Abstract
The pathophysiology of dengue virus infection remains poorly understood, although secondary infection is strongly associated with more severe disease. In the present study, we performed a nested, case-control study comparing the responses of pre-illness peripheral blood mononuclear cells between children who would subsequently develop either subclinical or symptomatic secondary infection 6–11 months after the baseline blood samples were obtained and frozen. We analyzed intracellular cytokine production by CD4+ and CD8+ cells in response to stimulation with dengue antigen. We found higher frequencies of dengue virus–specific TNFα, IFNγ-, and IL-2–producing T cells among schoolchildren who subsequently developed subclinical infection, compared with those who developed symptomatic secondary dengue virus infection. Although other studies have correlated immune responses during secondary infection with severity of disease, to our knowledge this is the first study to demonstrate a pre-infection dengue-specific immune response that correlates specifically with a subclinical secondary infection.
Dengue is an important global health problem, and the dengue viruses (DENV) are the most prevalent arthropod-borne viruses in the tropics and subtropics today. There are an estimated 50–100 million DENV infections annually worldwide [1]. The spectrum of disease ranges from subclinical infection to classical dengue fever to the more severe dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). DHF and DSS, characterized by significant plasma leakage and occasionally hemorrhage and shock, account for an estimated half million cases and >20,000 deaths annually [2].
There are 4 distinct serotypes of dengue virus (DENV 1–4); infection with 1 serotype is believed to confer lifelong immunity to that serotype, whereas protective immunity to heterologous serotypes is short-lived, probably on the order of months [3]. One of the unique features of dengue is the role of heterologous infection in disease severity. Both clinical and epidemiological studies have suggested that the immune response plays a major role in the pathogenesis of severe dengue disease. Subsequent infection with a heterologous serotype predisposes patients toward more severe disease; studies in Southeast Asia, done both in the 1980s [4, 5] and more recently [6, 7], have shown that DHF is associated with secondary infection, implying that other mechanisms in addition to viral replication could be the cause of DHF.
Pre-existing heterotypic immunity is believed to increase disease severity in secondary DENV infection and, thus, the risk for DHF through 2 broad hypothetical (and not necessarily mutually exclusive) pathways. The Antibody Dependent Enhancement hypothesis proposes that pre-existing heterotypic anti-DENV IgG antibodies acquired during a previous infection can, under certain conditions, facilitate uptake of virus into macrophages and other immune cells through the binding of virus-antibody complexes to Fcγ receptors. The consequent increase in receptor-mediated endocytosis leads to higher viral loads, which in turn, triggers a host inflammatory cascade that leads to DHF [8]. The second hypothesis involves cross-reactive memory T cells, which are activated during heterologous secondary infection. This leads to altered effector responses, including the generation of cytokines that may create an immunologic imbalance in response to infection and, thus, play either a protective or harmful role in the development of sequelae of infection [9].
Our model of T cell–mediated immunopathogenesis of DENV infection postulates that the relative production of vasoactive cytokines by activated dengue-specific memory T lymphocytes influences the severity of dengue illness [10]. To our knowledge, no previous studies have looked at host-immune factors that specifically correlate with subclinical dengue infection. We hypothesized that the memory T cell response generated during primary DENV infection is a major determinant of cytokine production during secondary infection and, therefore, influences the severity of symptoms experienced during secondary infection. Specifically, we predicted that the frequencies of dengue-specific, cytokine-producing T cells in peripheral blood mononuclear cells (PBMCs) obtained before secondary infection would correlate with the severity of disease seen in secondary infection.
To address this hypothesis, we tested PBMCs from Thai schoolchildren enrolled in a prospective cohort study [11]. The unique aspects of this study included the prospective scheduled collection and cryopreservation of PBMCs and the active surveillance for all febrile illnesses. This cohort provided an opportunity to study the predictive value of pre-infection cellular immune profiles with disease phenotype after subsequent DENV infection. From PBMCs obtained before secondary infection, we measured dengue-specific CD4+ and CD8+ T cell cytokine responses to dengue antigens to assess whether these responses predicted future disease severity. Because of the short interval between the collection of the baseline and follow-up blood samples, we believe that the frequencies of the DENV-specific cells at baseline reflect, as accurately as is possible in a large-scale prospective cohort, the host immune status with respect to DENV at the time of secondary infection.
