Abstract
Background. Early weaning may reduce human immunodeficiency virus (HIV) transmission but may have deleterious consequences for uninfected children. Here we evaluate effects of early weaning on diarrhea morbidity and mortality of uninfected children born to HIV-infected mothers.
Methods. HIV-infected women in Lusaka, Zambia, were randomly assigned to breastfeeding for 4 months only or to continue breastfeeding until the mother decided to stop. Replacement and complementary foods were provided and all women were counseled around feeding and hygiene. Diarrhea morbidity and mortality were assessed in 618 HIV-uninfected singletons alive and still breastfeeding at 4 months. Intent-to-treat analyses and comparisons based on actual feeding practices were conducted using regression methods.
Results. Between 4 and 6 months, diarrheal episodes were 1.8-fold (95% confidence interval (CI), 1.3–2.4) higher in the short compared with long breastfeeding group. Associations were stronger based on actual feeding practices and persisted after adjustment for confounding. At older ages, only more severe outcomes, including diarrhea-related hospitalization or death (relative hazard [RH], 3.2, 95% CI, 2.1–5.1 increase 4–24 months), were increased among weaned children.
Conclusions. Continued breastfeeding is associated with reduced risk of diarrhea-related morbidity and mortality among uninfected children born to HIV-infected mothers in this low-resource setting despite provision of replacement and complementary food and counseling.
Clinical Trials Registration. NCT00310726.
The benefits of breastfeeding in protecting against gastrointestinal infections are well established and have been demonstrated in both developed and developing countries [1–4]. These benefits are particularly critical in low-resource settings, as the burden of diarrheal morbidity is substantial and mortality associated with diarrhea is high [5]. However, human immunodeficiency virus (HIV)-infected women must balance the benefits of breastfeeding with the ongoing risk of transmitting HIV to their uninfected infants.
We have reported that early weaning at 4 months does not improve HIV-free survival in the first 2 years of life [6]. Rather, all-cause mortality among HIV-exposed, uninfected infants was significantly elevated with early weaning [7], and this excess mortality outweighed the reductions in HIV transmission [8]. We have also reported that continued breastfeeding was associated with improved growth into the second year of life among children who remained uninfected [9]. Determining the optimal duration of breastfeeding for children born to HIV-infected women is an important area of clinical and public health research. Here we evaluate the effects of early weaning on diarrheal morbidity and mortality.
METHODS
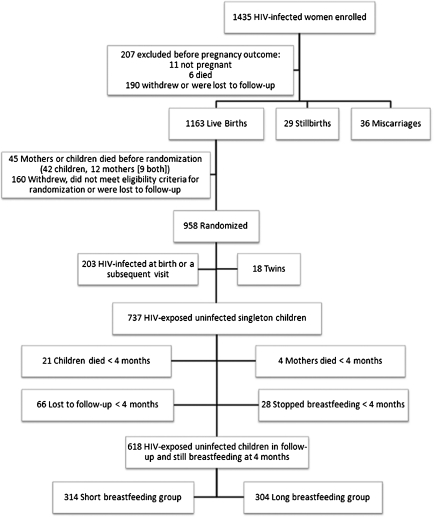
We conducted a randomized clinical trial, described in detail elsewhere [6, 10, 11], investigating the effect of short- vs long-duration breastfeeding on infant and young child mortality and vertical transmission of HIV. HIV-infected women attending 2 antenatal clinics in Lusaka, Zambia, from May 2001 to September 2004 were enrolled. In brief, 1435 HIV-infected pregnant women at <38 weeks gestation who intended to breastfeed were recruited and were given single-dose nevirapine for prevention of perinatal HIV transmission. A total of 958 mothers whose newborn was alive and being breastfed were randomized when their child reached 1 month of age to either abrupt cessation of breastfeeding at 4 months (short breastfeeding group) or continued exclusive breastfeeding to 6 months with gradual introduction of complementary foods and continued breastfeeding for a duration determined by personal choice (long breastfeeding group). Figure 1 presents the flow of subjects from enrollment through randomization. All women, regardless of randomization group, were encouraged to exclusively breastfeed for 4 months. Infant formula milk and fortified cereal were provided for 3 months to all children in the short breastfeeding group at the time of weaning. Preparation for weaning included education about hygiene and correct preparation of formula and weaning foods. Antiretroviral therapy only became available near the time of study completion. The study was approved by the institutional review boards of the investigators’ institutions. Written informed consent was obtained from all participants.
Figure 1.
Flow chart detailing the number of enrolled, randomized, and excluded subjects with reasons for their exclusion and the distribution of included subjects by randomization group.
Upon enrollment, sociodemographic information, medical and obstetric history, hemoglobin and CD4 T cell counts, and HIV-1 RNA quantity (Roche Amplicor® 1.5, Roche) were obtained. Mothers and infants were followed at study visits at 1, 2, 3, 4, 4.5, 5, and 6 months postpartum and every 3 months thereafter until 24 months. Blood samples were collected at each visit to determine the child’s HIV status by HIV DNA polymerase chain reaction [12]. Child feeding practice, the exact age at which breastfeeding cessation occurred, and information on morbidity, including diarrheal episodes, were assessed at each visit by maternal self-report using a detailed questionnaire. The questionnaire was administered by a separate member of the study team to the person conducting the education and counseling. Information about hospitalizations and circumstances surrounding deaths was ascertained through review of hospital records and interview with family members. Children who died were assumed to have been breastfed up to the date of death unless there was documentation from prior study visits indicating otherwise. Data on maternal and child characteristics and socioeconomic factors were collected at baseline, and mothers were weighed at each visit. To account for season, we defined the rainy season as October to April, when mean monthly rainfall was 119.8 mm, with the remainder of the year as the dry season, when mean monthly rainfall was 0.76 mm [13].
