Abstract
Background. End points used to detect influenza in vaccine efficacy trials have varied. Both the inactivated and live attenuated influenza vaccines are efficacious; however, failure to protect occurs.
Methods. We compared characteristics of influenza A (H3N2) and B cases from 3 years of a comparative placebo-controlled trial of inactivated and live attenuated vaccines, and we evaluated the laboratory end points used to determine efficacy.
Results. Although illness duration and reported symptoms did not differ by intervention, subjects with influenza in the inactivated vaccine group were less likely than those in the placebo group to report medically attended illnesses. All influenza type A (H3N2) and B cases isolated in cell culture were also identified by real-time polymerase chain reaction (rtPCR). However, only 69% of type A (H3N2) cases identified by rtPCR also were isolated in cell culture. Isolation frequency was lowest among live attenuated vaccine failures, a reflection of lower specimen viral loads. Among cases of rtPCR identified influenza A (H3N2), 90% of placebo and 87% of live attenuated vaccine recipients but only 23% of inactivated vaccine recipients demonstrated serologic confirmation of infection.
Conclusions. In influenza vaccine efficacy studies, virus identification using rtPCR is the ideal end point. Isolation in cell culture will miss cases, and a serologic end point alone will overestimate inactivated vaccine efficacy.
Inactivated influenza vaccine was developed >60 years ago in response to concerns about military readiness in World War 2. Over many years, vaccine trials in the US military estimated that protective efficacy was 70%–90% [1]. Live attenuated influenza vaccine has a somewhat shorter history, especially as a licensed trivalent preparation. Although studies of young children demonstrated high efficacy, in excess of 90% [2, 3], efficacy appears to be lower in young adults [4–6]. Thus, both vaccines are efficacious; however, failure to protect still occurs. Some have suggested that vaccinated persons with symptomatic influenza (vaccine failures) may have milder illnesses and less virus shedding, although this has not been clearly demonstrated [2, 3,7–9].
Although the standard methodology used to determine efficacy of both vaccines has been the placebo-controlled trial, end points have differed. Some studies used virus isolation in cell culture or embryonated eggs to confirm illness etiology [2, 3, 10], others used a serologic outcome—that is, an increase in serum antibody titer [11–13]. The validity of using serologic confirmation of infection rather than virus identification to determine vaccine efficacy has been questioned [10, 14]. Virus isolation can be compromised by varying sensitivity of the systems to support influenza virus [15–17]. Successful isolation in cell culture may also vary by influenza subtype and influenza season [18]. Within the past 10 years, real-time polymerase chain reaction (rtPCR) assays have been developed and used to identify influenza virus in respiratory specimens [19]. These assays can be configured with selected primers and probes to identify influenza viruses by type and by subtype and can be processed with reasonably high throughput.
Beginning in the 2004–2005 influenza season, we performed a series of annual studies to estimate the absolute and relative efficacies of licensed inactivated and live attenuated vaccines [4–6]. This randomized, placebo-controlled, community-based trial enrolled healthy adults aged <50 years and was conducted over a 4-year period. Multiple end points were used to confirm symptomatic illnesses as influenza, including virus isolation in cell culture, rtPCR assays, and serologic confirmation of infection. The study was designed with unbalanced randomization; for each individual receiving placebo, 5 participants received one or the other vaccine. This strategy was adopted to promote study participation and to increase the number of subjects in the study who had influenza infection in spite of vaccination. We now examine the clinical, virologic, and serologic characteristics of the influenza cases identified during the 2004–2005, 2005–2006, and 2007–2008 influenza seasons, the 3 study years when interventions were given, and compare these characteristics by intervention group. Our objectives were to determine whether influenza illnesses in the vaccine failures were different than illnesses in the placebo group and to examine the value of each laboratory assay in confirming illnesses as influenza.
METHODS
Study Design
Subjects eligible for the comparative vaccine trial were healthy men and women aged 18–49 years. Persons with any health condition for which the inactivated vaccine was specifically recommended and persons for whom either vaccine was contraindicated were excluded [20]. Subjects were recruited from the community at study sites located on university campuses in Michigan. The study was approved by the institutional review board at the University of Michigan Medical School, and written informed consent was obtained from all participants before enrollment. In study years 1, 2, and 4, participants were vaccinated based on random assignment of intervention (inactivated influenza vaccine or matching placebo administered by intramuscular injection, or live attenuated influenza vaccine or matching placebo administered by intranasal spray). Participants and nurses administering interventions were not aware of whether vaccine or placebo was administered, but they were aware of the route of administration. In study year 3, no interventions were given, and the duration of protection provided by vaccines administered in study year 2 was evaluated. Subjects enrolled in study year 1 were encouraged to return for study years 2 and 3 with supplemental recruitment and randomization taking place in year 2. In study year 4, subjects were newly recruited and randomized.
