Abstract
We previously found that CD4+CD25+FoxP3+ regulatory T cells (Tregs) expand in response to Mycobacterium tuberculosis infection in individuals who are healthy tuberculin reactors, but not in tuberculin-negative individuals. We also found that the M. tuberculosis mannose-capped lipoarabinomannan and prostaglandin E2 produced by monocytes are involved in Treg expansion. In this study, we found that Tregs expanded from CD4+CCR4+ cells but not from CCR4− cells. However, introduction of CCR4 small interfering RNA (siRNA) into CD4+ cells only marginally reduced expansion of Tregs. Using siRNA and neutralizing antibodies, we found that expansion of Tregs by M. tuberculosis required expression of programmed death1 (PD-1) and expression of the signaling molecule, cytokine inducible SH2-containing protein (CISH). Anti-PD-1 siRNA inhibited expression of CISH by expanded Tregs. M. tuberculosis–expanded Tregs produced transforming growth factor β and interleukin 10 and reduced the frequency of interferon γ–producing autologous CD8+ cells. We conclude that M. tuberculosis infection induces development of Tregs from CCR4+ cells through a process that depends on PD-1and CISH.
Regulatory CD4+ T cells (Tregs) that express CD25 and FoxP3 [1] constitute 5%–10% of CD4+ T cells in mice and humans and are essential to maintain peripheral tolerance and homeostasis. Several studies have demonstrated that Tregs can prevent autoimmunity, inhibit transplant graft rejection, suppress the immune response to tumors, and play a role in infectious diseases [1–5].
In Mycobacterium tuberculosis infection, Tregs proliferate and accumulate at sites of infection [6, 7] and prevent bacillary clearance in mice [8]. In patients with tuberculosis, T cell production of interferon γ (IFN-γ) in response to mycobacterial antigen is reduced compared with that in healthy tuberculin reactors [9]. Patients with tuberculosis have increased numbers of Tregs that inhibit IFN-γ production by bacille Calmette-Guérin–stimulated CD4+CD25− T cells, and depletion of Tregs enhances M. tuberculosis–induced IFN-γ production by peripheral blood mononuclear cells (PBMCs), suggesting that Tregs inhibit effective immunity [10, 11]. Tregs suppress antigen-specific human memory γδT cell responses to M. tuberculosis [12]. We found that Tregs expand in response to M. tuberculosis in healthy tuberculin reactors and that expanded Tregs inhibit IFN-γ production by T cells [11], suggesting that Tregs may limit tissue inflammation and destruction. However, the cellular mechanisms that mediate expansion of M. tuberculosis–induced Tregs are unknown.
In the present study, we found that M. tuberculosis infection induces development of Tregs from CCR4+ cells, and that this process depends on programmed death 1 (PD-1) and cytokine inducible SH2-containing protein (CISH).
MATERIALS AND METHODS
Patient Population
After obtaining informed consent, blood was obtained from 20 healthy persons with positive QuantiFERON-TB Gold test results, which is indicative of latent tuberculosis infection. All donors were 18–65 years old; did not have a history of tuberculosis, AIDS, or human immunodeficiency virus (HIV) infection; and were not receiving therapy with immunosuppressive drugs. All studies were approved by the institutional review board of the University of Texas Health Science Center at Tyler.
Antibodies and Other Reagents
For flow cytometry, we used fluorescein isothiocyanate (FITC) anti-CD4, allophycocyanin (APC) anti-CD25, phycoerythrin (PE) anti-FoxP3, PE-Cy5 anti-FoxP3 (all from eBioscience); and FITC anti-CD14, FITC anti-CD8, PE anti-PD1, and PE anti-CD127 (all from BD Biosciences). For neutralization, we used monoclonal antibodies to PD-1 and interleukin 12Rβ2 (IL-12Rβ2; both at a concentration of 10 μg/mL; R&D systems); and inducible T cell costimulator molecule (ICOS) and cytotoxic T lymphocyte associated antigen 4 (CTLA-4; both at a concentration of 10 μg/mL; eBioscience). We obtained γ-irradiated M. tuberculosis H37Rv J. Belisle (Colorado State University, Fort Collins, CO).
Isolation of Cell Subpopulations
PBMCs were isolated by differential centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech). CD14+ cells were isolated by positive immunomagnetic selection (Miltenyi Biotec) and were >95% CD14+, as measured by flow cytometry. CD4+CD25− cells were isolated from PBMCs by use of the Treg isolation kit (Miltenyi Biotec), as described elsewhere [11]. To isolate PD-1+ cells, CD14+, CD25+, and CD8+ cells were depleted from PBMCs and the remaining cells were labeled with PE-conjugated anti-PD-1, incubated with anti-PE-microbeads, and isolated by positive selection. To obtain CCR4+ cells, CD4+CD25− cells were treated with multisort release enzyme to release CD4 microbeads (Miltenyi Biotec). Next, cells were labeled with PE-conjugated antibodies and incubated with anti-PE microbeads (Miltenyi Biotec), then isolated by positive selection, with a purity of ∼90%.
