Abstract
Experimental evidence suggested the existence of unidentified leprosy susceptibility loci in the human leukocyte antigen (HLA) complex. To identify such genetic risk factors, a high-density association scan of a 1.9-mega-base (Mb) region in the HLA complex was performed. Among 682 single-nucleotide polymorphisms (SNPs), 59 were associated with leprosy (P <.01) in 198 Vietnamese single-case leprosy families. Genotyping of these SNPs in an independent sample of 292 Vietnamese single-case leprosy families replicated the association of 12 SNPs (P <.01). Multivariate analysis of these 12 SNPs showed that the association information could be captured by 2 intergenic HLA class I region SNPs (P = 9.4 × 10−9)—rs2394885 and rs2922997 (marginal multivariate P = 2.1 × 10−7 and P = .0016, respectively). SNP rs2394885 tagged the HLA-C*15:05 allele in the Vietnamese population. The identical associations were validated in a third sample of 364 patients with leprosy and 371 control subjects from North India. These results implicated class I alleles in leprosy pathogenesis.
Leprosy is an infectious disease of the skin and peripheral nerves that is caused by Mycobacterium leprae. Both the development of the disease and its subforms (ie, paucibacillary or multibacillary) are dependent on genetic factors, as demonstrated by the concordance rates among monozygotic twins [1] and segregation studies [2, 3]. Several candidate genes (IL10, VDR, SLC11A1) have been associated with leprosy and/or its subforms [4, 5], and recently, positional cloning has identified 2 risk loci (ie, PARK2/PACRG [6] and LTA [7]). Therefore, forward genetic dissection of the human–M. leprae interaction is a powerful strategy to further the understanding of this ancient affliction [8].
Numerous studies have implicated genetic variation in human leukocyte antigen (HLA) genes with altered risk of leprosy (reviewed in [9, 10]). Two linkage studies have linked chromosome region 6p21 (HLA complex) to leprosy susceptibility, including our own genome-wide scan in 86 Vietnamese multi-case leprosy families (multipoint lod-score = 2.62) [11, 12]. An association scan of a 10.4-Mb region underlying the linkage-peak at 39.48-Mb (build 37.1) indentified a functional SNP in the HLA class III gene lymphotoxin-α (LTA) as a risk factor for early-onset leprosy in the Vietnamese and North Indian populations [7]. However, we showed that this association was independent of HLA-DRB1, an established susceptibility gene [13–17], and at least 3 other potential leprosy-associated SNPs, suggesting that the HLA complex harbors multiple unidentified risk loci. Therefore, we set to identify additional genetic risk factors for leprosy in this chromosome region.
A high-resolution association scan in 198 single-case leprosy families from Vietnam, followed by step-wise replication, in an independent family-based sample from Vietnam and a population-based sample from North India identified 8 intergenic HLA class I region SNPs—tagged by rs2394885 and rs2922997 —as novel leprosy risk factors.
METHODS
Ethics Statement
The study was approved by review boards and health authorities in Ho Chi Minh City, Vietnam; the All India Institute of Medical Sciences, New Delhi, India; and the McGill University Health Centre, Montreal, Canada. Written informed consent was obtained from all participants.
Samples
We enrolled a total of 2205 individuals in this study. The 198 single-case Vietnamese leprosy families (Vietnam-1; 52% multibacillary) and the additional 292 single-case Vietnamese leprosy families (Vietnam-2; 54% multibacillary) were identified from records available at the Dermato-Venereology Hospital in Ho Chi Minh City, Vietnam. The criterion for enrollment was the availability of both parents for genetic analysis. The 364 patients with leprosy (70% multibacillary) were identified from records available at the Central JALMA Institute of Leprosy and Other Infectious Diseases in Agra, India. The 371 healthy control subjects (ie, no reported infectious/inflammatory disease) from North India were identified from blood donor clinics.
The diagnosis and classification of subtype were based on clinical and histological criteria [18]. However, the phenotype studied here was leprosy per se (ie, independent of specific clinical manifestations).
Previously, Vietnam-1 was used in the study of PARK2 [6], LTA [7], and MRC1 [19]. Vietnam-2 was a replication sample for the investigation of 3 nonsynonymous SNPs in MRC1 [19].
