Abstract
Objective
Weight loss and maintenance can be particularly challenging for postmenopausal women given the changes in body composition, metabolism, and lifestyle that can accompany the menopausal transition. Peptides mediating energy homeostasis (i.e., ghrelin, leptin, adiponectin, and insulin) may play an important role in the weight and body composition changes of postmenopausal women, and may in turn be affected by hormone therapy (HT) use. This study examines how success with weight loss may be related to peptides mediating energy homeostasis and HT use.
Methods
The present analysis involves 200 women from a lifestyle intervention trial in overweight, postmenopausal women for whom data on the peptides ghrelin, leptin, adiponectin, and insulin were collected at 0 and 18 months. Peptide levels were compared to changes in weight from 0-18 and18-30 months.
Results
Baseline peptide levels were not significantly related to future weight change. From 0-18 months, ghrelin (p=0.0005) and adiponectin (p=<0.0001) levels increased, while leptin (p=<0.0001), and insulin (p=0.0003) decreased with increasing amount of weight loss. However, only leptin change was related to 18-30 month weight change. Women who were on HT at 0 months but discontinued by 18 months had a greater increase in ghrelin from 0-18 months compared to women with continuous HT use or non-use.
Conclusions
In overweight, postmenopausal women, changes in energy homeostasis peptides relate to both concurrent and future weight change. Future studies should continue to address the how ghrelin, leptin, insulin, and adiponectin contribute to body composition changes and weight loss maintenance after menopause.
Keywords: weight loss, energy homeostasis, peptide therapy, postmenopause
Introduction
Weight gain after menopause is common1 and is associated with an increased likelihood of chronic health problems.2 With the decline of estrogen levels, a postmenopausal woman’s body often changes to include a higher composition of visceral fat.1, 3 These changes in weight and body composition may be related to changes in levels of certain energy homeostasis peptides that also may occur with menopause.4-6 Adverse changes in the energy homeostasis peptides in certain women may make it difficult for these women to lose weight and maintain weight loss, even with an intensive behavioral weight loss program.7-8
The current study evaluated levels of four peptides that mediate energy homeostasis: ghrelin, leptin, and adiponectin, and insulin.9-12 Ghrelin increases food intake and decreases energy expenditure,13-14 while leptin has the opposite effect.15-17 Adiponectin appears induce weight loss primarily by increasing energy expenditure;18-20 it also decreases insulin resistance.21 These peptides could influence weight change in different ways. Unfavorable changes in peptide levels with weight loss (for example, increased ghrelin or decreased leptin) might make it more difficult to maintain weight loss.
Furthermore, it may be possible that the estrogen (+/− progesterone) hormone therapy (HT) administered to some postmenopausal women alters levels of the energy homeostasis peptides. Studies to date have shown that HT may impact energy homeostasis peptides, but results are inconsistent across studies.22 For example, HT has been shown to decrease leptin levels in one population of postmenopausal women,23 but was shown to have no effect in others.24,25 To our knowledge, there have been no studies on the effects of initial administration and then discontinuation of HT on the energy homeostasis peptides and weight change.
In this study, we sought to understand specifically how energy homeostasis peptides relate to increased body weight and difficulty with weight loss. We examined fasting blood levels of ghrelin, leptin, adiponectin, and insulin (at 0 and 18 months) in 200 overweight, postmenopausal women, to determine whether baseline or 0-18 month change in these peptides are related to 0-18 (concurrent) and/or 18-30 (future) month weight and/or body composition changes. In addition, we examined potential relationships between the energy homeostasis peptides and HT use patterns.