MATERIALS AND METHODS
Study Design
We performed a nested, case-control study of 33 schoolchildren who were known to have developed secondary infection as part of a prospective study of primary school students in Kamphaeng Phet Province, Thailand. Using PBMCs obtained at the beginning of the enrollment period when all participants were healthy, we compared the frequency of intracellular cytokine-producing cells after stimulation with dengue antigens between 2 groups of children: one that had symptomatic dengue infection and a control group that had subclinical infection. The design of the prospective study has been reported elsewhere [11]. In brief, blood samples were collected from all participants in January 1998; plasma samples and PBMCs were cryopreserved and stored in liquid nitrogen. In addition, blood samples were collected from all participants in June, August, and November and were tested by hemagglutination inhibition (HI) to identify subclinical DENV infection, as measured by a ≥4-fold increase in HI titer from June through August or August through November.
Study participants were monitored for school absences because of fever during the period associated with peak dengue transmission (June–November). Those participants with fever or history of fever in the prior 7 days were further evaluated with collection of acute and convalescent (>15 day) blood samples. DENV infection was identified by virus isolation and/or reverse-transcriptase polymerase chain reaction (RT-PCR) of acute serum samples. The infecting DV serotype was identified from acute-phase serum samples by use of a serotype-specific RT-PCR with use of previously published methods [12]. Virus isolation in Toxorhnychites splendens mosquitoes was performed with plasma samples from all symptomatic patients with use of methods described elsewhere [13]. Serologic examination to determine primary or secondary infection used dengue IgG/IgM enzyme-linked immunosorbent assay (ELISA) and HI assays against all 4 dengue serotypes [14]. All donors during symptomatic illness had their blood samples tested by both PCR and virus isolation.
A case was considered to be symptomatic if they had a school absence with a fever or history of fever and serologic findings consistent with acute dengue infection. Subclinical DENV infection was defined as a 4-fold increase in HI antibody against any DENV serotype between 2 sequential serum samples obtained during the surveillance months (June, August, or November) without a febrile illness identified during active surveillance during the period that seroconversion occurred. Serum samples were tested concurrently for Japanese encephalitis virus (JEV)-specific HI antibody to exclude JEV infection and antibody cross-reactivity as a cause for an increase in dengue antibody.
All laboratory-confirmed virus infections were characterized according to World Health Organization guidelines for dengue fever and DHF [15]. Informed consent was obtained from all patients or their parents, and these studies were approved by the institutional review boards at the University of Massachusetts Medical School; the Ministry of Public Health, Thailand; and the Human Subjects Research Review Board for the Commanding General of the US Army Medical Research and Material Command.
From the larger cohort, we identified a group of patients who, by serologic testing, were known to have developed secondary DENV infections in 1998. From this group, 33 samples were randomly selected for analysis. The persons selected represented 6 of the 12 Kamphaeng Phet schools that participated in the study. Of the samples tested, 4 were eliminated from the analysis because of inadequate cell events (<50,000 events/tube). Two patients were excluded because the pre-illness serologic findings showed no detectable (titer, <1:10) neutralizing antibodies to any of the 4 DENV serotypes; one of these patients had a neutralizing antibody titer of 1:35 to JEV, and the other had no detectable neutralizing antibody to JEV. We compared 10 samples from children who developed symptomatic infection with 17 samples from children who had subclinical infection.
Antigen Preparation and Controls
Inactivated DENV-infected Vero cell lysates were prepared as described elsewhere [16]. Control antigens were prepared in a similar manner with use of uninfected Vero cells. The following viruses were used: DENV-1 Hawaii, DENV-2 New Guinea C, DENV-3 CH53489, and DENV-4 814669. Control antigen was a lysate of uninfected Vero cells. Phorbol Myristate Acetate at 20 μg/mL and ionomycin at 100 μg/mL were added as a positive control. A further negative control included cells incubated in media alone. All assays used a single lot of antigens, and each lot was tested for reactivity with monotypic DENV-immune donors [16].
Cytokine Flow Cytometry
Laboratory assays and analysis of flow cytometry data was done under code, with the investigator blinded to the clinical information. With use of previously established methods [16], PBMCs were stimulated with antigen at an antigen dilution of 1:20 for 18 h, at which time Brefeldin A was added; surface and intracellular cytokine staining was performed 6 h later. Cell phenotype was determined by the following surface stains: CD3+ Pacific Blue, CD8+ PERCP-Cy5.5, and CD 14+ & CD 19+ PE-CY7 (BD Biosciences) and CD4+ Alexa 700 (eBiosciences). Cell viability was measured using the surface stain Live-Dead Aqua (Invitrogen). Intracellular cytokine production was measured using the following stains: TNF-α APC (BD Biosciences), IFN-γ APC-Alexa 750 (Caltag Laboratories), and IL-2 PE (eBiosciences). Data were acquired using FACS - ARIA flow cytometer. Positive responses were measured by subtracting the background percentage of cytokine-positive T cells in the control antigen tube; those tubes with an equal or smaller percentage of cells than that of the control antigen were considered to have no response. The data were analyzed using FlowJo, version 6.2.4 (Tree Star). Live-Dead- CD3+ CD14/19- cells (both CD4+ and CD8+) were gated and analyzed for cytokine production.