This analysis includes 618 HIV-uninfected singleton children who were still alive, in follow-up and breastfeeding at 120 days (Figure 1). Children who stopped breastfeeding before 120 days were excluded because all study participants were counseled to breastfeed exclusively to 4 months and stopping before this time was a rare and unusual occurrence. Two separate analyses were conducted: (1) by randomization group (intent-to-treat analysis) and (2) by actual feeding practice in the interval before the visit (as practiced analysis). In the as practiced analysis, subjects were considered to have stopped breastfeeding if they were not breastfeeding for more than half of the time period since the last attended visit. Breastfeeding during the first 6 months was further classified as nonexclusive or exclusive. Nonexclusive breastfeeding was defined as ingesting breastmilk along with complementary foods or liquids while exclusive breastfeeding was defined as ingesting only breast milk, vitamins, and prescribed medication since the previous visit.
Three indicators of diarrhea morbidity were assessed by means of standard questionnaire at each visit. In order of severity, the least severe outcome was maternal report of any diarrheal episode, followed by a diarrheal episode resulting in the child being seen by a healthcare worker, and report of a prolonged diarrheal episode. A diarrheal episode was defined as 3 or more loose or watery stools per day lasting 2 or more days. Episodes of diarrhea lasting ≥7 days were classified as “prolonged” since most acute diarrheal episodes resolve within a week [14]. In addition, hospitalization or death related to diarrhea was determined by verbal autopsy or hospital record review.
First, we examined the occurrence of each outcome at each scheduled study visit separately. Comparisons between groups at each visit were made using X2 test or Fisher exact test if cell sizes were small. Then, we examined combined visits over the 4–6-month and 7–24-month periods separately using generalized estimating equation (GEE) logistic regression models to account for the longitudinal nature of the data. An autoregressive correlation structure was used to account for repeated measurements on the same subject. Univariate analysis was conducted to identify characteristics associated with diarrheal morbidity. Variables that were significant at P < .2 in the univariate analysis were tested in a multivariable GEE model and those that remained significant at P < .05 were retained in the final model [15]. In the case of multicolinear variables, the one with the strongest P value was retained in the model.
The rates of diarrhea-related hospitalization or death were assessed as a single combined outcome at each of 4 time periods: 4–6, 7–12, 13–18, and 19–24 months. Data from children were censored at the time of death or loss to follow-up. The first hospital admission was selected and subsequent hospitalizations in the same child were excluded. Period-specific rate ratios were computed by randomization group and actual feeding practice. Mantel-Haenszel summary rate ratios [16] were used to investigate the overall effect while controlling for child’s age. Cox regression analysis was used to assess time to the first diarrhea-related hospitalization or death as a single combined outcome, censoring children who died of causes other than diarrhea. All variables tested in the GEE model were also tested in the Cox model, and the same model selection process was used. Breastfeeding status, season, maternal death, and maternal body mass index (BMI) were tested as time-dependent variables in the Cox model. Statistical analyses were performed using SAS software version 9.2 (SAS Institute).
RESULTS
Of 618 uninfected children, 314 (50.8%) had been randomized to short-duration breastfeeding. Child and maternal characteristics such as female sex (46.8% short-duration group vs 47.4% long-duration group), birthweight, maternal health, delivery events, and socioeconomic factors were distributed evenly between the 2 groups. Distribution of characteristics within this subset was similar to those of the full sample with no differences between the 2 randomization groups [6]. Antiretroviral treatment within 24 months of delivery was rare and did not differ between the 2 groups (10.5% short-duration group vs 12.5% long-duration group). The median duration of breastfeeding was 4.5 months among mothers randomized to the short-duration group and 16.2 months for mothers in the long-duration group. Sixty-three percent of women randomized to the short-duration group weaned during the fourth month.
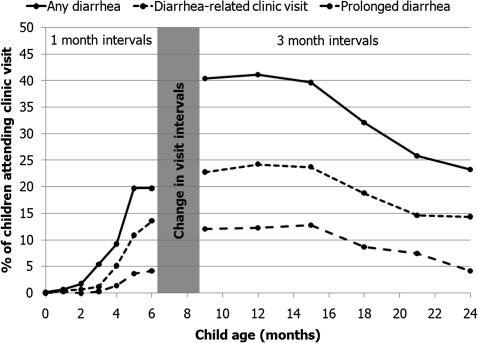
Diarrheal morbidity of differing degrees of severity is presented in Figure 2. Diarrhea was rare in the first 3 months and increased from 4 to 6 months. The frequency of diarrheal morbidity was highest from 6 to 15 months and subsequently declined to 24 months. During 24 months of study follow-up, 84 children were hospitalized with diarrhea (8 per 100 child-years) and 39 children died from a diarrhea-related cause (4 per 100 child-years).
Figure 2.
Frequency of maternal report of any diarrhea, diarrhea-related clinic visits, and prolonged diarrhea (longest episode lasting ≥7 days) since the last visit among 618 human immunodeficiency virus (HIV)-exposed uninfected infants. Frequency at 5 months includes events reported at 4.5 months.
