Abstract
Background. Posaconazole is a triazole with anti-Aspergillus activity. However, little is known about the utility of posaconazole as primary therapy for invasive pulmonary aspergillosis.
Methods. An in vitro model of the human alveolus was used to study the impact of minimum inhibitory concentrations (MIC) on exposure-response relationships. The pharmacokinetic-pharmacodynamic relationships of posaconazole were examined in an inhalational murine model of invasive pulmonary aspergillosis. A mathematical model was fitted to the entire data set. This model was then used to describe the relationship between drug exposure, quantified in terms of the area under the concentration time curve to MIC (AUC:MIC) and the observed antifungal effect.
Results. The posaconazole MIC was an important determinant of exposure-response relationships and accounted for a portion of the observed variance. Murine pharmacokinetics were linear for dosages 1–20 mg/kg/day. There was a dose-dependent decline in serum galactomannan concentrations, with near-maximal suppression following 20 mg/kg/day. The murine pharmacokinetic-pharmacodynamic data were well described by the mathematical model. An AUC:MIC ratio of 167 was associated with half-maximal antifungal effect.
Conclusions. These results provide the experimental foundation for the selection of candidate posaconazole regimens for the primary treatment of invasive pulmonary aspergillosis in profoundly neutropenic hosts.
Invasive aspergillosis is a frequently lethal infectious syndrome [1]. There are still relatively few therapeutic options compared with many microbial pathogens [2]. A detailed understanding of antifungal exposure-response relationships represents a first critical step for optimizing the clinical use of currently available compounds. One way this can be achieved is the use of preclinical infection models and mathematical modeling techniques that allow the experimental findings to be bridged to humans [3].
Posaconazole is a triazole with broad-spectrum antifungal activity [4]. The anti-Aspergillus activity of this compound has been demonstrated in laboratory animal models [5]. A number of clinical trials have examined its use for the prevention and treatment of invasive aspergillosis [6–8]. The clinical use of posaconazole is potentially compromised by increasing reports of Aspergillus isolates that are less susceptible to triazoles [9, 10]. Currently, there is little information on the magnitude of drug exposure that is associated with a high probability of therapeutic success in humans with invasive pulmonary aspergillosis.
Here, we use an in vitro model of the human alveolus to study the impact of minimum inhibitory concentrations (MICs) on the exposure-response relationships of posaconazole against A. fumigatus. The pharmacokinetic and pharmacodynamic relationships for posaconazole were further defined using an inhalational murine model of invasive pulmonary aspergillosis. A mathematical model was used to directly link serum posaconazole concentrations with the observed antifungal effect, which was quantified by circulating galactomannan concentrations. We then used this model to identify the magnitude of drug exposure that is associated with near-maximal antifungal activity. These results can be used to identify candidate regimens for the primary treatment of invasive pulmonary aspergillosis.
METHODS
Organisms and Minimum Inhibitory Concentrations
The initial experiments in an in vitro model of the alveolus were performed using a green fluorescent protein (GFP)-expressing strain of A. fumigatus, which has been described elsewhere [11]. Subsequently, 3 additional clinical isolates from our culture collection (F11628, F14532, and F16216) with a range of posaconazole MICs and previously described amino acid substitutions in the triazole target Cyp51 were studied [9]. The challenge strain for the murine inhalational model was A1163, as described elsewhere [12]. Posaconazole MICs were determined using EUCAST methodology [13]. MICs were performed a minimum of 8 times in separate experiments. Both the modal value and an expectation of MICs values were determined. The latter was calculated by multiplying the absolute MIC value by the fraction of total experiments in which that particular MIC value was obtained. An expectation was then obtained by summation of these products.