Data Analysis
All data were analyzed using Stata software. Comparison of median frequencies of DENV-specific cytokine-producing CD4+ and CD8+ T cells between symptomatic and subclinical infection groups was done using the Wilcoxon rank sum test. The number of patients with detectable (above negative control) DENV-specific T cell cytokine responses between the symptomatic and subclinical groups for each DENV serotype was compared using Fisher's exact test.
RESULTS
Cohort Characteristics
Table 1 shows the characteristics of both groups defined on the basis of the clinical outcome during secondary infection: persons who experienced a febrile illness (ie, symptomatic group) and persons who had serologic evidence of infection without a recognized febrile illness (ie, subclinical group). Of the 10 patients in the symptomatic group, only 1 was hospitalized (this patient did not meet criteria for DHF), 6 had RT-PCR positive for DENV (5 with DENV-3 and 1 with DENV-1); the virus isolation results matched these findings.
Table 1.
Serological Characteristics of the Study Population
| Plaque Reduction Neutralization Titers (PRNT50) |
Acute Illness |
||||||||
| Group | Subject | Den 1 | Den 2 | Den 3 | Den 4 | JE | PCR + | Viral Culture | Den IgM/IgG |
| Symptomatic | |||||||||
| 105 | 3443 | 7456 | 1445 | 162 | 1 | No | - | <1 | |
| 230 | 946 | 441 | 2657 | 210 | 323 | No | - | <1 | |
| 247 | 7925 | 129 | 8232 | 1 | 17 | No | - | <1 | |
| 592 | 1 | 152 | 1 | 1 | 1 | Yes | D1 | <1 | |
| 1136 | 745 | 21 | 176 | 56 | 1 | Yes | D3 | <1 | |
| 1250 | >10240 | 471 | 1962 | 1 | 71 | Yes | D3 | <1 | |
| 1285a | 1 | 299 | 150 | 1 | 1 | Yes | D3 | <1 | |
| 1307 | 211 | 898 | 3015 | 1 | 79 | Yes | D3 | <1 | |
| 1413 | 1 | 1 | 14 | 1 | 1 | No | - | <1 | |
| 1484 | 1 | 377 | 1 | 1 | 21 | Yes | D3 | <1 | |
| Subclinical | |||||||||
| 143 | 1081 | 482 | 979 | 51 | 1 | ||||
| 177 | 545 | 1030 | 219 | 1 | 1 | ||||
| 209 | 562 | 110 | 452 | 112 | 64 | ||||
| 431 | 130 | 478 | 71 | 53 | 1 | ||||
| 585 | 4456 | 715 | 2619 | 1 | 1 | ||||
| 628 | 2129 | 1 | 1674 | 1 | 1 | ||||
| 637 | 1107 | 686 | 2226 | 1 | 42 | ||||
| 963 | >10240 | 211 | 465 | 15 | 1 | ||||
| 982 | 2031 | 1 | 90 | 1 | 1 | ||||
| 1281 | 636 | 332 | 2344 | 1 | 80 | ||||
| 1294 | 1 | 1013 | 1 | 1 | 1 | ||||
| 1327 | 364 | 435 | 210 | 1 | 1 | ||||
| 1342 | 879 | 321 | 630 | 1 | 1 | ||||
| 1374 | 2658 | 441 | 451 | 11 | 42 | ||||
| 1402 | 1 | 261 | 1 | 1 | 53 | ||||
| 1557 | 1036 | 191 | 4598 | 1 | 1 | ||||
| 1577 | 16 | 906 | 124 | 1 | 1 | ||||
NOTE. The PRNT50 data refer to serum taken from all subjects as part of routine serologic surveillance in January 1998. The latter three columns refer to laboratory data taken at the time of acute illness. A PRNT50 value of 1 indicates an undetectable antibody response. An IgM/IgG ratio of <1.8 indicates secondary infection, while a ratio >1.8 indicates primary infection. The PCR, Viral culture, and DENV IgM data were unavailable for the subclinical group as these are routine tests during symptomatic infection.