Genotyping Methods
The 854 SNPs spanning the 1.9-Mb targeted interval on chromosome 6 (6p21.33-p21.32) were selected on the basis of their proximity to or location in known genes in the interval and tag-SNP information publicly available from the International HapMap project database (www.hapmap.org/). Included in our panel were 130 previously determined tag-SNPs for classical HLA class I and class II gene alleles in the Han Chinese in Beijing, China (CHB) population [20]. These SNPs were genotyped on one of the following platforms: (1) direct sequencing on an ABI PRISM 3100 genetic analyzer; (2) polarized fluorescence TaqMan Assay [21]; (3) the high-throughput GenomeLab SNPstream platform (formerly Orchid SNPstream UHT), which uses a single-base pair extension (SBE) method to incorporate fluorescently labeled terminal nucleotides, which are then detected by a specialized imager [22]; (4) the high-throughput SEQUENOM MassARRAY platform, which uses the iPLEX assay to incorporate mass-modified terminal nucleotides in the SBE step, which are then detected by MALDI-TOF MS [23]; and (5) the ultra-high throughput Illumina platform. This platform uses the GoldenGate assay, followed by a bead-based technology, to resolve individual SNP genotypes [24].
After genotyping, we excluded 172 SNPs from the analysis for the following reasons: 39 could not be genotyped, 3 could not be placed unambiguously on the sequence map, 19 showed deviations (P < .01) from Hardy-Weinberg equilibrium (HWE), 103 were noninformative or had a minor allele frequency (MAF) < 5%, 3 had > 10 Mendelian errors, and 5 were identified as “problem SNPs” (Table A1; online only).
All 52 SNPs genotyped in the Vietnam-2 sample were genotyped on the high-throughput SEQUENOM MassARRAY platform [23] (Table A2; online only). All 12 SNPs genotyped in the Indian sample were genotyped on one of the following platforms: (1) direct sequencing on an ABI PRISM 3100 genetic analyzer, (2) the high-throughput GenomeLab SNPstream platform (formerly Orchid SNPstream UHT) [22], or (3) the high-throughput SEQUENOM MassARRAY platform [23].
The HLA DRB1 gene was genotyped in the Vietnam-1 sample and the 371 control subjects from North India with use of standard methods [25]. HLA-C genotyping was performed using high-resolution (4-digit) sequence-based typing protocols recommended by the 13th International Histocompatibility Workshop [26].
Statistical Methods
We estimated population allelic frequencies and pairwise LD (r2) between SNPs from parental or control data using the algorithm implemented in Haploview 4.1 [27]. Bins of SNPs were constructed on the basis of the pairwise r2 and were defined as a not necessarily contiguous set of SNPs in which at least one SNP has r2 > 0.50 with all the other SNPs of the bin [28]. For the 1.9-Mb targeted interval (HCG27 → COL11A2), we uploaded all SNP genotype data for the CHB population from HapMap Data Rel 24 into Haploview 4.1. We reset the pairwise comparison threshold to 2 Mb, and using the Tagger function, we force included our 682 successfully genotyped SNPs and calculated the tagging efficiency by dividing the number of captured bins by the total number of bins (including single-SNP bins) with use of an r2 cutoff of 0.50.
Family-based association studies were performed in the Vietnam-1 and -2 samples with use of a classical transmission disequilibrium test, as implemented in FBAT, version 2.0.3 [29]. Alleles for which there was evidence of association were also analyzed by conditional logistic regression, as described elsewhere [30]. This approach permitted providing odds ratio (OR) estimates and made it possible to perform multivariate stepwise regression tests.
We performed a population-based association study in the North Indian sample, using classical multivariate logistic regression techniques as implemented in the LOGISTIC procedure of SAS, version 9.1 (SAS Institute). All population-based analyses were adjusted for sex, a classical risk factor for leprosy.
RESULTS
High-density SNP Association Scan of 6p21 in 198 Families with Leprosy from Vietnam
On the basis of our own results that provided evidence for the presence of additional leprosy susceptibility genes in the HLA class III region [7] and from numerous published studies implicating the HLA class II genes but not the class I genes [4,13–17], we focused our study on the HLA class II and III regions. However, because a small portion of the class I region fell within the 95% confidence interval (CI) of the linkage-peak from our previous genome-wide linkage study [11], we also selected the corresponding chromosomal segment (HCG27 → MICB) for high SNP coverage. Overall, an interval extending from 31.2 Mb to 33.1 Mb (build 37.1) on chromosome 6 (6p21.33-p21.32) was selected for a high-density association scan in 198 single-case leprosy families from Ho Chi Minh City, Vietnam (Vietnam-1). This 1.9-Mb interval includes 13 genes at the centromeric end of the HLA class I region (HCG27 → MICB), the 76 genes in the HLA class III region (PPIAP9 → NOTCH4), and the 34 genes in the HLA class II region (C6orf10 → HLA-DPA3). Complementing the target interval, 130 previously determined tag-SNPs for classical HLA class I and class II gene alleles in the CHB population were included [20]. Although some HLA allele tag-SNPs lie telomeric to the main targeted interval (Figure 1), these SNPs further increased the overall coverage of the HLA complex. In total, 854 SNPs were selected (Table A1; online only).