Breastfeeding Practice and Diarrhea
Table 1 displays the frequency of diarrhea morbidity of differing degrees of severity (any diarrhea episode, diarrhea-related clinic visit, and prolonged diarrhea) by randomization group (intent-to-treat analysis) and by actual feeding behavior. In the intent-to-treat analysis, children who were randomized to the short-duration breastfeeding group had significantly more episodes of diarrhea and diarrhea-related clinic visits between 4 and 6 months of age than did children randomized to the long-duration group. Episodes of diarrhea were increased 3-fold in the short-duration group at 4.5 months (relative risk [RR]; 95% CI, 1.6–5.4) and increased to a lesser extent at 5 months (RR [95% CI], 1.7 [1.1–2.6]) and 6 months (RR [95% CI], 1.6 [1.1–2.2]). Overall during the 4–6-month period, the risk of diarrhea episodes was increased 1.8-fold (95% CI, 1.3–2.4) in the short compared with the long breastfeeding group. The increase in diarrhea observed at 15 months most likely reflects weaning among women in the long breastfeeding group. Diarrhea-related clinic visits were also elevated in the short duration group with 2-fold increases at 5 and 6 months (P = .02 and .0007, respectively). Differences in prolonged diarrhea by randomization group, although in the same direction, were not statistically significant.
Table 1.
Frequency of Reported Diarrhea Among 618 Human Immunodeficiency Virus (HIV)-exposed Uninfected Infants by Random Group Assignment (Panel A) and by Actual Breastfeeding (BF) Practice (Panel B)
| A. As Randomized |
||||||
| Any Diarrhea No. (%) |
Diarrhea-Related Clinic Visit No. (%) |
Prolonged Diarrheaa No. (%) |
||||
| Visit (month) | Short BF Group | Long BF Group | Short BF Group | Long BF Group | Short BF Group | Long BF Group |
| 0 | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 3 (1.0) | 1 (0.4) | 1 (0.3) | 2 (0.7) | 2 (0.7) | 0 (0) |
| 2 | 5 (1.7) | 5 (1.8) | 2 (0.7) | 2 (0.7) | 0 (0) | 0 (0) |
| 3 | 18 (6.1) | 13 (4.6) | 2 (0.7) | 5 (1.8) | 1 (0.3) | 1 (0.4) |
| 4 | 28 (9.9) | 24 (8.6) | 17 (6.0) | 12 (4.3) | 3 (1.1) | 5 (1.8) |
| 4.5 | 38 (15.2)* | 13 (5.2)* | 15 (6.0) | 9 (3.6) | 4 (1.6) | 3 (1.2) |
| 5 | 48 (18.0)* | 30 (10.8)* | 30 (11.2)* | 16 (5.7)* | 12 (4.5) | 5 (1.8) |
| 6 | 69 (24.0)* | 44 (15.4)* | 53 (18.4)* | 25 (8.7)* | 17 (5.9) | 7 (2.4) |
| 9 | 108 (40.4) | 103 (40.4) | 61 (22.8) | 58 (22.7) | 35 (13.1) | 28 (11.0) |
| 12 | 115 (44.1) | 93 (38.0) | 65 (24.9) | 58 (23.6) | 31 (11.9) | 31 (12.7) |
| 15 | 82 (34.3)* | 98 (45.6)* | 56 (23.4) | 52 (24.2) | 24 (10.0) | 34 (15.8) |
| 18 | 70 (29.5) | 78 (34.8) | 44 (18.6) | 43 (19.1) | 17 (7.2) | 23 (10.3) |
| 21 | 56 (25.0) | 58 (26.6) | 31 (13.8) | 34 (15.5) | 12 (5.4) | 21 (9.6) |
| 24 | 49 (21.4) | 56 (25.1) | 30 (13.1) | 35 (15.7) | 7 (3.1) | 12 (5.4) |
| B. As Practiced |
||||||
| Any Diarrhea No. (%) |
Diarrhea-Related Clinic Visit No. (%) |
Prolonged Diarrheaa No. (%) |
||||
| Visit (month) | Stopped BF | Still BF | Stopped BF | Still BF | Stopped BF | Still BF |
| 0 | - | 1 (0.2) | - | 0 (0) | - | 0 (.0) |
| 1 | - | 4 (.7) | - | 3 (.5) | - | 2 (.3) |
| 2 | - | 10 (1.8) | - | 4 (.7) | - | 0 (0) |
| 3 | - | 31 (5.4) | - | 7 (1.2) | - | 2 (0.3) |
| 4 | - | 52 (9.2) | - | 29 (5.2) | - | 8 (1.4) |
| 4.5 | 34 (21.5)* | 17 (5.0)* | 14 (8.9)* | 10 (2.9)* | 4 (2.5) | 3 (0.9) |
| 5 | 40 (22.1)* | 38 (10.4)* | 28 (15.5)* | 18 (4.9)* | 11 (6.1)* | 6 (1.6)* |
| 6 | 61 (30.0)* | 52 (14.1)* | 48 (23.5)* | 30 (8.1)* | 15 (7.4)* | 9 (2.4)* |
| 9 | 93 (43.3) | 118 (38.4) | 58 (27.0) | 61 (19.9) | 36 (16.7)* | 27 (8.8)* |
| 12 | 99 (43.8) | 109 (38.9) | 66 (29.1)* | 57 (20.4)* | 36 (15.9) | 26 (9.3) |
| 15 | 91 (38.1) | 89 (41.4) | 62 (25.9) | 46 (21.4) | 33 (13.8) | 25 (11.6) |
| 18 | 103 (33.9) | 45 (28.7) | 63 (20.7) | 24 (15.2) | 32 (10.5) | 8 (5.1) |
| 21 | 103 (27.5) | 11 (16.4) | 55 (14.6) | 10 (14.9) | 32 (8.5)* | 1 (1.5)* |
| 24 | 98 (22.6) | 7 (38.9) | 62 (14.3) | 3 (16.7) | 17 (3.9) | 2 (11.1) |
NOTE. Three measures of diarrhea morbidity are shown in order of severity: any diarrhea, diarrhea-related clinic visit, and prolonged diarrhea as reported at each of the visits between 0 and 24 months. Asterisks indicate a statistically significant difference between groups (P < .05 using Fisher exact test).