Culture of CD4+CD25− Cells and Monocytes
CD4+CD25− cells were isolated as outlined above and cultured in 12-well plates at 2 × 106 cells per well in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% heat-inactivated human serum, with 2 × 105 autologous monocytes per well. CD4+CD25− cells and monocytes were cultured with or without γ-irradiated M. tuberculosis (10 μg/mL) for 4 d at 37°C. In some cases, 10 μg/mL neutralizing antibodies to IL-12Rβ2, PD-1, CTLA-4, or ICOS was added on days 0 and 2.
Isolation of Expanded CD4+CD25+CD127− and CD4+CD25−CD127+ Cells
For microarray analyses, CD4+ cells and autologous monocytes were cultured with γ-irradiated M. tuberculosis H37Rv (10 μg/mL) for 4 d. CD4+ cells were negatively selected, then incubated with anti-CD127-conjugated magnetic beads. From the CD127− cells, CD25+ cells were immunomagnetically selected. FoxP3+ cells comprised 70% of the CD4+CD25+CD127− cells and 2% of the CD4+CD25−CD127+ cells
Immunolabeling of Intracellular FoxP3
Surface staining to detect CD4+, CD25+, and CD127+ cells and intracellular staining to detect FoxP3+ cells was performed using the Cytofix/Cytoperm Plus kit (eBioscience). Controls for each experiment included cells that were unstained, cells to which FITC-conjugated, APC-conjugated, or PE-conjugated rat immunoglobulin (IgG) had been added, and cells that were single stained for either the surface marker or FoxP3. We gated on CD4+ lymphocytes and determined the percentages of CD25+ and FoxP3+ cells. For some experiments, we gated on CD127low cells to detect FoxP3+ cells, using a FACSCalibur instrument (BD Biosciences).
Culture of Monocytes, CD8+ Cells, and Tregs
Freshly isolated CD8+ cells (2 × 106 cells per well) from healthy tuberculin reactors were cultured with 2 × 105 autologous monocytes per well. In some wells, 2 × 105 autologous Tregs, isolated from CD4+ cells and monocytes cultured with γ-irradiated M. tuberculosis, were added. Cells were cultured for 2 d, CD8+ cells were isolated by positive immunomagnetic selection, and the frequency of IFN-γ-producing cells was determined by enzyme-linked immunospot assay (ELISPOT) [11].
Frequency of CD4+CD25+ and CD4+CD25− Cells Producing Transforming Growth Factor β and Interleukin 10
CD4+CD25+ and CD4+CD25− cells from cultured CD4+ cells were isolated using the Miltenyi Biotech Treg isolation kit. Aliquots of CD4+CD25+ and CD4+CD25− cells were placed on ELISPOT plates, and the numbers of cells that produced latent transforming growth factor β (TGF-β) and interleukin 10 (IL-10) were detected by ELISPOT (R&D Systems and eBioscience, respectively).
Transfection of Monocytes and CD4+ Cells
CD4+CD25− cells were transfected with small interfering RNA (siRNA) for CISH or cAMP response element modulator (CREM) or with scrambled siRNA (Santa Cruz Biotechnology). Briefly, 106 CD4+CD25− cells were transfected with 50 μmol/L siRNA in 200 μL of transfection medium. After 6 h, 200 μL of RPMI complete medium was added, and cells were cultured overnight in a 12-well plate. Autologous monocytes were then added, with or without γ-irradiated M. tuberculosis. After 4 d, the percentages of CD4+CD25+FoxP3+ cells were determined by flow cytometry. In some cases, to determine the efficiency of siRNA knockdown, cells were washed after 24 h and protein was extracted to perform Western blots.
Western Blot Analysis
Protein was extracted from M. tuberculosis–expanded Tregs and quantified by the bicinchoninic acid method (Pierce). Ten micrograms from each sample was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with antibodies to CISH, β-actin, or glyceraldehyde 3-phosphate dehydrogenase (all from Santa Cruz Biotechnology). After washing, the membranes were incubated with horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology), and binding was detected by electrochemiluminescence (GE Healthcare).
Statistical Analysis
Results are given as the mean (± SE). Comparisons between groups were performed with a paired or unpaired t test, as appropriate.
RESULTS
Microarray Analysis of Expanded Tregs
To identify the intracellular signaling molecules that mediate expansion of Tregs from M. tuberculosis–stimulated CD4+ cells, we compared gene expression profiles of expanded Tregs and CD4+ non-Tregs, using microarray analysis (supplemental data Table S1). CD4+ cells and autologous monocytes from 2 healthy tuberculin reactors were cultured with γ-irradiated M. tuberculosis H37Rv. Tregs express low levels of the interleukin 7 receptor, CD127 [13, 14]. After 3 d, we isolated CD4+CD127+ cells (non-Tregs; <2% FoxP3+) and CD4+CD127− cells, as described in the Materials and Methods. From the latter subpopulation, CD25+ cells were positively selected (Tregs; 70% FoxP3+). Total RNA was obtained, and microarray analysis was performed for 1,200 genes that are involved in immune responses. In the samples from both donors, messenger RNA (mRNA) expression for 49 genes was >2-fold higher in Tregs than in non-Tregs (supplemental data Table S1). Of these genes, we selected IL-12Rβ2, chemokine receptor CCR4, ICOS, CTLA-4, and the intracellular signaling molecules, CISH and CREM, for further study. CCR4 antagonists block accumulation of human Tregs during administration of a vaccine encoding M. tuberculosis antigens [15]. IL-12Rβ2 promotes development of Tregs [16]. ICOS and CTLA-4 are expressed by Tregs in different experimental systems [17, 18], CISH is associated with Th2 responses [19], and CREM is linked to reduced T cell activation in systemic lupus erythematosus [20]. We first studied expansion of Tregs from CD4+CD25− cells expressing CCR4 receptor.
