilepsy, but additional areas of slowed white matter development would be identified as well; (ii) discrete areas of grey matter volume over- and under-expansion would be identified in the children with epilepsy, but abnormalities in white matter expansion rate would remain the key abnormality; (iii) abnormalities in prospective neurodevelopmental change would be identified in subcortical structures with the thalamus among the most likely affected structure; and (iv) differences in prospective neurodevelopmental change would be more pronounced in children with IGE compared to children with LRE.
Finally, there is evidence that the rate of prospective neurodevelopmental change is related to baseline anatomic characteristics and abnormalities that may vary across different brain regions (Shaw et al., 2008). Therefore, more precise estimates of group differences in prospective neurodevelopmental change will result if there is consideration and control of group differences in baseline brain status. To that end, we identified and then controlled for baseline group differences as a spatially varying covariate in the analyses of prospective brain development in testing these hypotheses.
Materials and methods
Subjects
Research participants included children with new/recent onset epilepsy (n = 44) and healthy first-degree cousin controls (n = 36), aged 8–18 years, all attending regular schools. Initial selection criteria included: (i) diagnosis of epilepsy within the past 12 months; (ii) chronological age between 8 and 18 years; (iii) no other neurological disorders; and (iv) normal clinical MRI. Epilepsy participants met criteria for classification of idiopathic epilepsy in that they had normal neurological examinations, no identifiable lesions on MRI and no other signs or symptoms indicative of neurological abnormality (Engel, 2001). The epilepsy group contained 24 children with LRE and 20 with IGE. Six children in the IGE group were diagnosed with childhood absence epilepsy and 14 with juvenile myoclonic epilepsy. The LRE group comprised six children with benign epilepsy of childhood with centrotemporal spikes, five with frontal lobe epilepsy, four with temporal lobe epilepsy and nine with focal epilepsy, not otherwise specified. Epilepsy diagnostic classification was determined by a paediatric epileptologist who reviewed all available medical records, including EEG studies and seizure semiology and was blinded to results from neuropsychological evaluation and MRI. For example, patients were determined to have juvenile myoclonic epilepsy if the following criteria were present: (i) 4–6 Hz polyspike and slow-wave generalized discharges; (ii) history of myoclonic jerks; and (iii) history of generalized tonic–clonic seizures. Benign epilepsy of childhood with centrotemporal spikes was diagnosed by the presence of either simple partial seizures during the waking hours or tonic–clonic convulsive seizures at night associated with the presence of centrotemporal spikes on EEG, occurring independently in the right and left centrotemporal regions. Activation of spike wave charges with sleep was also considered a feature suggestive of this syndrome. Similar rigorous application of electroclinical criteria was used in the characterization of the other LRE (temporal lobe, frontal lobe) and generalized syndromes (childhood and juvenile absence epilepsy).
Control participants were age and gender-matched first-degree cousins. Criteria for controls included no histories of (i) any initial precipitating event (e.g. simple or complex febrile seizures); (ii) seizure or seizure-like episode; (iii) diagnosed neurological disease; (iv) loss of consciousness >5 min; or (v) other family history of a first-degree relative with epilepsy or febrile convulsions. Of the initial cohort, 94% was retained and returned for prospective evaluation.
This study was reviewed and approved by the Institutional Review Board of study institution and on the day of study participation families and children gave informed consent and assent, and all procedures were consistent with the Declaration of Helsinki (World Medical Association, 1991).
Table 1 provides the demographic characteristics of the research participants at baseline. Healthy children group was marginally older than LRE group (P = 0.07; t-test) and marginally younger than the IGE group (P = 0.04; t-test); however, the group age difference between LRE and IGE was statistically significant (P = 0.001; t-test). There were no significant differences between the epilepsy and control groups in gender (all P > 0.76; Fisher’s exact test). Although there was no significant difference between the duration of epilepsy between LRE and IGE groups, children with LRE had significantly earlier onset age compared to the ones with IGE (P = 0.002; t-test).
Table 1.
Demographic features of study groups
| Healthy | IGE | LRE | |
|---|---|---|---|
| n | 36 | 20 | 24 |
| Baseline age (years) | 12.4 (2.98)* | 14.3 (3.6)* | 11.0 (2.6)* |
| Gender (F/M) | 18/18 | 11/9 | 11/13 |
| Age of onset (years) | – | 13.5 (3.7)* | 10.5 (2.6)* |
| Duration, months | – | 8.6 (3.95)* | 7.1 (3.9)* |
| Verbal IQ | 108.8 (13.8)* | 99.0 (13.3)* | 102.8 (10.6)* |
| Performance IQ | 108.5 (12.3)* | 99.2 (11.5)* | 101.9 (14.3)* |
| Full-scale IQ | 109.8 (12.4)* | 99.6 (11.1)* | 102.6 (13.1)* |
| Percent with attention deficit/hyperactivity disorder | – | 25 | 37.5 |
| Percent on an antiepileptic drug | – | 100 | 70.8 |
*Mean (standard deviation).
The IQ scores for all groups fell in the average range. Statistically, the LRE and IGE groups were not significantly different (i.e. all P > 0.32; t-test), while the LRE group was marginally lower than the controls (P = 0.06 for verbal IQ, P = 0.06 for performance IQ and P = 0.04 for full-scale IQ; t-test), and the IGE group scored significantly lower than controls (P = 0.01 for verbal IQ, P = 0.008 for performance IQ, and P = 0.004 for full-scale IQ; t-test), but again all scores were average.
The LRE and IGE groups were not statistically different in terms of percentage of children with attention-deficit/hyperactivity disorder (P = 0.29; Fisher’s exact test). Finally, the proportion of children with LRE on an anti-epileptic drug was lower compared to the IGE group (P = 0.009; Fisher’s exact test). In the current sample, there are no differences in volumes between those not treated with valproate and those treated with valproate, commonly the first-line treatment of choice in IGE (Sullivan and Dlugos, 2004). In addition, no volume differences were evident between children whose first medication was valproate and children that valproate was not used as the first anti-epileptic drug. If anti-epileptic drug effects were present, they were uniform across subjects and not dependent upon drug type. It should also be noted that the IGE subjects had a relatively short exposure to anti-epileptic drugs (i.e. 1–12 months) at baseline (Publisher et al., 2010).