Figure 1.
High-density SNP association scan of 6p21 in the Vietnam-1 sample. Evidence for association with leprosy of 682 SNPs in 198 Vietnamese single-case families (Vietnam-1) is plotted as –log10P on the y-axis. The location of the SNPs, in Mb, is indicated on the x-axis (dbSNP129). The HLA class I, class III and class II regions are indicated by solid lines. The low SNP coverage in the class I regions corresponds to tag-SNPs for class I and class II alleles that are located outside the target area. The thin dotted line indicates the P = .01 significance threshold. Three different genetic models were tested for each SNP (i.e., additive and fully dominant/recessive) and the P-values for the best model are reported here (Pmin). The HLA-A, -B and -C genes are indicated at the top of the graph. SNPs rs2394885 and rs2922997 are squared and circled, respectively, to indicate their chromosomal locations and to highlight the modest association of these SNPs in the discovery sample.
After excluding 172 SNPs (see Methods; Table A1; online only), the tagging efficiency (ie, proportion of tagged bins [28]; see Methods) of the entire 1.9-Mb interval using an r2 cutoff of 0.50 was 0.80 in the CHB population (HapMap Data Rel 24). By univariate analysis, 64 of the 682 suitable SNPs were significantly associated with leprosy at the 0.01 level (Figure 1). Expectedly, rs2239704 (LTA+80), the previously reported LTA promoter risk variant [7], was among the 64 SNPs with a P < .01. We included LTA+80 to identify and exclude highly correlated SNPs from subsequent replications. Indeed, 4 SNPs were in strong linkage disequilibrium (LD; r2 ≥ 0.80) with LTA+80. As such, only the 59 non-LTA+80 correlated SNPs were targeted for replication (Table A2; online only).
Of note, when we performed age-stratified analysis of the rs2394885 bin SNPs, we observed that the overall risk effect was exclusively because of individuals aged >15 years at the time of diagnosis (109 families; rs2394885; P> 15 = .006 and P≤ 15 = .371). Of interest, we previously showed that the effect of the LTA+80 A-allele on leprosy susceptibility was mainly a result of individuals aged ≤15 years at the time of diagnosis (89 families) [7].
Replication Study: 292 Families with Leprosy from Vietnam
We enrolled a second independent sample of 292 single-case leprosy families from Ho Chi Minh City, Vietnam (Vietnam-2), to identify false-positive associations in the Vietnam-1 sample. We genotyped 52 of the 59 non-LTA+80–correlated SNPs with P < .01. Seven SNPs were excluded from the panel for the following reasons: 3 SNPs had a HWE P < .05 in the Vietnam-1 sample, 3 SNPs were redundant (r2 ≥ 0.99 with an included SNP), and 1 SNP failed primer design (Table A2; online only). By univariate analysis, 12 of the 49 suitable SNPs (3 SNPs could not be genotyped) were significantly associated with leprosy at the 0.01 level in the Vietnam-2 sample (with concordant risk alleles) (Table 1). Of the 12 replicated SNPs, 11 were located in the centromeric end of the HLA class I region (between HCG27 and MICA), and 1 SNP was located in the adjacent HLA class III region (between PPIAP9 and RPL15P4).
Table 1.