CCR4+ Cells Expand to Tregs, but CCR4 Is Not Essential for Expansion
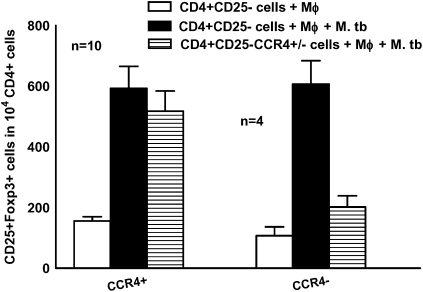
Previously we demonstrated that Tregs expand in response to M. tuberculosis in healthy tuberculin reactors, but not in tuberculin-negative individuals, and M. tuberculosis–expanded Tregs arose from CD4+CD25− cells, not from CD4+CD25+FoxP3+ natural Tregs [11]. This finding suggests that M. tuberculosis–expanded Tregs are induced Tregs. In the present study, we asked whether CD4+CD25− T cells expressing CCR4 selectively expand to Tregs in response to M. tuberculosis. Using positive immunomagnetic selection, we isolated CCR4+ cells from freshly isolated CD4+CD25− cells of healthy tuberculin reactors and cultured them with monocytes and γ-irradiated M. tuberculosis for 4 d. In cells from 10 healthy tuberculin reactors, M. tuberculosis converted some CCR4+ cells into FoxP3+ cells (593 ± 72 vs 157 ± 14 cells per 104 CD4+ cells, respectively; P < .001) (Figure 1). In contrast, Tregs did not expand from CCR4− cells (Figure 1). To determine whether expanded FoxP3+ cells express CCR4, we cultured CD4+ cells from 6 healthy tuberculin reactors with autologous monocytes and γ-irradiated M. tuberculosis. After 4 d, 90% of CD4+FoxP3+ cells were CCR4+ (data not shown).
Figure 1.
Selective expansion of CD4+CD25−CCR4+ cells to CD4+CD25+FoxP3+ cells by Mycobacterium tuberculosis (M. tb). CD25+ cells were immunomagnetically depleted from peripheral blood mononuclear cells of healthy tuberculin reactors, and CD4+ cells were isolated by positive selection. From the CD4+CD25− cells, CCR4+ cells and CCR4− cells were isolated as outlined in the Materials and Methods and cultured with autologous monocytes (Mφ) in medium alone or with γ-irradiated M. tuberculosis H37Rv (10 μg/mL). After 4 d, the percentages of CD4+CD25+FoxP3+ cells were determined by flow cytometry and the results were expressed as the number of cells per 104 CD4+ cells. Mean values and SEs are shown.
To determine whether CCR4 is required to expand Tregs, CD4+CD25− cells from healthy tuberculin reactors were transfected with CCR4 siRNA, scrambled siRNA, or no siRNA and cultured with autologous monocytes and γ-irradiated M. tuberculosis. After 4 d, CD4+CD25+FoxP3+ cells were quantified by flow cytometry. CCR4 siRNA significantly inhibited CCR4 expression, as determined by Western blot analysis (data not shown). In cells from 10 healthy tuberculin reactors, CCR4 siRNA reduced Treg expansion, compared with scrambled siRNA (472 ± 24 vs 551 ± 31 cells per 104 CD4+ cells; P < .01), but this decrease of only 15% is of uncertain biologic significance. We conclude that CCR4 is a marker of CD4+ cells that expand to Tregs, but that other molecules are more important in controlling M. tuberculosis–induced Treg expansion.