Methods
Study Design
To explore relationships between weight change and peptides mediating energy homeostasis, data from the Women on the Move through Activity and Nutrition (WOMAN) study were examined. The WOMAN study is a randomized clinical trial of a non-pharmacological lifestyle intervention of diet and physical activity to modify or reduce subclinical markers of cardiovascular disease (CVD). The WOMAN study design, including a description of the health education (HE) and lifestyle change (LC) groups, has been previously reported.26
Primary goals for women in the LC group were to achieve 150 minutes/week of moderate physical activity, to follow an eating pattern low in total fat (17%) and saturated fat (<4%), and to achieve 10% weight loss. The study meal pattern emphasized grains, fruits, and vegetables as well as the use of functional foods, such as stanol-ester-containing margarine, increased sources of omega-3 fatty acids, and foods high in soluble fiber. There were significant differences in weight, waist circumference, nutrient intake, physical activity and CVD risk factors between the HE and LC groups at 18 months.27 The total duration of the WOMAN study was 48 months, although the lifestyle intervention was stopped at approximately 36 months due to lack of continued funding.
Study Population
Five hundred and eight postmenopausal women were recruited for the WOMAN study, primarily through direct mailing from selected zip codes in Allegheny County, Pennsylvania from April 2002 to October 2003. Eligibility criteria for enrollment into the study included waist circumference ≥ 80 cm, body mass index (BMI) between 25 – 39.9 kg/m2, not currently taking lipid lowering drugs and having a low density lipoprotein cholesterol (LDL-C) level between 100 – 160 mg/dL, no physical limitations that would preclude walking, no known diabetes, and no diagnosed psychotic disorder or depression. The current analyses involve a convenience sample of women with data on ghrelin, leptin, adiponectin, and insulin at 0 and 18 months (N = 200, approximately the first 200 women enrolled in the study). Due to funding constraints, we did not perform ghrelin, leptin, and adiponectin assays for the remaining 308 women in the WOMAN study.
The first 200 women enrolled in the study are different from the subsequent 308 women enrolled in that a significantly greater percentage of the initial enrollees were on HT at baseline (79.5% versus 47.1%, respectively; p<0.0001). After the results of the Women’s Health Initiative (WHI) study were released,28 new WOMAN participants were not required to have been on HT at the time of randomization. However, most of these women had been on HT within six months prior to randomization. Also, more of the women in the present study were Caucasian (93% vs. 85%; p=0.01). The women in the present study are similar to the remaining participants in terms of baseline weight (177.8 vs. 181.8 lb; p=0.09), waist circumference (106.0 vs. 105.8 cm; p=0.8), age (57.1 vs. 56.9 years; p=0.3), and percentage in lifestyle intervention group (49.5% vs. 50.7%; p=0.8).
All participants provided written informed consent and all protocols were approved by the institutional review board at the University of Pittsburgh.
Measurement of Peptides Mediating Energy Homeostasis
Peptides were measured from fasting blood samples at baseline and 18 months; peptides assessed include ghrelin, leptin, adiponectin, and insulin. Total ghrelin levels were measured by commercial radioimmunoassay (RIA) (Linco Research, Inc.). This assay utilizes 125I labeled ghrelin and a ghrelin antiserum to determine the level of total ghrelin by the double antibody/PEG technique. The limit of sensitivity is 100 pg/ml and the response is linear to 10ng/ml; the intra-assay coefficient of variation is 5.5%. Although only acylated ghrelin is bioactive, levels of total ghrelin may be a reasonable surrogate of the bioactive form, because the ratio of total to bioactive ghrelin appears to remain fairly constant under conditions that affect ghrelin.29 Adiponectin, leptin, and insulin levels were also assayed using commercial RIA kits (Linco Research, Inc.). For leptin, the intra-assay and inter-assay coefficients of variation were 6.6+0.8% and 5.5+0.9%, respectively. Total (rather than high molecular weight) adiponectin levels were measured. Changes in peptide levels were calculated by subtracting baseline values from values measured at 18 months. We also calculated proportional changes in peptide levels by dividing the changes in peptide levels by the baseline values.