Magnetic resonance image acquisition
Baseline and 2-year follow-up magnetic resonance brain images for each participant were obtained on a 1.5 T GE Signa magnetic resonance scanner. Sequences acquired for each participant included: T1-weighted, three-dimensional (3D) spoiled gradient recalled acquisition with the following parameters: echo time = 5 ms, repetition time = 24 ms, flip angle = 40°, NEX = 1, slice thickness = 1.5 mm, slices = 124, plane = coronal, field of view = 200 mm, matrix = 256 × 256. A required change to another 1.5 T GE scanner was made during the course of the study, but only the baseline imaging data and an equal proportion of children with epilepsy (and LRE and IGE) and healthy controls were involved in this change. In addition, phantom studies revealed <1% volume difference between the GE machines. A supplementary analysis (i.e., voxel-wise analysis of baseline DBM data) of healthy children yield no statistically significant bias due to change in scanner.
Preprocessing of the structural magnetic resonance images
The skull, scalp, extra-cranial tissue, meninges and brainstem (at the level of the diencephalon) were removed from each magnetic resonance brain image data using the automated Brain Surface Extractor software (Shattuck and Leahy, 2002) followed by a quality check. The remaining image volume was then corrected for intensity inhomogeneity using the non-parametric non-uniform intensity normalization (N3) technique (Sled et al., 1998).
Creation of maps of differences in baseline neurodevelopmental state
A large deformation mapping technique based on fluid-flow warping was used to spatially normalize baseline brain images to a template image. The baseline brain image of a healthy male child of age 12 years (i.e. mean age of the study population) was selected as the template image rather than a group average in order to retain the finest anatomical structures in the template for accurate registration (Kochunov et al., 2002). The first step in the baseline spatial normalization was to affine align the baseline brain images and the reference brain image to adjust for global differences in brain positioning and scale across individuals. We then generated non-linear inverse-consistent fluid-flow deformation between each affine aligned baseline brain image and the reference image (Joshi et al., 2004). The Jacobian matrix of the deformation field was estimated at each voxel. The determinant of the Jacobian matrix was calculated to estimate the fractional volume contraction or expansion at each voxel, quantifying the baseline differences in neurodevelopmental state for each participant relative to the reference brain image.
Creation of maps of prospective neurodevelopmental change within participants
Each participant’s brain image at 2-year follow-up was coregistered with a six-parameter transformation to the participant’s baseline brain scan at the time of diagnosis. The fluid-flow-based non-linear inverse-consistent deformation algorithm was used to create deformation field maps of prospective neurodevelopmental change between scans at the time of diagnosis and 2-year follow-up scans of each participant. The Jacobian matrix of the deformation field was estimated at each voxel. The determinant of the Jacobian matrix was calculated to estimate the fractional volume contraction or expansion as a measure of prospective neurodevelopmental change at each voxel evaluated in the baseline brain image space. Resulting individual prospective neurodevelopmental change maps were then mapped from the participant’s baseline image space to the reference image space by first applying the affine transformation matrix and then the fluid-flow deformation field estimated for the baseline spatial normalization.
Statistical analysis