Prolonged diarrhea is defined as a diarrheal episode lasting at least 7 days.
In as practiced analyses, weaning was strongly associated with increased diarrhea and diarrhea-related clinic visits at 4.5, 5, and 6 months. Children who had stopped breastfeeding had a 4.3-fold increased risk of diarrhea at 4.5 months (95% CI, 2.5–7.5), a 2.1-fold increased risk at 5 months (95% CI, 1.4–3.2) and a 2.1-fold increased risk at 6 months (95% CI, 1.5–3.0) compared with children who were still breastfeeding at these time points. Overall, between 4 and 6 months, weaning was associated with a 3.5-fold increased risk of diarrhea episodes (95% CI, 2.6–4.7). Breastfeeding cessation was also associated with a 3-fold increased risk of a diarrhea-related clinic visit at 4.5 months (95% CI, 1.4–6.7), a 3.1-fold increased risk at 5 months (95% CI, 1.8–5.5), and a 2.9-fold increased risk at 6 months (95% CI, 1.9–4.4). In contrast to the less severe outcomes, the association between weaning and prolonged diarrhea continued to older ages, showing significant associations at 5, 6, 9, and 12 months.
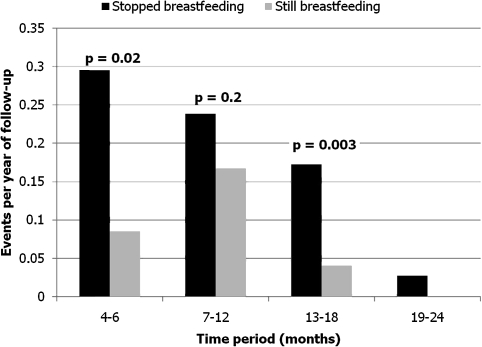
Rates of diarrhea-associated hospitalization or death stratified by actual feeding status and child age are presented in Figure 3. Weaning was associated with diarrhea hospitalization and death across all age strata. Overall between 4 and 24 months, weaning was associated with a 3-fold increase in rates of diarrhea-related hospitalizations or death (relative hazard [RH], = 3.2, 95% CI, 2.1–5.1). In the 4–6-month period, the increase was 3.8-fold (95% CI, 1.2–11.9). The increase was attenuated in intent-to-treat analysis (RH [95% CI], 1.5 [0.5–4.1]).
Figure 3.
Rates of diarrhea-related hospital admission or death among 618 human immunodeficiency virus (HIV)-exposed uninfected infants by actual breastfeeding practice and by age.
Other Risk Factors for Diarrhea
We also compared rates of diarrhea during 4–6 months between nonexclusive breastfeeding, exclusive breastfeeding, and the weaned groups. Compared to exclusive breastfeeding, not breastfeeding was associated with a 3.5-fold increased risk (95% CI, 2.6–4.7) and nonexclusive breastfeeding with a 2.5-fold increase (95% CI, 1.7–3.8) of diarrhea episodes. For diarrhea-related clinic visits and prolonged diarrhea, the associations were stronger (Table 2). Child age, maternal BMI, parity, and maternal employment were associated with diarrhea morbidity at 4–6 months. Adjustment for these confounders slightly attenuated the associations between weaning and diarrhea morbidity, but the associations remained significant. For diarrhea-related clinic visits, breastfeeding cessation was associated with a 3.4-fold increased risk (95% CI, 2.4–5.1) relative to exclusive breastfeeding, adjusting for child age, maternal BMI, and full-time employment. The strongest associations were observed with prolonged diarrhea, where stopping breastfeeding posed a 3.8-fold increased risk (95% CI, 2.1–7.2) compared with exclusive breastfeeding, adjusting for child age and maternal BMI (Table 3).
Table 2.
Odds Ratios From Generalized Estimating Equation Regression Models Assessing Risk Factors for Diarrhea Morbidity Among HIV-exposed Uninfected Infants During the Period 4 to 6 months
| Category | Any Diarrhea |
Diarrhea-Related Clinic Visit |
Prolonged Diarrheaa |
|||
| UnadjustedOR (95% CI) | AdjustedOR (95% CI) | UnadjustedOR (95% CI) | AdjustedOR (95% CI) | UnadjustedOR (95% CI) | AdjustedOR (95% CI) | |
| Feeding practice: | ||||||
| Stopped breastfeeding vs exclusive breastfeeding | 3.5 (2.6–4.7)* | 3.1 (2.3–4.2)* | 4.2 (2.9–6.0)* | 3.5 (2.4–5.1)* | 4.6 (2.5–8.5)* | 3.8 (2.1–7.2)* |
| Still breastfeeding nonexclusively vs exclusive breastfeeding | 2.5 (1.7–3.8)* | 2.2 (1.4–3.3)* | 3.1 (1.9–5.0)* | 2.5 (1.5–4.3)* | 3.6 (1.6–7.9)* | 3.1 (1.3–7.2)* |
| Maternal characteristics: | ||||||
| Plasma viral load ≥100,000 vs < 100,000 copies/mL | 1.2 (0.8–1.6) | - | 1.3 (0.9–2.0) | - | 1.5 (0.8–3.0) | - |
| BMI <18.5 vs ≥18.5 kg/m2 | 1.6 (1.2–2.3)* | 1.6 (1.1–2.2)* | 1.5 (1–2.3)* | - | 2.8 (1.4–5.6)* | 2.8 (1.4, 5.6)* |
| Sociodemographic factors: | ||||||
| ≥ Second child vs first child | 1.3 (0.8–2.0) | - | 1.9 (1.0–3.5)* | 1.9 (1.0–3.4)* | 0.5 (0.1–1.7) | - |
| ≥1 child ≥5 years old in the household vs none | 1.2 (0.9–1.6) | - | 1.1 (0.8–1.5) | - | 0.9 (0.5–1.8) | |
| High school or more (≥8 years) vs <8 years | 1 (0.8–1.4) | - | 0.9 (0.7–1.3) | - | 1.2 (0.6–2.3) | - |
| Electricity in the home | 1 (0.8–1.4) | - | 1 (0.7–1.4) | - | 0.7 (0.4–1.4) | - |
| Full-time paid job vs part-time job or unemployed | 1.7 (1.1–2.8)* | - | 2.4 (1.4–4.0)* | 2.1 (1.2–3.6)* | 0.5 (0.1–2.0) | - |
| ≥1 day without food at home 1 month before baseline | 1.1 (0.8–1.6) | - | 1 (0.6–1.5) | - | 1 (0.4–2.4) | - |
| Rainy season vs dry season | 1.1 (0.8–1.4) | - | 1.1 (0.8–1.5) | - | 1.5 (0.8–2.8) | - |
NOTE. Separate regression analyses are shown for each of the 3 outcomes: any diarrhea, diarrhea-related clinic visit, and prolonged diarrhea. Table displays odds ratios (ORs) and 95% confidence intervals (CIs) from generalized estimating equation (GEE) models. ORs marked with an asterisk are significant at P < .05. The adjusted ORs are adjusted for the other factors shown in the adjusted column of the table as well as for age.