Univariate Analysis of 12 HLA SNPs in the Vietnam-1, Vietnam-2, Combined Vietnamese, and North-Indian Samples
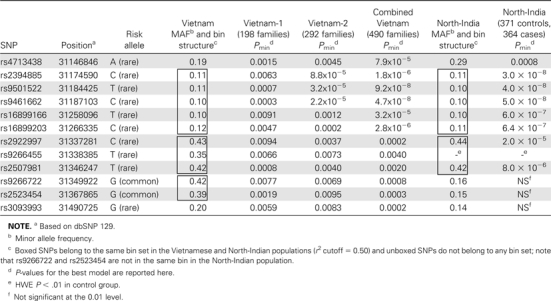 |
When we combined the Vietnam-1 and Vietnam-2 samples (490 single-case leprosy families), the evidence for association for each of the 12 replicated SNPs was very strong (Table 1). However, because not all redundant SNPs were excluded, extensive LD among the 12 SNPs in the combined parental dataset (ie, 980 unrelated Vietnamese individuals) was still observed (Table A3; online only). Specifically, the 12 SNPs could be assigned to 5 bins with use of an r2 cutoff of 0.50 (Table 1). Consequently, stepwise multivariate conditional logistic regression analysis of the 12 SNPs in the combined sample showed that the association information could be captured by a model (P = 9.4 × 10−9) that included 2 HLA class I intergenic SNPs—rs2394885 and rs2922997—each with an additive genetic effect and marginal multivariate P of 2.1 × 10−7 (OR for CC vs CG = CG vs GG = 2.32; 95% CI, 1.62–3.33) and 0.0016 (OR for CC vs CT = CT vs TT = 1.42; 95% CI, 1.14–1.76), respectively (Table 2). Multivariate analysis generated additional models representing different combinations of 2 SNPs from the 2 associated bins that were statistically indistinguishable from the one presented here. We choose to present the aforementioned 2-SNP model because rs2394885 is a reported tag-SNP for HLA-C*0401 in the CHB population [20]. To confirm that SNP rs2394885 was similarly a tag-SNP for HLA-C in the Vietnamese population, we tested for LD between rs2394885 and HLA-C in 200 unrelated Vietnamese individuals and detected a correlation between rs2394885 and HLA-C*15:05 (r2 = 0.69). Therefore, the HLA-C*15:05 allele is a strong candidate for the functional variant explaining the association of rs2394885-tagged bin.
Table 2.
Univariate and Multivariate Analysis of the Eight Replicated HLA SNPs in the Combined Vietnamese and North-Indian Samples
 |
Replication of HLA Class I SNPs Associated in the Genome-Wide Association Study of Leprosy
Recently, the first genome-wide association study (GWAS) of leprosy in the Chinese population was published [31, 32]. Two association signals in the HLA complex—in the class I and II regions—were observed. In the class I region, the 5 most strongly associated SNPs in the primary scan (P < .00001) were rs7759127, rs2524070, rs2853930, rs2524132, and rs9264868, but they could not be replicated in the subsequently tested samples. We tested the association of these 5 SNPs in our Vietnam-1 sample (198 single-case families). Among these SNPs, 4 (rs7759127, rs2524070, rs2524132, and rs9264868) were associated with leprosy (P ≤ .05), and 1 SNP (rs2853930) had a P of .08. LD analysis in the Vietnam-1 parental dataset (ie, 396 unrelated Vietnamese individuals) revealed that all 5 SNPs were in strong LD and belonged to a single bin (r2 cutoff = 0.5). Of interest, we noticed that the 5 GWAS SNPs were associated with HLA-C gene expression (cis-effect) in the CEU-CHB-JPT populations [33]. For multivariate analysis, we included a single SNP (ie, tag-SNP) from each of the 3 aforementioned associated bins (Table 1) and a tag-SNP from the GWAS bin: rs2394885, rs2922997, rs2523454, and rs2524132 (GWAS bin). Owing to LD between rs2523454 and rs2524132 (r2 = 0.25), rs2524132 captured the association information of rs2523454. This suggested that the evidence of association observed for the SNPs in the rs2523454-tagged bin and the GWAS bin may be explained by a regulatory effect on HLA-C gene expression.
Validation Study: 364 Patients with Leprosy and 371 Control Subjects from North India
To validate the observed associations with leprosy in a geographically and ethnically different population, we enrolled a third sample of 364 unrelated individuals with leprosy and 371 unrelated healthy control subjects from Agra, North India. We successfully genotyped the 12 replicated SNPs. By univariate logistic regression, 8 of the 11 suitable SNPs (HWE P for rs9266455 was <0.01 among control subejcts) were significantly associated with leprosy at the 0.01 level (with concordant risk alleles and genetic models as compared with the combined Vietnamese sample) (Table 1). That neither rs9266722 nor rs2523454 were associated with leprosy in the North Indian sample was not surprising, given that, in this population, unlike in the Vietnamese population, these SNPs are not in LD with the GWAS SNPs that impact on HLA-C transcription (r2 between rs2524132 and rs9266722 and rs2523454 was .067 and .074, respectively). Identical to the combined Vietnamese sample, multivariate analysis of the 8 associated SNPs showed that the best model (P = 7.6 × 10−9) included the same 2 SNPs—rs2394885 and rs2922997—with marginal multivariate P values of 6.9 × 10−8 (OR for CC vs CG = CG vs GG = 2.23; 95% CI, 1.61–3.09) and 0.0038 (OR for CC vs CT = CT vs TT = 1.45; 95% CI, 1.15–1.83), respectively (Table 2).