Weight and Other Anthropometric Data
Weight, height, and waist circumference were measured at clinic visits at baseline, 18 months, and 30 months. Women were divided into quartiles of weight change for each time interval of interest in the study (0-18, 18-30 months). From 0-18 months, the quartiles of weight change were: Quartile 1 = 0 – 18 pounds gained; Quartile 2 = 0 – 7.6 pounds lost; Quartile 3 = 7.6 – 18 pounds lost; and Quartile 4 = 18 – 77.5 pounds lost. From 18-30 months, the quartiles of weight change were: Quartile 1 = 9.2 – 22 pounds gained; Quartile 2 = 4.7 – 9.2 pounds gained; Quartile 3 = 0 – 4.7 pounds gained; and Quartile 4 = 0 – 30 pounds lost.
Measurement of body composition
All subjects in the WOMAN study underwent a total body Dual Energy X-ray Absorptiometry (DXA) scan at 0 and 18 months. Total body DXA scan was performed using the pencil beam technology (QDR 1500, Hologic, Waltham, MA, USA; enhanced whole body, software version 5.71). A standard soft tissue examination includes total body and regional measurements of trunk, arms and legs to analyze body composition according to a three-compartment model (fat mass, lean tissue and bone mineral content). We computed change in total body fat mass and percent body fat between 0 and 18 months.
Hormone Therapy Use
Hormone therapy (HT) use and type of HT used were obtained through self-report at each clinic visit. In addition, participants were classified as being either current HT users or non-users at 0 and 18 months. Some women in this study discontinued HT after the Women’s Health Initiative reports,28 and variations in HT status at 0 and 18 months were compared to change in energy homeostasis peptides from 0 to 18 months. Study participants were classified as continuous HT users (on/on; n=68), continuous HT non-users (off/off; n=38), or women who discontinued HT during the 18 months of follow up (on/off; n=91); most HT discontinuation occurred within the first 6 months following baseline. Three women who started HT between 0 and 18 months were excluded from this part of the analysis.
Other Variables
Demographic characteristics of participants, including age and race, were collected at baseline.
Statistical Analyses
Baseline characteristics of the population studied were obtained by calculating mean values or percentages. Differences in peptides across quartiles of weight loss (at 0-18 and 18-30 months) were evaluated using the nonparametric Jonkheere-Terpstra test and the linear trend test. The same analysis was performed with women stratified by baseline HT use status. Similarly, quartiles of body fat composition and percentage of body fat changes were analyzed by the Jonkheere-Terpstra and linear trend tests. Differences in HT status changes from 0-18 months (on/on, on/off, off/off) for the energy homeostasis peptides were analyzed using ANOVA. Additional exploratory analyses on the 18-30 month weight change data which were restricted only to women who lost ≥ 10 lbs from 0-18 months (N=85) were also performed.
Multiple linear regression models were used to predict change in energy homeostasis peptide (dependent variable) due to quartiles of weight change at 18 months (primary independent variable) and possible confounders (baseline energy homeostasis peptide value, age, baseline weight, race, treatment group (i.e., lifestyle change vs. health education)), HT status at baseline, and change in HT status from 0-18 months. Another multivariable model was created to explore how much of the variation in weight change 0-18 months (dependent variable) was explained by changes in each of the peptides. Peptides were added to the model in a sequential fashion, starting with the peptide with the lowest p-value as determined from univariable regression models. Following addition of the peptides, the model was further adjusted for age, baseline weight, race, treatment group, and HT use, which were entered as a block of variables.
All statistical analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, North Carolina).
Results
Baseline peptides and weight change
At baseline, the mean BMI was 30.3, weight was 177.8 pounds, and waist circumference was 106.0 cm (Table 1). Baseline peptide levels were unrelated to weight change. There were no significant relationships seen between baseline levels of ghrelin, leptin, adiponectin, or insulin and successive quartiles of weight change from 0-18 or 18-30 months (data not shown). Results were the same when weight change was expressed as a continuous variable (data not shown).
Table 1.