Prolonged diarrhea is defined as a diarrheal episode lasting at least 7 days.
Table 3.
Odds Ratios From Generalized Estimating Equation Regression Models Assessing Risk Factors for Diarrhea Morbidity Among HIV-exposed Uninfected Infants During the Period 7–24 Months
| Category | Any Diarrhea |
Diarrhea-Related Clinic Visit |
Prolonged Diarrheaa |
|||
| UnadjustedOR (95% CI) | AdjustedOR (95% CI) | UnadjustedOR (95% CI) | AdjustedOR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Feeding practice: | ||||||
| Stopped breastfeeding vs still breastfeeding | 0.9 (0.7–1.0) | - | 1 (0.8–1.3) | - | 1.2 (0.9–1.6) | 1.7 (1.3–2.3)* |
| Maternal characteristics: | ||||||
| Plasma viral load ≥100,000 vs <100,000 copies/ml | 1.1 (0.9–1.4) | - | 1.2 (0.9–1.6) | - | 1.1 (0.8–1.5) | - |
| BMI <18.5 vs ≥18.5 kg/m2 | 1.2 (1–1.6) | - | 1.4 (1–1.8)* | - | 1.8 (1.2–2.5)* | 1.7 (1.2, 2.4)* |
| Sociodemographic factors: | ||||||
| ≥ Second child vs first child | 1.3 (1–1.7)* | - | 1.6 (1.1–2.4)* | 1.6 (1–2.4)* | 0.6 (0.3–1.0)* | 1.8 (1.1–3.1)* |
| ≥1 child ≥5 years old in the household vs none | 1.2 (1–1.5)* | 1.3 (1.1–1.6)* | 1.2 (0.9–1.5) | - | 1.1 (0.8–1.5) | - |
| High school or more (≥8 years) vs <8 years | 0.8 (0.7–1.0) | - | 0.8 (0.6–1.0) | - | 0.8 (0.6–1.1) | - |
| Electricity in the home | 0.7 (0.6–0.9)* | 0.7 (0.6–0.9)* | 0.7 (0.6–.9)* | 0.8 (0.6–1.0)* | 0.7 (0.5–1.0)* | - |
| Full-time paid job vs part time job or unemployed | 1 (0.7–1.4) | - | 1.1 (0.7–1.7) | - | 1 (0.6–1.6) | - |
| ≥1 day without food at home 1 month before baseline | 1.2 (1–1.5) | - | 1.5 (1.1–1.9) | 1.4 (1–1.8) | 1.3 (0.9–1.8) | - |
| Rainy season vs dry season | 1.5 (1.3–1.8)* | 1.5 (1.3–1.8)* | 1.7 (1.4–2.0)* | 1.7 (1.4–2.1)* | 1.7 (1.3–2.3)* | 1.7 (1.3–2.2)* |
NOTE. Separate regression analyses are shown for each of the 3 outcomes: any diarrhea, diarrhea-related clinic visit, and prolonged diarrhea. Table displays odds ratios (ORs) and 95% confidence intervals (CIs) from generalized estimating equation (GEE) models. ORs marked with an asterisk are significant at P < .05. The adjusted ORs are adjusted for the other factors shown in the adjusted column of the table as well as for age.
Prolonged diarrhea is defined as a diarrheal episode lasting at least 7 days.
During the 7–24-month period, the associations between weaning and the less severe outcomes of any diarrheal episodes or diarrhea-related clinic visits were attenuated. However, there remained a significant association between weaning and prolonged diarrhea (Table 3). Factors related to diarrhea morbidity at 7–24 months differed from those observed in the 4–6-month period. The rainy season, higher parity, more children in the home, lack of electricity, and food insecurity at baseline were associated with the less severe diarrhea outcomes in this age group. Stopping breastfeeding continued to be associated with a significant 1.7-fold increased risk (95% CI, 1.3–2.3) of prolonged diarrhea relative to continued breastfeeding, after adjustment for child age, season, maternal BMI, and parity.
Breastfeeding cessation was significantly associated with diarrhea-related hospitalizations and deaths at 4–24 months after adjustment for potential confounders. Children who stopped breastfeeding had a 2.2-fold (95% CI, 1.4–3.3) increased risk of a diarrhea-related hospitalization or death after adjusting for number of children in the home, season, and maternal death, which were the other factors related to this outcome in multivariable analysis.
