Abstract
LH receptor knockout (LhrKO) male mice exhibit a bilateral cryptorchidism resulting from a developmental defect in the gubernaculum during the inguinoscrotal phase of testis descent, which is corrected by testosterone replacement therapy (TRT). In vivo and in vitro experiments were conducted to investigate the roles of the androgen receptor (AR) and RXFP2 signals in regulation of gubernacular development in LhrKO animals. This study demonstrated that AR and RXFP2 proteins were expressed in the gubernaculum during the entire postnatal period. TRT normalized gubernacular RXFP2 protein levels inLhrKO mice. Organ and primary cell cultures of gubernacula showed that 5α-dihydrotestosterone (DHT) upregulated the expression of Rxfp2 which was abolished by the addition of an AR antagonist, flutamide. A single s.c. testosterone injection also led to a significant increase in Rxfp2 mRNA levels in a time-dependent fashion in LhrKO animals. DHT, natural and synthetic insulin-like peptide 3 (INSL3), or relaxin alone did not affect proliferation of gubernacular mesenchymal cells, while co-treatments of DHT with either INSL3 or relaxin resulted in an increase in cell proliferation, and they also enhanced the mesenchymal cell differentiation toward the myogenic pathway, which included a decrease in a mesenchymal cell marker, CD44 and the expression of troponin. These effects were attenuated by the addition of flutamide, siRNA-mediated Rxfp2 knockdown, or by an INSL3 antagonist. Co-administration of an INSL3 antagonist curtailed TRT-induced inguinoscrotal testis descent in LhrKO mice. Our findings indicate that the RXFP2 signaling pathway plays an important role in mediating androgen action to stimulate gubernaculum development during inguinoscrotal testis descent.
Introduction
Undescended testis is by far one of the most common congenital malformations in humans (Main et al. 2009). Testicular descent can be anatomically and temporally divided into two distinct stages, i.e. transabdominal and inguinoscrotal phases (Hutson et al. 1997, Amann & Veeramachaneni 2007, Foresta et al. 2008, Hughes & Acerini 2008). Both insulin-like peptide 3 (INSL3), which is also known as relaxin-like factor (RLF), and androgen signaling pathways play important roles in the control of the transabdominal phase. The first stage of testis descent is believed to be the result of regression of the cranial suspensory ligament (CSL) and outgrowth of the gubernaculum (Hutson et al. 1997). Dissolution of the CSL is an androgen-dependent process. Persistence of the CSL has been found in androgen receptor (AR) mutant (tfm) mice that are completely androgen-insensitive, and rats that were prenatally exposed to an androgen antagonist, flutamide (van der Schoot 1992, Barthold et al. 1994). Proliferation and differentiation of the gubernaculum during embryogenesis is now known to depend on INSL3. Congenital mutation, targeted disruption, and transgenic insertion of the Insl3 or its cognate receptor gene, Rxfp2 (also known as GREAT or Lgr8), in human and mouse cause the failure of gubernacular development during embryogenesis. As a result, males have bilateral cryptorchidism in which the testes were either freely moving or attached to the lower pole of the kidney (Zimmermann et al. 1999, Marin et al. 2001, Overbeek et al. 2001, Gorlov et al. 2002, Tomiyama et al. 2003). By contrast, the mechanism that regulates the inguinoscrotal phase is not completely understood, even though the incidence of this defect is much more common clinically (Elder 2002). It has been proposed that androgen is involved in regulation of the second phase of testis descent (Hutson et al. 1997). The underlying mechanism of androgenic action, however, has not yet been established. The site of androgenic action during the second phase is still controversial (Husmann & Levy 1995, Hutson & Hasthorpe 2005). Although never experimentally tested, it has been suggested that the RXFP2 signaling pathway may also play a role during the inguinoscrotal phase of testis descent (Hrabovszky et al. 2002, Bogatcheva & Agoulnik 2005).
Targeted disruption of LH receptor knockout (LhrKO) in mouse impaired the development of adult-type Leydig cells which led to a dramatic reduction of Insl3 mRNA and testosterone levels. LhrKO males exhibited a bilateral cryptorchid phenotype resulting from a defect in inguinoscrotal testis descent (Lei et al. 2001, 2004, Yuan et al. 2006). Histology, morphometry, and cell proliferation and differentiation analyses demonstrated that the defect was due to a reduction in gubernacular cell division and differentiation into cremaster muscle cells during the second stage. Morphometric measurement indicates that the areas of gubernacular cord and cremaster muscle in 2-week-old wild-type (WT) mice are comparable to those of adult LhrKO males. Although the expression of several genes in the gubernaculum that are known to be involved in gubernacular development, such as Hoxa10, Hoxa11, Wt1, Dll1, and Desrt, were not altered in mutant mice, Rxfp2 mRNA levels drastically decreased compared with age-matched WT siblings. Postnatal testosterone replacement therapy (TRT) completed testicular descent into the scrotum and concomitantly normalized Rxfp2 mRNA levels in the gubernaculum. Remedy of the defect by TRT was neither dependent on recovery of adult-type Leydig cells nor on the genitofemoral nerve (GFN). These findings collectively suggest that both the androgen and RXFP2 signals are necessary for the inguinoscrotal phase of testis descent (Yuan et al. 2006).
In the present study, in vivo and in vitro experiments demonstrated that androgen-induced gubernacular Rxfp2 expression and activation of the RXFP2 signaling pathway by either INSL3 or relaxin stimulated gubernacular cell proliferation and myogenic differentiation during the second phase of testis descent in LhrKO animals. Thus, the RXFP2 signal mediates a direct action of androgen in the gubernaculum constituting an essential pathway to regulate inguinoscrotal testis descent.
Results
Expression of Ar in the gubernaculum
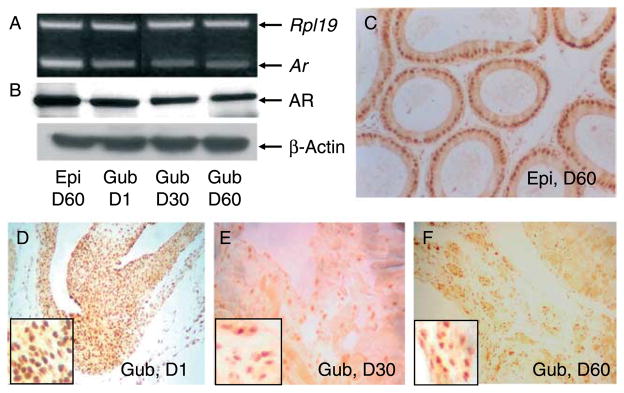
The gubernaculum throughout neonatal and adulthood contained Ar mRNA and 110 kDa protein (Fig. 1A and B). A classical androgen target tissue, epididymis, was used as a positive control. Immunohistochemistry revealed that AR protein was localized in the nuclei of interstitial and cremaster muscle cells in the gubernaculum (Fig. 1D, E and F) as well as in the nuclei of epididymal cells (Fig. 1C). The mRNA and protein levels of AR in the gubernaculum appeared to decrease from postnatal days 30 to 60. It is not clear whether the decrease in gubernacular AR during this period is relevant to the completion of testicular descent.
Figure 1.
RT-PCR (A) and western blot (B) demonstrate that neonatal (D1), pubertal (D30) and adult (D60) gubernacula (Gub), similar to adult epididymis (Epi), contain Ar mRNA (A) and 110 kDa protein (B). Rpl19 and β-actin were used as loading controls for RT-PCR and western blot, respectively. Immunohistochemistry shows that AR protein was localized in the nuclei of gubernacular mesenchymal and cremaster muscle cells (D–F) and epididymal epithelium (C).Representative pictures show one of three mice in each age group. Mag. C–F=×150, Inset in F=×600.