PD-1 is Necessary for M. tuberculosis–induced Treg Expansion
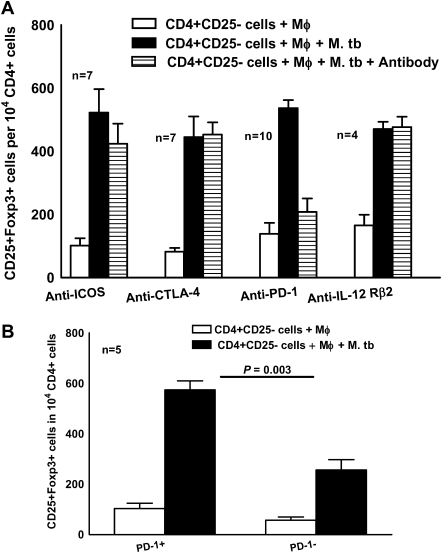
Studies in other experimental systems found that signaling via IL-12Rβ2 controls the number and functional maturity of Tregs [16], that ICOS and CTLA-4 mediate Treg function, and that PD-1 down-regulates activated T cells. To determine the role of these molecules in Treg expansion, we cultured CD4+CD25− cells and autologous monocytes from PBMCs of healthy tuberculin reactors, with γ-irradiated M. tuberculosis, in the presence of antibodies to IL-12Rβ2, ICOS, CTLA-4, or PD-1 (10 μg/mL). Only anti-PD-1 reduced expansion of Tregs (537 ± 26 vs 208 ± 43 cells per 104 CD4+ cells; P = .01) (Figure 2A). In cells from 4 healthy tuberculin reactors, isotype control antibodies to IL-12Rβ2 and ICOS (IgG1), CTLA-4 (IgG2a), and PD-1 (IgG2b) did not affect M. tuberculosis–mediated Treg expansion (515 ± 70, 550 ± 51, and 560 ± 54 vs 583 ± 39 CD25+FoxP3+cells per 104 CD4+ cells, respectively).
Figure 2.
Contribution of programmed death 1 (PD-1) to Mycobacterium tuberculosis (M. tb)–induced expansion of regulatory T cells (Tregs). A, Inhibition of Treg expansion by anti-PD-1. CD4+CD25− cells from peripheral blood mononuclear cells of healthy tuberculin reactors were cultured with autologous monocytes (Mφ) in medium alone or with γ-irradiated M. tuberculosis H37Rv (10 μg/mL) in the presence or absence of antibodies to interleukin 12Rβ2 (IL-12Rβ2), inducible T cell costimulator molecule (ICOS), cytotoxic T lymphocyte associated antigen 4 (CTLA-4), or PD-1. After 4 d, the percentages of CD4+CD25+FoxP3+ cells were determined by flow cytometry and the results were expressed as the number of cells per 104 CD4+ cells. Mean values and SEs are shown. B, Expansion of CD4+CD25−PD-1+ cells to Tregs. PD-1+ and PD-1− cells were isolated as outlined in the Materials and Methods and cultured with autologous monocytes in medium alone or with γ-irradiated M. tuberculosis H37Rv (10 μg/mL). After 4 d, the percentages of CD4+CD25+FoxP3+ cells were determined by flow cytometry and the results were expressed as the number of cells per 104 CD4+ cells. Mean values and SEs are shown.
PD-1+ Cells Expand to Tregs
We next asked whether CD4+CD25−PD-1+ T cells expand to Tregs in response to M. tuberculosis. In samples from 4 healthy donors, all PD-1+ cells also expressed CCR4. Using immunomagnetic selection, we isolated PD-1+ and PD-1− cells from freshly isolated CD4+CD25− cells of healthy tuberculin reactors and cultured them with autologous monocytes and γ-irradiated M. tuberculosis for 4 d. In samples from 5 healthy tuberculin reactors, more FoxP3+ cells were obtained from PD-1+ cells than from PD-1− cells (574 ± 36 vs 256 ± 42 cells per 104 CD4+ cells, respectively; P = .003) (Figure 2B).
CISH Contributes to M. tuberculosis–induced Treg Expansion
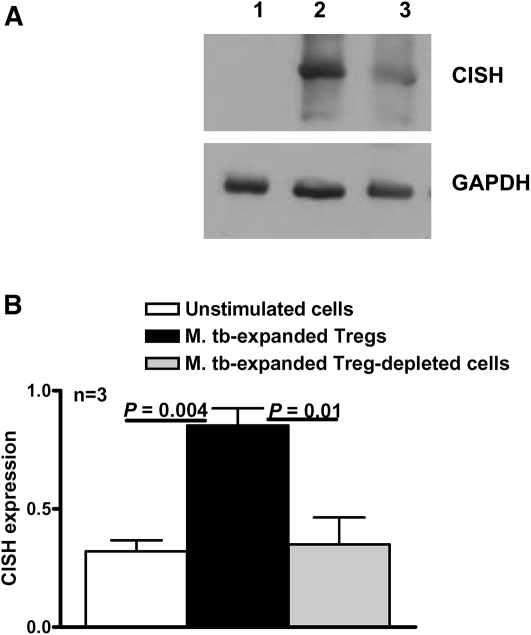
Microarray analysis showed that CISH mRNA expression was up-regulated in M. tuberculosis–expanded Tregs. To measure CISH protein expression, we cultured CD4+ cells and autologous monocytes from healthy tuberculin reactors, with or without γ-irradiated M. tuberculosis. After 4 d, we isolated CD4+CD25+ Tregs and CD4+CD25− cells and prepared cellular extracts. Western blot analysis showed that γ-irradiated M. tuberculosis significantly up-regulated CISH expression only by expanded Tregs (Figure 3).
Figure 3.
