Abstract
This review summarizes the work from our laboratory investigating mechanisms of opioid analgesia using the Northern grass frog, Rana pipiens. Over the last dozen years, we have accumulated data on the characterization of behavioral effects after opioid administration on radioligand binding by using opioid agonist and antagonist ligands in amphibian brain and spinal cord homogenates, and by cloning and sequencing opioid-like receptor cDNA from amphibian central nervous system (CNS) tissues. The relative analgesic potency of mu, delta, and kappa opioids is highly correlated between frogs and other mammals, including humans. Radioligand binding studies using selective opioid agonists show a similar selectivity profile in amphibians and mammals. In contrast, opioid antagonists that are highly selective for mammalian mu, delta, and kappa opioid receptors were not selective in behavioral and binding studies in amphibians. Three opioid-like receptor cDNAs were cloned and sequenced from amphibian brain tissues and are orthologs to mammalian mu, delta, and kappa opioid receptors. Bioinformatics analysis of the three types of opioid receptor cDNAs from all vertebrate species with full datasets gave a pattern of the molecular evolution of opioid receptors marked by the divergence of mu, delta, and kappa opioid receptor sequences during vertebrate evolution. This divergence in receptor amino acid sequence in later-evolved vertebrates underlies the hypothesis that opioid receptors are more type-selective in mammals than in nonmammalian vertebrates. The apparent order of receptor type evolution is kappa, then delta, and, most recently, the mu opioid receptor. Finally, novel bioinformatics analyses suggest that conserved extracellular receptor domains determine the type selectivity of vertebrate opioid receptors.
Keywords: Bioinformatics, Analgesia, Selectivity, Domain
1. Introduction
The reasons for using amphibians in opioid research are varied and numerous. They range from philosophical and ethical considerations, to scientific and comparative aspects, to economic and aesthetic issues. The use of an amphibian model for biomedical research is also a matter of personal choice, reflecting a pioneering spirit and supports a diversity of biological models in the face of decreased research funding and increased emphasis on mammalian models. The goal of this review is to briefly summarize nociceptive pathways in amphibians and provide an overview of our work on developing an alternative model of opioid and pain research using amphibians. The last half of the review summarizes the results of cloning and sequencing opioid receptors in amphibians and bioinformatics analysis that lead to novel hypotheses on the molecular evolution of vertebrate opioid receptors.
2. Nociception and pain in amphibians
The first issue to be considered in talking about a nonmammalian model for pain and opioid research is one of semantics. The word pain, in its strictest sense, is not correctly applied to nonhuman animals. Pain refers to a complex perceptual and emotional experience in humans that has a prominent subjective component [18,22]. Purists would refer to what we commonly call pain in animals as nociception and balk at using terms like pain and analgesia with animal models. Nociception is used to describe the transmission of noxious stimuli and subsequent processing up to the point in the brain whereby the pain experience, if present, is mediated. However, for ease of use and to make research findings accessible to a general audience, the term pain is generally used instead of nociception, and analgesia is used instead of antinociception. This is not a trivial point, as our attitudes about animals are reflected by the language we use in describing them [5]. Pain researchers, in an attempt to use less jargon, have accepted this less-than-precise usage of the word pain, but this does not necessarily imply that these researchers believe that the animals they use experience pain as we do. It also does not mean that researchers using nonhuman animals believe that animals do not feel pain. In this regard, we must remain agnostic, using the best available science to guide our judgment. The terms pain and analgesia are used here for convenience and, in doing so, make no assumptions as to the capacity of nonmammalian animals to experience pain.
Nerve endings that transduce noxious stimuli are called nociceptors. In contrast to the variety of specialized end organs on mammalian afferent fibers, only three groups of afferent nerve endings have been identified in amphibians. These include one type of expanded tip ending primarily located in the superficial level of the dermis and two groups of free nerve endings: one in the epidermis, the other in the deeper layers of the dermis [12,39,46]. Nociception is most readily associated with the free nerve endings of the deeper dermis, which primarily transduce temperature. It is likely that, under normal physiological conditions, these nerve endings also transduce noxious mechanical stimuli, such as pinching and pin pricks [74]. Early electrophysiological work suggested that the detection of all noxious sensory stimuli, including chemical stimuli, was mediated exclusively by the same free nerve endings associated with thermal sensitivity in amphibians [1].
Amphibians, like mammals, possess both myelinated and unmyelinated afferent fibers running concurrently in mixed-fiber peripheral sensory nerves. These afferent fibers have been delineated into three general classes based on morphology, conduction velocities, latency of response, and action potential characteristics: large, heavily myelinated A fibers; small, thinly myelinated B; and small, unmyelinated fibers C fibers. Their characteristics correlate well with those observed in corresponding classes of somatic sensory afferents in mammals: Aβ, Aδ, and C fibers, respectively [61]. In studies that separated fibers both by conduction velocity and fiber diameter in amphibians, small, slowly conducting fibers transmitted the majority of all impulses induced by noxious heat, pinching, pin pricks, and the application of dilute acetic acid to the skin [1,25]. A recent study closely examined the afferent fibers excited in the acetic acid test (AAT; see below) and found that concentrations producing the behavioural response to the cutaneously applied acid solution evoked both Aβ and C fibers to an equal extent [15].
There is a controversy over the central terminations of primary afferent fibers within the amphibian spinal gray matter [33], although most studies indicate that sensory afferents from the skin terminate in areas of the dorsal field of the frog spinal cord that correspond to mammalian laminae I–IV. Some studies describe small-diameter primary afferent fibers that carry information of a noxious nature, terminating primarily within the dorsal field and, to a lesser extent, in the central field surrounding the central canal [33]. Other studies suggest that the end branches of small caliber fibers associated with nociception terminate exclusively in the superficial dorsal laminae (the substantia gelatinosa in mammals), while only larger fibers associated with transmission of nonnoxious information penetrate to the deeper laminae [62]. Electrophysiological evidence supports the existence of primary afferent synapses on motoneuron dendrites within the amphibian dorsal horn. It appears that in more recently evolved vertebrates, primary afferent fibers have become more restricted to the superficial laminae of the spinal dorsal horn [20]. It is not known if this is the case for specific classes of primary afferents (e.g., nociceptive afferent fibers) throughout vertebrate phylogeny.
The primary afferent nerve fibers release pain signaling neurotransmitters such as substance P, calcitonin gene-related peptide, and glutamate in mammals. Using immunohistochemical techniques, these substances are readily identified in abundance in the spinal dorsal horn of amphibians [19,24]. In mammals, substance P and glutamate excite second-order neurons, which have their cell bodies in the dorsal horn and send long fibers upward to form the ascending pain pathway. Such second-order neurons within the dorsal horn that receive direct primary afferent input have not been identified in amphibians [44]. Also present in the mammalian spinal cord are endorphinergic neurons that release metenkephalin, an endogenous opioid peptide that acts presynaptically to inhibit the release of substance P and postsynaptically to decrease the firing of the second-order pain neurons. Likewise, metenkephalin, substance P, and other putative pain transmitters, as well as opioid binding sites, are noted in the amphibian spinal cord [47,55]. Additionally, using in situ hybridization to identify neurons expressing mRNA for metenkephalin, endorphinergic neurons have been identified throughout the brain and spinal cord of Rana pipiens [41]. Thus, while the most detailed studies have only been performed in mammals, the existence of these key pain neurotransmitters and endogenous opioid peptides suggests that basic mechanisms of pain transmission within the spinal cord are common in both amphibians and mammals. Ascending pathways carrying nociceptive information from the spinal cord to the brain in vertebrates are considered in Section 3.