DISCUSSION
Early breastfeeding cessation had an adverse effect on diarrheal morbidity and mortality among HIV-exposed uninfected children. The adverse effect of not breastfeeding was strongest in the 4–6-month period when children transitioned from exclusive breastfeeding to no breastfeeding, but the increased risk of prolonged diarrhea and diarrhea-related hospitalizations and deaths persisted into the second year of life. Our results are consistent with many studies over past decades among children born to HIV-uninfected mothers that have consistently reported that the absence of breastfeeding is a major risk factor for diarrheal morbidity [17–19] and diarrhea-related mortality [20–23].
Our results are also consistent with findings from other studies of uninfected children born to HIV-infected mothers. In 3 independent cohorts of HIV-infected women in Malawi, Uganda, and Kenya, women were advised to stop breastfeeding earlier than usual [24–26]. All observed noticeably higher rates of diarrheal morbidity and mortality after weaning. In one cohort, a water safety intervention was implemented. Despite good uptake, there was no impact on rates of diarrhea among weaned children [26]. An analysis from Malawi compared the incidence of gastroenteritis in HIV-exposed uninfected children enrolled in 2 trials of postexposure prophylaxis [27]. In one trial, women were counseled to stop breastfeeding early; in the other no specific advice was given about breastfeeding duration. There was increased incidence of gastroenteritis in the cohort counseled to stop breastfeeding at 6 months compared with the prior cohort not advised to wean early. Our study included intensive counseling and education around replacement food preparation and hygiene. Although we provided infant formula, our staff strongly encouraged use of a fortified weaning cereal that required cooking to minimize risks of contamination associated with formula. All children received cotrimoxazole between 6 weeks and 12 months. Despite these interventions, the absence of breast milk was associated with significant hazards. In addition, in this analysis, we made conservative assumptions that would bias findings toward the null hypothesis (ie, weaning is not associated with diarrhea-related mortality) and would minimize misclassification attributable to reverse causality (ie, mothers stopping breastfeeding as a result of the child’s illness). An example of this is the assumption that children who died had breastfed until the date of death unless there was a record that they had stopped prior to the illness that preceded their death.
Acute and persistent diarrhea renders a child susceptible to death [14, 28, 29] and long-term morbidity. Enteric pathogens that trigger early childhood diarrhea affect nutrient absorption and have a lasting impact on growth, recovery from malnutrition, and development [30]. In a multicountry analysis, the odds of stunting at age 24 months increased multiplicatively with each diarrheal episode and with each day of diarrhea before 24 months of age [31].
We found that nonexclusive breastfeeding at 4–6 months conferred a higher risk of acute and prolonged diarrhea than did exclusive breastfeeding. Exclusive breastfeeding has been reported to be more protective against diarrheal morbidity and mortality than nonexclusive breastfeeding in some [17, 19] but not all prior studies [21, 22, 32]. In low-resource settings, introducing non-breast-milk items to a breastfed child may diminish the protective effects of breastfeeding through bacterial exposure from unprotected water sources, contaminated foods, or unhygienic sanitation [33–35]. Several studies have noted that the association between breastfeeding and diarrheal disease declines as the child gets older [19, 20, 23], consistent with our findings of high relative risks associated with weaning in the 4–6-month age group. Although in our data benefits of breastfeeding were attenuated after 6 months, older children whose mothers stopped breastfeeding still had significantly higher rates of more severe diarrhea than did those whose mothers continued to breastfeed. During these later months, all breastfeeding is nonexclusive; thus the comparison is nonexclusive breastfeeding vs fully weaned. All children, regardless of whether they are breastfed, are exposed to the contaminated environment. Exposure increases with age due to behavioral development and with introduction of complementary foods and liquids. Our findings of persistent benefits of breastfeeding at older ages suggest that benefits of breastfeeding are explained both by a reduction in exposure to environmental contaminants and by protective components of human milk. When breastfeeding is no longer exclusive, immunologic factors are likely to be the predominant explanation for breastfeeding’s benefits. Many factors in breast milk, including secretory antibodies, oligosaccharides, glycoconjugates, lactoferrin, leukocytes, and cytokines, have been found to have anti-infective, anti-inflammatory, and immunoregulatory functions [36].
Seasonal patterns in diarrheal morbidity and mortality are expected and have been observed in similar settings. They are typically attributed to increased fecal contamination of drinking water, contamination of foods washed by contaminated water, higher bacterial growth due to high temperatures, and peaks in the incidence of infectious diseases such as cholera [37, 38]. In our study, an increased frequency of diarrhea during the rainy season occurred only among children older than 6 months. The protective benefits of breastfeeding persisted after adjustment for season. The environmental risks to HIV-exposed uninfected children were exemplified in an outbreak of diarrhea in Botswana after a period of heavy rains, in which not breastfeeding dramatically increased the risk of diarrhea-related morbidity and mortality [39, 40].
Maternal full-time paid employment was associated with an increase in infant diarrhea at 4–6 months of age. Other factors related to food insecurity, hygiene, low socioeconomic status, low maternal BMI, other children in the household, and high parity were associated with diarrheal morbidity at older ages only. Use of an unprotected water source is cited as a typical risk factor for diarrheal morbidity, especially after the period of exclusive or predominant breastfeeding [37, 41]; however, we did not find any effect of water source that could not be explained by confounding. This is likely due to the fact that the majority of subjects got their water from protected sources, and none reported using an unprotected water source.