RXFP2 protein expression in the gubernaculum during postnatal development
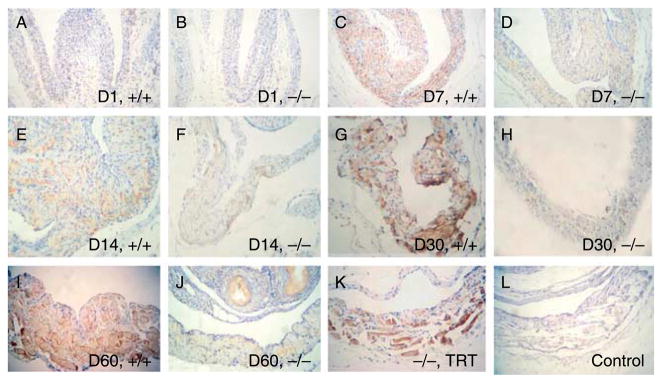
To better understand the role of RXFP2 in the second phase of testis descent, we first determined the spatial and temporal expression of RXFP2 protein in the gubernaculum during postnatal development. Immunohistochemical staining showed that RXFP2 protein was located in both mesenchymal and cremaster muscle cells of the gubernaculum (Fig. 2A–K). The immunostaining was not detected when RXFP2 antibody was pre-adsorbed with excess RXFP2 peptide (Fig. 2L), suggesting that the immunostaining observed was specific. Based on the results of three animals in each age and genotype group evaluated independently by two researchers, weak RXFP2 immunostaining was observed in 1-day-old WT and LhrKO mice (Fig. 2A and B). Thereafter, the immunostaining intensity gradually increased with age except for a slight drop on day 14, and the highest levels were detected in cremaster muscle cells of 30–60-day-old WTanimals (Fig. 2C, E, G and I). The LhrKO mice, on the other hand, failed to show the postnatal increase of RXFP2 immunostaining (Fig. 2D, F, H, and J). TRT of 30-day-old Lhr null mice resulted in a restoration of RXFP2 immunostaining intensity similar to that of the age-matched WT animals (Fig. 2K).
Figure 2.
RXFP2 immunostaining in neonatal (D1), premature (D7 and D14), pubertal (D30), and adult (D60) gubernacula of wild-type (+/+) and LhrKO (−/−) mice, and LhrKO mice placed on testosterone replacement therapy (−/−, TRT). Immunohistochemistry shows that RXFP2 protein was localized in gubernacular mesenchymal and cremaster muscle cells. Panel (L) is a procedural control, in which the primary antibody was pre-adsorbed with RXFP2 peptide. All sections were counterstained with Gill’s hematoxylin. Representative pictures show one of three mice in each group. Mag. A–L=×160.
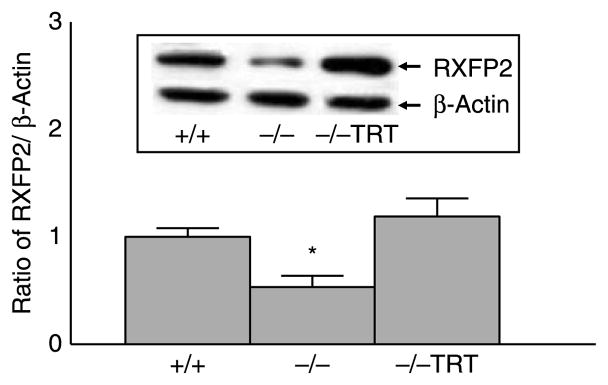
Western blot analysis revealed the presence of a 95 kDa RXFP2 protein in the gubernacular tissues of 60-day-old WT mice (Fig. 3). This protein was absent when the blot was incubated with RXFP2 antiserum pre-adsorbed with a RXFP2 peptide (data not shown). In agreement with the RT-PCR and immunohistochemistry results, the immunoblotting results revealed that RXFP2 protein expression was significantly reduced in Lhr null mice compared with the age-matched WT mice (Fig. 3). TRT completely restored RXFP2 protein levels in LhrKO mice to that in WT mice (Fig. 3).
Figure 3.
Gubernacular RXFP2 protein levels in 60-day-old animals (n=6) were significantly lower in LhrKO (−/−, *P<0.05) than the age-matched WT (+/+) as determined by western blotting. TRT (−/−, TRT) of LhrKO mice restored RXFP2 to WT levels. Results are the mean±S.E.M.
In vitro and in vivo androgen modulation of gubernacular Rxfp2 mRNA levels
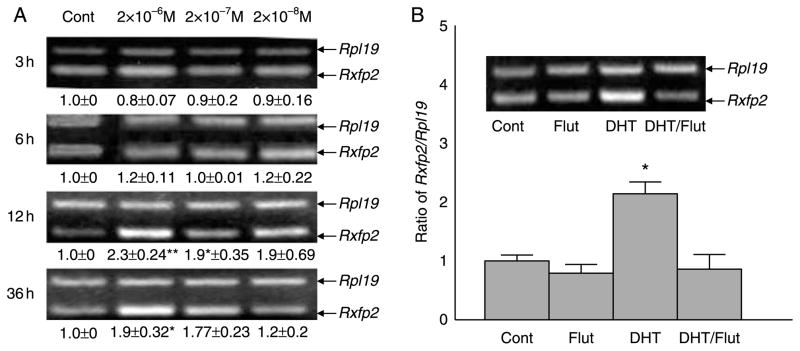
To determine whether testosterone directly regulates Rxfp2 expression at the mRNA level, we performed gubernacular organ cultures of 22-day-old WT animals and treated them with a potent androgen, 5α-dihydrotestosterone (DHT). RT-PCR results demonstrated that DHT increased gubernacular Rxfp2 mRNA levels in a time-dependent manner (Fig. 4A). The maximal stimulation was 2×10−6 M DHT at 12 h of incubation (Fig. 4A). It must be emphasized that this is a pharmacological dose of DHT in vitro. We are uncertain whether the local concentrations of androgens in the gubernaculum could reach such high levels in vivo. Nevertheless, the upregulatory effect of DHT was completely blocked by co-treatment with flutamide (Fig. 4B), and flutamide alone had no effect (Fig. 4B), suggesting that this was an AR-mediated action.
Figure 4.
Effect of DHT on Rxfp2 mRNA levels in organ cultures of gubernacula from 22-day-old WT mice as determined by semi-quantitative RT-PCR. (A) Time-dependent upregulation of Rxfp2 expression by DHT. (B) Flutamide (Flut) blocked the DHTeffect. Results are the mean±S.E.M. for five independent experiments, and the gubernacular tissues used in each experiment were pooled from two to three mice. *P<0.05, **P<0.001 compared with controls.
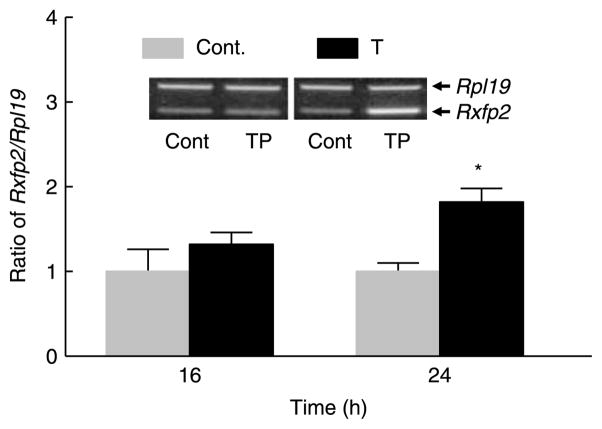
Our previous report showed that Rxfp2 mRNA levels dramatically decreased in LhrKO mice, and 21-day TRT of 30-day-old mice restored the levels to that of the age-matched WT animals (Yuan et al. 2006). To assess the acute effect of androgen on Rxfp2 expression in vivo, 30-day-old LhrKO mice were given a single injection of testosterone propionate (TP), and were killed at 16 and 24 h later, respectively. Semi-quantitative RT-PCR demonstrated that while TP had no effect at 16 h, it was able to significantly increase the Rxfp2 mRNA levels in the gubernaculum at 24 h compared with that of vehicle-treated mice (Fig. 5).
Figure 5.
Effect of a single s.c. injection of testosterone propionate (TP) on gubernacular Rxfp2 mRNA levels at 16 and 24 h in 30-day-old LhrKO mice (n=5) as determined by semi-quantitative RT-PCR. Results are the mean±S.E.M. *P<0.05 compared with control.