3. Comparative neurology of pain
There is a critical need for data from studies of comparative neurology to help resolve issues of animal welfare and the potential for pain [8]. Nociceptive information in vertebrates is parallel-processed throughout the nervous system, with separate pathways for the sensory–discriminative and motivational–affective aspects of pain. At present, there are only adequate neurological data of nociceptive transmission in mammals [16,73], but insofar as nociceptive pathways are contained within sensory tracts in lower vertebrates, an examination of ascending sensory tracts and their brain connections can provide an initial basis for comparison. For example, as outlined above, nociceptors in the skin and afferent fibers, which transmit nociceptive information to the spinal cord, are identified in amphibians, but the location of second-order neurons in the spinal cord and the route of their fibers that carry this information to the brain are unknown [46]. Functional studies of evoked responses in supraspinal sites after specific noxious stimulation of peripheral fibers are lacking. A single study found that electrical stimulation of the sciatic nerve produces evoked potentials in posterior thalamic nuclei and primordial hippocampal structures in frogs [67]. The following neuroanatomical description is a simplification of the findings from a number of comparative studies [10,17,21,34].
The sensory–discriminative pathway originating from sensory tracts from spinal neurons makes direct connections with neuron groups farther towards the front of the brain in correlation with the phylogeny of vertebrates. For example, fish and amphibians have direct spinal connections to the brainstem; reptiles and birds to the brainstem and dorsal thalamus in the midbrain; and mammals to the brainstem, thalamus, and primary cortex. Pathways that appear to contribute to the motivational–affective dimension of pain follow a similar evolutionary pattern, except that more medial target sites are contacted in the brain: the medial thalamic nuclei in amphibians and the limbic cortex in mammals. Throughout phylogeny, all the target sites of spinal tracts in the brain increase in complexity, specialization, and number of neurons, suggesting that even nociceptive messages to the thalamus may not be comparable in amphibians and mammals.
Comparing amphibian and mammalian brains, there are significant differences in both the discriminative and affective pathways. While sensory–discriminative information from the spinal cord does indirectly reach the dorsal thalamus in frogs by way of the brainstem–thalamus tract, amphibian thalamic nuclei are diffuse and are not entirely organized as they are in mammals. In amphibians, there are only scant fibers coursing from the thalamus to an unspecialized area of the forebrain olfactory lobe, whereas thalamic projections to cerebral cortex in mammals are large and prominent. There is no strong thalamus-to-cortex connection in the frog because the cerebral cortex, which evolves from the olfactory (telencephalic) area, does not appear until class Reptilia. Even this primordial cortex in reptiles is primitive and lacks the complex laminar structure seen in mammals. Amphibians simply have a brain without a cerebral cortex. The phylogenetic development of the medial pathway correlated with motivational–affective aspects of pain is similar to the discriminative pathway such that, in amphibians, the most rostral projection reaches to a diffuse olfactory area with little organization of neurons. In mammals, the most rostral target of this pathway is the highly organized limbic cortex. Again, the beginning of even a rough laminar organization of the limbic area does not appear until class Reptilia. The amphibian brain also does not contain a limbic cortex.
Cortical tissue, whether in limbic or cerebral regions, is a highly complex and laminated structure, which is a relatively recent development in the evolution of the vertebrate nervous system. We know from human experience that decreasing the activity of cortical neurons by anesthesia or surgical lesion results in a loss of the full appreciation of pain or nociception; the patient reports an awareness of pain “but it no longer bothers them” [69]. Recent studies using positron imaging techniques also show specific areas of the cortex activated by noxious stimuli in awake humans [64]. For these reasons, there is widespread agreement among various scientific organizations that an intact cortex is needed for the appreciation of pain [2,45,66]. It is likely that amphibians, without either a cerebral or limbic cortex, have a vastly diminished potential for the appreciation of pain. As noted above, even the most rudimentary cerebral and limbic cortex does not appear until class Reptilia. In this sense, the use of amphibians may represent a ‘purer’ model system for the study of nociception, possibly without the additional factors of learning and conditioning, which can interfere with the accurate measurement of analgesia in mammals [3].
4. Behavioural assay of nociceptive thresholds (NTs) in amphibians
The Northern grass frog or leopard frog (R. pipiens) is available from commercial suppliers where they have been previously collected from Minnesota, Wisconsin, or more rarely from Northeastern states in the United States. Animals with a snout vent length of 5–7 cm (about 25–35 g of body weight) are used, as this size is generally available on a year-round basis. Upon arrival, animals are placed in groups of 48 in large terrestrial/aquatic enclosures with free-flowing water and a 12-h light cycle. Animals are fed three times a week with live crickets, also obtained from a commercial source. At least 2 days before the start of an experiment, frogs are transported into the laboratory and randomly assigned to individual plastic cages with soft mesh lids for acclimatization to laboratory conditions. Animals are generally used only once, although pilot experiments are carried out in recycled animals. Frogs cost about one-sixth the price of rats and about one-fourth the price of mice, and per-diem charges are not incurred or are minimal. R. pipiens are a robust and healthy species with little susceptibility to disease. Unlike rodent and other mammalian species, there are no known zoonoses that may affect researchers handling amphibians, as well as no danger of bites or scratches. There are no reported cases of allergies to amphibians—a problem noted to occur among labworkers handling rodents [11]. Amphibians in the laboratory do not emit odor, and fecal material is minimal and easily washed away with the changing of the cage water. Finally, R. pipiens in the laboratory setting are aesthetically pleasing with varied and bright coloration, and melodic vocalizations in the evening.
The author’s Master’s thesis advisor, Dr. Paul D. Pezalla, first developed a method to assess NT in frogs using dilute concentrations of cutaneously applied acetic acid [35]. The AAT used to determine NT in frogs consists of 11 concentrations of acetic acid serially diluted from glacial acetic acid (two parts acid:one part water). The concentrations are given a code number from 0 to 10, with the lowest code number equal to the lowest concentration of acetic acid. The actual molar concentration of acetic acid per each solution is given by the equation:
Nociceptive testing is done by placing, with a Pasteur pipette, a single drop of acid on the dorsal surface of the frog’s thigh. Testing begins with the lowest concentration and proceeds with increasing concentrations until the NT is reached. The NT is defined as the code number of the lowest concentration of acid that causes the frog to vigorously wipe the treated leg with either hindlimb. To prevent tissue damage, the acetic acid is immediately wiped off with a gentle stream of distilled water once the animal responds, or after 5 s if the animal fails to respond. If the animal fails to respond, testing continues on the opposite hindlimb. An animal that fails to respond to the highest concentration (no. 10) is assigned the cutoff value of 11. The wiping response in frogs, like the tail flick in rodents, remains intact after a high spinal transection, demonstrating sufficient circuitry in the spinal cord to mediate this behavior [13]. This response has not been observed in the laboratory in the absence of noxious stimuli and appears specific for assessing nociception. The wiping response is also the basis for much research on the motor systems of the amphibian spinal cord [14].
The AAT uses a brief and mild noxious stimulus, with stimulus control possible by use of the experimental animal. Stimulus control in this sense means that the animal terminates the noxious stimulus by exhibiting a behavior that signals nociception to the investigator and, in this case, the dilute acid is rinsed immediately by a stream of water. The AAT measures the behavioral response to an acute chemical noxious stimulus. The comparable test in rodents is the writhing test (also called the abdominal constriction test) whereby chemical irritants are injected into the intraperitoneal cavity and the number of abdominal constrictions (writhes) is counted over a given interval, usually 30 min. However, due to the lack of stimulus control by the animal, the writhing test may not be sanctioned by pain research guidelines [76].
5. Opioid analgesia in amphibians
Initial studies of the analgesic effects of opioid administration in amphibians were conducted using nonselective opioid agonists, endogenous opioid peptides, and antagonists [36,56–58]. These studies showed that both exogenous opioid agonists and endogenous opioid peptides could raise the NT in amphibians by an action at an opioid receptor. Tolerance to the analgesic effects of daily morphine administration was documented [52] and stress-induced release of endogenous opioids was shown to produce analgesia, which was potentiated by enkephalinase inhibitors [60]. Other behavioral studies include an investigation of the effects of opioids on noxious and nonnoxious sensory modalities [70,71], and an examination of agents acting on alpha2 adrenergic receptors after systemic and spinal administration [7,51].