Jacobian maps encoding group differences in baseline status and prospective change over the 2-year period were smoothed with a Gaussian spatial kernel with a maximum filter width of 12-mm full-width half-maximum in the reference image space. Using these spatially normalized and smoothed Jacobian maps, we performed voxel-wise statistical tests between groups. The Jacobian maps for children with epilepsy (i.e. IGE and LRE groups separately) were compared to those for healthy children using voxel-wise tests. Specifically, the Jacobian map of prospective anatomic change was regressed using a general linear model at every brain tissue voxel against a categorical variable coding group membership as a predictor and age and gender as spatially constant covariates and Jacobian maps of baseline status as a spatially varying covariate. Intracranial volume was not accounted for in the general linear model since Jacobian maps of baseline status were estimated using affine aligned brain images. As supplementary results, the effects of epilepsy on baseline status and prospective 2-year change were tested using separate general linear models with age and gender as covariates. Voxel-wise tests were individually controlled for multiple comparison with a false discovery rate of q < 0.05 (Benjamini and Hochberg, 1995), and at each voxel, we evaluated the significance level of group differences using a two-sample t-test with unequal variance. The resulting P-values were displayed as maps to visualize patterns of significant group differences throughout the brain.
Results
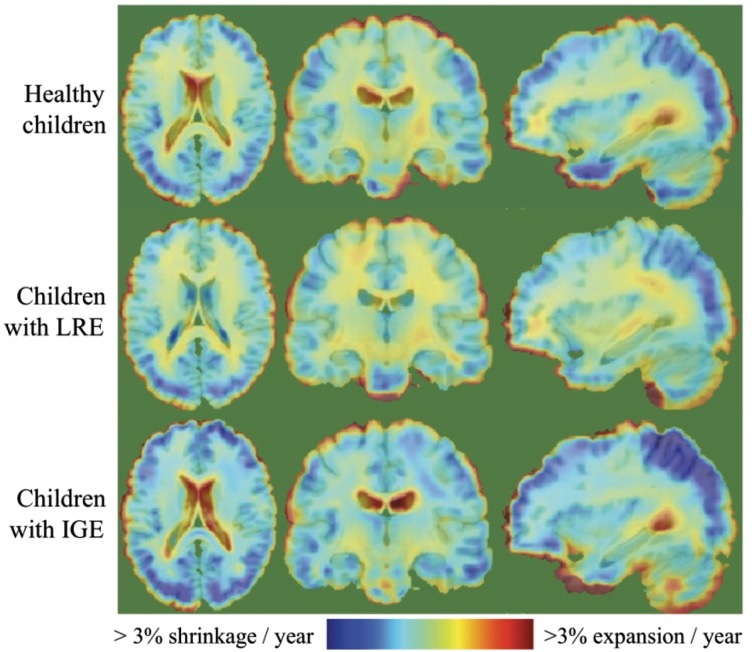
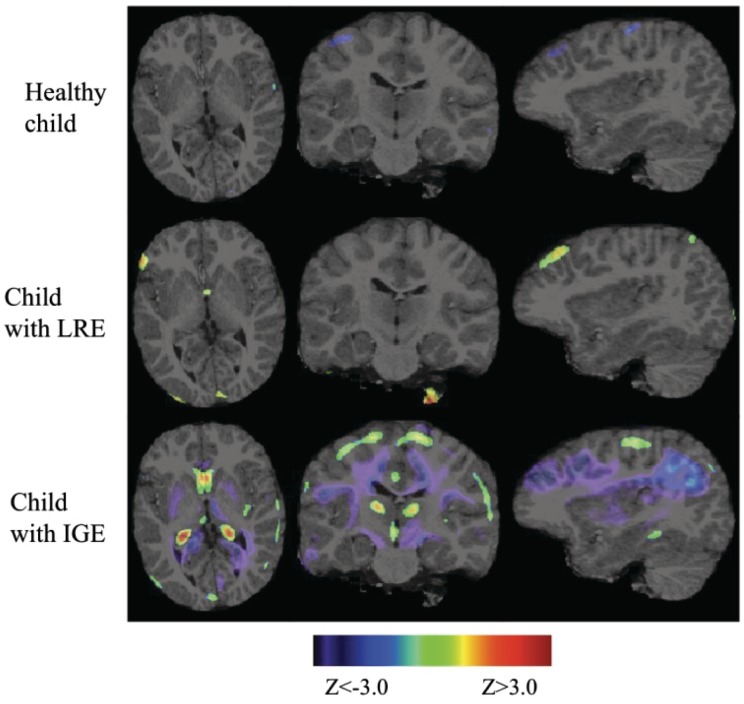
Figure 1 illustrates the average prospective neurodevelopmental changes for healthy children, children with LRE and children with IGE, separately. Over a 2-year period, healthy children displayed reduction in grey matter volume throughout the cortex, more pronounced in the frontal, parietal and temporal regions, and increase in white matter volume throughout the brain. Regionally specific deviations from these normal neurodevelopment rates were observed in both LRE and IGE groups as shown in the middle and bottom panels of Fig. 1, with the details of these deviations specified in the following sections. Uncorrected z-score maps (thresholded at |z| > 3.0; 99.9% cumulative probability) of single subject’s neurodevelopmental rates against healthy children’s neurodevelopmental rates are also provided for sample subjects in Fig. 2. These figures demonstrate how these sample subjects’ neurodevelopmental rates deviate from the mean neurodevelopmental rates observed in the healthy children population. While slowed grey matter tissue loss localized to the frontal and parietal cortical regions was observed in the sample child with LRE, widespread slowed grey matter tissue loss and slowed rates of white matter expansion was evident in the sample child with IGE.
Figure 1.
Within group average neurodevelopmental rates.
Figure 2.
Uncorrected z-score maps (thresholded at |z| > 3.0) of single subject’s neurodevelopmental rates against healthy children’s neurodevelopmental rates.
Localization-related epilepsies prospective neurodevelopmental change compared to healthy children
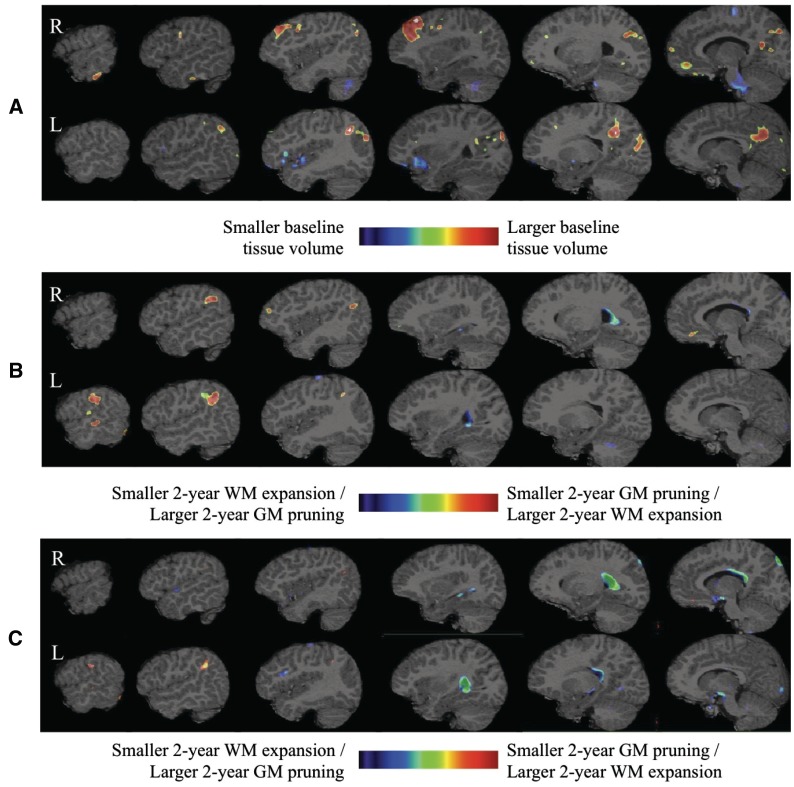
The top panel of Fig. 3 characterizes baseline brain status and illustrates larger grey matter volume in the right inferior temporal, right middle frontal, right superior frontal, right medial orbito-frontal, bilateral precuneus, bilateral posterior cingulate, bilateral cuneus, and left inferior parietal regions as well as the smaller left insular, left lateral fronto-orbital, and left inferior frontal grey matter. Also evident is smaller right cerebellum white matter and pons tissue volumes in the LRE group compared to healthy controls. The middle panel in Fig. 3 shows the corrected statistical maps at q = 0.05 for prospective 2-year brain change in LRE compared to healthy controls overlaid on the reference magnetic resonance brain image. Grey matter tissue loss was slowed in the left superior frontal, left middle frontal, left lateral fronto-orbital, left middle temporal, right inferior parietal, bilateral superior parietal, and left supramarginal regions, with slowed ventricular and bilateral hippocampal volume expansion and moderate slowing of left cerebellar white matter and corpus callosum expansion. After accounting for the LRE-control baseline brain status differences, the bottom panel of Fig. 3 reveals slower grey matter tissue loss in the LRE group only in the left superior parietal and left middle frontal regions. After accounting for the baseline brain status differences, the LRE group also exhibited marked reductions in the ventricular, hippocampal and corpus callosum volume expansion rates.
Figure 3.
Healthy children versus children with LRE differences in baseline status (A) and in prospective neurodevelopmental change when baseline findings are not controlled for (B) and when they are controlled (C) in the model. GM = grey matter; WM = white matter.