In vitro proliferative effect of MA-10 cell-conditioned medium and relaxin on gubernacular cells in the presence of androgen
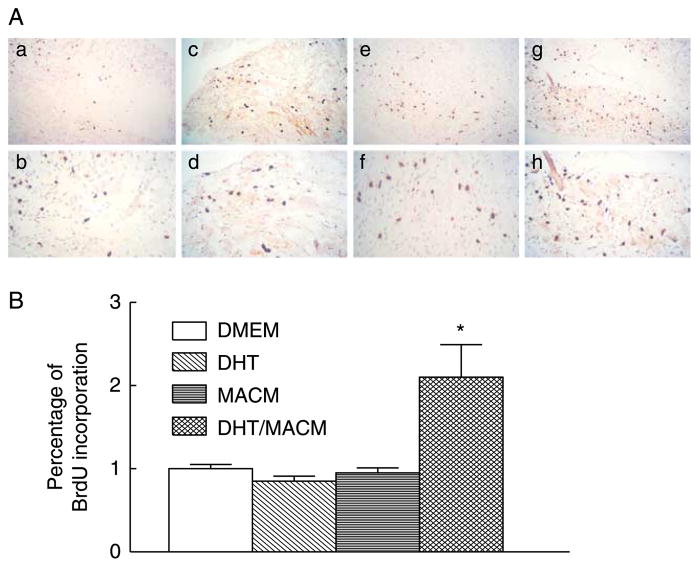
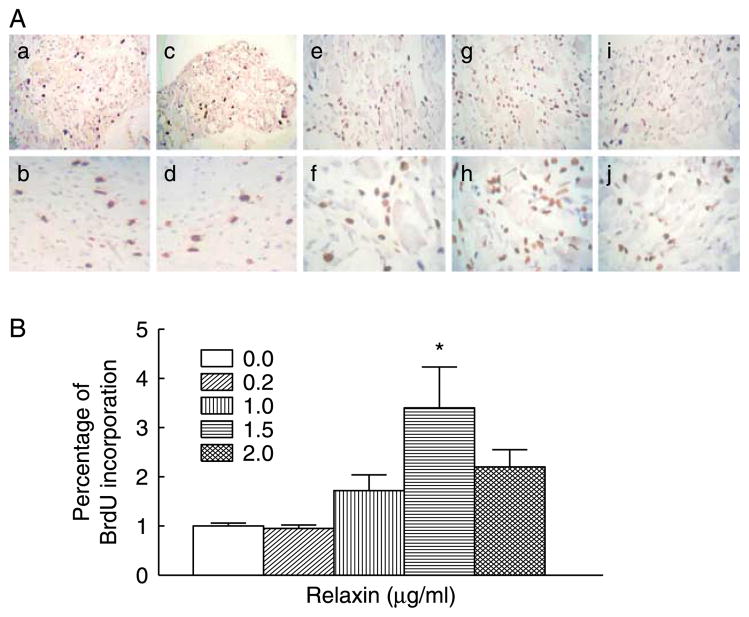
To evaluate cell proliferative effect of the RXFP2 signal pathway during postnatal development, the gubernacular organ cultures from 30-day-old LhrKO animals were incubated with MA-10 cell-conditioned medium (MACM), which served as a source of natural INSL3, or recombinant human relaxin as an alternative ligand of RXFP2 (Ivell & Bathgate 2002, Kubota et al. 2002, Hsu et al. 2005). MA-10 cell line is known to synthesize and secrete INSL3 but not endogenous androgens due to a lack of 17α-hydroxylase (Payne 1990, Houk et al. 2004, Sadeghian et al. 2005). While treatment with DHT or MACM alone had no effect, the combined treatment significantly increased BrdU incorporation compared with control cultures (Fig. 6A and B). In the presence of DHT, a similar growth-promoting effect was also observed in organ cultures treated with relaxin at a concentration of 1.5 μg/ml. However, a higher concentration of relaxin did not further stimulate cell proliferation (Fig. 7A and B). The relaxin-induced cell proliferation appeared to be mediated by RXFP2 but not by RXFP1 (also known as LGR7). Published results as well as our unpublished observations indicated that Rxfp1 transcripts were not detectable by RT-PCR in the gubernaculum (Kubota et al. 2002, Bathgate et al. 2006).
Figure 6.
Effects of DHT and MA10 cell-conditioned medium (MACM) on the cell proliferation in organ cultures of gubernacula from 30-day-old LhrKO mice. (A) Immunohistochemical detection of BrdU incorporation in control (a and b), DHT (c and d), MACM (e and f), and combined treatment of DHT and MACM (g and h). (B) Quantitative analysis of the percentage of BrdU-positive nuclei in the gubernacular tissues. Results are the mean±S.E.M. for five independent experiments, and the gubernacular tissues used in each experiment were pooled from two to three mice. *P<0.05 compared with control. Mag. a, c, e and g=×160, b, d, f and h=×600.
Figure 7.
Effect of relaxin on the cell proliferation in organ cultures of gubernacula from 30-day-old LhrKO mice in the presence of DHT. (A) Immunohistochemical detection of BrdU incorporation in control (a and b), 0.2 (c and d), 1.0 (e and f), 1.5 (g and h), and 2.0 (i and j) μg/ml relaxin. (B) Quantitative analysis of the percentage of BrdU-positive nuclei in the gubernacular tissues. Results are the mean±S.E.M. for five independent experiments, and the gubernacular tissues used in each experiment were pooled from two to three mice. *P<0.05 compared with control. Mag. a, c, e, g and i=×160, b, d, f, h and j=×600.
Androgen-AR and INSL3–RXFP2 in regulation of gubernacular cell proliferation
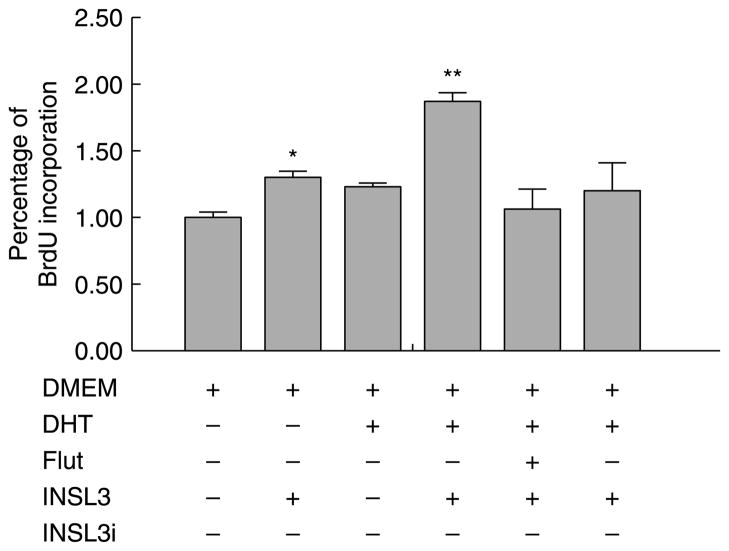
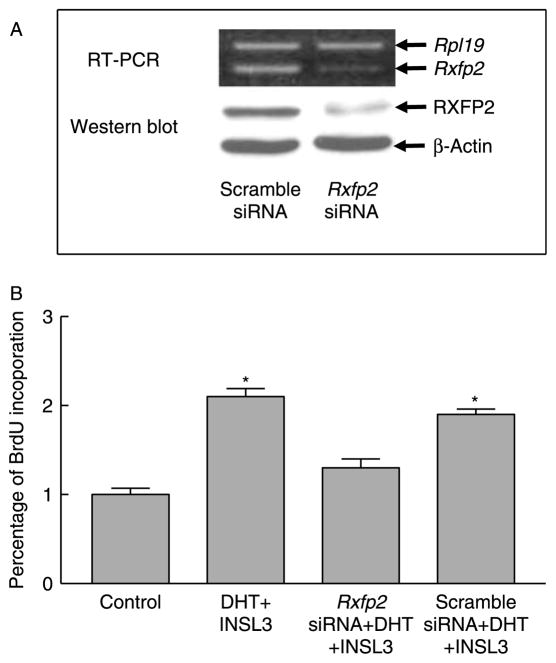
Gubernacular organ cultures showed that DHT, MACM, or relaxin alone did not affect gubernacular cell proliferation. To further assess the roles of androgen-AR and INSL3–RXFP2 signal pathways in regulating cell division of postnatal gubernacular cells, we also performed gubernacular primary cell cultures. The AR signal pathway was stimulated by DHT and inhibited by flutamide (Fig. 8). The RXFP2 signal pathway was activated by chemically synthesized INSL3 and repressed by either NSL3 inhibitor (INSL3i; Fig. 8) or Rxfp2 siRNA (Fig. 9). Immunocytochemistry demonstrated that the gubernacular cells isolated from neonatal WT gubernacula contained RXFP2 protein. Primary cell cultures allowed us to effectively transfect Rxfp2 siRNA to knock down Rxfp2 mRNA and protein as determined by semi-quantitative RT-PCR and western blot, respectively (Fig. 9A).