Later results of systematic studies examining the antinociception of selective mu, delta, or kappa opioid agonists administered by different routes yielded an important finding: The relative antinociceptive potency of mu, delta, or kappa opioid agonists after systemic, intraspinal, or intracerebroventricular administration in amphibians was highly correlated to that observed in typical mammalian models and to the relative analgesic potency of opioid analgesics in human clinical studies [50,53,59]. This data established the amphibian model as a robust and predictive adjunct or alternative model for the testing of opioid analgesics [48,49]. Table 1 provides a summary of the agonists used in behavioral studies of opioid analgesia following systemic, spinal, and supraspinal routes of administration.
Table 1.
Characteristics of opioid agonists assessed with the AAT in amphibians
| Opioid agonist | Type | Routea | Dosage rangeb | ED50c |
|---|---|---|---|---|
| Fentanyl | Mu | s.c. | 0.1–30 | 1.4 |
| CI-977 (Enadoline) | Kappa | s.c. | 1–30 | 5.8 |
| Levorphanol | Mu | s.c. | 1–100 | 7.5 |
| U50488H | Kappa | s.c. | 1–100 | 8.5 |
| Methadone | Mu | s.c. | 10–300 | 19.9 |
| Bremazocine | Kappa | s.c. | 10–100 | 44.4 |
| Morphine | Mu | s.c. | 10–300 | 86.3 |
| Buprenorphine | Mu | s.c. | 30–300 | 99.1 |
| Meperidine | Mu | s.c. | 30–1000 | 128.1 |
| Codeine | Mu | s.c. | 10–1000 | 140.3 |
| Nalorphine | Kappa | s.c. | 100–300 | 320.9 |
| Dermorphin | Mu | i.s. | 0.003–0.3 | 0.04 |
| DAMGO | Mu | i.s. | 0.03–1 | 0.13 |
| DSLET | Delta | i.s. | 0.01–3 | 0.13 |
| DADLE | Delta/mu | i.s. | 0.03–3 | 0.14 |
| Fentanyl | Mu | i.s. | 0.3–10 | 0.94 |
| Morphine | Mu | i.s. | 0.3–10 | 2.26 |
| DPDPE | Delta | i.s. | 1–10 | 3.29 |
| Deltorphin | Delta | i.s. | 0.3–100 | 13.50 |
| CI-977 (Enadoline) | Kappa | i.s. | 0.3–30 | 13.51 |
| Bremazocine | Kappa | i.s. | 3–100 | 22.89 |
| U50488H | Kappa | i.s. | 3–100 | 36.82 |
| Nalorphine | Kappa | i.s. | 3–100 | 43.13 |
| DADLE | Delta/mu | i.c.v. | 0.1–10 | 1.3 |
| Morphine | Mu | i.c.v. | 0.1–10 | 2.0 |
| DPDPE | Delta | i.c.v. | 1–30 | 8.3 |
| Fentanyl | Mu | i.c.v. | 0.3–100 | 23.0 |
| CI-977 (Enadoline) | Kappa | i.c.v. | 3–100 | 35.5 |
| U50488H | Kappa | i.c.v. | 6–100 | 61.5 |
Route of administration: s.c.= subcutaneous; i.s.= intraspinal; i.c.v.= intracerebroventricular (data from Refs. [50,53,59]).
Effective dosage range: in nanomoles per gram for s.c. administration; in nanomoles per frog for i.s. and i.c.v. administration.
Dose that gives 50% analgesic effects: in nanomoles per gram for s.c. administration; in nanomoles per frog for i.s. and i.c.v. administration.
The most common highly selective opioid antagonists used in mammalian studies are β-funaltrexamine (β-FNA; for mu) [37], naltrindole (NTI; for delta) [63], and nor-binaltorphimine (nor-BNI; for kappa) [68]. However, the lack of selectivity of these antagonists was shown in initial behavioral studies using R. pipiens [54]. After spinal administration, each of the highly selective mu, delta, or kappa opioid antagonists (β-FNA, NTI, and nor-BNI, respectively) decreased the antinociceptive effects produced by all three types of selective mu, delta, or kappa opioid agonists in amphibians. It was hypothesized that only one type of opioid receptor mediated the antinociceptive effects of mu-, kappa-, and delta-selective opioids, called the opioid unireceptor [54]. As discussed below, more recent radio-ligand binding and molecular cloning studies suggest that all three types of opioid receptors are expressed in amphibian central nervous system (CNS), but that these opioid receptors may be less selective than those found in mammals.
6. Opioid binding sites in amphibian brain and spinal cord
The first studies involving opioid receptor purification used amphibian brain tissue, as it was recognized to be a rich source of opioid receptors [43]. More recent biochemical and receptor isolation studies using amphibian brain tissues demonstrate a high proportion of opioid receptors compared to mammals [6,43,72,75]. These studies show that amphibian brain expresses mostly kappa-like opioid binding sites, as the competitive binding profile was most correlated to the selectivity profile of the mammalian kappa opioid receptor. The main difference observed was a greater affinity of mu- and delta-selective opioids for the amphibian kappa-like site, and a lesser affinity for kappa-selective opioids, compared to mammalian kappa opioid receptors [6,27,55]. These results provided the first hint from radio-ligand studies that opioid receptors in amphibians may be less selective than their mammalian counterparts.
In recent studies from our laboratory, the nonselective opioid ligand [3H]diprenorphine bound to R. pipiens brain tissue homogenates with a Kd value of 0.65 nM and a Bmax value of 287.7 fmol/mg protein [30]. Using the nonselective opioid antagonist [3H]naloxone, binding studies using brain and spinal cord membranes from R. pipiens showed a single high-affinity site (Kd value of 7.1 nM in the brain), which was displaced by mu-, delta-, and kappa-selective opioid agonists with apparent affinities ranging from 1.86 nM to 31 μM. Surprisingly, the highly selective opioid antagonists (β-FNA, nor-BNI, and NTI) displaced [3H]naloxone binding with equal affinity to opioid receptors in brain and spinal cord tissues, each with a Ki of about 3.0 nM [31,32]. This finding was consistent with behavioral studies showing nonselectivity of these ‘selective’ antagonists and also supports the hypothesis that opioid receptors from earlier-evolved vertebrates are less selective than mammalian receptors (see below). Upon examination of Ki values, the overall trend of [3H]naloxone displacement in R. pipiens shows a rank potency of mu>delta≥kappa opioids. This activity profile is consistent with the relative affinity of naloxone for mammalian mu, delta, and kappa opioid receptors [38,42] as well as the observed relative potency of opioid analgesia in amphibians [50,53,59].
Most recently, using the selective opioid agonist radio-ligands [3H]DAMGO (mu), [3H]U65953 (kappa), and [3H]DPDPE (delta), three distinct opioid binding sites were identified based on different binding densities and selective competitive displacement of agonist radioligand by mu, delta, and kappa opioid ligands [29]. With agonist radioligand binding, selectivity was observed such that cognate ligands were potent displacers of the selective agonist radioligand and the rank order of mu, delta, and kappa selectivity was similar to that observed in mammals. As opposed to their equipotent displacement of [3H]naloxone binding, the highly selective opioid antagonists (β-FNA, nor-BNI, and NTI) were highly selective in displacing the binding of selective mu, delta, and kappa opioid agonist radioligands [29].