Hospitalized; did not meet WHO criteria for DHF.
DENV-Specific CD4+ T Cell Cytokine Responses
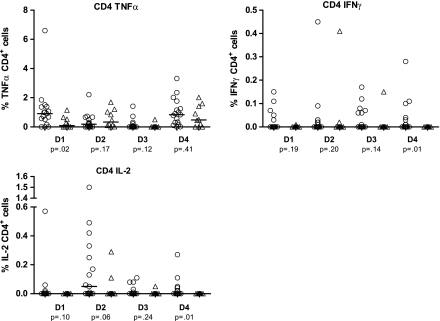
Figure 2 shows the frequency of DENV-specific cytokine-producing cells (TNFα, IFNγ, and IL-2) in response to stimulation with each of the 4 DENV antigens in the PBMCs collected before the index secondary DENV infection. Regardless of clinical outcome, the majority of patients tested displayed a higher frequency of TNFα-producing cells after antigen stimulation, regardless of serotype, whereas the frequencies of IFNγ and IL-2 producing cells were much smaller for both groups.
Figure 2.
CD4+ intracellular cytokine production. TNFα, IFNγ, and IL-2 production was measured in response to antigen stimulation with 4 dengue serotypes of presecondary infection PBMC from 27 Thai schoolchildren. Comparison groups are 10 children (triangles) who developed symptomatic infection and 17 children (circles) who had subclinical infection established retrospectively through dengue IgG ELISA and HI titers. Median percentage of cytokine-producing cells and P values to detect significant median differences using the Wilcoxon rank sum test are shown.
The gating strategy is shown in Figure 1. Overall (ie, across serotypes and cytokines), the frequencies of DENV-specific cytokine-producing CD4+ T cells were higher in the subclinical group than in the symptomatic group. The percentage of IL-2–producing cells was higher in the subclinical group after in vitro stimulation with DENV-1, DENV-2, and DENV-4 antigens, although the finding was statistically significant for only DENV-4 (P = .01). The percentage of IFNγ-producing cells responding to DENV-4 antigen was significantly higher in the subclinical group, as was the percentage of TNFα-producing cells responding to DENV-1 antigen.
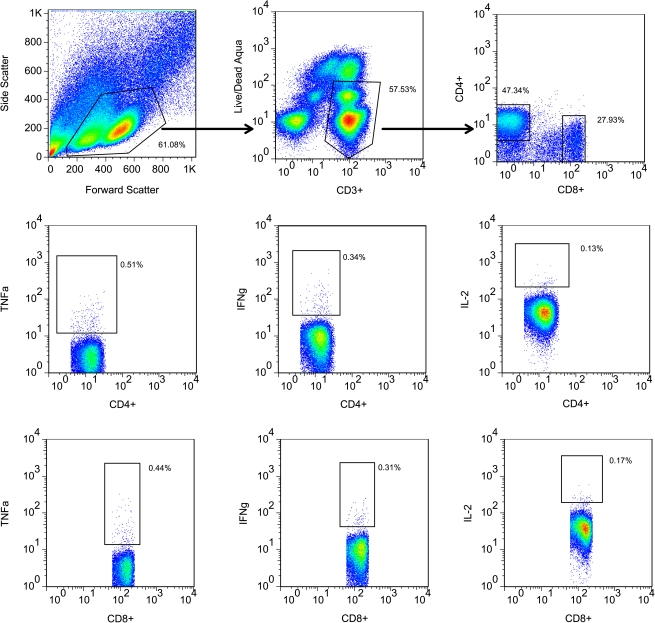
Figure 1.
Gating strategy. Shown in the top row are gated lymphocytes in forward versus side scatter (left); CD3+, live/dead negative gated cells (center); and CD4+ or CD8+ gated cells (right). In the middle row are CD4+ cells producing TNFα (left), IFNγ (center), or IL-2 (right); the bottom row shows the same arrangement for CD8+ cells. The frequencies of cytokine-producing cells were determined by subtraction of the background percentage of cytokine-producing antigen cells in the control antigen tube. This figure represents the PBMC responses from a patient with subclinical secondary infection; the antigen used to stimulate PBMCs here were derived from DENV-3.
The number of patients who showed measurable CD4+ T cell cytokine responses to DENV antigen stimulation is shown in Table 2. For individual serotypes, there was a significantly higher proportion of subclinical IL-2 and IFNγ responders after stimulation with DENV-4. An additional serotype-specific association among responders was also seen in TNFα (to DENV-2), although this finding did not reach statistical significance.