There are a number of limitations to our study. By virtue of the success of our study intervention to encourage women in both study arms to exclusively breastfeed for 4 months [10], evaluation of the association between the exclusivity of breastfeeding and diarrhea was limited by the relatively small number of nonexclusively breastfed infants. It was also not possible to evaluate the effect of breastfeeding on persistent diarrhea using the standard definition of more than 14 days’ duration [42] due to the scarcity of such cases. This too may be a byproduct of participation in the clinical trial in which mothers were encouraged to seek medical treatment for ill infants. We were also not able to identify the proximal causes of diarrhea and are unable to determine whether the reductions in diarrhea due to breastfeeding differed across etiologic agents and noninfectious causes.
Poor caretaker recall of childhood diarrheal incidence is well documented [43–45] and likely led to an underestimate of diarrheal frequency in this study. In addition, as only 63% of women randomized to the short-duration breastfeeding group actually weaned at 4 months, findings in intent-to-treat analyses were diluted. Analyses based on actual practices may have been influenced by unmeasured or residual confounding. We did not collect data on sanitation practices, typically cited as strong risk factors for diarrhea [17]. Finally, women were drawn from a socioeconomically disadvantaged urban population, limiting the generalizability of our findings.
Overall our data demonstrate that early weaning poses substantial risk for HIV-exposed uninfected infants and young children even in a setting with adequate education, counseling, and provision of replacement and complementary foods. The clear protective benefits of exclusive breastfeeding on diarrheal morbidity exhibited during the first 6 months of life underscores the importance of supporting exclusive breastfeeding for HIV-infected mothers in low-resource settings to prevent HIV transmission and reduce morbidity among uninfected infants. The continued benefits of breastfeeding on reducing the severe outcomes of prolonged diarrhea and diarrhea-related hospitalization and deaths after 6 months of age underscore the importance of promoting continued breastfeeding after 6 months for HIV-infected women, a recommendation recently adopted by the WHO [46]. Although there is a possibility of ongoing HIV transmission with continued breastfeeding, this risk needs to be viewed in the light of the serious morbidity, mortality, and growth risks for HIV-uninfected infants associated with prematurely truncating the usual duration of breastfeeding.
Funding
This research was supported by the National Institute of Child Health and Human Development (HD 57161, HD 39611, and HD 40777).
References
- 1.Feachem RG, Koblinsky MA. Interventions for the control of diarrhoeal diseases among young children: promotion of breast-feeding. Bull World Health Organ. 1984;62:271–91. [PMC free article] [PubMed] [Google Scholar]
- 2.Monterrosa EC, Frongillo EA, Vasquez-Garibay EM, Romero-Velarde E, Casey LM, Willows ND. Predominant breast-feeding from birth to six months is associated with fewer gastrointestinal infections and increased risk for iron deficiency among infants. J Nutr. 2008;138:1499–504. doi: 10.1093/jn/138.8.1499. [DOI] [PubMed] [Google Scholar]
- 3.Myers MG, Fomon SJ, Koontz FP, McGuinness GA, Lachenbruch PA, Hollingshead R. Respiratory and gastrointestinal illnesses in breast- and formula-fed infants. Am J Dis Child. 1984;138:629–32. doi: 10.1001/archpedi.1984.02140450011003. [DOI] [PubMed] [Google Scholar]
- 4.Sethi D, Cumberland P, Hudson MJ, et al. A study of infectious intestinal disease in England: risk factors associated with group A rotavirus in children. Epidemiol Infect. 2001;126:63–70. doi: 10.1017/s0950268801005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn L, Aldrovandi G, Sinkala M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–41. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn L, Sinkala M, Semrau K, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50:437–44. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn L, Aldrovandi GM, Sinkala M, et al. Differential effects of early weaning for HIV-free survival of children born to HIV-infected mothers by severity of maternal disease. PLoS One. 2009;4:e6059. doi: 10.1371/journal.pone.0006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arpadi S, Fawzy A, Aldrovandi GM, et al. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90:344–53. doi: 10.3945/ajcn.2009.27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn L, Sinkala M, Kankasa C, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS One. 2007;2:e1363. doi: 10.1371/journal.pone.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thea D, Vwalika C, Kasonde P, et al. Issues in the design of a clinical trial with a behavioral intervention—the Zambia exclusive breast-feeding study. Control Clin Trials. 2004;25:353–65. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh MK, Kuhn L, West J, et al. Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol. 2003;41:2465–70. doi: 10.1128/JCM.41.6.2465-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambia Meteorological Department. World Weather Information Service–Lusaka. http://worldweather.wmo.int/040/c00150.htm. Accessed 10 October 2008. [Google Scholar]
- 14.Black R. Persistent diarrhea in children of developing countries. Pediatr Infect Dis J. 1993;12:751–61. doi: 10.1097/00006454-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 16.Honda Y, Macaluso M, Brill I. A SAS program for the stratified analysis of follow-up data. J Occup Health. 1998;40:154–7. [Google Scholar]
- 17.Ahiadeke C. Breast-feeding, diarrhoea and sanitation as components of infant and child health: a study of large scale survey data from Ghana and Nigeria. J Biosoc Sci. 2000;32:47–61. [PubMed] [Google Scholar]