7. Cloning of amphibian opioid receptors
The number of full-length cDNA sequences for opioid receptors in nonmammalian vertebrates deposited in Gen-Bank is surprisingly small. At present, there are no databank sequences for any type of opioid receptor from any species of Reptilia or Aves. From class Pisces, there was an initial entry of a mu opioid-like receptor sequences expressed in the white suckerfish Castomus commersoni [9] and, more recently, the triad of mu, delta, and kappa opioid-like receptor cDNAs was cloned from the zebrafish Danio rerio [4,40]. The cloning of full-length opioid-like receptors in R. pipiens was particularly needed given that there is a corresponding dataset of opioid behavioral and binding data. Other nonmammalian species do not have a corresponding dataset of opioid binding and behavioral studies to integrate molecular cloning and in vitro results with whole animal behavioural data on opioid analgesia.
As fully detailed elsewhere (Stevens and Brasel, in preparation), three full-length clones of opioid-like receptors were obtained by reverse transcription polymerase chain reaction (RT-PCR) amplification of R. pipiens brain and spinal cord cDNA. On the basis of high homologies to existing vertebrate sequences, these clones were named rpMOR (GenBank accession no. AF530571), rpDOR (GenBank accession no. AF530572), and rpKOR (GenBank accession no. AF530573) as novel orthologs to mammalian mu, delta, and kappa opioid receptors. The percent identity of amino acids in rpMOR compared to the zebrafish mu opioid-like receptor was 79%, and ranges from 83% to 84% compared to mammalian mu opioid receptors. The delta opioid-like receptor rpDOR had 77% identity with the zebrafish ortholog and 73% identity compared to mammalian delta opioid receptors. Mammalian kappa opioid receptors were 70–71% identical to rpKOR and the zebra-fish kappa opioid-like receptor was 64% identical to rpKOR. Comparable phylogenetic homology was noted when similarity values were compared (Stevens and Brasel, in preparation).
8. Bioinformatics of vertebrate opioid-like receptors
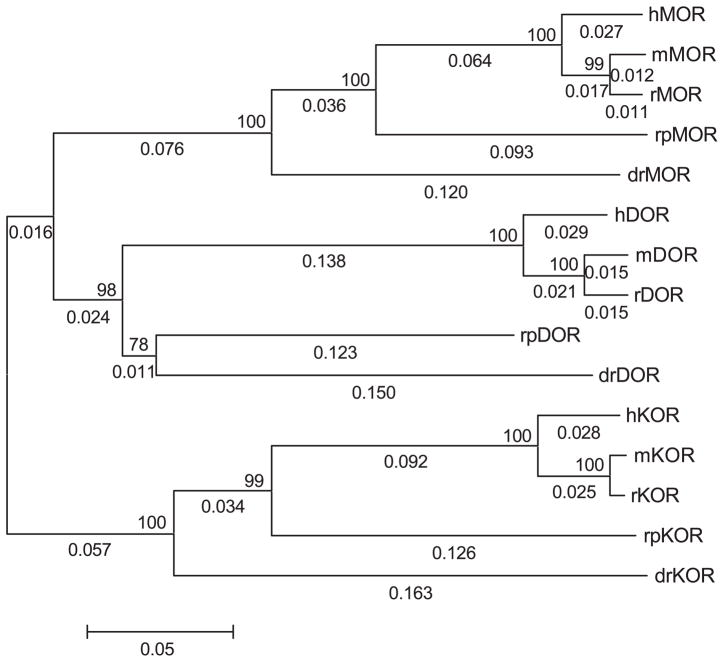
The bioinformatics analysis of the amphibian opioid-like receptor used existing matched datasets of mu, delta, and kappa opioid receptors from other vertebrate species. Somewhat surprising, there are only four vertebrate species, excluding the amphibian data, which have full-length sequences for all three types of opioid receptor cDNA deposited in GenBank. These species are the zebrafish (D. rerio), mouse (Mus muscularis), rat (Rattus norvigecus), and human (Homo sapiens). Phylogenetic dendrograms (trees) were constructed using existing alignment (CLUS-TALW) and molecular evolution (MEGA2) software. The nearest-neighbor joining trees for each opioid receptor type gave the accepted evolutionary relationship (see Fig. 1). As shown, the three groups of opioid receptor types did not arise from a single, common ancestral sequence, but rather the group of kappa opioid receptors shared a common ancestral sequence to the branch bifurcating later to the mu and delta opioid receptor groups (Fig. 1).
Fig. 1.
Molecular evolution of vertebrate opioid receptors. Phylogenetic dendrogram (tree) was generated using the neighbor-joining method. Numbers above internal branches represent the bootstrap value (percentage giving the same topology at that node with 1000 bootstrap runs). Numbers along branches are branch length. Scale bar shows length in proportional difference (e.g., 0.05 equals 5% difference or divergence in amino acid sequence). Prefixes refer to the animal species: h=human; r=rat; m=mouse; rp=R. pipiens (frog); and dr=D. rerio (fish). MOR is the mu opioid receptor, DOR is delta opioid receptor, and KOR is the kappa opioid receptor.
The results of pairwise BLAST analysis for the three opioid-like receptor sequences within each species yielded a rank order of divergence such that in earlier-evolved vertebrates, mu, delta, and kappa opioid-like sequences were more closely related to each other than in humans and other mammals. For the percent similarity comparison, these values reached statistical difference such that the mean values of similarity of mu, delta, and kappa opioid-like receptors in nonmammals were greater than the similarity of mu, delta, and kappa opioid receptors in mammals (Table 2). Insofar as divergence of molecular sequence is related to the greater type selectivity of opioid receptors, this finding gives rise to the novel hypothesis that opioid receptors are more type-selective in mammals than in nonmammalian species.
Table 2.
Homology of MOR, DOR, and KOR receptors within different vertebrate speciesa
| Species | MOR vs. DOR | MOR vs. KOR | DOR vs. KOR | Mean (S.E.M.)b |
|---|---|---|---|---|
| Identity | ||||
| Zebrafish | 68 | 62 | 68 | 66.0 (2.0) |
| Frog | 73 | 65 | 63 | 67.0 (3.1) |
| Rat | 66 | 61 | 61 | 62.7 (1.7) |
| Mouse | 61 | 60 | 61 | 60.7 (0.3) |
| Human | 62 | 60 | 59 | 60.3 (0.9) |
| Similarity | ||||
| Zebrafish | 80 | 78 | 82 | 80.0 (1.2)** |
| Frog | 85 | 81 | 77 | 81.0 (2.3)* |
| Rat | 76 | 75 | 74 | 75.0 (0.6) |
| Mouse | 71 | 75 | 73 | 73.0 (1.2) |
| Human | 73 | 73 | 72 | 72.7 (0.3) |
Percent identity and similarity determined by BLAST-P (version 2.2.4) at NCBI website (WWW.NCBI.NLM.NIH.GOV/BLAST) settings: matrix=Blossum62, gap open=11, gap extension=1, x dropoff=50, expect=10.00, wordsize=3, and filter off (from Stevens and Brasel, in preparation).
Standard error of the mean.
Significantly different than rat, mouse, and human mean values (p<0.05, one-way ANOVA followed by post-hoc Newman–Kuels test).