Each year, blood specimens were collected for serologic assays from participants immediately prior to vaccination, ∼30 days after receipt of vaccine/placebo and at the end of the influenza season. From November through April each year, participants reported influenza-like illnesses meeting a case definition (≥1 respiratory symptom [cough or nasal congestion] plus ≥1 systemic symptom [fever or feverishness, chills, or body aches]). Throat swab specimens were collected for virus identification, and participants were followed for collection of data on illness characteristics. Aliquots of serum and original illness specimen material were held in freezer repositories under appropriate long-term storage conditions.
Illness Severity Assessments
Participants with influenza-like illnesses reported illness onset date, noted symptoms and graded their severity as 1 (mild: “it didn't affect how I did my usual activities at all”), 2 (moderate: “it affected how I did my usual activities, but I was still able to do them”), or 3 (severe: “I could not work or do my usual activities”) at multiple points during illness, reported whether the illness was medically attended (health care provider contact—visit or phone contact), and reported the date of illness resolution. For this analysis, indicators of illness severity included whether both fever/feverishness and cough were reported, whether any illness symptom was considered severe, whether the illness was medically attended, and the duration of illness symptoms. All illnesses were also characterized by interval (in days) from illness onset to specimen collection.
Laboratory Assays
Throat swab specimens collected from participants with influenza-like illnesses were cultured in primary rhesus monkey kidney cells to identify isolation-positive specimens. Specimens were also tested by means of rtPCR assays using the Taqman system (Applied Biosystems); primers and probes used in this assay were developed by the Centers for Disease Control and Prevention’s Influenza Division for universal detection of influenza A and B viruses, and subtype identification of influenza A viruses. Cycle threshold (Ct) values from rtPCR assays are indicators of the amount of virus in a specimen, with lower Ct values indicating higher viral loads [21]; specimens with Ct values ≥40 were considered negative for influenza. For this analysis, all specimens that were initially laboratory confirmed as influenza by isolation in cell culture and/or identification in rtPCR assay were retested by rtPCR in a limited number of batches with optimized primers and probes to reduce Ct value variability between rtPCR runs. Specimens were run in duplicate and average values determined. Resulting Ct values were categorized to identify specimens with high (Ct , <25.0), medium (Ct, 25.0–30.0) and low (Ct, >30.0 to <40.0) specimen viral loads [21].
Serum samples obtained from participants immediately prior to receipt of vaccine/placebo, ∼30 days after receipt, and at the end of the influenza season were tested with the hemagglutination inhibition (HAI) assay, using vaccine and circulating influenza A (H3N2) and B virus strains from appropriate seasons as antigens [4–6]. Participants with serologic evidence of response to intervention (≥4 fold increase in HAI titer to vaccine strains between pre- and post-intervention specimens) were identified, as were participants with serologic evidence of influenza infection (≥4 fold increase in HAI titer to vaccine and/or circulating strains between post-intervention [preseason] and postseason specimens).
Statistical Analyses
Categorical data were analyzed with an appropriate χ2 test or, when necessary, the Fisher exact test; continuous values (eg, illness duration) were analyzed using Wilcoxon rank sum tests. Statistical analyses were conducted with the use of SAS software, version 9.2 (SAS Institute). A P value of < .05 was considered to indicate statistical significance. No correction for multiple testing was considered. Analyses presented here were limited to data from subjects with symptomatic illnesses that were laboratory confirmed as influenza A (H3N2) or influenza B by isolation in cell culture and/or identification in rtPCR assay, and that were identified during the 3 study years when interventions (vaccine or placebo) were administered (2004–2005, 2005–2006, and 2007–2008). Influenza A (H1N1) was rarely identified during these study years ( n = 2) and is not considered here. Results for influenza A and B cases are considered separately.