Idiopathic generalized epilepsies prospective neurodevelopmental change compared to healthy controls
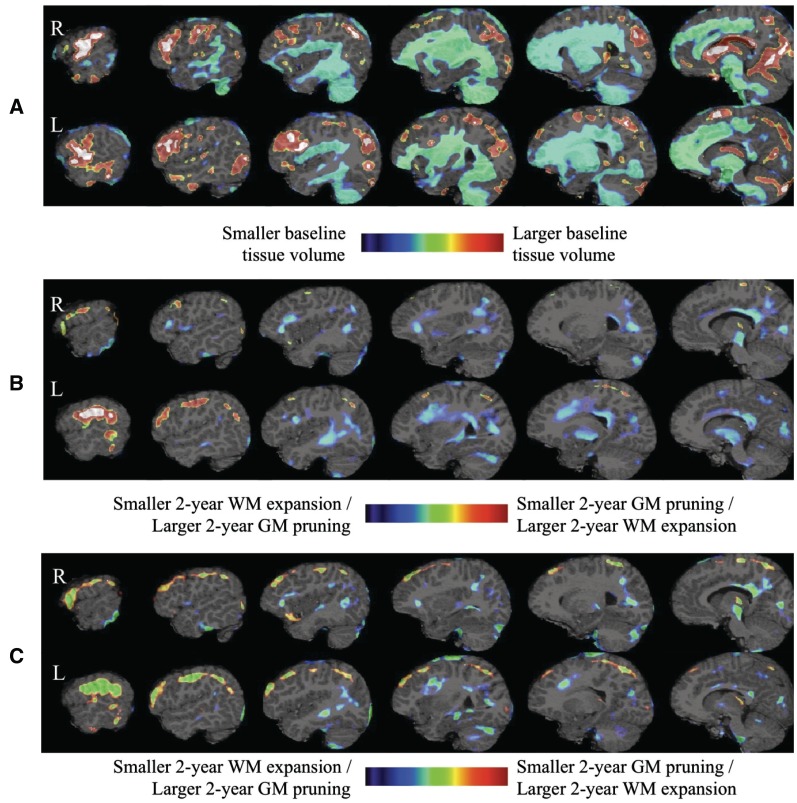
The top panel of Fig. 4 shows the baseline status of the children with IGE compared to the healthy control group. As can be seen, larger grey matter tissue volumes and smaller white matter tissue volumes were evident throughout the frontal, parietal, and temporal regions including subcortical structures with smaller grey matter tissue volumes in the medial orbito-frontal region in the IGE group compared to the healthy group. In addition, there were baseline abnormalities in the cerebellum including larger cerebellar grey matter tissue volume in the right anterior lobe and left VIIA and VIIB posterior lobes, with smaller cerebellar grey matter and white matter tissue volumes in the tonsil, and VIIIA, VIIIB, IX, X posterior lobes bilaterally. The middle panel in Fig. 4 shows the corrected statistical maps for the 2-year neurodevelopmental change comparison of IGE and healthy groups overlaid on the reference magnetic resonance brain image. Over this interval, the rate of grey matter tissue loss in IGE was slower than controls in regions of the frontal lobe (precentral, middle fronto-orbital, middle frontal, and inferior frontal predominantly in the left hemisphere) and parietal lobe (postcentral and superior parietal in the left hemisphere). Rates of white matter expansion were also significantly slowed in IGE compared to controls, affecting frontal as well as temporal and parietal regions and corpus callosum. Conversely, greater volume decline in IGE compared to controls was evident in the grey matter of cerebellum (right VI posterior lobe, left IV and V anterior lobes, and left IX posterior lobe), brainstem, pons, and several subcortical structures (thalamic nuclei, putamen, hippocampus, caudate, and amygdala). Finally, additional areas of greater grey matter volume loss were evident in discrete cortical regions including bilateral cuneus, left precuneus, left posterior cingulate, right inferior temporal, left lingual, left fusiform, left parahippocampal, bilateral inferior parietal and bilateral inferior occipital regions.
Figure 4.
Healthy children versus children with IGE differences in baseline status (A) and in terms of prospective change when baseline findings are not controlled for (B) or are controlled for (C) in the model. GM = grey matter; WM = white matter.
After accounting for the baseline differences, prospective neurodevelopmental change differences between the IGE and control groups were more pronounced including regions with slowed rate of reduction in grey matter volume reflected in the bottom panel of Fig. 4. Still evident was significantly slowed white matter expansion but with a smaller spatial extent (middle and bottom panels of Fig. 4). In addition, by including the baseline differences in the regression model, we also detected regions in left cerebellar white matter with slowed expansion.
Discussion
Four primary findings result from this examination of baseline and prospective differences in brain structure in children with new and recent onset idiopathic epilepsies compared to healthy controls. First, children with epilepsy demonstrate differences from controls in baseline grey and white matter volumes suggesting anomalies in brain development antecedent to the onset of seizures, as well as abnormal patterns of prospective brain development that involve not only slowed white matter expansion as reported previously, but now shown here to be more extensive with involvement of both greater and lesser volume changes in the cortical grey matter regions. Second, these abnormal neurodevelopmental changes extend outside the cortex and involve several subcortical structures (thalamus, putamen, caudate, amygdala, and hippocampus) as well as corpus callosum, cerebellum, brainstem, pons and ventricles. Third, there are significant differences between the epilepsy syndromes (LRE and IGE) in the evident patterns of altered brain development, the IGE group showing a more disrupted pattern of neurodevelopmental change over the 2-year interval. Finally, as predicted, controlling for baseline abnormalities alters the view of prospective brain changes. Specifically, controlling for baseline differences attenuates prospective abnormalities for the LRE group but not for the IGE group. This observation suggests static hit to neurodevelopment of LRE group at the disease onset that simply persists and does not worsen over time while the disease display a dynamic effect on IGE group. These findings will be discussed below.
Changes in white matter expansion and grey matter contraction
The longitudinal deformation-based morphometry technique used in this study demonstrated the expected white matter expansion and grey matter contraction as well as changes in subcortical structures and cerebellum in normal neurodevelopment in the healthy controls (top panel of Fig. 1). Against this backdrop, abnormalities in prospective brain development were evident among the children with epilepsy in their patterns of white matter expansion and grey matter tissue loss.
