Abstract
(See the editorial commentary by Flanigan and Beckwith, on pages 1201–3.)
Background. Although some studies have identified impressive clinical gains for incarcerated HIV-seropositive injection drug users (IDUs) undergoing antiretroviral therapy (ART), the effect of incarceration on adherence to ART remains undetermined.
Methods. We used data from a long-term community-recruited cohort of HIV-seropositive IDUs, including comprehensive ART dispensation records, in a setting where HIV care is free. We estimated the relationship between the cumulative burden of incarceration, measured longitudinally, and the odds of <95% adherence to ART, with use of multivariate modeling.
Results. From 1996 through 2008, 490 IDUs were recruited and contributed 2220 person-years of follow-up; 271 participants (55.3%) experienced an incarceration episode, with the number of incarcerations totaling 1156. In a multivariate model, incarceration had a strong dose-dependent effect on the likelihood of nonadherence to ART: 1-2 incarceration events (adjusted odds ratio [AOR], 1.49; 95% confidence interval [95% CI], 1.03–2.05), 3–5 events (AOR, 2.48; 95% CI, 1.62–3.65), and > 5 events (AOR, 3.11; 95% CI, 1.86–4.95).
Conclusions. Among HIV-seropositive IDUs receiving ART, an increasing burden of incarceration was associated with poorer adherence in a dose-dependent fashion. Our findings support improved adherence support for HIV-seropositive IDUs experiencing incarceration.
Modern antiretroviral therapy (ART) is associated with dramatic improvements in survival and life expectancy among HIV-infected individuals [1]. Although ART has been shown to confer similar survival benefits among individuals who use injection drugs (IDU) [2], numerous studies have demonstrated that HIV-seropositive IDUs are less likely to be prescribed ART, typically begin therapy at later clinical stages [3], and have poor survival profiles, compared with other HIV-positive groups [4].
A primary determinant of survival for HIV-seropositive individuals is adherence to ART. High levels of adherence are required to guarantee durable clinical benefits, such as suppression of plasma HIV-1 RNA load and reconstitution of immunologic function [1]. IDUs are known to frequently have lower levels of adherence [5]; several behavioral factors have been identified as barriers to adherence to ART, including higher-intensity drug use [6], concern about adverse effects [7], and lower adherence self-efficacy [8]. Although social- and structural-level exposures are increasingly appreciated as important determinants of many forms of drug-related harm [9, 10], most studies of HIV treatment adherence and disease progression have focused on individual-level factors [11].
Imprisonment is a common experience for IDUs [12, 13]. In recent years, some optimism has been expressed that correctional facilities can serve as important sites for detecting infections and initiating treatment [14, 15]. In the United States, where ∼10% of all HIV-seropositive individuals are thought to cycle through a correctional setting every year [16], jails and prisons are the de facto primary site for HIV care for persons who lack access to community-based treatment [17]. Thus, the quality of prison-based care and the effect of imprisonment on HIV disease is of central importance to the health of the most vulnerable HIV-seropositive groups, such as the poor, illicit drug users, and ethnic minorities. Although impressive clinical gains have been observed among HIV-infected prisoners engaged in treatment in some state-run prison systems [14], the general effect of incarceration on HIV outcomes among IDUs remains equivocal, with some studies identifying a heightened risk of ART discontinuation associated with incarceration [18] and failure to suppress viral load [19]. We are unaware of any studies of community-recruited IDUs that have considered the effects of the typical patterns of incarceration on adherence to ART over the long term. Thus, in the present study, we aimed to estimate the effect of the cumulative burden of incarceration, measured longitudinally, on ART nonadherence with use of data from a long-running prospective cohort of illicit drug users in a Canadian setting.