In vitro Model of the Human Alveolus
An in vitro model of the human alveolus was used to examine the impact of MIC on the posaconazole exposure-response relationships. A cellular bilayer was constructed as described elsewhere [11]. This model simulates the following pulmonary compartments that are relevant to the pathogenesis of invasive pulmonary aspergillosis: (1) the alveolar compartment (the airspace), (2) the cellular bilayer comprising a monolayer of alveolar epithelial cells and pulmonary endothelial cells (the alveolar-capillary barrier), and (3) the endothelial compartment (the pulmonary capillary). The alveolar compartment contained cell culture medium without protein (EBM-2; Lonza), while the endothelial compartment contained EBM-2 supplemented with 2% fetal bovine serum (FBS; Lonza) to simulate human protein concentrations.
Pure posaconazole powder (Schering-Plough) was dissolved in DMSO (Sigma) to produce a stock solution at 1600 mg/L. This was then serially diluted in DMSO, followed by final single-step 1:100 dilution into EBM-2 supplemented with 2% FBS to achieve a range of concentrations. Posaconazole was administered to the endothelial compartment 6 h following the inoculation of Aspergillus conidia into the alveolar compartment. The inoculum was prepared as described elsewhere [11]. The in vitro models were incubated at 37°C in 5% CO2 for 18 h. Samples from the endothelial and alveolar compartments were obtained and stored at −80oC for measurement of posaconazole and galactomannan concentrations.
Inhalational Murine Model of Invasive Pulmonary Aspergillosis
All animal experiments included in this study were part of ongoing studies performed under UK Home Office project license PPL40/3101 and had received ethical clearance from The University of Manchester. Male CD1 mice (Charles River Ltd) weighing 22–25 g were used. The mice were housed in vented HEPA-filtered cages, and food and water were provided ad libitum.
A previously described [14] persistently neutropenic inhalational murine model of invasive pulmonary aspergillosis was used to examine the in vivo posaconazole pharmacokinetic and pharmacodynamic (PK-PD) relationships. This model results in ∼50% mortality at 7-days, with mice beginning to succumb 4–5 days after inoculation [12]. Mice were rendered neutropenic on days minus 2 and +3 with cyclophosphamide 200 mg/kg intraperitoneally (i.p.), which resulted in profound and persistent neutropenia for 6 days. Cortisone acetate (Sigma) 250 mg/kg subcutaneously (s.c.) was also administered on days minus 2 and + 3 to impair the function of pulmonary alveolar macrophages and enable invasive infection to be established. A conidial suspension containing 1 × 109 conidia/mL was prepared from 7-day old cultures on Sabouraud glucose agar (Scientific Laboratory Supplies) by flooding the plate with phosphate buffered saline (Invitrogen) with 0.05 Tween 80 (Sigma). Mice (n = 6 per cage) were exposed to 12 mL of this solution, which was nebulized (Hudson RCI, High Wycombe, UK) at 1 bar for 1 h. The desired inoculum was verified by quantitative culture.
The clinical formulation of posaconazole was diluted to the desired concentration in 20% (2-Hydroxypropyl)-β-cyclodextrin (Sigma) to ensure the active compound remained in solution and was administered by gavage. Therapy was initiated 24 h post-exposure and was subsequently administered at 48 and 72 h post-exposure. Mice were killed throughout the study period for both pharmacokinetic and pharmacodynamic studies.
Pharmacokinetic and Pharmacodynamic Studies
Groups of mice (n = 36 for each cohort) received 1, 2.5, and 20 mg/kg. These dosages were chosen on the basis of preliminary data that indicated they encompassed the informative portion of the exposure-response relationship. Groups (n = 4) were sacrificed throughout the first and third dosing interval at 1, 3, 6, and 24 h post-dose. Serum samples were obtained by cardiac puncture after terminal anaesthesia with 5% isoflurane (Baxter). For the pharmacodynamic studies, galactomannan concentrations were initially determined at 24, 48, 72, and 96 h and then subsequently using the same sampling times as were used for the pharmacokinetic studies.