A major finding is the slowed pattern of white matter expansion in the children with epilepsy over the 2-year interval, an effect observed prominently in children with IGE. As hypothesized, the slowed white matter expansion was evident in the frontal regions, but shown here to be more widespread than initially reported (Hermann et al., 2010) with abnormalities detected in the corpus callosum and temporal and parietal regions, prominent in the left hemisphere. This altered expansion of cerebral white matter in children with new and recent onset epilepsy is an important finding for both clinical and theoretical reasons. Abnormalities in cerebral white matter volumes have been reported in adults with chronic childhood/adolescent onset temporal lobe epilepsy, a finding we suggested reflected neurodevelopmental vulnerability associated with an early insult and/or recurrent seizures to the developing brain (Hermann et al., 2002; Seidenberg et al., 2005). Cross-sectional investigations of specific white matter tracks such as the corpus callosum demonstrated that volumetric abnormalities are more notable in adults with childhood versus later onset of recurrent seizures (Hermann et al., 2003; Weber et al., 2007; Riley et al., 2010), but a direct confirmation of this effect remained to be demonstrated.
There is now direct evidence of white matter abnormalities in children with chronic epilepsy. Recent investigations of white matter microstructure using diffusion tensor imaging have characterized distributed abnormalities in the context of lateralized LRE (Govindan et al., 2008), reported decreased anistropy in all four white matter tracts investigated (corticospinal tract and the uncinate, arcuate, and inferior longitudinal fasciculi) both ipsilateral and contralateral to side of seizure onset in children with left temporal lobe epilepsy. In addition, an association was detected between duration of epilepsy with 3 of 4 tracks ipsilateral to side of onset suggesting a progressive component. Nilsson et al. (2008) reported bilaterally increased parallel and perpendicular diffusivity with preserved fractional anisotropy in the white matter of temporal lobe and cingulate gyrus in children with temporal lobe epilepsy. Lastly, we recently reported microstructural abnormalities in new onset childhood epilepsy (Hutchinson et al., 2010), suggesting that these abnormalities were evident early in the course of epilepsy. Interestingly, radial but not axial diffusivity was altered in some areas of interest (e.g. posterior corpus callosum). Although there is no definitive microstructural change certain to underlie this pattern, there are a growing number of diffusion weighted MRI studies relating altered radial diffusivity to myelination abnormalities (Song et al., 2005; Budde et al., 2007) and showing a reduction of radial diffusivity during normal white matter development in humans thought to correspond to compacting fibres and myelination (Snook et al., 2005; Alexander et al., 2007; Hasan et al., 2009).
Also as suspected, we identified differences in the pattern of grey matter thinning in the children with epilepsy compared to controls, again with many similarities but some differences between LRE and IGE groups. The IGE group in particular showed cortical regions expressing both larger tissue loss (most pronounced in the left parietal and temporal regions) as well as smaller tissue loss (in bilateral frontal and parietal regions) than expected compared to healthy controls. These areas were not detected in the previous report where only volumes of total lobar grey matter volumes were examined. Localization of greater grey matter tissue loss in the left temporal and parietal lobes is particularly interesting given the role of this brain region in language and evidence for both mild diffuse cognitive and linguistic impairment, as well as academic underachievement exhibited by children with epilepsy regardless of epilepsy syndrome (Hermann et al., 2006; Caplan et al., 2009a, b). The between group differences in the rate of grey matter tissue loss in the frontal and parietal regions might reflect the role these regions play in linguistic competence (Thiebaut de Schotten et al., 2005).
We observed fairly asymmetric areas of abnormality in baseline brain status with a larger left hemisphere effect in the LRE group. Compared to pronounced smaller baseline cerebellar grey matter tissue volume in IGE group, we observed more modest effects prospectively even after accounting for baseline neurodevelopmental stage differences. The abnormal patterns of grey matter thinning in IGE group are more limited in cerebral cortex and are bilateral in nature affecting the frontal, parietal and temporal regions. In contrast, the children with LRE, consisting of children with benign epilepsy of childhood with centrotemporal spikes and temporal and frontal epilepsies, show more anterior prospective grey matter abnormalities.
Developmental abnormalities in subcortical structures and posterior fossa
Extremely interesting, and unanticipated, were the abnormal developmental trajectories in several subcortical structures and especially cerebellum. The finding of abnormal cerebellar development is particularly interesting. Cerebellar atrophy is often noted in adults with chronic epilepsy, a finding reported in neuropathological investigations of institutionalized epilepsy patients dating back to 1825 (Honavar and Meldrum, 2002) and clearly evident prior to the availability of phenytoin. However, with the introduction of phenytoin it quickly became evident that cerebellar atrophy could be linked to drug effects in both human and animal investigations (Finkleman and Arieff, 1942; Utterback et al., 1958; Kokenge et al., 1965; del Cerro and Snider, 1967; McLain et al., 1980; Lindvall and Nilsson, 1984). However, none of the children with IGE were treated with phenytoin.
Dam and colleagues and others refocused interest on the role of seizures and seizure-induced hypoxia in the aetiology of cerebellar atrophy, marshalling both animal and human data to support this view (Dam, 1966, 1970; Dam and Nielsen, 1970; Dam et al., 1984; Nielsen et al., 1971). Further suggestion of direct seizure-related mechanisms came from investigations of patients with frequent partial epilepsies where hypoxia was uncommon, yet who exhibited cerebellar atrophy (Salcman et al., 1978; Ney et al., 1994). However, these children with IGE do not have particularly frequent or severe seizures.
Considered infrequently were the potential effects of initial aetiological insults and/or early neurodevelopmental factors on cerebellar development (Gessaga and Urich, 1985; Ney et al., 1994). Very few investigations of childhood epilepsy have examined the cerebellum (Lawson et al., 2000a, b), none in new/recent onset children and none in a prospective fashion. The baseline abnormalities in cerebellar structure, as well as the further abnormalities in cerebellar development, especially evident in the posterior inferior and anterior inferior cerebellar regions, were most notable in the children with IGE. More generally, the findings clearly indicate a neurodevelopmental contribution to this commonly noted anatomic abnormality.
Volumetric abnormalities of the thalamus have been reported in children and adults with IGE (for review see Pulsipher et al., 2009), but little has been known regarding the origin of this abnormality and its development over time. Studies of children with new onset epilepsy have indicated that abnormalities in thalamocortical connectivity are present early in the course of the disorder and here we show further prospective abnormalities in thalamic development. This early and then progressive change may underlie the thalamic abnormalities detected later in the course of IGE (Bernhardt et al., 2009). How thalamic abnormalities contributes to further alterations in cognition in children with IGE remains to be determined.