METHODS
Data for these analyses was accessed from the AIDS Care Cohort to Evaluate Exposure to Survival Services (ACCESS), an ongoing prospective observational cohort of HIV-seropositive illicit drug users in Vancouver, Canada. Described in detail elsewhere [2], the cohort was populated through snowball sampling and extensive street outreach beginning in 1996 in the city's Downtown Eastside neighbourhood, a postindustrial area with an open drug market and high levels of IDU, poverty, and HIV infection [20, 21]. Individuals are eligible for ACCESS if they are aged ≥18 years, are HIV seropositive, have used illicit drugs other than cannibinoids in the previous month, and provide written informed consent. At baseline and at every 6-month follow-up interview, participants answer a standardized interviewer-administered questionnaire, are examined by a study nurse, and provide blood samples for serologic analysis. The ACCESS study has been approved by the University of British Columbia/Providence Healthcare Research Ethics Board.
The information on sociodemographic characteristics, drug use, and other behavioral characteristics gathered at each interview is augmented with data on HIV treatment and outcomes from the British Columbia Centre for Excellence in HIV/AIDS, as described elsewhere [2]. In brief, information from this province-wide centralized ART pharmacy provides complete information on all antiretroviral medications dispensed to all participants during the study period, both during periods of incarceration, and while in community settings. In addition, data from the Centre's HIV/AIDS monitoring lab supplies a complete prospective clinical profile, including CD4 cell counts for every participant.
In this study, we included all participants who were ART exposed at recruitment or who initiated ART during the study period. The outcome of interest was nonadherence to ART. Information on exposure to ART was obtained through a confidential linkage to the records at the comprehensive provincial dispensary described above [2]. We measured adherence to therapy in each 6-month period as the number of days for which ART was dispensed over the number of days that the individual was eligible for ART. For example, if an individual was dispensed 90 days of medications and was eligible for treatment for the entire 6-month period (ie, 180 days) prior to the interview, adherence was 0.5 (50%). We defined nonadherence to ART as any level <95% adherence. Although therapy for individuals in this study was not directly observed, we have previously demonstrated the clinical validity of this pharmacy refill data and shown that it reliably predicts virologic suppression [22–24] and survival [2, 25]. Of note, in British Columbia, all ART delivered to correctional and noncorrectional environments is dispensed through the British Columbia Centre for Excellence in HIV/AIDS. Thus, our outcome measure is complete and includes both community- and prison-based adherence patterns.
The primary explanatory variable was the burden of incarceration during the study period. This was measured by assessing the number of times that individuals had been held overnight or longer in youth detention, local jails, provincial prisons, or federal penitentiaries during each 6-month period before each semi-annual follow-up visit. For these analyses, this repeated measure was converted into a cumulative sum of incarceration events up to the current interview, updated at each interview period. To aid in interpretation, we converted this variable into a categorical factor with 4 levels: no incarceration events, 1–2 incarceration events, 3–5 incarceration events, and >5 incarceration events.
To best estimate the relationship between the burden of incarceration and nonadherence, we also considered secondary explanatory variables that we hypothesised might confound this relationship. These included demographic and socioeconomic characteristics, such as age (per year older), sex (female vs male), aboriginal ancestry (yes vs no), educational attainment (no high school diploma vs at least high school diploma), formal employment (yes vs no), and homelessness (yes vs no). All variables except sex and aboriginal ancestry were time updated; formal employment referred to having salaried or temporary work at any time during the previous 6 months. Information on aboriginal ancestry was included as a possible confounder because of previous work identifying elevated incidence of HIV infection [26], lower levels of ART uptake among aboriginal IDUs [27], and overrepresentation of aboriginal individuals in Canadian correctional facilities [28]. The variable used was dichotomized from an open-ended question asked during the baseline interview about the individual's ethnic group or family background. Any response of “First Nations,” “Métis,” “Aboriginal,” or “Inuit” was coded as aboriginal ancestry. Consistent with Canadian government research guidelines [29], representatives of local aboriginal groups are involved in the ongoing work of the ACCESS cohort through a community advisory board. Homelessness referred to living on the street or having no fixed address at the time of the interview. In addition, we included the individual-level behavioral variables: injection cocaine use (at least daily vs less than daily), injection heroin use (at least daily vs less than daily), inhalation methamphetamine use (at least daily vs less than daily), inhalation crack cocaine use (at least daily vs less than daily.) We also included self-reported public drug use (yes vs no) and participation in the sex trade, defined as any exchange of money, drugs, or other goods for sex (yes vs no). These variables were time updated, referred to the 6-month period before the interview, and were consistent with previous analyses [30]. Clinical variables included were the CD4 cell count (per 100 cells/mm3) and plasma HIV-1 RNA load (per log10.) For both measures, we used the mean of all available observations in the previous 6 months; if none were available, we used the most recent observation. Plasma HIV-1 RNA load was measured using the Roche Amplicor Monitor assay (Roche Molecular Systems). We also included the time since ART initiation, measured in months.