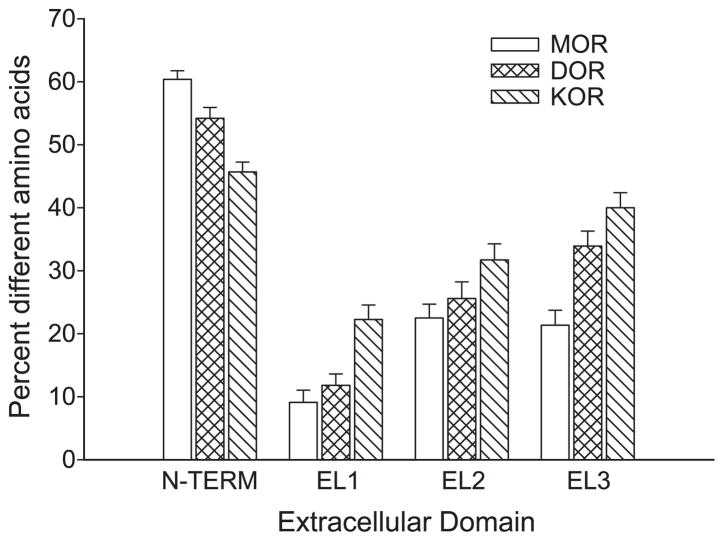
To assess the divergence of extracellular domains of the amphibian and other vertebrate opioid-like receptors, amino acid sequences for the N-terminal (N-TERM), extracellular loop 1 (EL1), extracellular loop 2 (EL2), and extracellular loop 3 (EL3) domains were analyzed by the average distance method for each type of opioid receptor across the five vertebrate species (see Fig. 2). The N-terminal sequence of all three types of opioid-like receptors is highly divergent, ranging from 32.9% to 36.4%. EL1 is the least divergent (most conserved) for mu opioid-like receptors (7.4% divergent), followed by EL3 (14.1%) and EL2 (19.4%). For delta and kappa opioid-like receptors, EL1 is also the least divergent extracellular domain (11.4% and 17.1%, respectively), but EL2 (18.4% and 25.8%) is less divergent than EL3 (27.2% and 31.5%). Among all the EL domains, the kappa opioid-like receptors show the most divergence; the mu opioid-like receptors show the least divergence and delta opioid-like receptors show intermediate divergence.
Fig. 2.
Plot of average divergence for extracellular opioid receptor domains by type. Each domain for all three receptors in five species was analyzed in an average distance matrix to yield mean (±S.E.) for each domain by type using the MEGA2 software.
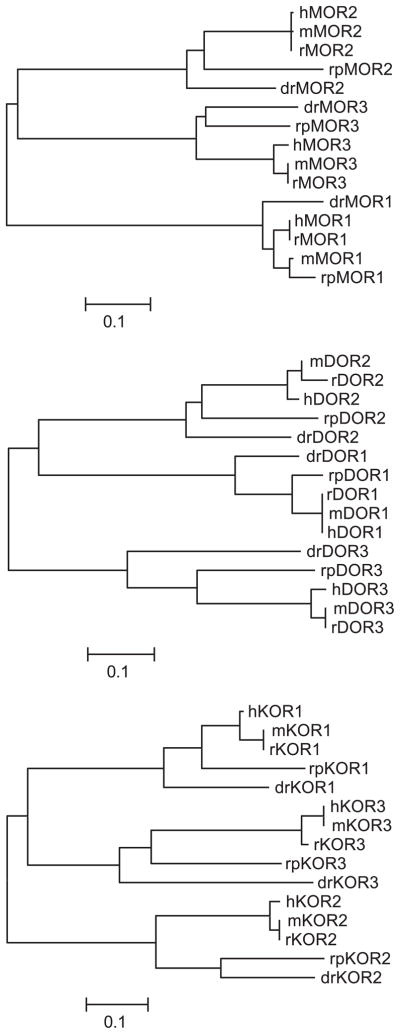
There is little evidence to suggest that EL domains are descended from a common, single EL sequence, although it is possible that an ancestral receptor-like protein might have only one EL domain. However, the tools of bioinformatics can be used to examine the sequence divergence and related sequences of aligned opioid receptor EL domains to gain insight into EL domain divergence. Neighbor-joining trees constructed with only the extracellular domains of vertebrate mu, delta, and kappa opioid-like receptor sequences are shown in Fig. 3. Phylogenetic analysis of the EL1 domains shows that the mu opioid-like sequences are most similar to each other and distinct from EL1 domains of delta and kappa opioid-like receptors (i.e., mu opioid EL1 domains form a single branch from unrooted ancestor, whereas delta and kappa sequences branch as one node from a common ancestor). For the EL2 domains, kappa opioid-like receptors are distinctly grouped separately from the mu and delta opioid-like EL2 domains. Trees generated for analysis of EL3 domains show that delta opioid-like receptor EL3 domains are distinct and separated from branches containing mu and kappa opioid-like EL3 domains.
Fig. 3.
Molecular evolution of EL domains. Neighbor-joining method used for the generation of EL domains by opioid receptor type. Bootstrap values and branch lengths are omitted in the plots for clarity, but the scale bar denotes 0.01% or 1% divergence in amino acid sequence.
9. Hypothesis of receptor domain selectivity
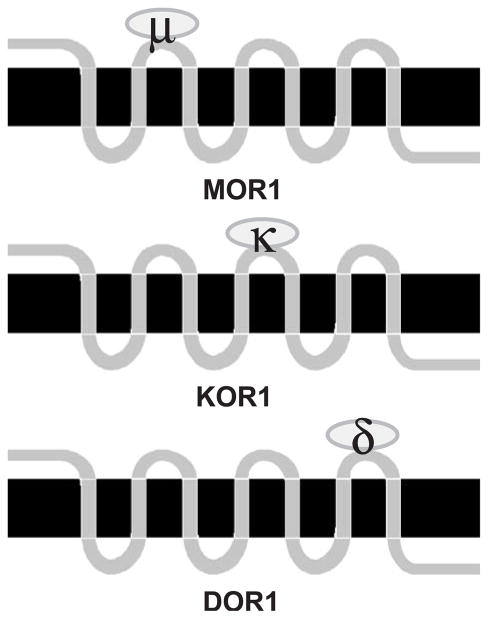
The simplest hypothesis that arises from Fig. 3 and previous site-directed mutagenesis and receptor chimera studies using opioid receptors is that each one of the three EL domains of opioid receptors determines selectivity for each type of receptor. Broadly stated, the data support the scenario illustrated in Fig. 4. As shown in the schematic, mu opioid receptors are selective due to conserved residues in the EL1, kappa receptors are selective due to conserved residues in EL2, and delta receptors are type-selective by virtue of conserved residues in EL3. Not shown is the idea that the other two (nonselective) EL domains for each type of opioid receptor (e.g., EL2 and EL3 for mu) contain amino acids that block noncognate ligands from binding, an idea called negative determinacy [26,28,65].
Fig. 4.
Schematic of the hypothesis of domain type selectivity in vertebrate opioid receptors. MOR1 is the generic vertebrate mu opioid receptor, KOR1 is the kappa opioid receptor, and DOR1 is the delta opioid receptor. Type selectivity is determined by the ligand binding characteristics, or filters, by the different EL domains for each type of opioid receptor. Ovals binding to EL domains represent type-selective opioid agonists. See text for further details.
Secondly, considering the phylogenetic relationships of both the complete receptor sequence and the extracellular domains individually, it is also hypothesized that amphibian mu, kappa, and delta opioid receptors may be less selective compared to mammalian opioid receptors. This hypothesis gains some support from the finding that nonselective opioids bind better than selective opioids to cell membranes transfected with a delta opioid-like receptor cloned from the zebrafish [40] and from previous findings of nonselectivity with highly selective opioid antagonists in behavioral and binding assays using amphibians [31,54].
Three types of opioid-like receptor cDNAs were cloned from amphibian brain and spinal cord that are highly homologous to mu, delta, and kappa opioid receptors in mammals. There are now complete coding sequence of mu, delta, and kappa opioid receptors from five different species from three classes of vertebrates. In spite of this limited dataset, phylogenetic analyses suggest that kappa opioid receptors were the first type differentiated from a common opioid receptor sequence and that the mu opioid receptor is the newest of the opioid receptor genes. Additionally, comparing amphibian opioid-like receptors to mammalian opioid receptors, rpKOR is the least homologous to mammalian kappa opioid receptors, whereas rpMOR is the most homologous to mammalian mu opioid receptors, with rpDOR homology to mammalian delta opioid receptors showing intermediate values. This would be expected if kappa opioid receptors are the most ancestral receptors and mu opioid receptors are the most recent; the earlier-evolved sequences would enjoy a longer evolutionary time for sequence divergence than later-evolved sequences. While it might be expected that amphibian opioid receptors would be more homologous to fish receptors than mammalian receptors, fish and amphibians diverged from a common ancestor long before amphibians and mammals.