Table 2.
Proportions of Subjects with Detectable Dengue-specific CD4+ T cell Cytokine Production from Presecondary Infection PBMC for Subclinical and Symptomatic Groups
| No. of responders: no. of nonresponders |
||||
| Cytokine | Antigen | SubclinicalSamples | Symptomatic Samples | P (by Fisher's exact test) |
| TNFα | D1 | 14:3 | 6:4 | .36 (NS) |
| D2 | 11:6 | 10:0 | .06 | |
| D3 | 7:10 | 1:9 | .19 (NS) | |
| D4 | 16:1 | 8:2 | .54 (NS) | |
| IFNγ | D1 | 5:12 | 1:9 | .36 (NS) |
| D2 | 8:9 | 2:8 | .23 (NS) | |
| D3 | 7:10 | 1:9 | .19 (NS) | |
| D4 | 8:9 | 0:10 | .01 | |
| IL-2 | D1 | 4:13 | 0:10 | .26 (NS) |
| D2 | 10:7 | 2:8 | .11(NS) | |
| D3 | 5:12 | 1:9 | .36 (NS) | |
| D4 | 8:9 | 0:10 | .01 | |
NOTE. NS, not statistically significant difference in proportions.
DENV-Specific CD8+ T Cell Cytokine Responses
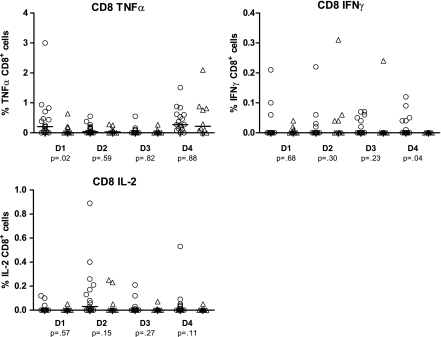
Figure 3 shows the frequency of DENV-specific cytokine-producing cells (TNFα, IFNγ, and IL-2) in response to stimulation with each of the 4 DENV antigens in the PBMCs collected before the index secondary DENV infection. Similar to the CD4+ T cell responses, stimulation with DENV antigens yielded a much higher frequency of TNFα-positive CD8+ T cells than for either IFNγ or IL-2.
Figure 3.
CD8+ intracellular cytokine production. TNFα, IFNγ, and IL-2 production was measured in response to antigen stimulation with 4 dengue serotypes of presecondary infection PBMCs from 27 Thai schoolchildren. Comparison groups are 10 children (triangles) who developed symptomatic infection and 17 children (circles) who had subclinical infection established retrospectively through dengue IgG ELISA and HI titers. Median percentage of cytokine-producing cells and P values to detect significant median differences using the Wilcoxon rank sum test are shown.
As seen in Figure 3, the subclinical group again showed overall higher median frequencies of cytokine-producing cells after stimulation with DENV antigens. As with the CD4+ T cells, TNFα production was higher in the subclinical group after stimulation with DENV-1 antigen alone, whereas IFNγ production was higher in the subclinical group after stimulation with DENV-4 antigen (P <.05). No statistically significant differences in the pre-infection CD8+ T cell characteristics between the symptomatic and subclinical groups were found with respect to IL-2 production.
The number of patients who generated CD8+ T cell cytokine responses after stimulation with DENV antigens is shown in Table 3. There was a significantly higher proportion of subclinical TNFα responders after stimulation with DENV-1 antigen (P = .01), in addition to a higher proportion of subclinical IFNγ responders to DENV-4, but this was not statistically significant (P = .06). No serotype-specific differences could be found with respect to IL-2.
Table 3.
Proportions of Patients with Detectable Dengue-specific CD8+ T cell Cytokine Production from Presecondary Infection PBMCs for Subclinical and Symptomatic Groups
| No. of responders: no. of nonresponders |
||||
| Cytokine | Antigen | Subclinical samples | Symptomatic samples | P (by Fisher's exact test) |
| TNFα | D1 | 14:3 | 3:7 | .01 |
| D2 | 11:6 | 6:4 | >.99 (NS) | |
| D3 | 4:13 | 3:7 | >.99 (NS) | |
| D4 | 15:2 | 8:2 | .59 (NS) | |
| IFNγ | D1 | 3:14 | 3:7 | .64 (NS) |
| D2 | 4:13 | 4:6 | .41 (NS) | |
| D3 | 6:11 | 1:9 | .20 (NS) | |
| D4 | 6:11 | 0:10 | .06 | |
| IL-2 | D1 | 3:14 | 1:9 | >.99 (NS) |
| D2 | 11:6 | 3:7 | .12 (NS) | |
| D3 | 7:10 | 2:8 | .41 (NS) | |
| D4 | 7:10 | 1:9 | .19 (NS) | |
NOTE. NS, not statistically significant difference in proportions.