Localization-related epilepsies versus idiopathic generalized epilepsies
Abnormalities in both baseline and prospective brain development were clearly more evident in the children with IGE than LRE. This may be due to the fact that the LRE group was composed of children with a diversity of localization-related syndromes (frontal, temporal, and benign epilepsy of childhood with centrotemporal spikes), which may have obscured the developmental abnormalities inherent in any one group. It is important to mention that the findings in IGE group represent primarily juvenile myoclonic epilepsy as 14 of 20 children with IGE were diagnosed with juvenile myoclonic epilepsy. We are currently in the process of expanding the size of the cohort so that finer investigation of specific syndromes within LRE and IGE can be examined in detail. However, the inclusion of the LRE group suggests that the more diffuse abnormalities observed in children with IGE are likely not due to the non-specific effects of medications or episodic seizures. That said, it might be the case that the generalized nature of the epileptic process and seizures in IGE, as well as possible compensatory changes made in reaction to this form of epilepsy, may be contributing directly to the diffuse anatomic findings.
The effects of anti-epileptic drugs on human brain structure and neurodevelopment remain to be clarified. There are several studies in the animal literature that suggest anti-epileptic drugs may affect cell proliferation, differentiation, and migration, as well as other processes such as myelination and they may cause neurodegenerative changes in regions such as the thalamus (Ikonomidou and Turski, 2010). It is unknown how well this research translates to individuals beginning anti-epileptic drug treatment in late childhood and adolescence, as there is no literature indicating anti-epileptic drug-induced grey matter loss in children with post-natal only exposure to anti-epileptic drugs.
However, case reports have noted pseudoatrophy and/or encephalopathy in persons with epilepsy or other conditions (McLachlan, 1987; Guerrini et al., 1998; Galimberti et al., 2006; Abreu et al., 2009). However, these were all average IQ children at baseline and there is no cognitive decline in the children with IGE or other children at follow-up.
In IGE, involvement of the precentral gyrus might reflect source and inferior frontal and superior temporal spread of IGE (Holmes et al., 2010). Widespread involvement in IGE is commensurate with this being a ‘generalized’ seizure disorder. Spread in LRE appears to be more anterior and into the limbic system. Involvement of frontal and temporal regions in IGE and LRE, and parietal in IGE could help to explain cognitive and linguistic deficits, as well as attention deficits found in IGE and LRE. Thalamus and cerebellum also play an important role in attention, language, and cognition.
Conclusion
In summary, this combined baseline and prospective investigation of brain structure in children with idiopathic LRE and IGE reveals abnormalities in brain structure detected shortly after diagnosis—suggesting anomalies in brain development occurring prior to the diagnosis of epilepsy. Monitoring of trajectories of brain development over the 2 years following the diagnosis of epilepsy reveals abnormally affected cerebral white matter expansion, over and under reduction or ‘pruning’ in cortical grey matter volume, and abnormal developmental trajectories for important subcortical structures (thalamus), cerebellum, and other neural areas. Several potential limitations as well as opportunities for future research should be mentioned.
While abnormal prospective trajectories were detected, the interval of follow-up was modest (2 years). Longer follow-up would be of particular interest, especially with regard to whether some children with epilepsy might eventually ‘catch up’ with controls as has been described in some neurodevelopmental disorders (Shaw et al., 2007). The abnormalities described here occur in the context of active epilepsy (ongoing recurrent seizures or control using anti-epilepsy medications). Rates of remittance are known to be favourable in cohorts such as these (Berg et al., 2001) and longer term follow-up with examination of brain development in relation to cessation of seizures and medications may prove extremely informative. Such long-term outcomes would address the issue of whether these represent reversible maturational lags.
Second, significant changes are occurring in the cognitive development of children within the age range represented in this investigation (8–18 years). Very little is known regarding the relationship between normal neurodevelopmental alterations in grey and white matter change and how they are linked to specific improvements in mental status in epilepsy. One would anticipate a degree of symmetry between patterns of neurocognitive change and age-appropriate brain development and this area of research remains to be addressed in childhood epilepsy.
Long-term follow-up (30+ years) of children with uncomplicated epilepsy, including those no longer treated with medications, has shown that such patients do not do as well in several aspects of adult life performance compared to matched healthy controls (Sillanpaa et al., 2004). Perhaps these very early neurodevelopmental abnormalities, even if reversible in part, may place some limitations on the development of important life skills.
Finally, group analyses presented in this work aim to identify common regions of the brain where there are statistically significant abnormalities; however, in disease many regions of the brain are involved, and involvement is heterogeneous and differs among subjects. Therefore, subtle changes in multiple regions, which might not be significant on a group analysis, might interact with each other to produce significant clinical effects. Further studies using single-subject analysis techniques need to be pursued in order to capture such subject-specific morphometric abnormalities.
Funding
This work was supported by the National Institutes of Health (NIH) through the NIH Roadmap for Medical Research, Grant No. U54 RR021813, Center for Computational Biology (CCB). Additional support was provided by the National Institutes of Health (NIH)/National Center for Research Resources Grant No. P41 RR013642 and grants NS32070 (RC), MH 67187 (RC), and RO1 44352 (NINDS).