As a first step, we examined the frequency and distribution of incarceration and nonadherence longitudinally and selected explanatory variables at baseline. We estimated univariate statistics for the relationships between nonadherence and all explanatory variables over the study period with use of generalized linear mixed-effects modeling. This form of regression modeling was used to account for the correlation between covariates gathered over time from the same individual and estimate the independent effect of incarceration on the likelihood of nonadherence in each individual. To account for possible confounding and calculate the best effect estimate, we constructed a multivariate model using an a priori–defined modeling strategy suggested by Greenland et al [31, 32]. First, we fit a full model, including the primary explanatory and all secondary explanatory variables. Using a manual stepwise approach, we constructed reduced models, each with one variable removed from the full set of secondary explanatory variables. Comparing the value of the coefficient for the primary explanatory in the full model and each of the reduced models, we removed the secondary explanatory corresponding to the smallest relative change. We continued this process until the maximum change from the full model exceeded 5%. This technique has been used successfully by several authors to estimate the independent relationship between an outcome of interest and a selected explanatory variable [31, 33, 34] by retaining secondary covariates with greater relative influence on the relationship between the outcome and the primary explanatory variable.
RESULTS
From May 1996 through September 2009, 490 ART-exposed individuals were recruited and included in these analyses, of whom 201 (41.0%) were female and 192 (39.2%) reported aboriginal ancestry. The median follow-up duration was 28.8 months (interquartile range [IQR], 0.0–64.0 months), contributing to 2220 person-years of follow-up. Select sociodemographic and clinical characteristics at baseline are presented in Table 1, stratified by the number of incarceration events during the study period (<1 vs ≥ 1). Compared with participants with no incarceration episodes over the study period, incarcerated individuals were more likely to be younger and not possess a high school diploma at baseline.
Table 1.
Selected Sociodemographic, Behavioral, and Clinical Characteristics at Baseline, Stratified by Burden of Incarceration Over the Study Period in ACCESS Among 490 Antiretroviral Therapy–Exposed Participants
| Characteristic | Burden of incarceration during study |
OR | 95% CI | |
| <1 event | ≥1 events | |||
| Total | 219 (44.7%) | 271 (55.3%) | ||
| Age | ||||
| Median (IQR) | 43.5 (37.7–49.3) | 35.6 (30.0–41.2) | 0.90 | 0.88–.92 |
| Sex | ||||
| Male | 128 (58.4) | 161 (59.4) | 1.00 | |
| Female | 91 (41.6) | 110 (40.6) | 0.96 | 0.67–1.38 |
| Aboriginal ancestry | ||||
| No | 136 (62.1) | 162 (59.8) | 1.00 | |
| Yes | 83 (37.9) | 109 (40.2) | 1.10 | 0.77–1.59 |
| Educational attainment | ||||
| Less than high school diploma | 116 (54.2) | 170 (63.7) | 1.00 | |
| At least high school diploma | 98 (45.8) | 97 (36.3) | 0.68 | 0.47–.97 |
| Current MMT | ||||
| No | 125 (57.3) | 164 (60.5) | 1.00 | |
| Yes | 93 (42.7) | 107 (39.5) | 0.88 | 0.61–1.26 |
| CD4 cell count | ||||
| Per 100 cells/mm3 | 2.8 (1.5–4.1) | 2.8 (1.7–3.9) | 1.01 | 0.93–1.10 |
| HIV-1 RNA load | ||||
| Per log10 increase | 4.3 (3.2–5.3) | 4.5 (3.9–5.1) | 1.44 | 1.17–1.77 |
NOTE. Abbreviations: CI, confidence interval; MMT, Methadone maintenance therapy; OR, odds ratio.