RESULTS
In the 2004–2005, 2005–2006, and 2007–2008 influenza seasons, there were 1247, 2058, and 1952 participants that received study interventions, respectively. There were 28, 32, and 106 participants with symptomatic influenza A (H3N2) (total, = 166 participants) and 19, 1, and 11 participants with symptomatic influenza B (total, = 31 participants) in the respective influenza seasons. Analyses for influenza B are limited to 29 cases, because for 2 cases, no original specimen material remained for rtPCR retesting. In the 2004–2005 season, antigenically drifted A (H3N2) viruses and both vaccine-like and variant type B viruses were circulating [4]. In the 2005–2006 and 2007–2008 influenza seasons, the circulating A (H3N2) viruses were similar to vaccine strains, and the circulating type B viruses were all from the lineage not included in the vaccine [5, 6].
Subject Age and Clinical Characteristics of Cases
Age, sex, and race distributions of the overall population enrolled in the trial were similar across intervention groups in each study year [4–6]. Table 1 presents participant mean age and illness severity assessments by intervention group for subjects with influenza A (H3N2) and with influenza B. The mean age of subjects with influenza A (H3N2) was 24.7 years, compared with 27.7 years for those with influenza B; for both subjects with influenza A and those with influenza B, those who experienced inactivated vaccine failures were slightly, but not significantly, older than those who experienced either live attenuated vaccine failure or received placebo. Reported mean duration of illness in vaccinated subjects with influenza A (H3N2) illness was only slightly less than that in placebo recipients; mean duration of illness for inactivated vaccine recipients with influenza type B illness was less than that reported by placebo cases (3.7 vs 9.4 days; P = .055), although not significantly. Report of fever/feverishness and cough was common and did not significantly differ by intervention group for either influenza A (H3N2) or B cases. Similarly, the majority of illnesses were self-characterized as severe, and this was similar in both vaccinated and placebo groups. In contrast, report of health care provider contact among subjects with influenza A (H3N2) differed between the vaccine and placebo recipients, with less frequent contact reported by vaccine recipients; in particular, individuals who experienced inactivated vaccine failure were significantly (20% vs 43%; P = .019) less likely to report contact. Health care provider contact was also less likely to be reported by inactivated vaccine recipients with type B illnesses.
Table 1.
Mean Age, Mean Duration of Illness and Illness Severity Assessments by Intervention Group for Laboratory-Confirmed Symptomatic Influenza Type A (H3N2) and Type B
| Group, characteristic | LAIV arm | TIV arm | Placebo arm | Total |
| Subjects with laboratory-confirmed symptomatic influenza A (H3N2) | ||||
| No. of subjects | 80 | 42 | 44 | 166 |
| Age, mean years ± SDa | 24.8 ± 8.9 | 26.8 ± 9.6 | 22.8 ± 7.1 | 24.7 ± 8.7 |
| Duration of illness, mean days ± SD | 6.5 ± 4.5 | 6.1 ± 5.7 | 7.1 ± 6.4 | 6.6 ± 5.4 |
| Reported symptoms of fever/feverishness and cough | 72 (90.0) | 35 (83.3) | 37 (84.1) | 144 (86.7) |
| Reported severe symptoms | 54 (67.5) | 24 (57.1) | 30 (68.2) | 108 (65.1) |
| Health care provider contactb | 27 (34.6) | 8 (19.5)d | 19 (43.2) | 54 (33.1) |
| Subjects with laboratory-confirmed symptomatic influenza B | ||||
| No. of subjects | 9 | 9 | 11 | 29 |
| Age, mean years ± SDc | 25.3 ± 11.5 | 31.0 ± 9.4 | 26.9 ± 8.7 | 27.7 ± 9.8 |
| Duration of illness, mean days ± SD | 8.4 ± 3.9 | 3.7 ± 3.1 | 9.4 ± 7.2 | 7.3 ± 5.6 |
| Reported symptoms of fever/feverishness and cough | 7 (77.8) | 8 (88.9) | 7 (63.6) | 22 (75.9) |
| Reported severe symptoms | 5 (55.6) | 7 (77.8) | 5 (45.5) | 17 (58.6) |
| Health care provider contactb | 4 (44.4) | 1 (12.5) | 4 (36.4) | 9 (32.1) |
NOTE. Data are no. (%) of subjects, unless otherwise indicated. LAIV, live attenuated vaccine; SD, standard deviation; TIV: trivalent inactivated vaccine.
Subjects were aged 18–48 years.
Numbers do not add to expected totals due to missing data.