Figure 8.
Effects of DHT and INSL3 on cell proliferation in the primary cultures of mesenchymal cells isolated from the gubernacula of neonatal WT mice. Cell proliferation was determined by the BrdU incorporation using a cell proliferation ELISA kit. Results are the mean±S.E.M. for five independent experiments, and the gubernacular tissues used for the preparation of mesenchymal cells in each experiment were pooled from three mice. *P<0.05 and *P<0.01 compared with DMEM (control).
Figure 9.
Effect of Rxfp2 siRNA on cell proliferation in the primary cultures of mesenchymal cells isolated from the gubernacula of neonatal WT mice. Representative pictures of RT-PCR and western blot from three replicates show that Rxfp2 siRNA but not scramble siRNA effectively knocks down Rxfp2 expression (A). Cell proliferation was determined by the BrdU incorporation using a cell proliferation ELISA kit (B). Results are the mean±S.E.M. for five independent experiments, and the gubernacular tissues used for the preparation of mesenchymal cells in each experiment were pooled from three mice. *P<0.01 compared with control.
Consistent with organ culture results, treatment with DHT alone had no effect on cell proliferation (Fig. 8). A modest increase was observed after treatment with INSL3 alone, and further enhancement was noted when INSL3 was combined with DHT (Fig. 8). Interestingly, this enhancement was completely blocked by co-treatment of flutamide, INSL3i (Fig. 8), or Rxfp2 siRNA but not by that of scramble siRNA (Fig. 9B).
The short-term in vivo administration of TP dramatically stimulated gubernacular cell division in Lhr null mice (Fig. 10). Co-injection with INSL3i significantly curtailed the proliferative effect (Fig. 10).
Figure 10.
Effects of daily injections of testosterone propionate (TP) and RXFP2 inhibitor (INSL3i) for 3 days on BrdU incorporation in the gubernacula of 30-day-old LhrKO mice (n=3). Results are the mean ±S.E.M. aP<0.01 compared with control; bP<0.05 compared with TP/INSL3i.
The influence of INSL3i on androgen-induced testis descent in Lhr null mice
As with our previous study (Lei et al. 2001), TRT induced 100% testicular descent in Lhr null mice. Co-treatment with INSL3i prevented testicular descent in 50% Lhr null mice placed on TRT.
The role of androgen-AR and INSL3–RXFP2 in gubernacular cell differentiation
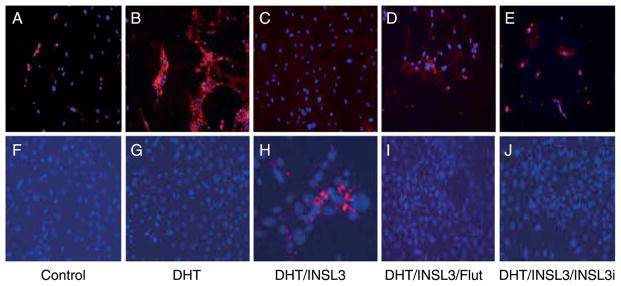
To evaluate whether androgen-AR and INSL3–RXFP2 signal pathways are involved in modulation of mesenchymal cell differentiation toward myogenic pathways, the gubernacular cells were cultured in a differentiation medium and treated with DHT, INSL3, flutamide, INSL3i, or a combination of these agents. Results of immunofluorescence staining performed in gubernaculum cells cultured with the differentiation medium revealed that the cells co-treated with DHT and INSL3 expressed troponin (Fig. 11). The cells treated with DHT alone, DHT/INSL3/flutamide, and DHT/INSL3/INSL3i, on the other hand, showed no troponin immunostaining (Fig. 11). Coincidentally, immunostaining of CD44 was markedly decreased in gubernacular cells treated with DHT and INSL3 compared with any other treatments, and this decrease was prevented by the combined treatment with INSL3i and flutamide (Fig. 11).
Figure 11.
Effects of DHT and INSL3 on cell differentiation in the primary cultures of mesenchymal cells isolated from the gubernacula of neonatal WT mice. Immunofluorescent staining of a mesenchymal cell marker, CD44 (A–E) and a muscle cell marker, troponin (F–J) showed that the combined treatment of DHT and INSL3 led to a reduction of CD44 (C) and appearance of troponin (H). Addition of either flutamide (Flut) or INSL3i abolished DHT and INSL3 effects (D, E, I and J). The nuclei are labeled with blue color by Hochest dye. Representative pictures show one of three independent experiments, and the gubernacular tissues used for the preparation of mesenchymal cells in each experiment were pooled from three mice. Mag. All=×300.
Discussion
It is generally recognized that androgens are required for inguinoscrotal testis descent (Hutson et al. 1997), but there is no consensus on whether androgens can directly act on the gubernaculum (Husmann & Levy 1995). Androgens have been proposed to affect the differentiation of the GFN and stimulate the secretion of a neurotransmitter, calcitonin gene-related peptide (CGRP), which somehow directs the inguinoscrotal testis descent (Hutson et al. 1997). However, it remains controversial whether androgens influence a sex dimorphic development of the GFN and stimulate or inhibit the release of CGRP (New & Mudge 1986, Popper & Micevych 1989, Popper et al. 1992, Barthold et al. 1994, Cain et al. 1995, Husmann & Levy 1995). TRT in the newborn, prepubertal, or even adult LhrKO mice fully restored the second phase of testis descent, and it was not affected by the transfection of the GFN, suggesting a direct action of androgen on the gubernaculum (Yuan et al. 2006). The present study, in line with numerous other reports, demonstrated that the gubernaculum, as other classic androgen target tissues such as epididymis, expressed AR during the entire postnatal development. Presence of AR in the postnatal gubernaculum allows for direct androgen action during the second phase of testicular descent.
The development of the gubernaculum during the first phase of testicular descent is known to be dependent on the RXFP2 signaling which is activated upon the binding of INSL3. RXFP2 is a G protein-coupled receptor that has been shown to express abundantly in the fetal gubernaculum (Bathgate et al. 2006). The promoter activity of human but not of mouse Rxfp2 gene has been shown to be upregulated by a transcription factor, SOX9, during the early stage of mouse development (Feng et al. 2007). Continuous expression of high levels of Rxfp2 in the postnatal gubernaculum and cremaster muscle demonstrated in this study is consistent with a previous report by Feng et al. (2009) and supports the previous speculation that RXFP2 signal may also play a role during the process of the second phase of testicular descent (Hrabovszky et al. 2002, Bogatcheva & Agoulnik 2005).