The results demonstrate that it is likely that all three types of opioid receptors are present in all vertebrate species. The kappa opioid receptor appeared to arise first in evolution, followed by the delta opioid receptor, with the mu opioid receptor the most recent and least divergent of all three types of opioid receptors. It is interesting to note that the target of most clinically used opioids is the mu opioid receptor, which is the newest or least divergent of the opioid receptor types and produces the most potent analgesia in humans and animal models. It appears that all vertebrates code for three types of opioid receptors, as in even the most primitive vertebrate Agnathan species (lamprey), three partial fragments that were mu, delta, and kappa-opioid-like were cloned [23]. If a single ancestral opioid receptor type (a true unireceptor?) existed in a primitive vertebrate species, it is likely that this species became extinct millions of years ago. There is no evidence of three opioid receptor types expressed in invertebrates species, nor of any protein that can be considered opioid receptor-like, although there is evidence of endogenous opioid peptides and behavioral effects of opioids in some invertebrates.
Phylogenetic analysis of the EL domains of opioid receptors provides support for the receptor domain selectivity hypothesis in that the first EL domain was most conserved for mu opioid receptors, the second EL domain for kappa opioid receptors, and the third EL was the most conserved extracellular domain for delta opioid receptors. These results lend credence to some of the earlier receptor mutagenesis and chimera studies, which suggested that specific extracellular domains were needed for mu, delta, and kappa opioid selectivity. However, phylogenetic analysis of receptor domains using natural evolutionarily selected sequences throughout vertebrate phylogeny overcomes the limitations of mutagen or chimera studies, which may introduce unknown secondary structures from hybrid receptors. This unique approach of using phylogenetic analysis with matched datasets of mu, delta, and kappa opioid-like receptor cDNAs will realize greater gains in the understanding of opioid analgesia and underlying mechanisms of opioid type selectivity with the addition of opioid receptors cloned from species of classes Reptilia and Aves.
10. Summary and conclusions
The amount of opioid research in nonmammalian species is limited. This brief review summarized the work from our research group investigating opioid analgesia, radioligand binding, and molecular cloning studies of opioid receptors using the Northern grass frog R. pipiens as a novel animal model. General aspects of using amphibians for pain and opioid research were highlighted and include a phylogenetic perspective on the mechanisms of opioid analgesia and opioid receptors. Other issues include the simplicity of the amphibian CNS, the economic advantage of using frogs, and ethical considerations of conducting pain research in nonmammalian vertebrate species. Chronologically, studies of opioid pharmacology in amphibians followed a broad hierarchical trend from the initial characterization of the behavioral effects after opioid administration, to radioligand binding studies to determine the affinity and density of opioid binding sites and the selectivity of opioid ligands in brain and spinal cord, to the most recent studies cloning opioid-like receptor cDNAs from amphibian CNS tissues. Behavioral studies revealed a high correlation of the relative analgesic potency of mu, delta, and kappa-selective opioid agonists in both amphibians and mammals. In contrast, highly selective opioid antagonists did not show the same selectivity in blocking the analgesic effects of cognate mu, delta, and kappa opioids in amphibians and mammals. Radioligand binding studies using [3H]naloxone suggested a single opioid binding site, unlike results found with naloxone binding in mammals. Displacement of the naloxone label by mu, delta, and kappa opioids was correlated to their relative analgesic potency. In contrast to mammalian studies, naloxone was displaced with equal apparent affinity by highly selective mu, delta, and kappa opioid antagonists. Binding studies using labeled mu-, delta-, and kappa-selective opioid agonists distinguished three distinct sites, and each selective opioid agonist radioligand was displaced most effectively by its cognate highly selective opioid antagonist. Cloning studies employing RT-PCR with degenerate oligonucleotide primers followed by full-length RACE reactions revealed three distinct mu-, delta-, and kappa-like receptor cDNAs expressed in amphibian CNS. Sequencing of the amphibian cDNA and phylogenetic analysis of opioid receptor cDNAs from all vertebrate species with available datasets of the three types of opioid receptors yield a pattern of the molecular evolution of vertebrates marked by a greater sequence similarity among mu, delta, and kappa opioid receptors in earlier-evolved vertebrates. These novel analyses suggest that kappa>delta>mu opioid receptors is the order of the evolution of opioid receptor types. Finally, these analyses suggest that conserved receptor domains, the three ELs, may determine the type selectivity of vertebrate opioid receptors.
Acknowledgments
We acknowledge research support from the National Institutes of Health (NIDA) and the Oklahoma Center for Advancement of Science and Technology (OCAST). Great appreciation is extended to Dr. Lynne U. Sneddon (University of Liverpool) for the symposium invitation.
References
- 1.Adrian ED, Cattell M, Hoagland H. Sensory discharges in single cutaneous nerve fibres. J Physiol. 1931;72:377–391. doi: 10.1113/jphysiol.1931.sp002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews EJ, Bennett BT, Clark JD, Houpt KA, Pascoe PJ, Robinson GW, Boyce JR. 1993 Report of the AVMA panel on euthanasia. J Am Vet Med Assoc. 1993;202:229–249. [PubMed] [Google Scholar]
- 3.Bardo MT, Hughes RA. Exposure to a nonfunctional hot plate as a factor in the assessment of morphine-induced analgesia and analgesic tolerance in rats. Pharmacol Biochem Behav. 1978;10:481–485. doi: 10.1016/0091-3057(79)90221-1. [DOI] [PubMed] [Google Scholar]
- 4.Barrallo A, González-Sarmiento R, Alvar F, Rodriguez RE. ZFOR2, a new opioid receptor-like gene from the teleost zebrafish (Danio rerio. Mol Brain Res. 2000;84:1–6. doi: 10.1016/s0169-328x(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 5.Beer C. Motives and metaphors in considerations of animal nature. In: Pfaff DW, editor. Considerations of Animal Nature. Springer-Verlag; New York: 1983. pp. 125–151. [Google Scholar]
- 6.Benyhe S, Varga E, Hepp J, Magyar A, Borsodi A, Wollemann M. Characterization of kappa1 and kappa2 opioid binding sites in frog (Rana esculenta) brain membrane. Neurochem Res. 1990;15:899–904. doi: 10.1007/BF00965909. [DOI] [PubMed] [Google Scholar]