Baseline Characteristics
| Body Mass Index | 30.3 (3.7) |
| Age (yrs) | 57.1 (2.9) |
| Weight (lbs) | 177.8 (25.1) |
| Waist Circumference (cm) | 106.0 (10.7) |
| Total Body Fat (kg) | 32.7 (6.8) |
| Total Body Fat % | 41.0 (4.0) |
| % White | 93.0% |
| % Intervention Group | 49.5% |
| % on HT at baseline | 79.5% |
| Ghrelin (pg/ml) | 866.4 (340.6) |
| Leptin (ng/ml) | 22.6 (8.3) |
| Adiponectin (ng/ml) | 18.2 (8.7) |
| CRP (μg/ml) | 4.9 (4.7) |
| Insulin (μU/ml) | 12.9 (6.6) |
| Glucose (mg/dl) | 93.9 (8.9) |
Data are presented as N% or as mean (SD)
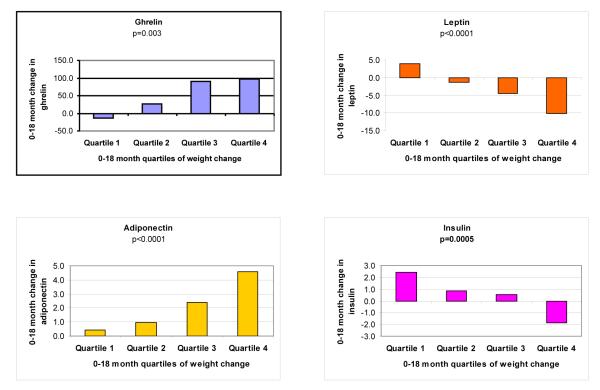
Change in peptides and weight change from 0-18 months
In contrast to baseline peptide levels, 0-18 month changes in peptides were significantly related to 0-18 month weight change quartiles (Figure 1). At 18 months, the average weight change was −10.5 lbs [range +18 lbs to −77.5 lbs]. Ghrelin and adiponectin levels increased, and leptin and insulin levels decreased with greater weight loss. Results were the same when we examined proportional changes in peptide levels and when weight change was expressed as a continuous variable (data not shown).
Figure 1.
Trends in Appetite Hormone Change for Quartiles of Weight Change (0-18 months)
*P-values determined by linear trend test (Jonkeere-Terpstra p-values also determined but not shown; all p-values from J-T test were <0.05 as well). Quartile 1 = 0 – 18 pounds gained; Quartile 2 = 0 – 7.6 pounds lost; Quartile 3 = 7.6 – 18 pounds lost; and Quartile 4 = 18 – 77.5 pounds lost.
Changes in peptides and body fat change
Change in leptin, adiponectin, and insulin from 0 to 18 months were each related to total body fat change (0 to 18 months) quartiles. Change in ghrelin was not significantly related to the total body fat quartiles (p=0.1 by linear trend test). Ghrelin, leptin, adiponectin, and insulin were all significantly related to quartiles of percentage total body fat change from 0 to 18 months (data not shown).
Effect of HT use on peptides and weight change
At baseline, 30.5% of the women in this study were on oral estrogen only, and 37% were taking oral estrogen and progesterone. At 18 months, 20% were taking oral estrogen, and 10% were taking estrogen and progesterone. In stratified analyses, baseline hormone use did not affect the relationships between energy homeostasis peptides and weight change observed in the study overall. Only change in ghrelin was found to be different between the three HT status change groups (i.e., on/on, on/off, off/off) (p=0.03), with women on/on having a 31.5 pg/ml increase, those on/off a 92 pg/ml increase, and those off/off a 3.5 pg/ml decrease in ghrelin levels.
Multivariable models
In multiple regression, weight change from 0-18 months remained associated with change in each energy homeostasis peptide after controlling for potential confounders (baseline energy homeostasis peptide value, age, baseline weight, race, treatment group, peptide therapy (HT) status at baseline, and change in HT status from 0-18 months) (Table 2). R2 values for the models ranged from 0.09 (ghrelin) to 0.48 (leptin). In the multivariable model examining predictors of weight change from 0-18 months, changes in leptin and adiponectin were found to be independently associated with weight change and remained so after potential confounders were added to the model. The R2 for the final model was 0.71.
Table 2.