Thus far, it is unclear how the expression of Rxfp2 in the postnatal gubernaculum is regulated. Our previous studies revealed that male LhrKO mice have a severe androgen deficit, and gubernacular Rxfp2 mRNA levels were dramatically reduced. TRT that completely corrected bilateral cryptorchid phenotype of LhrKO mice also recovered gubernacular Rxfp2 mRNA and protein levels (Yuan et al. 2006). Our current study demonstrated that both AR and RXFP2 were in the mesenchymal and muscle cells of the gubernaculum and cremaster sac in newborn, prepubertal, and adult mice. There is a steady high level of Rxfp2 expression when androgen levels are substantially elevated during postnatal sexual maturation in mice. These findings suggest that Rxfp2 may be a downstream target gene of androgen. Indeed, DHT treatments increased Rxfp2 mRNA levels in organ cultures of 22-day-old gubernacula which was abolished by co-incubation of an androgen inhibitor, suggesting that it is an AR-mediated event in the gubernaculum. Moreover, gubernacular Rxfp2 expression was rapidly responsive to a single administration of androgen in vivo which occurred within the time before growth and organogenesis, thus it would reasonably be a direct target gene of androgen. Feng et al. (2006) previously showed that the effect of the synthetic AR agonist R1881 on the Rxfp2 mRNA levels did not reach statistical significance in neonatal gubernacular cells. This inconsistency with our findings may be due to the differences in the dose and time being used. It has been well established that androgens exert most of their effects by interacting with AR, a member of the nuclear receptor superfamily. The activated AR binds to specific recognition sequences, androgen response elements (AREs) located in androgen-regulated genes, known as classical genomic actions (Gelmann 2002, Nantermet et al. 2004). During analysis of genomic DNA sequences within 5 kbp of the first known transcribed nucleotide of the mouse Rxfp2 gene using the transcription element search software (TESS; University of Pennsylvania, http://www.cbil.upenn.edu/cgi-bin/tess/tess), three potential AREs were found within the 1.9 kbp fragment upstream of the predicted transcription start site. Whether these three putative AREs may physically interact with AR to regulate the Rxfp2 gene expression requires further investigation.
Although the mandatory requirement of the RXFP2 signaling for transabdominal testis descent is unequivocally demonstrated, the role of the RXFP2 signal during the process of the inguinoscrotal phase is quite elusive. Using an organ culture system, treatment of DHT alone did not significantly enhance postnatal gubernacular cell division in the absence of RXFP2 ligands despite the fact that it markedly increased Rxfp2 transcripts. The mouse Leydig tumor cell line MA-10 is known to secrete RXFP2 cognate ligand INSL3 but not testosterone, and human relaxin is capable of activating RXFP2 signaling (Hsu et al. 2002, Bathgate et al. 2006). However, BrdU incorporation studies showed that neither relaxin nor MACM alone stimulated cell proliferation in the postnatal gubernacular tissues from LHR null mice, unless they were co-treated with DHT. Indeed, relaxin significantly increased cell division when DHT was present. Remarkably, blockade of AR activity by flutamide, similar to suppression of Rxfp2 expression by siRNA or squelch of RXFP2 signaling by a competitive INSL3i, abolished INSL3-induced cell proliferation. Taken together, these data suggest that androgen regulation of inguinoscrotal testis descent is mediated by induction of gubernacular Rxfp2 expression and then activation of the RXFP2 signaling pathway, which in turn, stimulates development of the gubernaculum. This notion is further supported by the result that postnatal co-administration of INSL3i and testosterone in vivo curtailed androgen induction in the inguinoscrotal testis descent in LhrKO mice. This competitive INSL3i has been shown to cause cryptorchidism in a dose-dependent fashion when it was administrated to mid-pregnant rats (Bullesbach et al. 2008). Testis descent in half of the LhrKO animals induced by TRT was not prevented by INSL3i combined treatment, which may be due to a suboptimal dose and/or route of administration of the inhibitor.
It is known that the differentiation of gubernacular mesenchymal cells into muscle cells and the growth of cremaster muscle (striated muscle in nature) are essential during inguinoscrotal testis descent (Wensing 1986, Hrabovszky et al. 2002). Although androgen signaling is required for appropriate formation of striated muscle, little is currently known of the mechanism by which androgen signaling influences differentiation of the mesenchymal cells to myofibers in the gubernaculum and ultimately to form cremasteric muscle during inguinoscrotal testis descent. The present study demonstrated that the combined treatment of DHT and INSL3, but not DHT alone, resulted in a disappearance of a mesenchymal cell marker CD44, and an appearance of a structural muscle-specific protein troponin (Shefer et al. 2004, Kadivar et al. 2006). More importantly, combined treatment with either flutamide or INSL3i with DHT and INSL3 effectively prohibited the differentiation of mesenchymal cells to myofibers. These observations provide the experimental evidence to support the speculation that the RXFP2 signaling plays a role in myogenesis (Tanyel 2004). Thus, we envision that androgen acts in part via the RXFP2 signaling pathway that stimulates not only mesenchymal cell proliferation but also differentiation into myofiber during the inguinoscrotal phase of testis descent.
The present study illustrates a direct action of androgen in the gubernaculum to maintain the expression of Rxfp2 that is essential for inguinoscrotal testis descent. It provides a possible explanation indicating that overexpression of Insl3 alone cannot correct the inguinoscrotal cryptorchid phenotype of Gnrhr mutant mice because these animals, the same as LhrKO mice, have severe androgen deficiency (Feng et al. 2006). Androgen is known to be a pleiotropic steroid hormone which has genomic and nongenomic actions (Gelmann 2002). This study does not preclude the possibility that androgen may exert broader effects. In fact, the effects of the RXFP2 signal on proliferation and differentiation of gubernacular mesenchymal cells required the presence of androgen, suggesting that the androgen signal pathway is necessary for sustaining the expression of Rxfp2 and may also work in collaboration with the RXFP2 signal pathway to regulate gubernacular development during inguinoscrotal testis descent.
Materials and Methods
Animals
Mice were maintained as required under the National Institutes of Health guidelines for the Care and Use of Laboratory Animals. All studies have been approved by the Animal Care and Use Committee of the University of Louisville. Generation of the LhrKO mice has previously been reported (Lei et al. 2001). Adult male and female heterozygous mice were mated to obtain WT (+/+), heterozygous (+/−), and homozygous (−/−) animals. The genotype was determined by PCR with tail genomic DNA as described previously (Yuan et al. 2006). All the animals were maintained on 12h light:12h darkness cycles with food and water provided ad libitum.
Male mice that were 1, 7, 14, and 22 days of age were killed by cervical dislocation. Thirty- and sixty-day-old male mice of WT, homozygote with or without TRT were killed using deep anesthesia via i.p. injection of sodium pentobarbital. The abdominal cavity was opened by a sagittal incision. The gubernacula and epididymides were harvested for subsequent experiments. Three mice in each age or treatment group were used to determine postnatal expression of AR and RXFP2 in the gubernaculum.
Testosterone treatment
Twenty-two-day-old WT male mice (n=5) received a single s.c. injection of 10 mg TP dissolved in sesame oil (Sigma). Control animals (n=5) received an injection of sesame oil. Mice were killed at 16 and 24 h after the injection, and the gubernacula were collected for RT-PCR analysis of Rxfp2 mRNA levels.
For TRT, 21-day time release pellets containing 5 mg testosterone (Innovative Research America, Sarasota, FL, USA) were subcutaneously implanted into Lhr null male animals (n=6) at 30 days of age, corresponding to puberty when circulating testosterone levels normally begin to rise. The pellets represent a ‘Matrix Driven Delivery’ system integrating the principles of diffusion, erosion, and concentration gradient resulting in a biodegradable matrix that effectively and continuously releases testosterone. The therapy resulted in an increase in serum testosterone levels to about 800±100 ng/dl (Lei et al. 2001). Null mice for controls (n=6) were implanted only with placebo pellets (Innovative Research America). The age of animals at the end of the therapy corresponded approximately to the age of sexual maturity (6–8 weeks), and the animals were killed to recover the gubernacula.