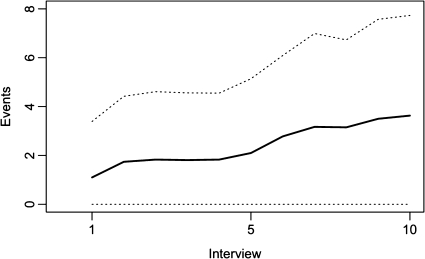
Figure 1 presents the mean number of incarceration events per participant by interview. More than half (271; 55.3%) of the participants were incarcerated during the study period, giving a crude incarceration rate of 52.5 per 100 person-years (95% confidence interval [CI], 49.6–55.7). Among those, the median number of incarceration episodes was 3 (IQR, 1–6). In total, there were 1156 incarceration episodes, of which 6 (0.5%) were in youth detention facilities, 621 (53.7%) were in local jails, 511 (41.2%) were in provincial prisons, and 18 (1.6%) were in federal penitentiaries. Over the entire study period, the median level of adherence to ART was 61.0% (IQR, 11.0%–100.0%). Of the 3731 follow-up periods, 1345 (36.0%) were characterized by <95% adherence to prescribed ART.
Figure 1.
Mean number of incarceration episodes by number of follow-up interviews among 490 ART-exposed IDUs in Vancouver, Canada. Solid line: Median number of incarceration episodes; dotted line: ± 1 standard deviation.
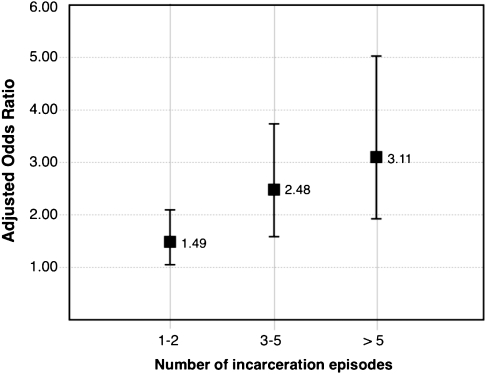
Table 2 presents the univariate estimates of the likelihood of nonadherence for each primary and secondary explanatory variable over the study period. The cumulative burden of incarceration, measured longitudinally, was a strong predictor of nonadherence to ART. Compared with individuals with no history of incarceration, participants with 1 or 2 incarceration events had almost double the odds of nonadherence at each follow-up period (odds ratio [OR], 1.91; 95% CI, 1.35–2.72). The odds increased to 2.85 (95% CI, 1.87–4.33) for individuals with 3–5 previous incarceration episodes and to 3.59 (95% CI, 2.12–6.09) for individuals with >5 incarceration events. This relationship persisted in the multivariate model after adjustment for possible confounders, including female sex, frequent cocaine use, engagement in methadone maintenance therapy, the number of months since ART initiation, and plasma HIV-1 RNA load. As presented in Figure 2, individuals with a burden of 1–2 incarceration events were 1.49 times more likely to be nonadherent in the previous 6 months (95% CI, 1.03–2.05), a burden of 3–5 incarceration events was independently associated with 2.48 times greater odds of nonadherence (95% CI, 1.62–3.65), and individuals with ≥5 incarceration events were 3.11 (95% CI, 1.85–4.95) times more likely to be nonadherent in comparison with individuals free of incarceration episodes, after adjustment for sociodemographic, behavioral and clinical confounders. Because newer antiretroviral regimes, including longer-acting protease inhibitors and nonnucleoside reverse-transcriptase inhibitors, have shifted the relationship between incomplete adherence and disease progression [35], we repeated our model building protocol using <85% adherence in the past 6 months as the outcome of interest. The results again showed a dose-response effect of incarceration on nonadherence (data available from corresponding author.)