Up-regulation of cytokine inducible SH2-containing protein (CISH) expression on Mycobacterium tuberculosis (M. tb)–expanded regulatory T cells (Tregs). A, CD4+CD25− cells from peripheral blood mononuclear cells of healthy tuberculin reactors were cultured with autologous monocytes in medium alone or with γ-irradiated M. tuberculosis (10 μg/mL). After 4 d, CD4+CD25+ (Tregs) and CD4+CD25− (Treg-depleted) cells were isolated and whole-cell lysates were prepared. Lysates were subjected to Western blot analysis with an antibody to CISH (upper panel). The blot was stripped and reprobed with an antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (lower panel). The result is representative of experiments performed on samples from 3 donors. Lane 1, Unstimulated cells; lane 2, M. tuberculosis–expanded Tregs; lane 3, M. tuberculosis–expanded Treg-depleted cells. B, CISH expression quantified by densitometry. Cells from 3 donors were prepared, and Western blot analysis was performed as detailed for panel A. Mean values and SEs are shown.
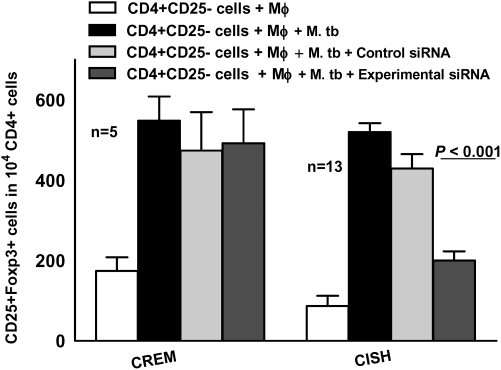
We used siRNA to determine whether CISH was necessary for expansion of Tregs. CISH siRNA significantly inhibited expression of CISH protein, as measured by Western blot, and CREM siRNA inhibited CREM mRNA expression, as determined by real-time polymerase chain reaction (data not shown). CD4+CD25− cells from healthy tuberculin reactors were transfected with CISH or CREM siRNA and then cultured with autologous monocytes and γ-irradiated M. tuberculosis. After 4 d, the percentages of CD4+CD25+FoxP3+ cells were measured by flow cytometry. CISH siRNA significantly inhibited Treg expansion, but CREM siRNA did not (Figure 4).
Figure 4.
Inhibition of regulatory T cell expansion by cytokine inducible SH2-containing protein (CISH) small interfering RNA (siRNA). CD4+CD25− cells from healthy tuberculin reactors were transfected with siRNA to cAMP response element modulator (CREM) or CISH or with scrambled siRNA and cultured with autologous monocytes (Mφ) in medium alone or with γ-irradiated Mycobacterium tuberculosis (M. tb) H37Rv (10 μg/mL). After 4 d, the percentages of CD4+CD25+FoxP3+ cells were determined by flow cytometry and the results were expressed as the number of cells per 104 CD4+ cells. Mean values and SEs are shown.
PD-1 Regulates CISH Expression
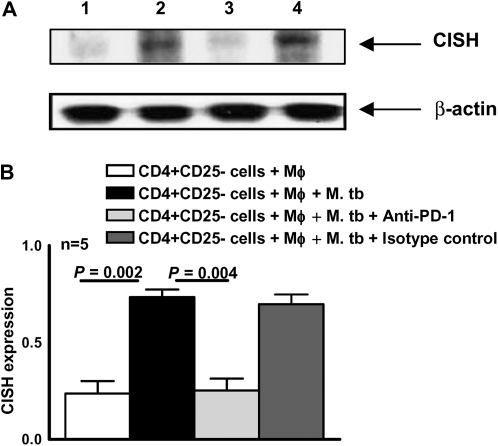
The above findings suggest that PD-1 and the intracellular signaling protein CISH mediate Treg expansion by M. tuberculosis. To delineate the relationship between these molecules, we asked whether blocking PD-1 affects CISH expression. We cultured CD4+CD25− cells and autologous monocytes from PBMCs of healthy tuberculin reactors, with γ-irradiated M. tuberculosis, in the presence or absence of antibodies to PD-1 (10 μg/mL) and isotype control antibodies for 4 days. Western blot analysis of cellular extracts showed that CISH expression was reduced by anti-PD-1 but not by isotype control antibodies (Figure 5).
Figure 5.
Programmed death 1 PD-1)–dependent expression of cytokine inducible SH2-containing protein (CISH). A, CD4+CD25− cells from healthy tuberculin reactors cultured with autologous monocytes (Mφ) in medium alone or with γ-irradiated Mycobacterium tuberculosis (M. tb; 10 μg/mL), with or without anti-PD-1 or isotype control antibody (10 μg/mL). After 4 d, whole-cell lysates were prepared and subjected to Western blot analysis with an antibody to CISH (upper panel). The blot was stripped and reprobed with an antibody to β-actin (lower panel). The result is representative of experiments performed on samples from 5 donors. Lane 1, Unstimulated cells; lane 2, M. tuberculosis–stimulated cells; lane 3, M. tuberculosis–stimulated cells with anti-PD-1; lane 4, M. tuberculosis–stimulated cells with isotype control antibody. B, CISH expression quantified by densitometry. Cells from 5 donors were prepared, and Western blot analysis was performed as detailed for panel A. Mean values and SEs are shown.