Multivariable Regression Models, with energy homeostasis peptide changes from 0-18 months as outcome variables and 0-18 month weight change quartiles as primary predictor variables
| Ghrelin | Leptin | Adiponectin | Insulin | |
|---|---|---|---|---|
|
Quartile 1 (0 -18 lbs
gained) |
−0.27# | 0.82+ | −0.56+ | 0.37# |
|
Quartile 2 (0 -1.6 lbs
lost) |
−0.19* | 0.55+ | −0.43+ | 0.23* |
|
Quartile 3 (7.6 - 18 lbs
lost) |
−0.04 | 0.32+ | −0.24# | 0.17 |
|
Quartile 4 (18 - 77.5 lbs
lost) (referent) |
0 | 0 | 0 | 0 |
| R2 | 0.09 | 0.48 | 0.23 | 0.14 |
Data presented are standardized effect sizes (β-coefficients) from linear regression models with energy homeostasis peptide changes from 0-18 months as the outcome variables; 0-18 month weight change quartiles are the primary predictor variables.
All models are controlled for baseline energy homeostasis peptide value, age, baseline weight, race, treatment group (i.e., lifestyle change vs. health education), hormone therapy (HT) status at baseline, and change in HT status from 0-18 months. Quartile 4 serves as the reference group in each model.
For each model, the R2 statistic refers to the amount of variation in the outcome variable accounted for by the model. For example, 48% in the variation of leptin is explained by the final model.
p<0.05
p<0.01
p<0.001.
Change in peptides and weight change from 18-30 months
We also evaluated 0-18 month change in peptides with 18-30 month weight change (Table 3). The quartiles were different for the 18-30 month weight change, since the majority of women gained weight and only those in the top quartile lost weight. It should also be noted that the women who lost weight from 18-30 months had lost the least amount of weight from 0-18 months (−15.9 lbs vs. −4.3 lbs were lost from 0-18 months for women in quartile 1 verses quartile 4 at 18-30 months; p<0.0001). In this analysis, only the 0-18 month change leptin was found to be significantly associated with 18-30 month weight change. Women who lost weight from 18-30 months had the smallest decrease in leptin from 0-18 months. Results were the same when we examined proportional changes in peptide levels and also when we controlled for baseline weight (data not shown). Additional analyses that were limited only to those women who lost ≥ 10 lbs from 0-18 months (N=85) revealed no clear pattern between weight change from 18-30 months and change in any peptide from 0-18 months (data not shown). Results were also the same when weight change was expressed as a continuous variable (data not shown).
Table 3.
Relationships between energy homeostasis peptide changes from 0-18 months and weight change from 18-30 months
| Quartile 1 (gained 22-9.2 lbs) N=47 |
Quartile 2 (gained 9.2- 4.7 lbs) N=47 |
Quartile 3 (gained 4.7-0 lbs) N=46 |
Quartile 4 (lost 0-30 lbs) N=48 |
P-value (linear trend) |
|
|---|---|---|---|---|---|
|
Avg. Change
Ghrelin |
55.89 (187.6) | 96.60 (200.0) | 67.15 (215.3) | −5.73 (190.1) | 0.1 |
|
Avg. Change
Leptin |
−4.25 (7.2) | −5.41 (7.4) | −3.24 (6.4) | −0.76 (7.9) | 0.008 |
|
Avg. Change
Adiponectin |
2.40 (4.0) | 2.67 (4.7) | 1.66 (3.7) | 1.26 (4.2) | 0.1 |
|
Avg. Change
Insulin |
−0.53 (6.5) | 0.92 (4.4) | −0.61 (4.9) | 1.30 (6.9) | 0.3 |
Conclusion
In this study of overweight postmenopausal women, we found that with increasing amounts of weight loss, levels of ghrelin, leptin, adiponectin, and insulin changed in a linear fashion. The changes in energy homeostasis peptides that occur with weight change in this population have not yet been as well characterized in the literature. Also, the effect of HT discontinuation on energy homeostasis peptides has not been previously reported to our knowledge. We found levels of ghrelin (but not the other peptides examined) increased more over 18 months in women who had discontinued HT over the same time period.