Risk Factors rs2394885 and rs2922997 are Independent from HLA-DRB1 and LTA
Given that HLA-DRB1 (HLA class II) and LTA (HLA class III) are known leprosy susceptibility genes in the Vietnamese [7, 17] and North Indian [7, 13] populations, we wanted to exclude the possibility that the observed association of leprosy with SNPs rs2394885 and rs2922997 could be attributable to long-range LD with LTA+80 or HLA-DRB1 alleles. Multivariate analysis in the Vietnam-1 sample (198 single-case families) showed that there was no statistically significant impact on the risk effect of SNPs rs2394885 (univariate P = .006) and rs2922997 (univariate P = .009) when we included LTA+80 and HLA-DRB1 alleles despite the loss of 22 families in the process (adjusted P = .008 and .04 for rs2394885 and rs2922997, respectively). Similarly, multivariate analysis in the North Indian sample showed that there was no noticeable impact on the risk effect of SNPs rs2394885 (univariate P = 3.1 × 10−8) and rs2922997 (univariate P = 2.1 x 10−5) when we included LTA+80 in the multivariate analysis (adjusted P = 6.9 × 10−8 and .0038 for rs2394885 and rs2922997, respectively), nor did we detect any r2 > 0.10 between either SNP and HLA-DRB1 alleles in the control group. These results demonstrate the independent association of SNPs rs2394885 and rs2922997 from the known HLA leprosy susceptibility genes in these 2 populations.
DISCUSSION
We report the discovery of HLA class I region SNPs as strong genetic risk factors for leprosy per se. This is in accordance with our previously reported genome-wide linkage study that found evidence of linkage between 6p21 region microsatellite markers and leprosy, irrespective of clinical subtype [11]. These findings are substantiated by the replication of association in 2 independent family-based samples from Vietnam and the subsequent validation in a population-based sample from North India. When comparing the combined Vietnamese sample against the North Indian sample, the strength of the genetic effects—under identical models—were very similar, and the risk alleles were consistent. The association of the reported HLA class I region risk factors between ethnically and geographically different patients groups emphasizes the importance of the underlying susceptibility gene(s) in leprosy pathogenesis.
To date, our stepwise approach to positional cloning has identified 3 replicated leprosy risk factors, in addition to HLA-DRB1 [17], underlying the linkage-peak on chromosome region 6p21: LTA+80 [7], rs2394885, and rs2922997. Our experience depicts a complex situation with multiple HLA region alleles impacting on mycobacterial susceptibility. These results serve to improve our understanding of linkage-peak architecture in the context of a complex disease. Here, we show that, in analogy to what has been described for linkage QTLs in experimental models [34–36], a cluster of multiple susceptibility loci can underlie the linkage-peak. The notion of “one linkage-peak = multiple risk loci” may prove to be a recurring observation in the genetic analysis of complex disease in humans. A recent genome-wide association study similarly revealed 3 independent loci in the HLA complex conferring risk of psoriasis [37].
As stated above (Results), models representing different combinations of 2 SNPs from the 2 associated bins are statistically indistinguishable. Analogous to most genetic studies, SNPs rs2394885 and rs2922997 are risk factors that likely tag the causal variants, identification of which requires ultra-fine LD mapping coupled with functional studies. However, the strong LD observed between rs2394885 and HLA-C*15:05 strongly implicates this class I allele in leprosy susceptibility. We also used the HapMap database (Data Rel 24) to screen a 1-Mb region around rs2394885 in the CHB population. Using an r2 cutoff of 0.40 (MAF, > 5%), 27 SNPs were correlated with rs2394885 (Table A4; online only). A single SNP—rs2233952—is a nonsynonymous coding SNP (PSORS1C2 leu83pro) but has no apparent functional relevance [38]. These data further support HLA-C*15:05 as leprosy susceptibility allele. We similarly screened a 1-Mb region around rs2922997 in the CHB population. Using an r2 cutoff of 0.40 (MAF, > 5%), 8 SNPs were correlated with rs2922997, none with any evidence of biological relevance (Table A4; online only).