Table 2.
Univariate and Multivariate Linear Mixed-Effects Analyses of Primary and Secondary Explanatory Variables and Nonadherence to Antiretroviral Therapy (ART) in ACCESS Among 490 ART-Exposed Participants
| Characteristic | OR | 95% CI | AOR | 95% CI |
| Incarceration eventsa | ||||
| 0 | 1.00 | 1.00 | ||
| 1–2 | 1.91 | 1.35–2.72 | 1.49 | 1.06–2.12 |
| 3–5 | 2.85 | 1.87–4.33 | 2.48 | 1.66–3.71 |
| > 5 | 3.59 | 2.12–6.09 | 3.11 | 1.93–5.03 |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 1.57 | 1.07–2.32 | 2.11 | 1.50–2.97 |
| Aboriginal ancestry | ||||
| No | 1.00 | |||
| Yes | 1.01 | 0.68–1.51 | ||
| Homelessb | ||||
| No | 1.00 | |||
| Yes | 1.51 | 0.97–2.35 | ||
| Educational attainment | ||||
| Less than high school diploma | 1.00 | |||
| at least high school diploma | 1.17 | 0.90–1.51 | ||
| Formal employmentb | ||||
| No | 1.00 | |||
| Yes | 0.73 | 0.48–1.11 | ||
| Cocaine use, injectionb | ||||
| Less than daily | 1.00 | 1.00 | ||
| At least daily | 1.54 | 1.21–1.97 | 1.23 | 0.94–1.62 |
| Heroin use, injectionb | ||||
| Less than daily | 1.00 | |||
| At least daily | 2.38 | 1.80–3.16 | ||
| Methamphetamine use, inhalationb | ||||
| Less than daily | 1.00 | |||
| At least daily | 2.64 | 0.75–9.28 | ||
| Crack cocaine use, inhalationb | ||||
| Less than daily | 1.00 | |||
| At least daily | 1.40 | 1.09–1.79 | ||
| Methadone maintenance therapy | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.41 | 0.32–.54 | 0.47 | 0.36–.62 |
| Sex-trade participationb | ||||
| No | 1.00 | |||
| Yes | 1.73 | 1.21–2.47 | ||
| Public drug useb | ||||
| No | 1.00 | |||
| Yes | 1.25 | 0.91–1.72 | ||
| CD4 cell count | ||||
| Per 100 cells/mm3 | 0.72 | 0.67–.77 | ||
| Plasma HIV-1 RNA load | ||||
| Per log10 unit increase | 5.41 | 4.74–6.17 | 5.41 | 4.73–6.20 |
| Time since ART initiation | ||||
| Per month | 1.00 | 1.00–1.01 | 1.01 | 1.00–1.01 |
NOTE. Abbreviations: AOR, adjusted odds ratio; CI, confidence interval;OR, odds ratio.
Cumulative number of incarceration events, time-updated.
Refers to six month period prior to interview
Figure 2.
Adjusted odds ratios for nonadherence to ART by number of incarceration episodes among 490 ART-exposed IDUs in Vancouver, Canada. Multivariate model adjusted for sex, daily injection cocaine use, number of months since ART initiation, methadone maintenance therapy, and plasma HIV-1 RNA load.