M. tuberculosis–induced CD4+CD25+FoxP3+ Cells are Functional Tregs
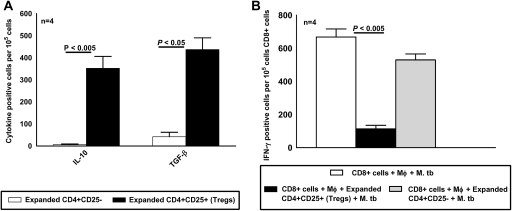
To confirm that the CD4+CD25+FoxP3+ cells derived from CD4+CD25− cells in the above experiments are functional Tregs, we measured their capacity to produce immunosuppressive cytokines and to inhibit IFN-γ production. We cultured CD4+CD25− cells and autologous monocytes from PBMCs of 4 healthy tuberculin reactors in medium alone or with γ-irradiated M. tuberculosis. After 3 d, expanded CD4+CD25+ Tregs and CD4+CD25− non-Tregs were isolated, as described in the Materials and Methods. The frequency of cells producing TGF-β and IL-10 was determined by ELISPOT. The frequency of IL-10-producing cells was much higher in M. tuberculosis–expanded CD4+CD25+ cells than in M. tuberculosis–expanded CD4+CD25− cells (352 ± 54 vs 6 ± 3 cells per 106 cells; P < .005) (Figure 6A). Similarly, the frequency of TGF-β-producing M. tuberculosis–expanded CD4+CD25+ cells was 10-fold higher than in CD4+CD25− cells (437 ± 53 vs 42 ± 20 cells per 106 cells; P < .05) (Figure 6A).
Figure 6.
Mycobacterium tuberculosis (M. tb)–induced CD4+CD25+FoxP3+ cells are functional regulatory T cells (Tregs). A, Cytokine production by expanded CD4+CD25+ (Tregs) and CD4+CD25+ cells. CD4+ cells and autologous monocytes (Mφ) from 4 healthy tuberculin reactors were cultured with γ-irradiated M. tuberculosis (10 μg/mL). After 48 h, CD4+CD25+ cells (Tregs) and CD4+CD25− cells were isolated as described in the Materials and Methods and incubated overnight in triplicate wells on an enzyme-linked immunospot assay (ELISPOT) plate to detect transforming growth factor β (TGF-β)–producing and interleukin 10 (IL-10)–producing cells. Mean values and SEs are shown. B, Reduction of the frequency of M. tuberculosis–responsive CD8+ interferon γ (IFN-γ)–producing cells by M. tuberculosis–expanded Tregs. Freshly isolated CD4+ cells and autologous monocytes from 4 healthy tuberculin reactors were cultured with γ-irradiated M. tuberculosis (10 μg/mL). After 72 h, Tregs were isolated and cultured with freshly isolated autologous CD8+ cells and CD14+ cells at a ratio of 1:9:1, with γ-irradiated M. tuberculosis (10 μg/mL). Then after 2 d, CD8+ cells were isolated and incubated overnight in triplicate wells on an ELISPOT plate to detect IFN-γ-producing cells.
Next, we determined the capacity of M. tuberculosis–expanded Tregs to inhibit IFN-γ production by CD8+ cells from 4 healthy tuberculin reactors. We used CD8+ cells to avoid contamination of CD4+ effector cells with Tregs. The frequency of IFN-γ-producing CD8+ cells was reduced by >80% by the addition of Tregs (113 ± 22 vs 668 ± 49 cells per 106 cells; P < .005) (Figure 6B). In contrast, M. tuberculosis–expanded CD4+CD25− cells, isolated as described in the Materials and Methods, had no effect on the frequency of IFN-γ-producing CD8+ cells (530 ± 36 vs 668 ± 49 cells per 106 cells; P > .05) (Figure 6B).
These results demonstrate that the cells designated as M. tuberculosis–induced Tregs in our experimental system produce immunosuppressive cytokines and can inhibit T cell cytokine production.
DISCUSSION
Recent animal and human studies found that Tregs expand in response to M. tuberculosis and inhibit effective immunity [6–12], but limited published information is available on the cellular mechanisms that mediate M. tuberculosis–induced Treg expansion. We found that M. tuberculosis induces some CD4+CCR4+ cells to express FoxP3, but this process did not require CCR4. Furthermore, when CD4+ cells were exposed to M. tuberculosis–activated mononuclear phagocytes, PD-1 up-regulated the expression of CISH and facilitated expansion of Tregs. Our study provides the first (to our knowledge) evidence that PD-1 and CISH contribute to Treg development during infection.