- 7.Brenner GM, Klopp AJ, Deason LL, Stevens CW. Analgesic potency of alpha adrenergic agents after systemic administration in amphibians. J Pharmacol Exp Ther. 1994;270:540–545. [PubMed] [Google Scholar]
- 8.Bullock TH. The future of comparative neurology. Am Zool. 1984;24:693–700. [Google Scholar]
- 9.Darlison MG, Greten FR, Harvey RJ, Kreienkamp H, Stuhmer T, Zwiers H, Lederis K, Richter D. Opioid receptors from a lower vertebrate (Catostomus commersoni): sequence, pharmacology, coupling to a G-protein-gated inward-rectifying potassium channel (GIRK1), and evolution. Proc Natl Acad Sci U S A. 1997;94:8214–8219. doi: 10.1073/pnas.94.15.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis SG, Melzack R. Perspectives on phylogenetic evolution of pain expression. In: Kitchell RL, Erickson HM, editors. Animal Pain: Perception and Alleviation. Waverly Press; Baltimore: 1983. pp. 151–160. [Google Scholar]
- 11.Enviro Control, Allergies Associated with Employment in Biomedical Research Laboratories. Office of Biohazard Safety; Bethesda: 1979. [Google Scholar]
- 12.Fox H, Whitear M. Observations of Merkel cells in amphibians. Biol Cell. 1978;32:223–232. [Google Scholar]
- 13.Fukson OI, Berkinblit MB, Feldman AG. The spinal frog takes into account the scheme of its body during the wiping reflex. Science. 1980;209:1261–1263. doi: 10.1126/science.7403886. [DOI] [PubMed] [Google Scholar]
- 14.Giszter SF, McIntyre J, Bizzi E. Kinematic strategies and sensorimotor transformations in the wiping movements of frogs. J Neurophysiol. 1989;62:750–767. doi: 10.1152/jn.1989.62.3.750. [DOI] [PubMed] [Google Scholar]
- 15.Hamamoto DT, Simone DA. Characterization of cutaneous primary afferent fibers excited by acetic acid in a model of nociception in frogs. J Neurophysiol. 2003;90:566–577. doi: 10.1152/jn.00324.2003. [DOI] [PubMed] [Google Scholar]
- 16.Hammond DL. New insights regarding organization of spinal cord pain pathways. NIPS. 1989;4:98–101. [Google Scholar]
- 17.Herrick CJ. The Brain of the Tiger Salamander. University of Chicago Press; Chicago: 1948. pp. 1–409. [Google Scholar]
- 18.IASP Subcommittee on Taxonomy. Pain. Suppl 3. 1986. Classification of chronic pain: description of chronic pain syndromes and definitions of pain terms; p. 5217. [PubMed] [Google Scholar]
- 19.Inagaki S, Senba E, Shiosaka S, Takagi H, Kawai Y, Takatsuki K, Sakanaka M, Matsuzaki T, Tohyama M. Regional distribution of substance P-like immunoreactivity in the frog brain and spinal cord: immunohistochemical analysis. J Comp Neurol. 1981;201:243–254. doi: 10.1002/cne.902010208. [DOI] [PubMed] [Google Scholar]
- 20.Joseph BS, Whitlock DG. The morphology of spinal afferent–efferent relationships in vertebrates. Brain Behav Evol. 1968;1:2–18. [Google Scholar]
- 21.Kicliter E, Ebbesson SOE. Organization of the “nonolfactory” telencephalon. In: Llinas R, Precht W, editors. Frog Neurobiology: A Handbook. Springer-Verlag; Berlin: 1976. pp. 946–972. [Google Scholar]
- 22.Kitchell RL, Erickson HM. What is pain? In: Kitchell RL, Erickson HM, editors. Animal Pain: Perception and Alleviation. Waverly Press; Baltimore: 1983. pp. vii–viii. [Google Scholar]
- 23.Li X, Keith DE, Jr, Evans CJ. Multiple opioid receptor-like genes are identified in diverse vertebrate phyla. FEBS Lett. 1996;397:25–29. doi: 10.1016/s0014-5793(96)01126-x. [DOI] [PubMed] [Google Scholar]
- 24.Lorez HP, Kemali M. Substance P, metenkephalin and somatostatin-like immunoreactivity distribution in the frog spinal cord. Neurosci Lett. 1981;26:119–124. doi: 10.1016/0304-3940(81)90336-0. [DOI] [PubMed] [Google Scholar]
- 25.Maruhashi J, Mizuguchi K, Tasaki I. Action currents in single afferent nerve fibres elicited by stimulation of the skin of the toad and the cat. J Physiol. 1952;117:129–151. doi: 10.1113/jphysiol.1952.sp004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzger TG, Ferguson DM. On the role of extracellular loops of opioid receptors in conferring ligand selectivity. FEBS Lett. 1995;375:1–4. doi: 10.1016/0014-5793(95)01185-h. [DOI] [PubMed] [Google Scholar]
- 27.Mollereau C, Pascaud A, Baillat G, Mazarguil H, Puget A, Meunier JC. Evidence for a new type of opioid binding site in the brain of the frog Rana ridibunda. Eur J Pharmacol. 1988;150:75–84. doi: 10.1016/0014-2999(88)90752-2. [DOI] [PubMed] [Google Scholar]
- 28.Moyle WR, Campbell RK, Myers RV, Bernard MP, Han Y, Wang X. Co-evolution of ligand–receptor pairs. Nature. 1994;368:251–254. doi: 10.1038/368251a0. [DOI] [PubMed] [Google Scholar]
- 29.Newman LC, Sands SS, Wallace DR, Stevens CW. Characterization of mu, kappa, and delta opioid binding in amphibian whole brain tissue homogenates. J Pharmacol Exp Ther. 2002;301:364–370. doi: 10.1124/jpet.301.1.364. [DOI] [PubMed] [Google Scholar]
- 30.Newman LC, Wallace DR, Stevens CW. Characterization of 3H-diprenorphine binding in Rana pipiens: observations of filter binding enhanced by naltrexone. J Pharmacol Toxicol Methods. 1999;41:43–48. doi: 10.1016/s1056-8719(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 31.Newman LC, Wallace DR, Stevens CW. Selective opioid agonist and antagonist displacement of [3H]-naloxone binding in amphibian brain. Eur J Pharmacol. 2000;397:255–262. doi: 10.1016/s0014-2999(00)00265-x. [DOI] [PubMed] [Google Scholar]
- 32.Newman LC, Wallace DR, Stevens CW. Selective opioid receptor agonist and antagonist displacement of [3H]-naloxone binding in amphibian spinal cord. Brain Res. 2000;884:184–191. doi: 10.1016/s0006-8993(00)02967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikundiwe AM, De-Boer van Huizen R, Ten Donkelaar HJ. Dorsal root projections in the clawed toad (Xenopus laevis) as demonstrated by anterograde labeling with horseradish peroxidase. Neuroscience. 1982;7:2089–2103. doi: 10.1016/0306-4522(82)90121-x. [DOI] [PubMed] [Google Scholar]
- 34.Northcutt RG. Evolution of the vertebrate central nervous system: patterns and processes. Am Zool. 1984;24:701–716. [Google Scholar]
- 35.Pezalla PD. Morphine-induced analgesia and explosive motor behavior in an amphibian. Brain Res. 1983;273:297–305. doi: 10.1016/0006-8993(83)90854-5. [DOI] [PubMed] [Google Scholar]
- 36.Pezalla PD, Stevens CW. Behavioral effects of morphine, levorphanol, dextrorphan and naloxone in the frog Rana pipiens. Pharmacol Biochem Behav. 1984;21:213–217. doi: 10.1016/0091-3057(84)90217-x. [DOI] [PubMed] [Google Scholar]
- 37.Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- 38.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned κ-, δ- and μ-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- 39.Roberts A, Hayes BP. The anatomy and function of free nerve endings in an amphibian skin sensory system. Proc R Soc Lond. 1977;196:415–420. doi: 10.1098/rspb.1977.0048. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez RE, Barrallo A, Garcia-Malvar F, McFadyen IJ, González-Sarmiento R, Traynor JR. Characterization of ZFOR1, a putative delta-opioid receptor from the teleost zebrafish. Neurosci Lett. 2000;288:207–210. doi: 10.1016/s0304-3940(00)01239-8. [DOI] [PubMed] [Google Scholar]