Discussion
Baseline energy homeostasis peptide levels were unrelated to weight change at 0-18 or 18-30 month weight change intervals. Langenberg et al11 have similarly reported that baseline ghrelin, leptin, and adiponectin levels do not predict long-term weight change, and they suggest that peptide levels actually follow an individual’s weight trajectory rather than governing it. We also found that change in each of the peptide levels from 0-18 months was related to simultaneous 0-18 month weight change. The trends were similar to previous studies,10, 13, 30 with ghrelin and adiponectin increasing and leptin and insulin decreasing with weight loss. This simultaneous change in peptide level may be a direct result of a decrease in adipose tissue and energy reserves associated with weight loss.
The increasing levels of ghrelin seen with greater 0-18 month weight loss (Figure 1) may be the body’s response to a decrease in baseline energy reserve level. With weight loss, the body assumes a state of starvation, and an increase in ghrelin leads to an increase in energy reserves. These actions by ghrelin may promote weight regain after weight loss. Also, the fact that ghrelin was the only peptide to not significantly change with total body fat change may be related to the fact that of the peptides studied it interacts less directly with adipose tissue.13,15, 31-32 Loss of adipose tissue with weight loss may explain the decrease in leptin values that were seen with increasing weight loss from 0 to 18 months 27-30 (Figure 1).
When examining 0-18 month energy homeostasis peptide changes in conjunction with 18-30 month weight change, only change in leptin was associated with 18-30 month weight change (change in ghrelin was of borderline significance) (Table 3). Women who lost weight from 18-30 months had the smallest decrease in leptin from 0-18 months. This suggests that maintaining relatively higher levels of the anorexogenic peptide leptin be associated with future weight loss, since a decrease in leptin may promote weight regain.33 A decrease in leptin when one is already resistant to its actions may make weight loss maintenance even more difficult.34-36 When analyses were restricted to only women who lost ≥ 10 lbs from 0-18 months, this pattern was not seen, which could suggest that initial weight loss (i.e., weight loss from 0-18 months) may be an equally or more important factor in weight regain than changes in leptin levels during the same period.
In our study, a woman’s baseline HT status did not appear to affect relationships between energy homeostasis peptides and weight loss. Also, only change in ghrelin from 0 to 18 months was related to the changes in HT use. The fact that women on HT at baseline but off at 18 months had the greatest increase in ghrelin (92 pg/ml) over 18 months is interesting, because it may suggest that HT favorably decreases ghrelin levels and that discontinuing HT leads to an increase in ghrelin. Ghrelin is known to promote food intake and decrease energy expenditure,9, 14, 20 and so the increase in ghrelin with discontinuation of HT could promote weight gain. Ghrelin has not been investigated in relation to HT as extensively as leptin, but Lambrinoudaki and colleagues found no relationship between HT administration and ghrelin levels in a study of 88 postmenopausal women.25 Our findings should be replicated in larger studies, as our findings with regard to ghrelin and HT could also be due to chance.
Changes in these energy homeostasis peptides with menopause and the decline in estrogen levels will be an interesting area for future research. For example, Hong et al have shown that a decline in estrogen correlates with changes in adipocytokines: increased TNF-α and adiponectin, and decreased leptin.6 Others likewise have shown a decline in leptin with decreased estrogen after menopause.37 Also, estrogen has been shown to enhance leptin sensitivity but decrease insulin sensitivity in the brain.38
This study has a few limitations. There is a lack of premenopausal controls to compare with for potential differences in the energy homeostasis peptides after menopause. Also, we do not have data on serum estrogen levels, which could have been directly compared to levels of energy homeostasis peptides. In addition, as mentioned previously, the 200 women in this study are a convenience sample of the 508 women in the WOMAN study overall; these women differ from the overall WOMAN population in terms of HT use, race, and perhaps in other, unmeasured ways. Due to our relatively small sample size, there is a chance that some of our findings are due to chance. Lastly, the results of this study are also not generalizable to populations other than postmenopausal women.