We have replicated and validated all leprosy susceptibility variants identified from linkage studies, including the 4 HLA region variants above, in geographically and ethnically different populations. This ease of replication implies that genetic heterogeneity in leprosy susceptibility is limited. Low genetic heterogeneity is in striking parallel to the low degree of diversity among M. leprae strains [39, 40] and the high degree of adaptation of the leprosy bacillus to its human host. We propose that low pathogen variability combined with high host-pathogen adaptation results in strong host genetic effects on leprosy susceptibility that are acting across ethnic groups and geographic locations. If this conclusion is correct, one would predict that, in infectious diseases, genetic heterogeneity mainly reflects differences in the infectious strains of the pathogen. Of interest, Mycobacterium tuberculosis strain-dependent host genetic effects have been described [41]. Together, these data suggest that a careful characterization of pathogens isolated from patients may be an effective way to overcome genetic heterogeneity in common infectious diseases.
Our results strongly implicate the HLA-C gene in leprosy susceptibility and motivate the future systematic analysis of class I genes in leprosy susceptibility. HLA-C alleles and promoter variants have previously been associated with inflammatory and infectious diseases. HLA-C*04 (OR,.39) and HLA-C*05 (OR, 2.92) were under- and over-represented in 70 patients with rheumatoid arthritis [42], respectively; HLA-Cw6 was identified as an early-onset risk factor for psoriasis [43], and HLA-C*05 was found to be protective against multiple sclerosis (P = 3.3 × 10−5) [44]. Likewise, the frequency of HLA-Cw4 was increased in patients with tuberculosis (28.9%), compared with control subjects (21.1%) [45], and a high-producing HLA-C promoter variant—rs9264942—was associated with low viral cutoff after HIV infection (P = 3.8 × 10−9) [46] and slower progression to AIDS [47]. As in the aforementioned studies, the association of HLA-C with leprosy susceptibility implicates natural killer (NK) cells—effectors of the innate response to viruses and intracellular pathogens—in the host response to M. leprae infection. This is supported by immunological assays that showed that M. leprae was able to induce NK cell–mediated cytotoxicity [48]. NK cell cytolytic activity and cytokine production is regulated by killer cell immunoglobulin-like receptors (KIRs) that bind MHC class I molecules, and HLA-C has emerged as a dominant ligand for KIRs [49]. Because of their pivotal role at the NK cell/HLA-C interface, and suggestive evidence of altered frequencies of KIR genes in patients with leprosy [50], the next step in the genetic dissection of leprosy is to target the KIR genes and their epistatic interaction with HLA-C alleles—an emerging area of study with much potential to expand our understanding of both leprosy pathogenesis and intracellular infection.
Supplementary Data
Supplementary data are available at http://jid.oxfordjournals.org online.
Funding
This work was supported by the Canadian Institutes of Health Research ( to ES) and MAGRALEPRE from l'Ordre de Malte (to AlA). The Laboratory of Human Genetics of Infectious Diseases is supported by grants from The Rockefeller University Center for Clinical and Translational Science grant number 5UL1RR024143-03 and The Rockefeller University. AnA holds a graduate studentship from the Natural Science and Engineering Research Council of Canada. AlA and LA are supported by the Assistance Publique-Hôpitaux de Paris, Programme de Recherche Fondamentale en Microbiologie Maladies Infectieuses et Parasitaires, and the Agence Nationale de la Recherche of the Ministère Français de l'Éducation Nationale de la Recherche et de la Technologie. ES is a Chercheur National du Fonds de la Recherche en Santé du Québec and an International Research Scholar of the Howard Hughes Medical Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank all patients and their families who participated in this study; A. Montpetit and A. Bélisle at the McGill University and Génome Québec Innovation Centre, for providing high-throughput genotyping service; L. Simkin, for assistance with genotyping data management; and L. Fuentes, for her assistance in HLA-C typing.