Subjects were aged 18-46 years.
For TIV vs placebo arms, - P < .05(by χ2 test).
Virologic Characteristics of Cases
Table 2 presents data on the timing of specimen collection relative to illness onset and shows the isolation frequency of type A (H3N2) and B viruses by intervention group. Also shown are Ct values associated with the rtPCR assays categorized based on specimen viral load from high (Ct, < 25) to low (Ct, >30). Specimens from > 60% of subjects with influenza A and B were collected ≤2 days after illness onset with no significant differences by intervention for type A cases; significant (P = .013) differences existed for type B cases driven by the fact that all specimens obtained from subjects who experienced live attenuated vaccine failure were collected early in illness. All influenza type A (H3N2) and B cases isolated in cell culture were also identified by rtPCR. However, only 69% of influenza A cases identified by rtPCR were also isolated in cell culture; frequency of isolation was highest among placebo recipients and lowest among subjects who experienced live attenuated vaccine failure (84% vs 58%; P = .002). Similarly, only 21% of subjects who experienced live attenuated vaccine failure had low Ct values, indicating high viral loads, compared with 41% of placebo recipients (P = .008). In contrast, all but 1 of the relatively small numbers of influenza type B cases identified by rtPCR were also isolated in cell culture. As with type A, fewer live attenuated vaccine type B failures were categorized in the lowest Ct (highest viral load) category. These findings were adjusted for timing of specimen collection relative to illness onset (0–2 days vs >2 days).
Table 2.
Timing of Specimen Collection, Plus Results from Laboratory Assays to Identify Illness Etiology as Influenza, by Intervention Group, for Laboratory-Confirmed Symptomatic Influenzatype A (H3N2) and Type B
| Group, characteristic | No. (%) of subjects |
|||
| LAIV arm | TIV arm | Placebo arm | Total | |
| Subjects with laboratory-confirmed symptomatic influenza A (H3N2) | ||||
| No. of subjects | 80 | 42 | 44 | 166 |
| Time from illness onset to specimen collectiona | ||||
| 0-2 days | 57 (71.3) | 23 (54.8) | 27 (62.8) | 107 (64.8) |
| >2 days | 23 (28.8) | 19 (45.2) | 16 (37.2) | 58 (35.2) |
| Isolation in cell culture | 46 (57.5)b | 31 (73.8) | 37 (84.1) | 114 (68.7) |
| rtPCR Ct values | ||||
| <25 | 17 (21.3)b | 13 (31.0) | 18 (40.9) | 48 (28.9) |
| 25–30 | 26 (32.5) | 10 (23.8) | 16 (36.4) | 52 (31.3) |
| >30 - <40 | 37 (46.3) | 19 (45.2) | 10 (22.7) | 66 (39.8) |
| Subjects with laboratory-confirmed symptomatic influenza B | ||||
| No. of subjects | 9 | 9 | 11 | 29 |
| Time from illness onset to specimen collection | ||||
| 0-2 days | 9 (100)b | 5 (55.6) | 4 (36.4) | 18 (62.1) |
| >2 days | 0 (0) | 4 (44.4) | 7 (63.6) | 11 (37.9) |
| Isolation in cell culture | 9 (100) | 9 (100) | 10 (90.9) | 28 (96.6) |
| rtPCR Ct values | ||||
| <25 | 1 (11.1) | 2 (22.2) | 3 (27.3) | 6 (20.7) |
| 25–30 | 2 (22.2) | 3 (33.3) | 3 (27.3) | 8 (27.6) |
| >30 to <40 | 6 (66.7) | 4 (44.4) | 5 (45.5) | 15 (51.7) |
NOTE. Ct, cycle threshold; LAIV, live attenuated vaccine; rtPCT, real-time polymerase chain reaction; TIV, trivalent inactivated vaccine.
Numbers do not add to expected totals due to missing data
For LAIV vs placebo arm, P < .05 (by χ2 test, with adjustment for timing of specimen collection).