Treatment with insulin-like factor 3 inhibitor (INSL3i, also known as RLFi)
LhrKO male mice (n=3) at 30 days of age received either a single s.c. injection of 2 mg TP or co-treatment of a single s.c. injection of 2 mg TP and a single i.p. injection of 0.5 μg INSL3i in the morning daily for 3 days. INSL3i, a human INSL3 A-chain derivative (Ades-1–8), was chemically synthesized and characterized as described in detail elsewhere (Bullesbach & Schwabe 2005). Control animals were injected with vehicles (sesame oil and PBS). On the fifth day, all animals were intraperitoneally injected with bromodeoxyuridine (BrdU, 100 mg/kg body weight, Sigma), and were killed 2 h later. The gubernacula were collected, fixed overnight in 10% buffered formalin, and embedded in paraffin. Serial 5 μm sections were cut for detection of BrdU incorporation by immunohistochemistry.
To determine whether INSL3i interfered with the androgen-induced inguinoscrotal phase of testis descent in LhrKO mice, 21-day time release pellets containing 5 mg of testosterone were subcutaneously implanted into 60-day-old Lhr null male mice as described above. These animals were separated into two groups. One group (n=4) received a daily i.p. injection of INSL3i at a concentration of 4 μg/kg body weight. The control group (n=4) received a daily i.p. injection of 120 μl PBS. The positions of the testes were checked daily. After 18 days, these mice were killed, and the positions of the testes were recorded.
Gubernacular organ culture and hormonal treatments
Organ cultures of gubernacula were performed according to Cooke et al. (1987) and Kubota et al. (2002). Briefly, gubernacula were harvested from 22-day-old WT and 30-day-old Lhr null male mice, and were cut into 1 mm3 pieces in a phenol red-free DMEM (Sigma) on ice. Five to ten tissue pieces were placed on a 0.45 μm filter (Millipore Corp., Bedford, MA, USA), and were cultured in a 35-mm well containing 1 ml serum- and phenol red-free DMEM supplemented with Gibco antibiotic–antimycotic solution (100 units/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B, Invitrogen), and 2 mM L-glutamine (Cellgro, Manassas, VA, USA) in a humidified incubator at 37 °C with 5% CO2 atmosphere.
After 3 h of pre-incubation, the tissue pieces were treated with 2×10−6, 2×10−7, and 2 ×10−8 M DHT (Sigma), respectively, for 3, 6, 12, and 36 h. In separate experiments, gubernacular tissue pieces were incubated with or without 2×10−6 M DHT for 12 h in the presence or absence of its antagonist flutamide (1.8×10−6 M, Sigma). The tissue pieces for controls were incubated with 0.02% ethanol. At the end of incubation, tissue pieces were collected and processed for RT-PCR analysis of Rxfp2 mRNA levels.
To determine the effect on cell proliferation in gubernacular organ cultures, the tissue pieces were incubated for 30 h with DHT (2×10−6 M), MACM, or DHT combined with MACM or variable concentrations of recombinant human relaxin (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA, USA). Then, BrdU (5×10−5 M) was added to the culture media for an additional 18 h before harvesting for determination of BrdU incorporation. The mouse MA-10 Leydig tumor cell line (a gift from Dr Ascoli, The University of Iowa, Iowa City, IA, USA) is known to produce and secrete INSL3 but not testosterone (Houk et al. 2004, Anand-Ivell et al. 2009). MACM was prepared by culturing MA-10 cells in a serum- and phenol red-free DMEM for 24 h, and the medium was collected for use in gubernacular organ cultures.
All these experiments were repeated five times, and the gubernacular tissues used in each experiment were pooled from two to three mice.
Gubernacular primary cell culture and Rxfp2 siRNA transfection
Mouse gubernacular primary cell cultures were performed according to Visser & Heyns (1995) and Boockfor et al. (2001). Briefly, gubernacula from prepubertal WT mice were removed and placed into DMEM, and were cut into 1 mm3 on ice. The tissue pieces were incubated in DMEM containing 1 mg/ml collagenase type 1 and 0.13 mg/ml DNase-1 (Sigma) for 1 h at 37 °C with constant agitation. The cell suspensions were then centrifuged for 5 min at 250 g. The dispersed cells were grown with DMEM supplemented with 10% fetal bovine serum (FBS), antibiotic–antimycotic solution (Invitrogen) in a humidified incubator at 37 °C with 5% CO2 and 95% air. The medium was then changed every other day.
The cells at about 50% confluence were used for Rxfp2 siRNA transfection. Rxfp2 siRNA was purchased from Santa Cruz Biotech Inc. (sc-40180; Santa Cruz, CA, USA) which consisted of a pool of three sequences raised against mouse Rxfp2 mRNA accession no. NM_080468 (strand A, 5′-CAGCAACGUAACAUUACUAtt-3′; strand B, 5′-CCUGUCUAGCAAUAUGAUAtt-3′; and strand C, 5′-GAAGUUCCUUGUCAUAGUAtt-3′). Typically, a mixture of 0.25 μg Rxfp2 siRNA, 100 μl Opti-MEM, and 4 μl Lipofectamine 2000 (Invitrogen) was added to the cells in a 15-mm well and incubated overnight. The transfection medium was replaced with 0.5 ml DMEM containing 10% FBS for an additional 24 h, and these cells were used for subsequent experiments. siGLO-green transfection indicator, a fluorescent oligonucleotide that localizes to the nucleus (D-001630, Dharmacon, Lafayette, CO, USA), was co-transfected with Rxfp2 siRNA to monitor the relative efficiency of transfection. A control siRNA (sc-36869, Santa Cruz Biotech Inc.) consisting of a scrambled RNA sequence was transfected in parallel with Rxfp2 siRNA as a specificity control for the Rxfp2 silencing. RT-PCR and western blotting were used to determine the effectiveness of Rxfp2 siRNA in suppression of the Rxfp2 mRNA and its protein levels in gubernacular cells.
Gubernacular cell proliferation assay
Gubernacular cells, cultured in 96-well plates (1.5×104 cells/well), were treated for 18 h with DHT (2×10−6 M), flutamide (2×10−6 M), INSL3 (1 ng/ml), and INSL3i (2 ng/ml) alone or co-treated with these compounds as indicated in Fig. 8. INSL3 was chemically synthesized and characterized as described in detail elsewhere (Bullesbach & Schwabe 2005). In siRNA experiments, the cells were transfected with Rxfp2 or scramble siRNA 24 h prior to co-treatment of DHT (2×10−6 M) and INSL3 (1 ng/ml). BrdU was then added to all the cultures, and incubation was continued for an additional 6 h. The cells were then processed for the determination of BrdU incorporation by a commercial kit following the procedure recommended by the manufacturer (Millipore Corp). This experiment was repeated five times, and three mice were used each time.
Gubernacular cells differentiation assessment
Gubernacular cells were cultured in chamber slides, and were treated with DHT, DHT/INSL3, DHT/INSL3/flutamide, and DHT/INSL3/INSL3i, respectively, in a serum- and phenol red-free DMEM for 24 h. The concentrations of these compounds were the same as in the cell proliferation assay. These cells were then incubated with a differentiation medium (phenol red-free DMEM containing 2% horse serum) for 3 days. The cells were fixed in methanol for immunostaining. This experiment was repeated three times, and three mice were used each time.
Immunostaining
The immunostaining procedures for RXFP2 and AR in the epididymis and gubernaculum tissue sections were conducted by an avidin–biotin complex (ABC) immunoperoxidase method (Vector Laboratories, Burlingame, CA, USA) as previously described (Yuan et al. 2006). Briefly, tissue sections were pre-treated with an antigen unmasking solution (Vector Laboratories) at 100 °C for 20 min, and formalin-fixed gubernacular cells were permeated with 0.3% Triton X-100 at room temperature for 20 min. Endogenous peroxidase activity was blocked by incubation of 3% H2O2 in methanol for 10 min. The slides were then incubated overnight at 4 °C with 1:250 diluted rabbit polyclonal antibody against mouse RXFP2 (LS-A4753, LifeSpan Biosciences Inc., Seattle, WA, USA) or 1:50 diluted polyclonal antibodies against AR (sc-816, Santa Cruz Biotech Inc). After rinsing in PBS, the slides were incubated with anti-rabbit IgG secondary antibody followed by ABC reagent and diaminobenzidine (DAB) chromogen (Santa Cruz Biotech Inc). RXFP2 antibody pre-adsorbed with excess corresponding antigen (LS-P4753, LifeSpan Biosciences Inc.) and omission of primary antibodies were used as procedural controls. Immunostained sections were assessed by a semi-quantitative method based on a five-level scale by two researchers independently. Positivity and positive cells were graded as negative (−), weak (+), moderate (++), strong (+++), or strongest (++++).