DISCUSSION
In the present study, we observed a dose-dependent association between the cumulative burden of incarceration and ART nonadherence. Because these findings are from a long-running observational cohort linked to complete ART dispensation records in a setting of universal access to free HIV care, these results are not under the influence of the confounding effect of financial ability or biased by the limitations of self-reported adherence [36]. Furthermore, unlike prison-based studies, our analysis considers the effect of incarceration in the course of HIV disease among community-recruited IDUs and clearly indicates that increasing number of cycles of imprisonment, release, and reincarceration is associated with poorer ART adherence in this population of IDUs.
As with all observational studies, the exposure of interest in these analyses was not randomly assigned, and thus, we cannot unequivocally conclude that a causal relationship between imprisonment and nonadherence exists. The possibility remains that the behaviors that led to arrest, such as illicit drug use, were a contributing cause of nonadherence. However, 3 major lines of evidence support the potential for a causal relationship between the burden of incarceration and patterns of adherence. First, our estimates for the effect of the burden of incarceration were derived from a multivariate model, which also adjusted for sex, intensive drug use, and engagement in methadone maintenance therapy, all previously associated with both access and adherence to ART [37–39]. Furthermore, the multivariate model was constructed to isolate the independent effect of incarceration on nonadherence by retaining and adjusting for explanatory variables with greater relative influence on that association. Second, support for a causal effect for incarceration on nonadherence can also be found in previous studies, which have reported that incarceration is associated with a greater risk of discontinuation and failure to achieve viral suppression among IDUs [40–42]. Similarly, prior studies have demonstrated that only a small minority of newly released prisoners typically manage to avoid HIV treatment interruptions [17], and any in-prison treatment gains appear to be short-lived [43, 44]. Finally, multiple prison-associated barriers to adherence were identified in an ethnographic investigation into in-prison HIV treatment in this setting [45], including a lack of medical care in short-term holding cells, the desire of participants to conceal HIV serostatus from other inmates, and the lack of continuity of care between community-based and in-prison providers.
Because of the tight link between nonadherence and HIV disease progression, our findings have direct relevance to public health efforts to reduce AIDS-related morbidity and mortality and continued viral transmission. Although our results do not entirely discount a role for correctional facilities in identification of HIV infection and initiation of treatment, they show an association between incarceration episodes and ART nonadherence and increased risk of HIV disease progression. To be sure, some small interventions have shown promise in improving HIV treatment outcomes during and after incarceration, especially when paired with substance abuse treatment [46]. In addition to programs seeking to minimize the adverse effects of incarceration on HIV treatment, future research might also investigate how social- and structural-level reforms, such as diverting nonviolent drug users from correctional settings, might improve HIV treatment outcomes.
This study has some limitations that should be noted. Although the cohort was recruited using street outreach and snowball sampling, no registries of HIV-seropositive individuals exist and random sampling is not possible. Thus, our results might not be representative of HIV-seropositive drug users in this settings or others. In addition, several measures, including incarceration, were self-reported by participants and may have been under the influence of social desirability bias. However, we believe that it is unlikely that this bias differentially effected the data by adherence level. In addition, the independent association between the primary explanatory variable and the outcome of interest may be the result of unobserved confounding rather than a causal association. However, as detailed above, evidence exists for a causal relationship between incarceration and poorer adherence patterns; a trial randomizing imprisonment for HIV-seropositive drug users receiving ART is ethically impossible. Finally, adherence is only a marker for plasma HIV-1 RNA suppression, and future studies should seek to examine the impact of incarceration experiences on viral load.
To conclude, we used data gathered from almost 15 years of follow-up of a community-recruited sample of HIV-seropositive IDUs and, using comprehensive antiretroviral dispensation records, observed a dose-dependent association between increasing burden of incarceration and ART nonadherence. Given the importance of correctional facilities in shaping the health of the vulnerable HIV-positive individuals, our findings should spur efforts to reform the delivery of in-prison HIV care and ease transitions to noncorrectional environments.