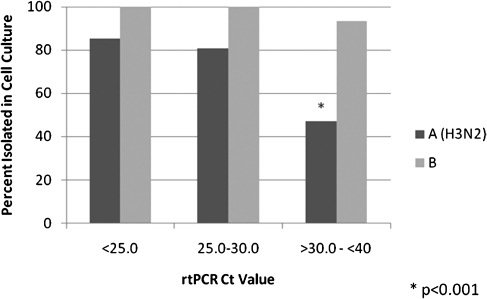
Figure 1 shows the isolation frequency of influenza type A (H3N2) and B viruses by rtPCR Ct value categories. For influenza A (H3N2) cases, the ability to isolate the virus in cell culture was related to the amount of virus in the clinical specimen; 85% of those with the higher viral quantity were positive by isolation, but only 47% of those with the lowest amount (P < .001). When stratified by time from illness onset to specimen collection (0–2 days vs >2 days) those with higher viral load, as determined by rtPCR, were more likely to be positive by isolation regardless of timing of specimen collection (data not shown).
Figure 1.
The proportion of cases confirmed as influenza types A (H3N2) or B that were isolated in cell culture by cycle threshold (Ct) category indicating high (Ct, < 25), medium (Ct, 25–30), and low (Ct, >30 to < 40) specimen viral loads. rtPCR, real-time polymerase chain reaction.
Serologic Characteristics of Cases
HAI tests conducted on blood specimens collected before and after the intervention and after influenza season were used to detect a serologic immune response to influenza strains included in each year's vaccine and to rtPCR-confirmed infection. Appropriate blood specimens for determination of vaccine and infection immune responses were available for 95% of influenza A (H3N2) cases and 83% of influenza B cases. Among cases of influenza A (H3N2) identified by rtPCR, 79% of inactivated vaccine recipients and 22% of live attenuated vaccine recipients (P < .001) had evidence of serologic response to vaccination with the A (H3N2) vaccine strain. Similarly, among cases of influenza type B identified by rtPCR, 63% of inactivated vaccine recipients and 22% of live attenuated vaccine recipients (P = .15) had evidence of serologic response to vaccination with the type B vaccine strain. As can be seen in Table 3, 90% of influenza A (H3N2) cases identified by rtPCR in the placebo group and 87% of cases in the live attenuated vaccine group demonstrated serologic confirmation of infection. In contrast only 23% of A (H3N2) cases in the inactivated vaccine group had serologic confirmation of infection (P < .001). Also shown are the number and proportion of infection immune responses examined by Ct categories; serologic confirmation of type A (H3N2) infection remained high even in those with a low specimen viral load (high Ct value) in the placebo and live attenuated vaccine groups, but it decreased in the inactivated vaccine group. It was not possible to see a similar result with influenza type B.
Table 3.
The Proportion of Influenza A (H3N2) and Influenza B Cases That Were Serologically Confirmed as Influenza for Each Intervention, Stratified by Real-Time Polymerase Chain Reaction (rtPCR) Cycle Threshold (Ct) Category (ndicating specimen viral load)
| Group, characteristic | No. (%) of subjects with serologic confirmation of influenza A (H3N2) or B infection No. (%) |
||
| Influenza A (H3N2) | LAIV arm | TIV arm | Placebo arm |
| No. of subjects | 77a | 39a | 41a |
| rtPCR Ct category | |||
| <25 | 15 (88.2) | 4 (30.8) | 17 (94.4) |
| 25–30 | 21 (84.0) | 4 (40.0) | 13 (92.9) |
| >30 to <40 | 31 (88.6) | 1 (6.3) | 7 (77.8) |
| Total | 67 (87.0) | 9 (23.1)b | 37 (90.2) |
| Influenza B | |||
| No. of subjects | 9 | 8a | 7a |
| rtPCR Ct category | |||
| <25 | 1 (100.0) | 2 (100.0) | 1 (50.0) |
| 25–30 | 2 (100.0) | 1 (33.3) | 0 (.0) |
| >30 to <40 | 6 (100.0) | 2 (66.7) | 2 (100.0) |
| Total | 9 (100.0)c | 5 (62.5) | 3 (42.9) |
NOTE. LAIV, live attenuated vaccine; TIV, trivalent inactivated vaccine.
Appropriate blood specimens necessary to determine infection immune response were not available for all subjects.
For TIV vs placebo arms, P < .001 (by χ2 test).
For LAIV vs placebo arms, P < .05 (by χ2 test).