For immunostaining of BrdU, the slides were sequentially exposed to 0.1% trypsin solution (Sigma) for 20 min and 2 M HCl for 30 min at 37 °C. After rinsing in PBS, the slides were incubated with 1:800 diluted BrdU antibody (B8434, Sigma) for 2 h followed by the secondary antibody (HRP-conjugated sheep anti-mouse Ig, GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) for 1 h at 37 °C. BrdU-positive nuclei were detected by incubation of the slides with DAB solution, and then the slides were counterstained with Gill’s hematoxylin to visualize BrdU-negative nuclei. Both BrdU-positive and -negative nuclei were counted in randomly selected four fields for each section (about 500–800 nuclei per section) under an Olympus microscope. BrdU incorporation index was determined by the ratio of BrdU-labeled cells to total cells.
For immunofluorescence, the chamber slides with gubernaculum cells attached were incubated with 1:50 diluted monoclonal antibodies against CD44 (sc-18849) and troponin (sc-48347) overnight at 4 °C (Santa Cruz Biotech Inc). Immunofluorescent staining was detected by incubation of the slides with a rhodamine-conjugated secondary antibody, and the slides were counterstained with Hochest dye (Sigma) to labeled nuclei and examined under an Olympus fluorescence microscope. Omission of primary antibodies was used as a procedural control. Immunostained sections were assessed by the semi-quantitative method as described above.
Semi-quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen), and was reverse transcribed using avian myeloblastosis virus reverse transcriptase (Promega) and random oligonucleotide hexamers (Invitrogen). PCR primers were designed according to the sequences obtained from GenBank (Ar, NM_013476 and Rxfp2, NM_080468) using the Vector NTI 9.0 computer program (InforMax, Frederick, MD, USA), and were synthesized by Operon Technologies (Alameda, CA, USA), which are listed below. The Ar primer set was designed to amplify a product of 303 bp that covered the exons 1–3 and the Rxfp2 primer set amplified a product of 332 bp that spanned the exons 16 and 17. The house keeping gene, ribosomal protein large subunit 19 (Rpl19), was co-amplified with Ar and Rxfp2 as an internal control for both complementary DNA (cDNA) quantity and quality.
Rxfp2: 5′-TTCCCTTTCAGCAACCTGCGC-3′ (forward),
5′-ACAGCCACCTCCTTCCCGATGT-3′ (reverse);
Ar: 5′-ATGGGACCTTGGATGGAGAA-3′ (forward),
5′-CCCTGCTTCATAACATTTCCG-3′ (reverse);
Rpl19: 5′-CTCAGGCTACAGAAGAGGCTT-3′ (forward),
5′-GGACAGAGTCTTGATGATCTC-3′ (reverse).
PCRs were run at 94 °C for 45 s, 57 °C for 45 s, then 72 °C for 75 s for 24 cycles which corresponded to the exponential phase of the amplification curve, and the reactions were followed by 72°C for 7 min for the last extension with the reaction mixture containing 1.0 μM forward and reverse primers, 2 mM MgCl2, 1 μl cDNA, 200 μM dNTPs, and 1.5 units of Taq DNA polymerase. The amplified products were electrophoresed in 1.5% agarose gel and stained with ethidium bromide. Densitometric analysis of the specific bands using TotalLab version 2.01 (Nonlinear USA, Durham, NC, USA) image analysis software normalized to Rpl19.
Immunoblotting
The epididymis, gubernacular tissues, and the cells were homogenized in a solution containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.5 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, and 5 μg/ml pepstatin. Aliquots of the lysates were separated by 8% SDS-PAGE and transferred to polyvinyldine fluoride membranes. After blocking with 3% nonfat dry milk, the membranes were incubated overnight at 4 °C with polyclonal antibodies against RXFP2 (1:1500) and AR (1:1000) respectively. The blots were then incubated with a peroxidase-labeled secondary antibody. The immune complexes were detected using a chemiluminescence detection system according to the procedure recommended by the manufacturer (GE Healthcare Bio-Sciences Corp). All the membranes were re-blotted with 1:2000 diluted MAB against β-actin for the sample loading control. RXFP2 antibody pre-adsorbed with excess corresponding antigen and omission of primary antibodies were used as procedural controls. Densitometric analysis of the specific bands using TotalLab version 2.01 (Nonlinear USA) image analysis software normalized to β-actin.
Statistical analyses
Statistical analysis was performed by one-way ANOVA followed by Tukey–Kramer multiple comparison post test or Fisher’s exact test, where appropriate using an Instat version 3.06 program (Graphpad Software, San Diego, CA, USA). A two-sided test with P values <0.05 was considered statistically significant.
Acknowledgments
Funding
This work was supported by Grant R01-HD057501 from the National Institutes of Health.
We thank Leta Weedman for editing the manuscript.
Footnotes
Declaration of interest
The authors declare that there are no conflicts of interests that could be perceived as prejudicing the impartiality of the research reported.
References
- Amann RP, Veeramachaneni DN. Cryptorchidism in common eutherian mammals. Reproduction. 2007;133:541–561. doi: 10.1530/REP-06-0272. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Heng K, Hafen B, Setchell B, Ivell R. Dynamics of INSL3 peptide expression in the rodent testis. Biology of Reproduction. 2009;81:480–487. doi: 10.1095/biolreprod.109.077552. [DOI] [PubMed] [Google Scholar]
- Barthold JS, Mahler HR, Newton BW. Lack of feminization of the cremaster nucleus in cryptorchid androgen insensitive rats. Journal of Urology. 1994;152:2280–2286. doi: 10.1016/s0022-5347(17)31658-0. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacological Reviews. 2006;58:7–31. doi: 10.1124/pr.58.1.9. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Agoulnik AI. INSL3/LGR8 role in testicular descent and cryptorchidism. Reproductive Biomedicine Online. 2005;10:49–54. doi: 10.1016/s1472-6483(10)60803-6. [DOI] [PubMed] [Google Scholar]
- Boockfor FR, Fullbright G, Bullesbach EE, Schwabe C. Relaxin-like factor (RLF) serum concentrations and gubernaculum RLF receptor display in relation to pre- and neonatal development of rats. Reproduction. 2001;122:899–906. doi: 10.1530/rep.0.1220899. [DOI] [PubMed] [Google Scholar]
- Bullesbach EE, Schwabe C. LGR8 signal activation by the relaxin-like factor. Journal of Biological Chemistry. 2005;280:14586–14590. doi: 10.1074/jbc.M414443200. [DOI] [PubMed] [Google Scholar]
- Bullesbach EE, Boockfor FR, Fullbright G, Schwabe C. Cryptorchid-ism induced in normal rats by the relaxin-like factor inhibitor. Reproduction. 2008;135:351–355. doi: 10.1530/REP-07-0330. [DOI] [PubMed] [Google Scholar]
- Cain MP, Kramer SA, Tindall DJ, Husmann DA. Flutamide-induced cryptorchidism in the rat is associated with altered gubernacular morphology. Urology. 1995;46:553–558. doi: 10.1016/S0090-4295(99)80272-6. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young PF, Cunha GR. A new model system for studying androgen-induced growth and morphogenesis in vitro: the bulbourethral gland. Endocrinology. 1987;121:2161–2170. doi: 10.1210/endo-121-6-2161. [DOI] [PubMed] [Google Scholar]
- Elder JS. Ultrasonography is unnecessary in evaluating boys with a nonpalpable testis. Pediatrics. 2002;110:748–751. doi: 10.1542/peds.110.4.748. [DOI] [PubMed] [Google Scholar]