Funding
The work was supported by the National Institutes of Health (R01DA021525), the Canadian Institutes of Health Research (MOP-79297, RAA-79918), Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research (CIHR; to T.K.), and doctoral research award from CIHR (to M.J.M.).
Acknowledgments
We thank the study participants for their contribution to the research and current and past researchers and staff and Deborah Graham, Tricia Collingham, Caitlin Johnston, Steve Kain, and Calvin Lai, for their research and administrative assistance.
Human participant protection: The ACCESS cohort is reviewed annually and has been approved by the University of British Columbia/Providence Healthcare Research Ethics Board.
References
- 1.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–4. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 2.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–4. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 3.Wood E, Hogg RS, Harrigan PR, Montaner JS. When to initiate antiretroviral therapy in HIV-1-infected adults: a review for clinicians and patients. Lancet Infect Dis. 2005;5:407–14. doi: 10.1016/S1473-3099(05)70162-6. [DOI] [PubMed] [Google Scholar]
- 4.Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–74. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 5.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35:261–8. doi: 10.1097/00126334-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Stein MD, Rich JD, Maksad J, et al. Adherence to antiretroviral therapy among HIV-infected methadone patients: effect of ongoing illicit drug use. Am J Drug Alcohol Abuse. 2000;26:195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- 7.Shannon K, Bright V, Duddy J, Tyndall MW. Access and utilization of HIV treatment and services among women sex workers in Vancouver's Downtown Eastside. J Urban Health. 2005;82:488–97. doi: 10.1093/jurban/jti076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Longshore D, Williams JK, et al. Substance abuse and medication adherence among HIV-positive women with histories of child sexual abuse. AIDS Behav. 2006;10:279–86. doi: 10.1007/s10461-005-9041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61:1026–44. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes T. Risk environments and drug harms: a social science for harm reduction approach. Int J Drug Policy. 2009;20:193–201. doi: 10.1016/j.drugpo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Krusi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Policy. 2010;21:4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Marlow E, White MC, Tulsky JP, Estes M, Menendez E. Recidivism in HIV-infected incarcerated adults: influence of the lack of a high school education. J Urban Health. 2008;85:585–95. doi: 10.1007/s11524-008-9272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baillargeon J, Binswanger IA, Penn JV, Williams BA, Murray OJ. Psychiatric disorders and repeat incarcerations: the revolving prison door. Am J Psychiatry. 2009;166:103–9. doi: 10.1176/appi.ajp.2008.08030416. [DOI] [PubMed] [Google Scholar]
- 14.Springer SA, Friedland GH, Doros G, Pesanti E, Altice FL. Antiretroviral treatment regimen outcomes among HIV-infected prisoners. HIV Clin Trials. 2007;8:205–12. doi: 10.1310/hct0804-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammett TM. HIV/AIDS and other infectious diseases among correctional inmates: transmission, burden, and an appropriate response. Am J Public Health. 2006;96:974–8. doi: 10.2105/AJPH.2005.066993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4:e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baillargeon J, Giordano TP, Rich JD, et al. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301:848–57. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr T, Marshall A, Walsh J, et al. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17:539–49. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 19.Palepu A, Tyndall MW, Li K, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. J Urban Health. 2003;80:667–75. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11:F59–65. doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–93. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 22.Low-Beer S, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23:360–1. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 23.Palepu A, Yip B, Miller C, et al. Factors associated with the response to antiretroviral therapy among HIV-infected patients with and without a history of injection drug use. AIDS. 2001;15:423–4. doi: 10.1097/00002030-200102160-00021. [DOI] [PubMed] [Google Scholar]