Glossary
Abbreviations
- IGE
idiopathic generalized epilepsies
- LRE
localization-related epilepsies
References
- Abreu L, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust N Z J Psychiatry. 2009;43:484–5. [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. Early development of intractable epilepsy in children: a prospective study. Neurology. 2001;56:1445–52. doi: 10.1212/wnl.56.11.1445. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage. 2009;46:373–81. doi: 10.1016/j.neuroimage.2009.01.055. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Li LM, Lopes-Cendes I, Guerreiro MM, Guerreiro CA, et al. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage. 2006a;32:498–502. doi: 10.1016/j.neuroimage.2006.04.174. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Lopes-Cendes I, Li LM, Guerreiro MM, Guerreiro CA, et al. MRI reveals structural abnormalities in patients with idiopathic generalized epilepsy. Neurology. 2006b;67:848–52. doi: 10.1212/01.wnl.0000233886.55203.bd. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Lopes-Cendes I, Li LM, Guerreiro MM, Guerreiro CA, et al. MRI volumetry shows increased anterior thalamic volumes in patients with absence seizures. Epilepsy Behav. 2006c;8:575–80. doi: 10.1016/j.yebeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57:688–95. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Sankar R, et al. Frontal and temporal volumes in Childhood Absence Epilepsy. Epilepsia. 2009a;50:2466–72. doi: 10.1111/j.1528-1167.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Levitt J. Language and fronto-temporal volumes in pediatric epilepsy. Epilepsia. 2009b;50:2466–72. doi: 10.1111/j.1528-1167.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Cormack F, Gadian DG, Vargha-Khadem F, Cross JH, Connelly A, Baldeweg T. Extra-hippocampal grey matter density abnormalities in paediatric mesial temporal sclerosis. Neuroimage. 2005;27:635–43. doi: 10.1016/j.neuroimage.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Daley M, Ott D, Blanton R, Siddarth P, Levitt J, Mormino E, et al. Hippocampal volume in childhood complex partial seizures. Epilepsy Res. 2006;72:57–66. doi: 10.1016/j.eplepsyres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Daley M, Siddarth P, Levitt J, Gurbani S, Shields WD, Sankar R, et al. Amygdala volume and psychopathology in childhood complex partial seizures. Epilepsy Behav. 2008;13:212–7. doi: 10.1016/j.yebeh.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam M. Organic changes in phenytoin-intoxicated pigs. Acta Neurol Scand. 1966;42:491–4. doi: 10.1111/j.1600-0404.1966.tb01200.x. [DOI] [PubMed] [Google Scholar]
- Dam M. Number of Purkinje cells in patients with grand mal epilepsy treated with diphenylhydantoin. Epilepsia. 1970;11:313–20. doi: 10.1111/j.1528-1157.1970.tb03895.x. [DOI] [PubMed] [Google Scholar]
- Dam M, Bolwig T, Hertz M, Bajorec J, Lomax P, Dam AM. Does seizure activity produce Purkinje cell loss? Epilepsia. 1984;25:747–51. doi: 10.1111/j.1528-1157.1984.tb03486.x. [DOI] [PubMed] [Google Scholar]
- Dam M, Nielsen M. Purkinje's cell density after diphenylhydantoin intoxication in rats. Arch Neurol. 1970;23:555–7. doi: 10.1001/archneur.1970.00480300077010. [DOI] [PubMed] [Google Scholar]
- de Araújo Filho GM, Jackowski AP, Lin K, Guaranha MSB, Guilhoto LMFF, da Silva HH, et al. Personality traits related to juvenile myoclonic epilepsy: MRI reveals prefrontal abnormalities through a voxel-based morphometry study. Epilepsy Behav. 2009;15:202–7. doi: 10.1016/j.yebeh.2009.03.011. [DOI] [PubMed] [Google Scholar]
- del Cerro M, Snider RS. Studies on dilantin intoxication. Neurology. 1967;17:452–66. doi: 10.1212/wnl.17.5.452. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Finkleman I, Arieff AJ. Untoward effects of phenytoin sodium in epilepsy. JAMA. 1942;118:1209–12. [Google Scholar]
- Galimberti CA, Diegoli M, Sartori I, Uggetti C, Brega A, Tartara A, et al. Brain pseudoatrophy and mental regression on valproate and a mitochondrial DNA mutation. Neurology. 2006;67:1715–7. doi: 10.1212/01.wnl.0000242882.58086.9a. [DOI] [PubMed] [Google Scholar]
- Gessaga EC, Urich H. The cerebellum of epileptics. Clin Neuropathol. 1985;4:238–45. [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, et al. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–70. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, et al. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proc Natl Acad Sci USA. 2008;105:15979–84. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–62. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Makki MI, Sundaram SK, Juhasz C, Chugani HT. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res. 2008;80:30–41. doi: 10.1016/j.eplepsyres.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–12. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Belmonte A, Canapicchi R, Casalini C, Perucca E. Reversible pseudoatrophy of the brain and mental deterioration associated with valproate treatment. Epilepsia. 1998;39:27–32. doi: 10.1111/j.1528-1157.1998.tb01270.x. [DOI] [PubMed] [Google Scholar]
- Guimaraes CA, Bonilha L, Franzon RC, Li LM, Cendes F, Guerreiro MM. Distribution of regional gray matter abnormalities in a pediatric population with temporal lobe epilepsy and correlation with neuropsychological performance. Epilepsy Behav. 2007;11:558–66. doi: 10.1016/j.yebeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Iftikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, et al. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Lin JJ, Jones JE, Seidenberg M. The emerging architecture of neuropsychological impairment in epilepsy. Neurologic Clinics. 2009;27:881–907. doi: 10.1016/j.ncl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, Hansen R, Seidenberg M, Magnotta V, O'Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003;18:284–92. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129 (Pt 10):2609–19. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, et al. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–71. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Dabbs K, Becker T, Jones JE, Myers y Gutierrez A, Wendt G, et al. Brain development in children with new onset epilepsy: A prospective controlled cohort investigation. Epilepsia. 2010;51:2038–46. doi: 10.1111/j.1528-1167.2010.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Quiring J, Tucker DM. Evidence that juvenile myoclonic epilepsy is a disorder of frontotemporal corticothalamic networks. Neuroimage. 2010;49:80–93. doi: 10.1016/j.neuroimage.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Honavar M, Meldrum B. Epilepsy. In: Graham DI, Lantos PL, editors. Greenfield's neuropathology. London: Edward Arnold; 2002. pp. 899–941. [Google Scholar]
- Hua X, Leow AD, Levitt JG, Caplan R, Thompson PM, Toga AW. Detecting brain growth patterns in normal children using tensor-based morphometry. Hum Brain Mapp. 2009;30:209–19. doi: 10.1002/hbm.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Pulsipher D, Dabbs K, Gutierrez AMy, Sheth R, Jones J, et al. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2010;88:208–14. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Antiepileptic drugs and brain development. Epilepsy Res. 2010;88:11–22. doi: 10.1016/j.eplepsyres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23(Suppl 1):S151–60. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JK, Koh SB, Lee SA, Lee JM, Kim SI, et al. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. Neuroimage. 2007;37:1132–7. doi: 10.1016/j.neuroimage.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, et al. An optimized individual target brain in the Talairach coordinate system. Neuroimage. 2002;17:922–7. [PubMed] [Google Scholar]