References
- 1.Chakravartti MR, Vogel FA. A twin study on leprosy. In: Becker PE, Lenz W, Vogel F, Wendt GG, editors. Topics in human genetics. Vol 1. Stuttgart: Georg Thieme; 1973. pp. 1–123. [Google Scholar]
- 2.Abel L, Demenais F. Detection of major genes for susceptibility to leprosy and its subtypes in a Caribbean island: Desirade island. Am J Hum Genet. 1988;42:256–66. [PMC free article] [PubMed] [Google Scholar]
- 3.Abel L, Vu DL, Oberti J, et al. Complex segregation analysis of leprosy in southern Vietnam. Genet Epidemiol. 1995;12:63–82. doi: 10.1002/gepi.1370120107. [DOI] [PubMed] [Google Scholar]
- 4.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 5.Mira MT. Genetic host resistance and susceptibility to leprosy. Microbes Infect. 2006;8:1124–31. doi: 10.1016/j.micinf.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Mira MT, Alcais A, Van Thuc N, et al. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature. 2004;427:636–40. doi: 10.1038/nature02326. [DOI] [PubMed] [Google Scholar]
- 7.Alcais A, Alter A, Antoni G, et al. Stepwise replication identifies a low-producing lymphotoxin-[alpha] allele as a major risk factor for early-onset leprosy. Nat Genet. 2007;39:517–22. doi: 10.1038/ng2000. [DOI] [PubMed] [Google Scholar]
- 8.Alcais A, Mira M, Casanova JL, Schurr E, Abel L. Genetic dissection of immunity in leprosy. Curr Opin Immunol. 2005;17:44–8. doi: 10.1016/j.coi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Orlova M, Schurr E. Genetic architecture of mycobacterial diseases. In: Mehra N, editor. The HLA complex in biology and medicine: a resource book. New Delhi: Jaypee Brothers Medical Publishers Ltd.; 2009. p. 365. [Google Scholar]
- 10.Mehra NK, Singh P, Sood P, Kaur G. MHC and Non-MHC genes in tuberculosis and leprosy. In: Mehra N, editor. The HLA complex in biology and medicine: a resource book. New Delhi: Jaypee Brothers Medical Publishers Ltd.; 2009. p. 386. [Google Scholar]
- 11.Mira MT, Alcais A, Van Thuc N, et al. Chromosome 6q25 is linked to susceptibility to leprosy in a Vietnamese population. Nat Genet. 2003;33:412–5. doi: 10.1038/ng1096. [DOI] [PubMed] [Google Scholar]
- 12.Miller EN, Jamieson SE, Joberty C, et al. Genome-wide scans for leprosy and tuberculosis susceptibility genes in Brazilians. Genes Immun. 2004;5:63–7. doi: 10.1038/sj.gene.6364031. [DOI] [PubMed] [Google Scholar]
- 13.Mehra NK, Rajalingam R, Mitra DK, Taneja V, Giphart MJ. Variants of HLA-DR2/DR51 group haplotypes and susceptibility to tuberculoid leprosy and pulmonary tuberculosis in Asian Indians. Int J Lepr Other Mycobact Dis. 1995;63:241–8. [PubMed] [Google Scholar]
- 14.Zerva L, Cizman B, Mehra NK, et al. Arginine at positions 13 or 70–71 in pocket 4 of HLA-DRB1 alleles is associated with susceptibility to tuberculoid leprosy. J Exp Med. 1996;183:829–36. doi: 10.1084/jem.183.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw MA, Donaldson IJ, Collins A, et al. Association and linkage of leprosy phenotypes with HLA class II and tumour necrosis factor genes. Genes Immun. 2001;2:196–204. doi: 10.1038/sj.gene.6363754. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Balamurugan A, Katoch K, Sharma SK, Mehra NK. Immunogenetics of mycobacterial infections in the North Indian population. Tissue Antigens. 2007;69(Suppl 1):228–30. doi: 10.1111/j.1399-0039.2006.77311.x. [DOI] [PubMed] [Google Scholar]
- 17.Vanderborght PR, Pacheco AG, Moraes ME, et al. HLA-DRB1*04 and DRB1*10 are associated with resistance and susceptibility, respectively, in Brazilian and Vietnamese leprosy patients. Genes Immun. 2007;8:320–4. doi: 10.1038/sj.gene.6364390. [DOI] [PubMed] [Google Scholar]
- 18.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–73. [PubMed] [Google Scholar]
- 19.Alter A, de Leseleuc L, Van Thuc N, et al. Genetic and functional analysis of common MRC1 exon 7 polymorphisms in leprosy susceptibility. Hum Genet. 2010;127:337–48. doi: 10.1007/s00439-009-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bakker PIW, McVean G, Sabeti PC, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–72. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LG, Connell CR, Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Res. 1993;21:3761–6. doi: 10.1093/nar/21.16.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell P, Chaturvedi S, Gelfand C, et al. SNPstream UHT: ultra-high throughput SNP genotyping for pharmacogenomics and drug discovery. Biotechniques. 2002;(Suppl 70-2, 74):76–7. [PubMed] [Google Scholar]
- 23.Griffin TJ, Smith LM. Single-nucleotide polymorphism analysis by MALDI-TOF mass spectrometry. Trends Biotechnol. 2000;18:77–84. doi: 10.1016/s0167-7799(99)01401-8. [DOI] [PubMed] [Google Scholar]