Chemokine receptors are critical for T cells to home to sites of infection and inflammation. Tregs expressing CCR4 are recruited to the site of disease in patients infected with the fungus Paracoccidiodes braziliensis [21]. Furthermore, CCR4 antagonists block accumulation of human Tregs during administration of a vaccine encoding M. tuberculosis antigens [15]. Our unpublished data indicate that M. tuberculosis stimulates monocytes to express the CCR4 ligands, CCL22 and CCL17, suggesting that CD4+CCR4+ Tregs migrate to the site of mycobacterial infection. We demonstrated that M. tuberculosis–induced Tregs expanded more effectively from CD4 cells expressing CCR4. However, CCR4 was not essential for Treg expansion, which was only marginally affected by CCR4 siRNA. The sum of these data suggest that CCR4 may be important for migration of Tregs to inflammatory sites but that expansion of Tregs depends on PD-1, rather than CCR4.
Multiple mechanisms have been identified for induction of Tregs in different experimental systems. Antigen-presenting cells, such as dendritic cells, are essential for expansion of Tregs through costimulatory molecules, such as B7 and ICOS [22, 23], and soluble factors, including interleukin 15, TGF-β, and prostaglandin E2 [24–26]. Cell surface receptors, such as Toll-like receptor 2, also control expansion and function of Tregs, thereby suppressing immunity to Candida albicans [27].
Substantial evidence indicates that interactions between PD-1 and its ligands, programmed death ligand 1 (PDL-1) and programmed death ligand 2, negatively regulate T cell proliferation and cytokine production [28, 29]. Blocking these interactions enhances graft rejection and immunity to intracellular pathogens, whereas PD-1 deficiency facilitates the development of autoimmunity [28]. However, limited information is available on the relationship between PD-1 and Tregs. Some investigators reported that PDL-1-negative dendritic cells failed to support induction of adaptive Tregs [30, 31], suggesting that PD-1/PDL-1 signaling is required for Treg expansion. In contrast, other investigators found that PD-1 limited proliferation of hepatic Tregs in patients with chronic hepatitis C virus infection by inhibiting interleukin 2–driven phosphorylation of signal transducer and activator of transcription 5 (STAT5) [32]. We found that blocking PD-1, but not ICOS or CTLA-4, markedly inhibits Treg expansion in response to M. tuberculosis (Figure 2). This suggests that interactions between PD-1 on activated T cells and its ligands on M. tuberculosis–infected monocytes are essential for expansion of Tregs.
Up-regulation of FoxP3 expression in Tregs is mediated through several signaling pathways, including p38 MAP kinase [33], linker for activation of T cells [34], and Notch1 [35, 36]. We provide evidence that an additional signaling molecule, CISH, contributes to expansion of Tregs in response to a microbial pathogen. CISH is a member of the family of suppressors of cytokine signaling (SOCS) proteins, which inhibit cytokine signaling by competing with Janus kinase and STAT proteins for binding to cytokine receptors [37]. SOCS-1 ameliorates colitis in mice and favors Treg development [38]. However, the role of CISH in the expansion of Tregs is unknown. We found that M. tuberculosis–expanded Tregs have increased expression of CISH and that CISH siRNA inhibits Treg expansion. We also found that PD-1 contributes to expansion of Tregs and that blocking PD-1 inhibits CISH expression. The cytoplasmic tail of PD-1 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM), which can act as docking sites for Src homology 2 domain containing protein tyrosine phosphatase 2 (SHP-2) and SHP-1. SOCS proteins, including CISH, have a conserved modular structure with pre–Src homology 2 (SH2) and SH2 domains, and SOCS3 binds to ITIM-like motifs via SH2 [39]. Our results and published information suggest that CISH may bind to the ITIM or ITSM of the PD-1 cytoplasmic tail, initiating downstream signaling that up-regulates FoxP3 expression and mediates expansion of Tregs. A potential pathway for this effect is through binding of CISH to protein kinase Cφ [19, 40], which enhances FoxP3 expression of natural Tregs by activating nuclear factor of activated T cells [41], which binds to the FoxP3 promoter and up-regulates gene expression [42–46].
Previous studies have found that Tregs are selectively enriched in the blood of patients with tuberculosis and at the sites of infection [11, 47, 48], but their role in disease pathogenesis remains controversial. Elegant experiments in mice suggest that Tregs inhibit immune responses to M. tuberculosis by delaying trafficking of effector T cells to the site of infection [49], whereas studies in primates indicate that Treg expansion occurs in response to inflammation during early tuberculosis [50], suggesting that they contribute to limiting tissue destruction. Using human cells cultured with M. tuberculosis in vitro, we previously showed that Tregs expand from CD4+ cells from healthy tuberculin reactors, but not from those from tuberculin-negative donors [11], indicating that Treg expansion is antigen-specific in the setting of exposure to mycobacterial antigen. A limitation of our system is that cells are cultured for a relatively short period in vitro with high antigen concentrations, which may not reflect conditions in the lung in vivo. Nevertheless, our present study provides insight into potential cellular mechanisms by which Tregs may expand in response to M. tuberculosis and should spur additional work to determine whether PD-1 and CISH contribute to Treg expansion from CCR4+ T cells in vivo in patients with tuberculosis.
Funding
This work was supported by the National Institutes of Health (grant numbers AI054629, A1085135 and AI073612 to R.V. and AI063514 to P.F.B.); the Cain Foundation for Infectious Disease Research; the Center for Pulmonary and Infectious Disease Control; the Potts Memorial Foundation grant to R.D. A.B. is supported by a postdoctoral fellowship awarded by the Council of Scientific and Industrial Research, India.