- 18.Bhandari N, Bahl R, Mazumdar S, Martines J, Black R, Bhan M. Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet. 2003;361:1418–23. doi: 10.1016/S0140-6736(03)13134-0. [DOI] [PubMed] [Google Scholar]
- 19.Brown K, Black R, Lopez de Romaña G, Creed de Kanashiro H. Infant-feeding practices and their relationship with diarrheal and other diseases in Huascar (Lima), Peru. Pediatrics. 1989;83:31–40. [PubMed] [Google Scholar]
- 20.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355:451–5. [PubMed] [Google Scholar]
- 21.Arifeen S, Black R, Antelman G, Baqui A, Caulfield L, Becker S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108:E67. doi: 10.1542/peds.108.4.e67. [DOI] [PubMed] [Google Scholar]
- 22.Bahl R, Frost C, Kirkwood B, et al. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: multicentre cohort study. Bull World Health Organ. 2005;83:418–26. [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon P, Black R, Moulton L, Becker S. Effect of not breastfeeding on the risk of diarrheal and respiratory mortality in children under 2 years of age in Metro Cebu, the Philippines. Am J Epidemiol. 1996;143:1142–8. doi: 10.1093/oxfordjournals.aje.a008692. [DOI] [PubMed] [Google Scholar]
- 24.Kourtis A, Fitzgerald D, Hyde L, et al. 14th CROI. Los Angeles: 2007. Diarrhea in uninfected infants of HIV-infected mothers who stop breastfeeding at 6 months: the BAN study experience 772. [Google Scholar]
- 25.Onyango-Makumbi C, Bagenda D, Mwatha A, et al. Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV prevention trials in Kampala, Uganda. J Acquir Immune Defic Syndr. 2010;53:20–7. doi: 10.1097/QAI.0b013e3181bdf68e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris JR, Greene SK, Thomas TK, et al. Effect of a point-of-use water treatment and safe water storage intervention on diarrhea in infants of HIV-infected mothers. J Infect Dis. 2009;200:1186–93. doi: 10.1086/605841. [DOI] [PubMed] [Google Scholar]
- 27.Kafulafula G, Hoover DR, Taha TE, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 28.Bhandari N, Bhan M, Sazawal S. Mortality associated with acute watery diarrhea, dysentery and persistent diarrhea in rural North India. Acta Paediatr Suppl. 1992;381:3–6. [PubMed] [Google Scholar]
- 29.Fauveau V, Henry F, Briend A, Yunus M, Chakraborty J. Persistent diarrhea as a cause of childhood mortality in rural Bangladesh. Acta Paediatr Suppl. 1992;381:12–4. doi: 10.1111/j.1651-2227.1992.tb12365.x. [DOI] [PubMed] [Google Scholar]
- 30.Petri WJ, Miller M, Binder H, Levine M, Dillingham R, Guerrant R. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–90. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Checkley W, Buckley G, Gilman R, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:814–30. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihrshahi S, Oddy W, Peat J, Kabir I. Association between infant feeding patterns and diarrhoeal and respiratory illness: a cohort study in Chittagong, Bangladesh. Int Breastfeeding J. 2008;3:28. doi: 10.1186/1746-4358-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks J, Ochieng J, Kumar L, et al. Surveillance for bacterial diarrhea and antimicrobial resistance in rural western Kenya, 1997–2003. Clin Infect Dis. 2006;43:393–401. doi: 10.1086/505866. [DOI] [PubMed] [Google Scholar]
- 34.Marino D. Water and food safety in the developing world: global implications for health and nutrition of infants and young children. J Am Diet Assoc. 2007;107:1930–4. doi: 10.1016/j.jada.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Motarjemi Y, Käferstein F, Moy G, Quevedo F. Contaminated weaning food: a major risk factor for diarrhoea and associated malnutrition. Bull World Health Organ. 1993;71:79–92. [PMC free article] [PubMed] [Google Scholar]
- 36.Morrow AL, Rangel JM. Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis. 2004;15:221–8. doi: 10.1053/j.spid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Mølbak K, Jensen H, Ingholt L, Aaby P. Risk factors for diarrheal disease incidence in early childhood: a community cohort study from Guinea-Bissau. Am J Epidemiol. 1997;146:273–82. doi: 10.1093/oxfordjournals.aje.a009263. [DOI] [PubMed] [Google Scholar]
- 38.Rowland M. The Gambia and Bangladesh: the seasons and diarrhoea. Dialogue Diarrhoea. 1986;26:3. [PubMed] [Google Scholar]
- 39.Creek TL, Kim A, Lu L, et al. Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana. J Acquir Immune Defic Syndr. 2006;53:14–9. doi: 10.1097/QAI.0b013e3181bdf676. [DOI] [PubMed] [Google Scholar]
- 40.Mach O, Lu L, Creek T, et al. Population-based study of a widespread outbreak of diarrhea associated with increased mortality and malnutrition in Botswana, January-March, 2006. Am J Trop Med Hyg. 2009;80:812–8. [PubMed] [Google Scholar]
- 41.Plate D, Strassmann B, Wilson M. Water sources are associated with childhood diarrhoea prevalence in rural east-central Mali. Trop Med Int Health. 2004;9:416–25. doi: 10.1111/j.1365-3156.2004.01200.x. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Geneva: World Health Organization; 1985. Diarrhoeal disease control programme. Persistent diarrhea in children-research priorities. [Google Scholar]
- 43.Alam N, Henry F, Rahaman M. Reporting errors in one-week diarrhoea recall surveys: experience from a prospective study in rural Bangladesh. Int J Epidemiol. 1989;18:697–700. doi: 10.1093/ije/18.3.697. [DOI] [PubMed] [Google Scholar]
- 44.Melo M, Taddei J, Diniz-Santos D, May D, Carneiro N, Silva L. Incidence of diarrhea: poor parental recall ability. Braz J Infect Dis. 2007;11:571–9. doi: 10.1590/s1413-86702007000600009. [DOI] [PubMed] [Google Scholar]
- 45.Ramakrishnan R, Venkatarao T, Koya P, Kamaraj P. Influence of recall period on estimates of diarrhoea morbidity in infants in rural Tamil Nadu. Indian J Public Health. 1998;42:3–6. [PubMed] [Google Scholar]
- 46.World Health Organization. Guidelines on HIV and infant feeding 2010. Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: WHO. 2010 [PubMed] [Google Scholar]