- 24.Fan J, Oliphant A, Shen R, et al. Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol. 2003;68:69–78. doi: 10.1101/sqb.2003.68.69. [DOI] [PubMed] [Google Scholar]
- 25.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–35. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 26. IHWC. Sequence based HLA typing. In: Hansen Ja, ed. Immunobiology of the human MHC (Proceedings of the 13th International histocompatibility Workshop and Conference). Vol 1. Seattle: IHWG Press, 2007: 304–416. [Google Scholar]
- 27.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 28.Hinds DA, Stuve LL, Nilsen GB, et al. Whole-genome patterns of common DNA variation in three human populations. Science. 2005;307:1072–9. doi: 10.1126/science.1105436. [DOI] [PubMed] [Google Scholar]
- 29.Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype–phenotype associations. Eur J Hum Genet. 2001;9:301–6. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- 30.Schaid DJ, Rowland C. Use of parents, sibs, and unrelated controls for detection of associations between genetic markers and disease. Am J Hum Genet. 1998;63:1492–506. doi: 10.1086/302094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schurr E, Gros P. A common genetic fingerprint in leprosy and Crohn's disease? N Engl J Med. 2009;361:2666–8. doi: 10.1056/NEJMe0910690. [DOI] [PubMed] [Google Scholar]
- 32.Zhang FR, Huang W, Chen SM, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–18. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 33.Stranger BE, Nica AC, Forrest MS, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–24. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legare ME, Bartlett FS, Frankel WN. A major effect QTL determined by multiple genes in epileptic EL mice. Genome Res. 2000;10:42–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Flint J, Mackay TFC. Genetic architecture of quantitative traits in mice, flies, and humans. Genome Res. 2009;19:723–33. doi: 10.1101/gr.086660.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay TFC, Stone EA, Ayroles JF. The genetics of quantitative traits: challenges and prospects. Nat Rev Genet. 2009;10:565–77. doi: 10.1038/nrg2612. [DOI] [PubMed] [Google Scholar]
- 37.Feng BJ, Sun LD, Soltani-Arabshahi R, et al. Multiple Loci within the major histocompatibility complex confer risk of psoriasis. PLoS Genet. 2009;5:e1000606. doi: 10.1371/journal.pgen.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YT, Tsai SF, Lin MW, et al. SPR1 gene near HLA-C is unlikely to be a psoriasis susceptibility gene. Exp Dermatol. 2003;12:307–14. doi: 10.1034/j.1600-0625.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- 39.Monot M, Honore N, Garnier T, et al. On the origin of leprosy. Science. 2005;308:1040–2. doi: 10.1126/science/1109759. [DOI] [PubMed] [Google Scholar]
- 40.Monot M, Honore N, Garnier T, et al. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat Genet. 2009;41:1282–9. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- 41.Caws M, Thwaites G, Dunstan S, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4:e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yen J-H, Moore BE, Nakajima T, et al. Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med. 2001;193:1159–68. doi: 10.1084/jem.193.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair RP, Stuart PE, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–51. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tai Wai Y, Philip LDJ, Simon GG, et al. A second major histocompatibility complex susceptibility locus for multiple sclerosis. Ann Neurol. 2007;61:228–36. doi: 10.1002/ana.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balamurugan A, Sharma SK, Mehra NK. Human leukocyte antigen class I supertypes influence susceptibility and severity of tuberculosis. J Infect Dis. 2004;189:805–11. doi: 10.1086/381689. [DOI] [PubMed] [Google Scholar]
- 46.Fellay J, Shianna KV, Ge D, et al. A Whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–7. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas R, Apps R, Qi Y, et al. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat Genet. 2009;41:1290–4. doi: 10.1038/ng.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaleab B, Ottenoff T, Converse P, et al. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol. 1990;20:2651–9. doi: 10.1002/eji.1830201219. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 2008;20:343–52. doi: 10.1016/j.smim.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franceschi DS, Mazini PS, Rudnick CC, et al. Association between killer-cell immunoglobulin-like receptor genotypes and leprosy in Brazil. Tissue Antigens. 2008;72:478–82. doi: 10.1111/j.1399-0039.2008.01127.x. [DOI] [PubMed] [Google Scholar]