Correlation Between Production of TNFα, IFNγ, and IL-2 at the Single-Cell Level
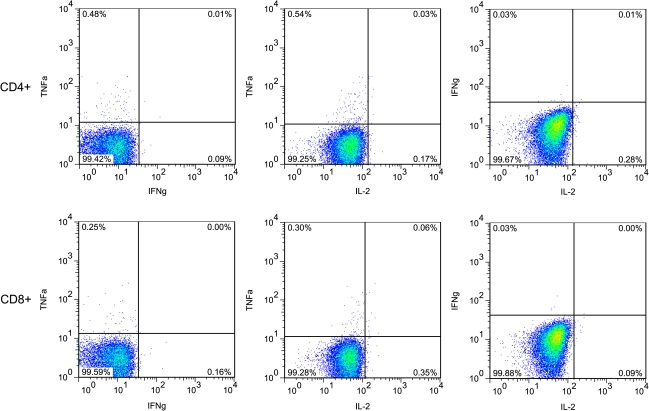
Because of the hypothesized importance of polyfunctional T cells in determining the outcome of the memory T cell response to virus infection, we examined the production of each of the 3 cytokines (TNFα, IFNγ, and IL-2) at the single cell level among DENV-specific T cell populations. After DENV antigen stimulation, the majority of responders generated monofunctional rather than polyfunctional cells. Only 2 donors, both in the subclinical group, had significant numbers of polyfunctional cells (Figure 4). In addition, there was no statistically significant association between production of any 2 cytokines after antigen stimulation, regardless of serotype (data not shown). This was true in both the CD4+ and CD8+ T cell populations.
Figure 4.
Lack of polyfunctionality in cytokine production following antigen stimulation. Twenty-five of the 27 donors had T cells that produced only 1 cytokine (upper left and lower right quadrants) but did not have cells that simultaneously produced 2 cytokines (upper right quadrants). The top row represents CD4+ cells, and the bottom row represents CD8+ cells. At left is TNFα (y-axis) versus IFNγ (x-axis); the center shows TNFα versus IL-2; the right shows IFNγ versus IL-2.
DISCUSSION
To our knowledge, this is the first article to describe the host immune response to DENV infection in relation to subclinical secondary infection and to do so using pre-illness samples collected as part of a prospective study. We report increased frequencies of DENV-specific, cytokine-producing CD4+ and CD8+ cells among schoolchildren who subsequently experienced subclinical, compared with symptomatic secondary DENV infections. Significantly higher frequencies with regard to IL-2 production were seen in the subclinical group after stimulation with DENV-4 antigen and higher frequencies after stimulation with DENV-1 and DENV-2, although these latter findings were not statistically significant. Differences between these subclinical and symptomatic groups in frequencies of DENV-specific IFNγ- and TNFα-producing CD4+ and CD8+ T cells was also present, with a significantly higher frequency of TNFα-producing T cells seen in the subclinical group after stimulation with DENV-1 antigen and a higher frequency of IFNγ-producing T cells after stimulation with DENV-4 antigen. We also noted a higher likelihood of being a responder in the subclinical group, particularly with respect to IFNγ and IL-2 production, although the small sample size of the study precludes making definitive conclusions.
The pattern of T cell responses observed suggests a protective role for heterologous T cell responses through antiviral cytokine (ie, IFNγ and IL-2) production. Other studies indicated that pro-inflammatory cytokines, such as TNFα, generated in the heterologous response could have a harmful effect; in a previous study based on this same prospective cohort (albeit, involving a different group of children), our group compared in vitro cytokine secretion after antigen stimulation in PBMCs collected before secondary infection between children who were hospitalized and those who were not hospitalized during the subsequent secondary DENV infection. We reported that the only TNFα responses were among children who were hospitalized [17]. That study did not include a separate subclinical infection group. In addition, the previous study measured cytokine secretion over a 6-day period after antigen stimulation. The longer assay involves in vitro expansion of memory T cells and measures total cytokine secretion in the culture supernatants rather than the frequency of cytokine-producing cells and may reflect different aspects of the T cell functional response than the overnight ex vivo flow cytometry assay. However, there was a tendency for increased IFNγ responses in the group with less severe symptoms, as seen in the present study.