In conclusion, this study suggests that differing success with weight loss in post-menopausal women may be related in part to changes in levels of energy homeostasis peptides. Future studies should continue to address how changes in peptide levels relate to simultaneous and future weight trajectories. In addition, postmenopausal women who discontinued HT after the release of WHI results may have some changes in energy homeostasis peptides such as ghrelin that could hinder the ability to lose weight. The relationships between menopause, hormone therapy, and peptides mediating energy homeostasis should continue to be better characterized, as weight loss and maintenance remain challenging and a healthy body weight is important to positive health outcomes in postmenopausal women.
Acknowledgements
The authors would like to acknowledge the contributions of the staff as well as the 508 dedicated participants of the WOMAN study. We also appreciate the programming assistance of Mr. Alhaji Buhari. This research was funded by National Heart, Lung, and Blood Institute contract R01-HL-66468. Dr. Conroy is supported by a NIH career development award (5K23HL085405).
Sources of Financial Support: This research was funded by National Heart, Lung, and Blood Institute contract R01-HL-66468. Dr. Conroy is supported by a NIH career development award (5K23HL085405).
Footnotes
No disclaimers.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tchernof A, Poehlman ET. Effects of the menopause transition on body fatness and body fat distribution. Obesity Res. 1998;6:246–254. doi: 10.1002/j.1550-8528.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 2001;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes. 1996;20:291–302. [PubMed] [Google Scholar]
- 4.Chu MC, Cosper P, Orio F, et al. Insulin resistance in postmenopausal women with metabolic syndrome and the measurements of adiponectin, leptin, resistin, and ghrelin. Am J OB/GYN. 2006;194:100–4. doi: 10.1016/j.ajog.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 5.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 6.Hong SC, Yoo SW, Cho GJ, et al. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause. 2007;14:835–840. doi: 10.1097/GME.0b013e31802cddca. [DOI] [PubMed] [Google Scholar]
- 7.Mosca L, et al. Evidence-based guidelines for cardiovascular disease prevention in women: America Heart Association scientific statement. Arteriosclerosis Thrombosis Vascular Biology. 2004 doi: 10.1161/01.ATV.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- 8.Kuller, et al. Lifestyle Intervention and Coronary Heart Disease Risk Factor Changes over 18 Months in Postmenopausal Women: The Women On the Move through Activity And Nutrition (WOMAN Study) Clinical Trial. Journal of Women’s Health. 2006;15(8):962–974. doi: 10.1089/jwh.2006.15.962. [DOI] [PubMed] [Google Scholar]
- 9.Campfield L, Smith F, Gulsez Y, Devos R, Burn P. Mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 10.Vendrell J, Broch M, Vilarrasa N, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obesity Research. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 11.Langenberg C, Bergstrom J, Laughlin G, Barrett-Connor E. Ghrelin, Adiponectin, and Leptin Do Not Predict Long-term Changes in Weight and Body Mass Index in Older Adults: Longitudinal Analysis of the Rancho Bernardo Cohort. American Journal of Epidemiology. 2005;162:1189–1197. doi: 10.1093/aje/kwi338. [DOI] [PubMed] [Google Scholar]
- 12.Goyenechea E, Parra M Dolores, Martinez J Alfredo. Weight regain after slimming induced by an energy-restricted diet depends on interleukin-6 and peroxisome-proliferator-activated-receptor-gamma2 gene polymorphisms. British Journal of Nutrition. 2006;96:965–972. doi: 10.1017/bjn20061901. [DOI] [PubMed] [Google Scholar]
- 13.Garcia J, Iyer D, Poston W, et al. Rise of plasma ghrelin with weight loss is not sustained with weight maintenance. Obesity. 2006;14:1716–1723. doi: 10.1038/oby.2006.197. [DOI] [PubMed] [Google Scholar]