DISCUSSION
In recent years, there has been substantial controversy about how well influenza vaccines protect groups such as the elderly population; much of this debate has involved observational studies without virologic end points [22, 23]. However, there has also been a longer-standing controversy about the precise efficacy of inactivated vaccine in healthy adults. That debate was mainly centered on the end points that were used in the trials conducted in the US military, studies that established that the inactivated vaccine was 70%–90% effective [10, 14]. These studies were performed before rtPCR assays were available, and outcomes typically involved serologic assessments (ie, using an increase in antibody titer to identify infections in the vaccinated group versus the placebo group). Some have suggested that this outcome might overestimate the efficacy of vaccines because of the concept of an “antibody ceiling”—that is, that once antibody titers were increased in response to the vaccine, they could go no higher in response to infection [14]. Despite this concern, the serologic end point has continued to be used in efficacy studies [11, 12], in part because it can be challenging to have subjects report illnesses and present for specimen collection for virus identification shortly after illness onset.
Serologic results from our recent comparative efficacy trials have demonstrated that, using only a serologic outcome, estimates will be biased in favor of overestimating inactivated vaccine efficacy [4–6]. In analyses presented here, only 23% of inactivated vaccine failures demonstrated serologic confirmation of rtPCR-confirmed A (H3N2) infection. Similar results were not found for cases of influenza B. The small numbers might be a reason; circulation of different type B lineages involved might also be an issue [4, 6]. The situation with estimating live attenuated vaccine efficacy using a serologic outcome is somewhat different. In the pivotal trials of live attenuated vaccine leading to licensure, a serologic end point was not used to determine efficacy [2, 3]. Data from our studies have paradoxically shown that using a serologic end point in the trials in children might have been successful, in part because the live attenuated vaccine does not produce major serologic antibody responses, and an increase in antibody produced by subsequent infection is easily demonstrated.
Use of rtPCR for detecting influenza infection has now become a validated standard. It was used, along with isolation in cell culture and serologic testing, to identify infection in our comparative vaccine efficacy study. The test is highly sensitive, so much so that concern has been raised that it might be detecting infections that are not clinically relevant. However, studies following influenza illness over time have shown that detectable virus does not persist, particularly in adults [24]. In our trials, rtPCR positivity was also associated with an increase in antibody titer in the live attenuated vaccine and placebo groups, even at high Ct values—a surrogate for low viral load. This and the fact that the illnesses met a symptomatic case definition indicate their clinical relevance. Low viral load, as estimated by Ct values, was also associated with failure to isolate the virus in cell culture. This applied only to A (H3N2) virus, which is known to be more difficult to isolate [18], and was particularly seen in live attenuated vaccine failures. These findings would suggest that rtPCR with appropriately designed primers and probes should be the primary end point used in future efficacy studies. Isolation in cell culture must still be used in those rtPCR positive to further characterize the viruses, but use of it alone as an end point could result in missed cases and biased results.
Studying the characteristics of vaccine failures is difficult when evaluating efficacious vaccines. Through use of an unbalanced randomization design, 83% of subjects in our efficacy trials received one or the other vaccine, but still there were relatively few influenza cases in the vaccinated group, particularly among those receiving the inactivated vaccine. That may be the reason why it was difficult to demonstrate evidence of milder illness in the vaccinated, with the exception of reduced health care use. Characterization of severity has always been a challenge in influenza illness studies, even in those involving antiviral treatment, for which it was the primary outcome [25, 26]. An interesting finding, which was most apparent in the more numerous live attenuated vaccine failures, was reduced viral shedding among vaccinated subjects, as estimated by Ct values; this finding was unrelated to timing of specimen collection relative to illness onset. If this can be confirmed in children, it might partially explain the mechanism of live attenuated vaccine in producing indirect protection in school children [27, 28].
There are clear problems with using serologic end points in studies involving inactivated vaccine, as well as with using isolation in cell culture without also performing rtPCR assays in studies involving both live attenuated and inactivated vaccines. How protective then are our currently licensed vaccines since one or the other of serology and isolation have been used in past efficacy studies? That is more easily answered for the inactivated vaccine, at least in terms of efficacy among young adults. In both years in which there was sufficient virus transmission in our studies, demonstrated protective efficacy, using rtPCR alone, was ∼70% [4, 6]. That may suggest that we should lower the usual description of vaccine efficacy from 70%–90% in healthy adults to closer to 70%; however, further confirmation by other studies is desirable. Given 70% efficacy in a population with 50% vaccine coverage, approximately one-quarter of influenza cases may occur among vaccinated persons, regardless of attack rate in a given year. This should be kept in mind when considering treatment strategies with influenza antivirals. In any event, these findings reinforce the need for improved influenza vaccines, perhaps even for young adults, the group in which the vaccine is thought to work best.