These differences in relative production of cytokines are consistent with other studies of DENV-specific memory T cells. We reported higher frequencies of TNFα- producing serotype–cross-reactive DENV-specific memory CD4+ T cells, compared with IFNγ-producing cells in PBMCs from recipients of candidate monovalent, live DENV vaccines [18]. By contrast, we also found a less consistent hierarchy of responses among individual DENV epitope–specific CD8+ T cells after primary infection [19], and Imrie et al [20] showed altered cytokine responses at the clonal level among CD8+ cells in Pacific Islanders after DENV-1 infection with a hierarchy of IFNγ, TNFα, and IL-2. In these studies, however, no associations could be drawn with regard to symptoms after subsequent secondary infection.
We cannot completely exclude the possibilities that the subclinical group had more recently experienced their previous DENV infection or had experienced 2 (or more) prior DENV infections, explaining both a higher frequency of DENV-specific antiviral cytokine-producing T cells and less symptomatic secondary infection. The narrow age range in the cohort and the absence of statistically significant differences between groups in the pre-illness PRNT data (Table 1) make this interpretation less likely, however, and our data still point to a relationship between the frequency of cytokine-producing T cells and subclinical infection. In addition to the immune response profile that we measured, a multiplicity of factors could influence the host immune response during secondary infection, resulting in the presence or absence of symptoms. For instance, we reported that certain HLA alleles were associated with disease severity in this population [21]. Although that study examined only symptomatic DENV-infected patients, it suggests that host genetic factors can be either protective or harmful in determining the presence or absence of symptoms in secondary infection. Other factors, including the immunologic similarities of DENV epitopes, the virus inoculum and/or the magnitude of the viremia during primary or secondary infection, the virulence of the strain [22], and dysregulation of the complement system [23], could contribute to differences in disease severity.
Statistically significant differences in cytokine-producing T cell frequencies after DENV-3 antigen stimulation were not prominent, although the majority of detectable infections in the symptomatic group involved DENV-3. Subgroup analysis of the patients with known DENV-3 infections did not provide further insights, largely because of small sample size. Although we cannot know with certainty the infecting serotype in the subclinical cases, our study and other data from Kamphaeng Phet province in that year indicate that DENV-3 was the predominant circulating serotype [24]. The response to other DENV serotypes detected in the flow cytometry assay, reflecting T cell responses generated by the earlier primary DENV infection, includes serotype–cross-reactive T cells that may be capable of blunting the severity of secondary infection with DENV-3.
Limitations of the current study include the use of an inactivated antigen preparation (Vero cell lysates) rather than overlapping peptides or known DENV epitopes. Although the drawbacks of this approach are that we could not identify specific immunodominant epitopes and that stimulation of CD8+ T cells by the inactivated antigen is probably suboptimal, the use of DENV antigen allowed us to evaluate the immunologic response to the entire proteome of all 4 DENV serotypes using the small number of PBMCs available from these pediatric patients. Another limitation is that the symptomatic group represented only mild infection. A separate group of patients with DHF would permit further definition of immunologic associations with disease. In addition, we only studied the production of IFNγ, TNFα, and IL-2. The production of other cytokines, such as IL-4 and IL-18, which have been implicated in dengue disease severity, might also be of interest [25, 26].
In summary, we believe that our study supports the model that cytokine imbalance created by stimulation of cross-reactive T cells contributes to dengue disease severity and that ours is the first study to evaluate the predictive value of cytokine flow cytometry among children who later experienced secondary DENV infection. However, the relationship between cross-reactive T cells and the role played by heterologous antibodies has yet to be examined. Another important line of inquiry would be to compare the findings of this study to those for genetically distinct human populations, such as in countries in the Western Hemisphere where dengue is endemic.
Establishing the relationships between antibody and T cell responses to dengue infection will be of particular value as vaccine development reaches fruition. The need to identify measurable correlates of protective immunity and to find immunologic markers predictive of such protection is critical. One recent study evaluated cell-mediated immunity during a phase 1 clinical trial of a chimeric tetravalent dengue vaccine [27], although data from phase 2 and 3 trials has yet to be analyzed. Because of the unique challenges in developing a tetravalent vaccine for dengue, witnessed by the difficulties encountered by vaccine manufacturers until now [28], a thorough understanding of the cellular immunology of secondary infection will be invaluable to evaluate the effectiveness of the several candidate vaccines currently in development.