References
- 1.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 2.Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–59. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Rouse BT, Suvas S. Regulatory cells and infectious agents: detentes cordiale and contraire. J Immunol. 2004;173:2211–5. doi: 10.4049/jimmunol.173.4.2211. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 6.Ordway D, Henao-Tamayo M, Harton M, et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179:522–31. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 7.Scott-Browne JP, Shafiani S, Tucker-Heard G, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kursar M, Koch M, Mittrucker HW, et al. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–5. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch CS, Toossi Z, Othieno C, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–73. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Lao SH, Wu CY. Increased frequency of CD4(+)CD25(high) Treg cells inhibit BCG-specific induction of IFN-gamma by CD4(+) T cells from TB patients. Tuberculosis (Edinb) 2007;87:526–34. doi: 10.1016/j.tube.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Garg A, Barnes PF, Roy S, et al. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008;38:459–69. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahan CS, Thomas JJ, Boom WH, Rojas RE. CD4(+) CD25(high) Foxp3(3+) regulatory T cells downregulate human Vdelta2(+) T-lymphocyte function triggered by anti-CD3 or phosphoantigen. Immunology. 2009;127:398–407. doi: 10.1111/j.1365-2567.2008.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–4. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Bayry J, Tchilian EZ, Davies MN, et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc Natl Acad Sci U S A. 2008;105:10221–6. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Z, Yu S, Fitzgerald DC, et al. IL-12R beta 2 promotes the development of CD4+CD25+ regulatory T cells. J Immunol. 2008;181:3870–6. doi: 10.4049/jimmunol.181.6.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL. Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol. 2008;180:2967–80. doi: 10.4049/jimmunol.180.5.2967. [DOI] [PubMed] [Google Scholar]
- 18.Tang AL, Teijaro JR, Njau MN, et al. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol. 2008;181:1806–13. doi: 10.4049/jimmunol.181.3.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Chen S, Xu X, et al. Cytokine-induced Src homology 2 protein (CIS) promotes T cell receptor-mediated proliferation and prolongs survival of activated T cells. J Exp Med. 2000;191:985–94. doi: 10.1084/jem.191.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenbrock K, Juang YT, Tolnay M, Tsokos GC. The cyclic adenosine 5′-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J Immunol. 2003;170:2971–6. doi: 10.4049/jimmunol.170.6.2971. [DOI] [PubMed] [Google Scholar]
- 21.Cavassani KA, Campanelli AP, Moreira AP, et al. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J Immunol. 2006;177:5811–8. doi: 10.4049/jimmunol.177.9.5811. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki S, Iyoda T, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akbari O, Freeman GJ, Meyer EH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–32. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baratelli F, Lin Y, Zhu L, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–90. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 26.Imamichi H, Sereti I, Lane HC. IL-15 acts as a potent inducer of CD4(+)CD25(hi) cells expressing FOXP3. Eur J Immunol. 2008;38:1621–30. doi: 10.1002/eji.200737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netea MG, Sutmuller R, Hermann C, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–8. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 28.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–25. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–6. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschini D, Paroli M, Francavilla V, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–64. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber S, Schrader J, Fritz G, et al. P38 MAP kinase signaling is required for the conversion of CD4+ PLoS One. 2008;3:e3302. doi: 10.1371/journal.pone.0003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J Exp Med. 2006;203:119–29. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samon JB, Champhekar A, Minter LM, et al. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood. 2008;112:1813–21. doi: 10.1182/blood-2008-03-144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ou-Yang HF, Zhang HW, Wu CG, et al. Notch signaling regulates the FOXP3 promoter through RBP-J- and Hes1-dependent mechanisms. Mol Cell Biochem. 2009;320:109–14. doi: 10.1007/s11010-008-9912-4. [DOI] [PubMed] [Google Scholar]
- 37.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 38.Horino J, Fujimoto M, Terabe F, et al. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunol. 2008;20:753–62. doi: 10.1093/intimm/dxn033. [DOI] [PubMed] [Google Scholar]
- 39.Orr SJ, Morgan NM, Elliott J, et al. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood. 2007;109:1061–8. doi: 10.1182/blood-2006-05-023556. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Anderson PO, Li L, Sjogren HO, Wang P, Li SL. Functional association of cytokine-induced SH2 protein and protein kinase C in activated T cells. Int Immunol. 2003;15:403–9. doi: 10.1093/intimm/dxg039. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–24. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 43.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 44.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–51. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantel PY, Ouaked N, Ruckert B, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 46.Takaki H, Ichiyama K, Koga K, et al. STAT6 inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem. 2008;283:14955–62. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 48.Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009;179:1061–70. doi: 10.1164/rccm.200804-529OC. [DOI] [PubMed] [Google Scholar]
- 49.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–20. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green AM, Mattila JT, Bigbee CL, Bongers KS, Lin PL, Flynn JL. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J Infect Dis. 2010;202:533–41. doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]