- 24.Wood E, Montaner JS, Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–61. [PMC free article] [PubMed] [Google Scholar]
- 25.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L. Ann Intern Med. 2003;139:810–6. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 26.Wood E, Montaner JS, Li K, et al. Burden of HIV infection among aboriginal injection drug users in Vancouver, British Columbia. Am J Public Health. 2008;98:515–9. doi: 10.2105/AJPH.2007.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood E, Kerr T, Palepu A, et al. Slower uptake of HIV antiretroviral therapy among Aboriginal injection drug users. J Infect. 2006;52:233–6. doi: 10.1016/j.jinf.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Aboriginal Issues Branch CSoC. Demographic overview of Aboriginal peoples in Canada and Aboriginal offenders in Federal corrections. Ottawa, Ontario: The Government of Canada; 1999. [Google Scholar]
- 29.Canadian Institutes of Health Research. CIHR guidelines for health research involving Aboriginal people. Ottawa, Ontario: Canadian Institutes of Health Research; 2007. [Google Scholar]
- 30.Wood E, Tyndall MW, Spittal PM, et al. Unsafe injection practices in a cohort of injection drug users in Vancouver: could safer injecting rooms help? CMAJ. 2001;165:405–10. [PMC free article] [PubMed] [Google Scholar]
- 31.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ, Greenland S. Modern Epidemiology. New York, New York: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 33.Lima V, Fernandes K, Rachlis B, Druyts E, Montaner J, Hogg R. Migration adversely affects antiretroviral adherence in a population-based cohort of HIV/AIDS patients. Soc Sci Med. 2009;68:1044–9. doi: 10.1016/j.socscimed.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 34.Marshall BD, Kerr T, Shoveller JA, Patterson TL, Buxton JA, Wood E. Homelessness and unstable housing associated with an increased risk of HIV and STI transmission among street-involved youth. Health Place. 2009;15:753–60. doi: 10.1016/j.healthplace.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulick RM. Adherence to antiretroviral therapy: how much is enough? Clin Infect Dis. 2006;43:942–4. doi: 10.1086/507549. [DOI] [PubMed] [Google Scholar]
- 36.Wood E, Kerr T, Hogg RS, Zhang R, Tyndall MW, Montaner JS. Validity of self-reported antiretroviral therapy use among injection drug users. J Acquir Immune Defic Syndr. 2006;41:530–1. doi: 10.1097/01.qai.0000199096.11215.79. [DOI] [PubMed] [Google Scholar]
- 37.Krusi A, Milloy MJ, Kerr T, et al. Ongoing drug use and outcomes from highly active antiretroviral therapy among injection drug users in a Canadian setting. Antivir Ther. 2010;15:789–96. doi: 10.3851/IMP1614. [DOI] [PubMed] [Google Scholar]
- 38.Tapp C, Milloy M-J, Kerr T, et al. Female gender predicts lower acess and adherence to antiretroviral therapy in a setting of free healthcare. BMC Infect Dis. doi: 10.1186/1471-2334-11-86. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uhlmann S, Milloy MJ, Kerr T, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105:907–13. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr T, Marshall A, Walsh J, et al. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17:539–49. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 41.Palepu A, Tyndall MW, Chan K, Wood E, Montaner JSG, Hogg RS. Initiating highly active antiretroviral therapy and continuity of HIV care: the impact of incarceration and prison release on adherence and HIV treatment outcomes. Antivir Ther. 2004;9:713–9. [PubMed] [Google Scholar]
- 42.Palepu A, Tyndall MW, Li K, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. J Urban Health. 2003;80:667–75. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38:1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 44.Stephenson BL, Wohl DA, Golin CE, Tien H-C, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Rep. 2005;120:84–8. doi: 10.1177/003335490512000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small W, Wood E, Betteridge G, Montaner J, Kerr T. The impact of incarceration upon adherence to HIV treatment among HIV-positive injection drug users: a qualitative study. AIDS Care. 2009;21:708–14. doi: 10.1080/09540120802511869. [DOI] [PubMed] [Google Scholar]
- 46.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health. 2010;87:592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]