Funding
National Institute of Allergy and Infectious Diseases (U01AI057853 to A.S.M) and an unrestricted grant from Sanofi Pasteur. ClinicalTrials.gov identifiers: NCT00133523 and NCT00538512.
References
- 1.Davenport FM. Control of influenza. Med J Aust. 1973;(Suppl):33–8. doi: 10.5694/j.1326-5377.1973.tb111174.x. [DOI] [PubMed] [Google Scholar]
- 2.Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza vaccine in children. N Engl J Med. 1998;338:1405–12. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 3.Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–75. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 4.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohmit SE, Victor JC, Teich ER, et al. Prevention of symptomatic seasonal influenza in 2005–-2006 by inactivated and live attenuated vaccines. J Infect Dis. 2008;198:312–7. doi: 10.1086/589885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–7. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 7.Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med. 1995;333:889–93. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- 8.Nichol KL, Mendelman PM, Mallon KP, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults. JAMA. 1999;282:137–44. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 9.Patriarca PA. A randomized controlled trial of influenza vaccine in the elderly; scientific scrutiny and ethical responsibility. JAMA. 1994;272:1700–1. [PubMed] [Google Scholar]
- 10.Edwards KM, Dupont WD, Westrich MK, et al. Randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169:68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Wilde JA, McMillan JA, Serwint J, et al. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA. 1999;281:908–13. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- 12.Govaert TM, Thijs CT, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–5. [PubMed] [Google Scholar]
- 13.Meiklejohn G, Kempe CH, Thalman WG, Lennette EH. Evaluation of monovalent influenza vaccines. II. Observations during an influenza A-prime epidemic. Am J Hyg. 1952;55:12–21. doi: 10.1093/oxfordjournals.aje.a119500. [DOI] [PubMed] [Google Scholar]
- 14.Langmuir AD, Henderson DA, Serfling RE. The epidemiological basis for the control of influenza. Am J Pub Health. 1964;54:563–71. doi: 10.2105/ajph.54.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petric M, Comanor L, Petti CA. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis. 2006;194:S98–110. doi: 10.1086/507554. [DOI] [PubMed] [Google Scholar]
- 16.Govorkova EA, Kodihalli S, Alymova IV, Fanget B, Webster RG. Growth and immunogenicity of influenza viruses cultivated in Vero or MDCK cells and in embryonated chicken eggs. Dev Biol Stand. 1999;98:39–51. [PubMed] [Google Scholar]
- 17.Oh DY, Barr IG, Mosse JA, Laurie KL. MDCK-SIAT1 cells show improved isolation rates for human influenza viruses compared to conventional MDCK cells. J Clin Micro. 2008;46:2189–94. doi: 10.1128/JCM.00398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumari K, Gulati S, Smith DF, Gulati U, Cummings RD, Air GM. Receptor binding specificity of recent human H3N2 influenza viruses. Virol J. 2007;4:42. doi: 10.1186/1743-422X-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Elden LJ, Nijhuis M, Schipper P. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2008;57/RR07:1-60. [PubMed] [Google Scholar]
- 21.Balish A, Warnes CM, Wu K, et al. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus - United States, 2009. MMWRMorb Mortal Wkly Rep. 2009;58:826–9. [PubMed] [Google Scholar]
- 22.Simonsen L, Taylor RJ, Viboud C, et al. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–66. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 23.Nichol KL. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine. 2009;27:6305–11. doi: 10.1016/j.vaccine.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients. Arch Intern Med. 2001;161:212–7. doi: 10.1001/archinte.161.2.212. [DOI] [PubMed] [Google Scholar]
- 26.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza. JAMA. 2000;283:1016–24. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 27.Rudenko LG, Slepushkin AN, Monto AS, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. J Infect Dis. 1993;168:881–7. doi: 10.1093/infdis/168.4.881. [DOI] [PubMed] [Google Scholar]
- 28.Clements ML, Snyder MH, Sears SD, et al. Evaluation of the infectivity, immunogenicity and efficacy of live cold-adapted B/Ann Arbor/1/86 reassortant virus vaccine in adult volunteers. J Infect Dis. 1990;161:869–77. doi: 10.1093/infdis/161.5.869. [DOI] [PubMed] [Google Scholar]