- 41.Rothe-Skinner KS, Stevens CW. Distribution of opioid-expressing neurons in the frog: an in situ hybridization study. Analgesia. 1995;1:683–686. [Google Scholar]
- 42.Satoh M, Minami M. Molecular pharmacology of the opioid receptors. Pharmacol Ther. 1995;68:343–364. doi: 10.1016/0163-7258(95)02011-x. [DOI] [PubMed] [Google Scholar]
- 43.Simon EJ, Hiller JM, Groth J, Itzhak Y, Holland MJ, Beck SG. The nature of opiate receptors in toad brain. Life Sci. 1982;31:1367–1370. doi: 10.1016/0024-3205(82)90383-6. [DOI] [PubMed] [Google Scholar]
- 44.Simpson JI. Functional synaptology of the spinal cord. In: Llinas R, Precht W, editors. Frog Neurobiology: A Handbook. Springer-Verlag; Berlin: 1976. pp. 728–749. [Google Scholar]
- 45.Smith JA, Boyd KM. Lives in the Balance. Oxford University Press; Oxford: 1991. pp. 1–295. [Google Scholar]
- 46.Spray DC. Cutaneous receptors: pain and temperature receptors of anurans. In: Llinas R, Precht W, editors. Frog Neurobiology: A Handbook. Springer-Verlag; Berlin: 1976. pp. 607–628. [Google Scholar]
- 47.Stevens CW. Opioid antinociception in amphibians. Brain Res Bull. 1988;21:959–962. doi: 10.1016/0361-9230(88)90034-2. [DOI] [PubMed] [Google Scholar]
- 48.Stevens CW. Alternatives to the use of mammals for pain research. Life Sci. 1992;50:901–912. doi: 10.1016/0024-3205(92)90167-n. [DOI] [PubMed] [Google Scholar]
- 49.Stevens CW. An amphibian model for pain research. Lab Anim. 1995;24:32–36. [Google Scholar]
- 50.Stevens CW. Relative analgesic potency of mu, delta and kappa opioids after spinal administration in amphibians. J Pharmacol Exp Ther. 1996;276:440–448. [PubMed] [Google Scholar]
- 51.Stevens CW, Brenner GM. Spinal administration of adrenergic agents produces analgesia in amphibians. Eur J Pharmacol. 1996;316:205–210. doi: 10.1016/s0014-2999(96)00681-4. [DOI] [PubMed] [Google Scholar]
- 52.Stevens CW, Kirkendall K. Time course and magnitude of tolerance to the analgesic effects of systemic morphine in amphibians. Life Sci. 1993;52:PL111–116. doi: 10.1016/0024-3205(93)90097-m. [DOI] [PubMed] [Google Scholar]
- 53.Stevens CW, Klopp AJ, Facello JA. Analgesic potency of mu and kappa opioids after systemic administration in amphibians. J Pharmacol Exp Ther. 1994;269:1086–1093. [PubMed] [Google Scholar]
- 54.Stevens CW, Newman LC. Spinal administration of selective opioid antagonists in amphibians: evidence for an opioid unireceptor. Life Sci. 1999;64:PL125–PL130. doi: 10.1016/s0024-3205(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 55.Stevens CW, Paul D. Opioid analgesia after spinal administration in amphibians: binding and behavioral studies. In: Harris LS, editor. Problems in Drug Dependence 1995, Meeting, NIDA Research Monograph; Bethesda. 1996; pp. 222–225. [Google Scholar]
- 56.Stevens CW, Pezalla PD. A spinal site mediates opiate analgesia in frogs. Life Sci. 1983;33:2097–2103. doi: 10.1016/0024-3205(83)90333-8. [DOI] [PubMed] [Google Scholar]
- 57.Stevens CW, Pezalla PD. Naloxone blocks the analgesic action of levorphanol but not of dextrorphan in the leopard frog. Brain Res. 1984;301:171–174. doi: 10.1016/0006-8993(84)90418-9. [DOI] [PubMed] [Google Scholar]
- 58.Stevens CW, Pezalla PD, Yaksh TL. Spinal antinociceptive action of three representative opioid peptides in frogs. Brain Res. 1987;402:201–203. doi: 10.1016/0006-8993(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 59.Stevens CW, Rothe KS. Supraspinal administration of opioids with selectivity for μ-, δ-and κ-opioid receptors produces analgesia in amphibians. Eur J Pharmacol. 1997;331:15–21. doi: 10.1016/s0014-2999(97)01026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens CW, Sangha S, Ogg BG. Analgesia produced by immobilization stress and an enkephalinase-inhibitor in amphibians. Pharmacol Biochem Behav. 1995;51:675–680. doi: 10.1016/0091-3057(94)00436-m. [DOI] [PubMed] [Google Scholar]
- 61.Stevens CW, Willenbring S. Pain sensation and analgesia in amphibians and reptiles. In: Ackerman L, editor. The Biology, Husbandry and Health Care of Reptiles and Amphibians. TFH Publications; Neptune City: 1997. pp. 309–324. [Google Scholar]
- 62.Szekely G. The morphology of motoneurons and dorsal root fibers in the frog’s spinal cord. Brain Res. 1976;103:275–290. doi: 10.1016/0006-8993(76)90799-x. [DOI] [PubMed] [Google Scholar]
- 63.Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Norbinaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- 64.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 65.Tian Y, Wu LH, Oxender DL, Chung FZ. The unpredicted high affinities of a large number of naturally occurring tachykinins for chimeric NK1/NK3 receptors suggest a role for an inhibitory domain in determining receptor specificity. J Biol Chem. 1996;271:20250–20257. doi: 10.1074/jbc.271.34.20250. [DOI] [PubMed] [Google Scholar]
- 66.Van Sluyters RC. Handbook for the Use of Animals in Neuroscience Research. Society of Neuroscience; Washington, DC: 1991. [Google Scholar]
- 67.Vesselkin NP, Agayan AL, Nomokonova LM. A study of thalamotelencephalic afferent systems in frogs. Brain Behav Evol. 1971;4:295–306. doi: 10.1159/000125439. [DOI] [PubMed] [Google Scholar]
- 68.Ward SJ, Portoghese PS, Takemori AE. Pharmacological profiles of beta-funaltrexamine (β-FNA) and beta-chlornaltrexamine (β-CNA) on the mouse vas deferens preparation. Eur J Pharmacol. 1982;80:377–384. doi: 10.1016/0014-2999(82)90083-8. [DOI] [PubMed] [Google Scholar]
- 69.White JC, Sweet WH. Pain and the Neurosurgeon. Thomas Publishers; Springfield: 1969. [Google Scholar]
- 70.Willenbring S, Stevens CW. Thermal, mechanical and chemical peripheral sensation in amphibians: opioid and adrenergic effects. Life Sci. 1996;58:125–133. doi: 10.1016/0024-3205(95)02265-1. [DOI] [PubMed] [Google Scholar]
- 71.Willenbring S, Stevens CW. Spinal μ, δ and κ opioids alter chemical, mechanical and thermal sensitivities in amphibians. Life Sci. 1997;61:2167–2176. doi: 10.1016/s0024-3205(97)00919-3. [DOI] [PubMed] [Google Scholar]
- 72.Wollemann M, Benyhe S, Simon J. The kappa opioid receptor: evidence for the different subtypes. Life Sci. 1993;52:599–611. doi: 10.1016/0024-3205(93)90451-8. [DOI] [PubMed] [Google Scholar]
- 73.Yaksh TL, Hammond DL. Peripheral and central substrates involved in the rostral transmission of nociceptive information. Pain. 1982;13:1–85. doi: 10.1016/0304-3959(82)90067-7. [DOI] [PubMed] [Google Scholar]
- 74.Yamashita Y, Ogawa H. Slowly adapting cutaneous mechanoreceptor afferent units associated with Merkel cells in frogs and effects of direct currents. Somatosens Motor Res. 1991;8:87–95. doi: 10.3109/08990229109144732. [DOI] [PubMed] [Google Scholar]
- 75.Zawilska J, Lajtha A, Borsodi A. Selective protection of benzomorphan binding sites against inactivation by N-ethylmaleimide. Evidence for kappa opioid receptors in frog brain. J Neurochem. 1988;51:736–739. doi: 10.1111/j.1471-4159.1988.tb01806.x. [DOI] [PubMed] [Google Scholar]
- 76.Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]