- Feng S, Bogatcheva NV, Truong A, Engel W, Adham IM, Agoulnik AI. Over expression of insulin-like 3 does not prevent cryptorchidism in GNRHR or HOXA10 deficient mice. Journal of Urology. 2006;176:399–404. doi: 10.1016/S0022-5347(06)00519-2. [DOI] [PubMed] [Google Scholar]
- Feng S, Bogatcheva NV, Truong A, Korchin B, Bishop CE, Klonisch T, Agoulnik IU, Agoulnik AI. Developmental expression and gene regulation of insulin-like 3 receptor RXFP2 in mouse male reproductive organs. Biology of Reproduction. 2007;77:671–680. doi: 10.1095/biolreprod.107.060442. [DOI] [PubMed] [Google Scholar]
- Feng S, Ferlin A, Truong A, Bathgate R, Wade JD, Corbett S, Han S, Tannour-Louet M, Lamb DJ, Foresta C, et al. INSL3/RXFP2 signaling in testicular descent. Annals of the New York Academy of Sciences. 2009;1160:197–204. doi: 10.1111/j.1749-6632.2009.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes, and environment in human cryptorchidism. Endocrine Reviews. 2008;29:560–580. doi: 10.1210/er.2007-0042. [DOI] [PubMed] [Google Scholar]
- Gelmann EP. Molecular biology of the androgen receptor. Journal of Clinical Oncology. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- Gorlov IP, Kamat A, Bogatcheva NV, Jones E, Lamb DJ, Truong A, Bishop CE, McElreavey K, Agoulnik AI. Mutations of the GREAT gene cause cryptorchidism. Human Molecular Genetics. 2002;11:2309–2318. doi: 10.1093/hmg/11.19.2309. [DOI] [PubMed] [Google Scholar]
- Houk CP, Pearson EJ, Martinelle N, Donahoe PK, Teixeira J. Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology. 2004;145:1269–1275. doi: 10.1210/en.2003-1046. [DOI] [PubMed] [Google Scholar]
- Hrabovszky Z, Di Pilla N, Yap T, Farmer PJ, Hutson JM, Carlin JB. Role of the gubernacular bulb in cremaster muscle development of the rat. Anatomical Record. 2002;267:159–165. doi: 10.1002/ar.10092. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Semyonov J, Park JI, Chang CL. Evolution of the signaling system in relaxin-family peptides. Annals of the New York Academy of Sciences. 2005;1041:520–529. doi: 10.1196/annals.1282.078. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Acerini CL. Factors controlling testis descent. European Journal of Endocrinology. 2008;159(Suppl 1):S75–S82. doi: 10.1530/EJE-08-0458. [DOI] [PubMed] [Google Scholar]
- Husmann DA, Levy JB. Current concepts in the pathophysiology of testicular undescent. Urology. 1995;46:267–276. doi: 10.1016/s0090-4295(99)80207-6. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Hasthorpe S. Testicular descent and cryptorchidism: the state of the art in 2004. Journal of Pediatric Surgery. 2005;40:297–302. doi: 10.1016/j.jpedsurg.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Hasthorpe S, Heyns CF. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocrine Reviews. 1997;18:259–280. doi: 10.1210/edrv.18.2.0298. [DOI] [PubMed] [Google Scholar]
- Ivell R, Bathgate RA. Reproductive biology of the relaxin-like factor (RLF/INSL3) Biology of Reproduction. 2002;67:699–705. doi: 10.1095/biolreprod.102.005199. [DOI] [PubMed] [Google Scholar]
- Kadivar M, Khatami S, Mortazavi Y, Shokrgozar MA, Taghikhani M, Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Temelcos C, Bathgate RA, Smith KJ, Scott D, Zhao C, Hutson JM. The role of insulin 3, testosterone, Mullerian inhibiting substance and relaxin in rat gubernacular growth. Molecular Human Reproduction. 2002;8:900–905. doi: 10.1093/molehr/8.10.900. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Molecular Endocrinology. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Ponnuru P, Li X, Yang ZW, Rao CV. Testicular phenotype in luteinizing hormone receptor knockout animals and the effect of testosterone replacement therapy. Biology of Reproduction. 2004;71:1605–1613. doi: 10.1095/biolreprod.104.031161. [DOI] [PubMed] [Google Scholar]
- Main KM, Skakkebaek NE, Toppari J. Cryptorchidism as part of the testicular dysgenesis syndrome: the environmental connection. Endocrine Development. 2009;14:167–173. doi: 10.1159/000207485. [DOI] [PubMed] [Google Scholar]
- Marin P, Ferlin A, Moro E, Rossi A, Bartoloni L, Rossato M, Foresta C. Novel insulin-like 3 (INSL3) gene mutation associated with human cryptorchidism. American Journal of Medical Genetics. 2001;103:348–349. [PubMed] [Google Scholar]
- Nantermet PV, Xu J, Yu Y, Hodor P, Holder D, Adamski S, Gentile MA, Kimmel DB, Harada S, Gerhold D, et al. Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. Journal of Biological Chemistry. 2004;279:1310–1322. doi: 10.1074/jbc.M310206200. [DOI] [PubMed] [Google Scholar]
- New HV, Mudge AW. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. Nature. 1986;323:809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- Overbeek PA, Gorlov IP, Sutherland RW, Houston JB, Harrison WR, Boettger-Tong HL, Bishop CE, Agoulnik AI. A transgenic insertion causing cryptorchidism in mice. Genesis. 2001;30:26–35. doi: 10.1002/gene.1029. [DOI] [PubMed] [Google Scholar]
- Payne AH. Hormonal regulation of cytochrome P450 enzymes, cholesterol side-chain cleavage and 17 alpha-hydroxylase/C17–20 lyase in Leydig cells. Biology of Reproduction. 1990;42:399–404. doi: 10.1095/biolreprod42.3.399. [DOI] [PubMed] [Google Scholar]
- Popper P, Micevych PE. The effect of castration on calcitonin gene-related peptide in spinal motor neurons. Neuroendocrinology. 1989;50:338–343. doi: 10.1159/000125243. [DOI] [PubMed] [Google Scholar]
- Popper P, Ulibarri C, Micevych PE. The role of target muscles in the expression of calcitonin gene-related peptide mRNA in the spinal nucleus of the bulbocavernosus. Brain Research. Molecular Brain Research. 1992;13:43–51. doi: 10.1016/0169-328x(92)90043-b. [DOI] [PubMed] [Google Scholar]
- Sadeghian H, Anand-Ivell R, Balvers M, Relan V, Ivell R. Constitutive regulation of the Insl3 gene in rat Leydig cells. Molecular and Cellular Endocrinology. 2005;241:10–20. doi: 10.1016/j.mce.2005.03.017. [DOI] [PubMed] [Google Scholar]
- van der Schoot P. Androgens in relation to prenatal development and postnatal inversion of the gubernacula in rats. Journal of Reproduction and Fertility. 1992;95:145–158. doi: 10.1530/jrf.0.0950145. [DOI] [PubMed] [Google Scholar]
- Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. Journal of Cell Science. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- Tanyel FC. The descent of testis and reason for failed descent. Turkish Journal of Pediatrics. 2004;46(Supplement):7–17. [PubMed] [Google Scholar]
- Tomiyama H, Hutson JM, Truong A, Agoulnik AI. Transabdominal testicular descent is disrupted in mice with deletion of insulinlike factor 3 receptor. Journal of Pediatric Surgery. 2003;38:1793–1798. doi: 10.1016/j.jpedsurg.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Visser JH, Heyns CF. Proliferation of gubernaculum cells induced by a substance of low molecular mass obtained from fetal pig testes. Journal of Urology. 1995;153:516–520. doi: 10.1097/00005392-199502000-00074. [DOI] [PubMed] [Google Scholar]
- Wensing CJ. Testicular descent in the rat and a comparison of this process in the rat with that in the pig. Anatomical Record. 1986;214:154–160. doi: 10.1002/ar.1092140208. [DOI] [PubMed] [Google Scholar]
- Yuan FP, Lin DX, Rao CV, Lei ZM. Cryptorchidism in LhrKO animals and the effect of testosterone-replacement therapy. Human Reproduction. 2006;21:936–942. doi: 10.1093/humrep/dei433. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Molecular Endocrinology. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]