- 14.Cummings D, Weigle D, Frayo S, et al. Plasma ghrelin levels after diet-induced weight loss of gastric bypass surgery. New England Journal of Medicine. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 15.Yildiz B, Suchard M, Wong M, McCann S, Lidnio J. Alteration in the dynamics of circulating ghrelin, leptin, and adiponectin in human obesity. Proceedings of the National Academy of Sciences of the United States. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods S, Lotter E, McKay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M, Boyko E, Kahn S, Ravussin E, Bogardus C. Reduced insulin secretion: Anindependent predictor of body weight gain. Journal of Clinical Endocrinology & Metabolism. 1995;80:1571–1576. doi: 10.1210/jcem.80.5.7745002. [DOI] [PubMed] [Google Scholar]
- 18.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nature Medicine. 2004 May;10(5):524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 19.Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007 Mar;50(3):634–42. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi T, Kamon J, Waki H, et al. The fat-derived peptide adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 21.Chu M, Cosper P, Nakhuda G, Lobo R. A comparison of oral and transdermal short-term estrogen therapy in postmenopausal women with metabolic syndrome. Fertility & Sterility. 2006;86(6):1669–1675. doi: 10.1016/j.fertnstert.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 22.Di Carlo C, Tommaselli GA, Nappi C. Effects of sex steroid hormones and menopause on serum leptin concentrations. Gynecol Endocrinol. 2002 Dec;16(6):479–91. [PubMed] [Google Scholar]
- 23.Cento RM, Proto C, Spada RS, Napolitano V, Ciampelli M, Cucinelli F, Lanzone A. Leptin levels in menopause: effect of estrogen replacement therapy. Horm Res. 1999;52(6):269–73. doi: 10.1159/000023493. [DOI] [PubMed] [Google Scholar]
- 24.Castracane VD, Kraemer RR, Franken MA, Kraemer GR, Gimpel T. Serum leptin concentration in women: effect of age, obesity, and estrogen administration. Fertil Steril. 1998 Sep;70(3):472–7. doi: 10.1016/s0015-0282(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 25.Lambrinoudaki IV, Christodoulakos GE, Economou EV, Vlachou SA, Panoulis CP, Alexandrou AP, Kouskouni EE, Creatsas GC. Circulating leptin and ghrelin are differentially influenced by estrogen/progestin therapy and raloxifene. Maturitas. 2008 Jan 20;59(1):62–71. doi: 10.1016/j.maturitas.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Kriska AM, Kinzel LS, et al. The clinical trial of Women On the Move through Activity and Nutrition (WOMAN) study. Contemp Clin Trials. 2007 Jul;28(4):370–381. doi: 10.1016/j.cct.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuller LH, Kinzel LS, Pettee KK, et al. Lifestyle Intervention and Coronary Heart Disease Risk Factor Changes over 18 Months in Postmenopausal Women: The Women On the Move through Activity and Nutrition (WOMAN Study) Clinical Trial. Journal of Women’s Health. 2006;15(8):964–976. doi: 10.1089/jwh.2006.15.962. [DOI] [PubMed] [Google Scholar]
- 28.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 29.Murakami N, Hayashida T, Kuroiwa T, et al. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. Journal of Endicrinology. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 30.Giannopoulou I, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism Clinical and Experimental. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Cummings D, Foster-Schubert K, Overduin J. Ghrelin and Energy Balance: Focus on Current Controversies. Current Drug Targets. 2005;6(2):153–169. doi: 10.2174/1389450053174569. [DOI] [PubMed] [Google Scholar]
- 32.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. New England Journal of Medicine. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 33.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 34.Caro JF, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–16. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 35.Bjorbaek C, Elmquist J, Frantz J, Shoelson S, Flier J. Identification of SOCS-3 as a potential mediator of central leptin resistance. Molecular Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 36.Wing R, Sinha M, Considine R, Lang W, Caro JF. Relationship between weight loss maintenance and changes in serum leptin levels. Peptide & Metabolic Research. 1996;28:698–703. doi: 10.1055/s-2007-979881. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, Mori M. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997;154:285–292. doi: 10.1677/joe.0.1540285. [DOI] [PubMed] [Google Scholar]
- 38.Clegg D, Brown L, Woods S, Benoit S. Gonadal peptides determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]