- Kokenge R, Kutt H, McDowell F. Neurological sequelae following Dilantin overdose in a patient and in experimental animals. Neurology. 1965;15:823–9. doi: 10.1212/wnl.15.9.823. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Cook MJ, Bleasel AF, Nayanar V, Morris KF, Bye AM. Quantitative MRI in outpatient childhood epilepsy. Epilepsia. 1997;38:1289–93. doi: 10.1111/j.1528-1157.1997.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Nguyen W, Bleasel AF, Pereira JK, Vogrin S, Cook MJ, et al. ILAE-defined epilepsy syndromes in children: correlation with quantitative MRI. Epilepsia. 1998;39:1345–9. doi: 10.1111/j.1528-1157.1998.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Burns L, McAnally L, et al. Predictors of hippocampal, cerebral, and cerebellar volume reduction in childhood epilepsy. Epilepsia. 2000a;41:1540–5. doi: 10.1111/j.1499-1654.2000.001540.x. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Bye AM. Cerebral and cerebellar volume reduction in children with intractable epilepsy. Epilepsia. 2000b;41:1456–62. doi: 10.1111/j.1528-1157.2000.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Lazaro L, Bargallo N, Castro-Fornieles J, Falcon C, Andres S, Calvo R, et al. Brain changes in children and adolescents with obsessive-compulsive disorder before and after treatment: a voxel-based morphometric MRI study. Psychiatry Res. 2009;172:140–6. doi: 10.1016/j.pscychresns.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leow AD, Klunder AD, Jack CR, Jr, Toga AW, Dale AM, Bernstein MA, et al. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. Neuroimage. 2006;31:627–40. doi: 10.1016/j.neuroimage.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Nilsson B. Cerebellar atrophy following phenytoin intoxication. Ann Neurol. 1984;16:258–60. doi: 10.1002/ana.410160218. [DOI] [PubMed] [Google Scholar]
- Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:1233–51. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan R. Pseudoatrophy of the brain with valproic acid monotherapy. Can J Neurol Sci. 1987 doi: 10.1017/s0317167100026640. [DOI] [PubMed] [Google Scholar]
- McLain LW, Martin JT, Allen JH. Cerebellar degeneration due to chronic phenytoin therapy. Ann Neurol. 1980;7:18–23. doi: 10.1002/ana.410070106. [DOI] [PubMed] [Google Scholar]
- Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–11. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney GC, Lantos G, Barr WB, Schaul N. Cerebellar atrophy in patients with long-term phenytoin exposure and epilepsy. Arch Neurol. 1994;51:767–71. doi: 10.1001/archneur.1994.00540200043014. [DOI] [PubMed] [Google Scholar]
- Nielsen MH, Dam M, Klinken L. The ultrastructure of Purkinje cells in diphenylhydantoin intoxicated rats. Exp Brain Res. 1971;12:447–56. doi: 10.1007/BF00234242. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Go C, Rutka JT, Rydenhag B, Mabbott DJ, Snead OC, 3rd, et al. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy Res. 2008;81:128–35. doi: 10.1016/j.eplepsyres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. Neuroimage. 2008;42:611–6. doi: 10.1016/j.neuroimage.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–11. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–47. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Pulsipher DT, Dabbs K, Tuchsherer V, Sheth R, Koehn MA, Hermann BP, et al. Thalamofrontal neurodevelopment in new-onset pediatric idiopathic generalized epilepsy. Neurology. 2011;76:28–33. doi: 10.1212/WNL.0b013e318203e8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher DT, Seidenberg M, Guidotti L, Tuchscherer VN, Morton J, Sheth RD, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009;50:1210–19. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–54. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, et al. Altered white matter integrity in temporal lobe epilepsy: Association with cognitive and clinical profiles. Epilepsia. 2010;51(4):536–45. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcman M, Defendini R, Correll J, Gilman S. Neuropathological changes in cerebellar biopsies of epileptic patients. Ann Neurol. 1978 doi: 10.1002/ana.410030104. Jan;3(1):10-9. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, et al. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46:420–30. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–42. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–54. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–9. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Pellock JM. Update on the epidemiology and prognosis of pediatric epilepsy. J Child Neurol. 2002;17(Suppl 1):S4–17. doi: 10.1177/08830738020170010201. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M, Haataja L, Shinnar S. Perceived impact of childhood-onset epilepsy on quality of life as an adult. Epilepsia. 2004;45:971–7. doi: 10.1111/j.0013-9580.2004.44203.x. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imag. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–73. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Blumenfeld R, Schuff N, Rosen HJ, Miller B, et al. Deformation tensor morphometry of semantic dementia with quantitative validation. NeuroImage. 2004;21:1387–98. doi: 10.1016/j.neuroimage.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Studholme C, Drapaca C, Iordanova B, Cardenas V. Deformation-based mapping of volume change from serial brain MRI in the presence of local tissue contrast change. Med Imag. IEEE Trans. 2006;25:626–39. doi: 10.1109/TMI.2006.872745. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Dlugos D. Idiopathic generalized epilepsy. Curr Treat Opt Neurol. 2004;6:231–42. doi: 10.1007/s11940-004-0015-6. [DOI] [PubMed] [Google Scholar]
- Tae WS, Hong SB, Joo EY, Han SJ, Cho JW, Seo DW, et al. Structural brain abnormalities in juvenile myoclonic epilepsy patients: volumetry and voxel-based morphometry. Korean J Radiol. 2006;7:162–72. doi: 10.3348/kjr.2006.7.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Kim SH, Joo EY, Han SJ, Kim IY, Kim SI, et al. Cortical thickness abnormality in juvenile myoclonic epilepsy. J Neurol. 2008;255:561–6. doi: 10.1007/s00415-008-0745-6. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–8. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–59. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utterback RA, Ojeman R, Malek J. Parenchymatous cerebellar degeneration with Dilantin intoxication. J Neuropathol Exp Neurol. 1958;17:516–9. [Google Scholar]
- van't Ent D, Lehn H, Derks EM, Hudziak JJ, Van Strien NM, Veltman DJ, et al. A structural MRI study in monozygotic twins concordant or discordant for attention/hyperactivity problems: evidence for genetic and environmental heterogeneity in the developing brain. NeuroImage. 2007;35:1004–20. doi: 10.1016/j.neuroimage.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, et al. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64:709–17. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- Weber B, Luders E, Faber J, Richter S, Quesada CM, Urbach H, et al. Distinct regional atrophy in the corpus callosum of patients with temporal lobe epilepsy. Brain. 2007;130:3149–54. doi: 10.1093/brain/awm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Krageloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki. J Law Med Ethics. 1991;19:264–5. [PubMed] [Google Scholar]