Funding
This work was funded by the National Institutes of Health (P01 AI034533) and the United States Army Medical Research and Materiel Command, Ft Detrick MD. Core resources were supported by the National Institutes of Health (Diabetes Endocrinology Research Center [DERC] grant DK32520]. ALR is a member of the UMMS DERC.
Acknowledgments
We thank the technicians and administrative personnel of the Department of Virology, Armed Forces Research Institute for Medical Sciences (AFRIMS), Thailand, for specimen collection and processing, data management, and serologic and virologic analyses; Marcia Woda, for assistance with flow cytometry; Jurand Janus, for antigen preparation; Kathryn Anderson, for assistance with serologic data interpretation; the subjects and their families, for study participation; Dr. Choorat Koosakulrat and his staff at the Office of Provincial Public Health, Kamphaeng Phet Province, for their support; and the clinical research team at AFRIMS and the support staff at the Kamphaeng Phet Field Station, for their efforts.
References
- 1.Kroeger A, Nathan M, Hombach J. Dengue. Nat Rev Microbiol. 2004;2:360–1. doi: 10.1038/nrmicro890. [DOI] [PubMed] [Google Scholar]
- 2.Dengue fever climbs the social ladder. Nature. 2007;448:734–5. doi: 10.1038/448734a. [DOI] [PubMed] [Google Scholar]
- 3.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Okuno Y, Fukunaga T, Srisupaluck S, Kasemsarn P, Dharakul C, Sangkawibha N. Serological and virological studies on patients with dengue hemorrhagic fever (DHF) in Chanthaburi province, Thailand. I. Serological studies on paired sera from DHF patients by neutralization (N), hemagglutination inhibition (HI) and staining tests. Biken J. 1980;23:113–21. [PubMed] [Google Scholar]
- 5.Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–69. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 6.Graham RR, Juffrie M, Tan R, et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995-1996. Am J Trop Med Hyg. 1999;61:412–9. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 7.Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 8.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–51. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 9.Kurane I, Rothman AL, Livingston PG, et al. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch Virol Suppl. 1994;9:59–64. doi: 10.1007/978-3-7091-9326-6_7. [DOI] [PubMed] [Google Scholar]
- 10.Rothman AL, Ennis FA. Immunopathogenesis of Dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 11.Endy TP, Chunsuttiwat S, Nisalak A, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 12.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–30. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 14.Innis BL. Antibody responses to dengue virus infection. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. New York: CAB International; 1997. pp. 221–43. [Google Scholar]
- 15.Dengue: Guidelines for Diagnosis, Treatment, Prevention Control. World Health Organization; 2009. [PubMed] [Google Scholar]
- 16.Mangada MM, Ennis FA, Rothman AL. Quantitation of dengue virus specific CD4+ T cells by intracellular cytokine staining. J Immunol Methods. 2004;284:89–97. doi: 10.1016/j.jim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Mangada MM, Endy TP, Nisalak A, et al. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J Infect Dis. 2002;185:1697–703. doi: 10.1086/340822. [DOI] [PubMed] [Google Scholar]
- 18.Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–83. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- 19.Bashyam HS, Green S, Rothman AL. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol. 2006;176:2817–24. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- 20.Imrie A, Meeks J, Gurary A, et al. Differential functional avidity of dengue virus-specific T-cell clones for variant peptides representing heterologous and previously encountered serotypes. J Virol. 2007;81:10081–91. doi: 10.1128/JVI.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens HA, Klaythong R, Sirikong M, et al. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Issue Antigens. 2002;60:309–18. doi: 10.1034/j.1399-0039.2002.600405.x. [DOI] [PubMed] [Google Scholar]
- 22.Rico-Hesse R. Microevolution and virulence of dengue viruses. Adv Virus Res. 2003;59:315–41. doi: 10.1016/s0065-3527(03)59009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nascimento EJ, Silva AM, Cordeiro MT, et al. Alternative complement pathway deregulation is correlated with dengue severity. PLoS One. 2009;4:e6782. doi: 10.1371/journal.pone.0006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endy TP, Nisalak A, Chunsuttitwat S, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 25.Bozza FA, Cruz OG, Zagne SM, et al. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC. Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2001;30:229–33. doi: 10.1111/j.1574-695X.2001.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 27.Guy B, Nougarede N, Begue S, et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine. 2008;26:5712–21. doi: 10.1016/j.vaccine.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Hatch S, Mathew A, Rothman A. Dengue vaccine: opportunities and challenges. IDrugs. 2008;11:42–5. [PubMed] [Google Scholar]