Measurement of Posaconazole Concentrations
Total posaconazole concentrations in cell culture media and mouse serum were measured using high-performance liquid chromatography (HPLC) with a Shimadzu Prominence (Shimadzu) and a TSK-gel ODS-80TS 4.6 × 150 mm column (Tosoh Bioscience). The injection volume was 100 μL. A standard curve encompassing 0.08–40 mg/L was constructed in the respective matrix, from stock solutions of posaconazole 1000 mg/L in methanol (Fisher Scientific). The internal standard was 4-nitro-1-napthylaminpurum 0.1 mg/L (Sigma). The mobile phase was 40% 0.02 mol/L potassium dihydrogen phosphate 60% acetonitrile (vol/vol) with an isocratic flow rate of 1.3 mL/min. Posaconazole and the internal standard were detected using UV 254 nm; they eluted after 4.3 and 2.7 min, respectively. The CV% was <8% over the concentration range 0.08–40 mg/L. The limit of detection was 0.08 mg/L. The intra- and inter-day variation was <7%.
Measurement of Galactomannan Concentrations
Galactomannan concentrations from the in vitro model and mouse serum were measured using a commercially available double sandwich enzyme immunoassay (Platelia, BioRad Laboratories), according to the manufacturer's instructions with a single modification. Because of the low volumes of experimental samples, 25 μL treatment solution was used to pretreat 75 μL of sample (rather than the recommended 100–300 μL). This modification did not have an impact upon estimates for the concentrations of galactomannan (data not shown).
Data Analysis and Mathematical Modeling
The posaconazole exposure response relationships in the in vitro model were modeled using an inhibitory sigmoid Emax model taking the following form:
where Econ is the galactomannan concentrations in the absence of therapy, Emax is the asymptotic reduction in galactomannan concentrations induced by posaconazole, E50 is the concentration of posaconazole that induced half-maximal effect, and H is the slope (or Hill) function. The model was fitted to the data using the pharmacokinetic program ADAPT 5 [15]. The mean galactomannan values from 3 individual in vitro models were calculated and the data were weighted by the observed variance.
The pharmacokinetic data were described using a 3-compartment pharmacokinetic model, which enabled the movement of drug from the endothelial compartment, through the cellular bilayer and into the alveolar compartment to be described (equations not shown). This pharmacokinetic model was then used to calculate the AUC:MIC in the endothelial compartment and therefore quantify the antifungal effect in both the endothelial and alveolar compartments in terms of the AUC:MIC. These calculations were performed using ADAPT 5 [15].
The murine pharmacokinetic and pharmacodynamic data were modeled using the big version of the population pharmacokinetic program nonparametric adaptive grid (BIG NPAG) [16]. The mathematical model is shown in the Appendix. The mean posaconazole and galactomannan concentrations were determined in each group of mice. The weighting for the mathematical modeling was determined using the maximum likelihood estimation module in ADAPT 5 [15], as described elsewhere [17].
Relationship between Drug Exposure and Anti-Aspergillus Effect
The mathematical model was used to determine the antifungal effect produced by various AUC:MIC values and expressed as a proportion of the theoretical maximum antifungal effect. This was achieved by determining the area under the galactomannan-time curve for incremental posaconazole dosages. Similarly, galactomannan concentrations at the end of the study period (96 h) following the administration of various dosages of posaconazole at 24, 48, and 72 h postinoculation were determined. The posaconazole and galactomannan AUCs were determined using integration within the simulation module of ADAPT 5 [15]. The posaconazole AUC:MIC was then calculated using the MIC of the study strain, which was 0.09 mg/L (see results below).
RESULTS
MICs
The estimates for the modal MIC values and expectation for GFP-expressing strain (Cyp51A genotype), F14532 (M220T), and F16216 (L98H+TR) were 0.06/0.12, 0.25/0.36, and 1/0.9, respectively. The modal MIC values and the expectation for the strain used in the in vivo experiments (A1163) was 0.06 and 0.09, respectively. Strain F11628 (G138C) consistently had an MIC >8 mg/L. A more precise estimate was not possible because of precipitation of posaconazole at these higher concentrations.
In vitro Pharmacokinetics and Pharmacodynamics
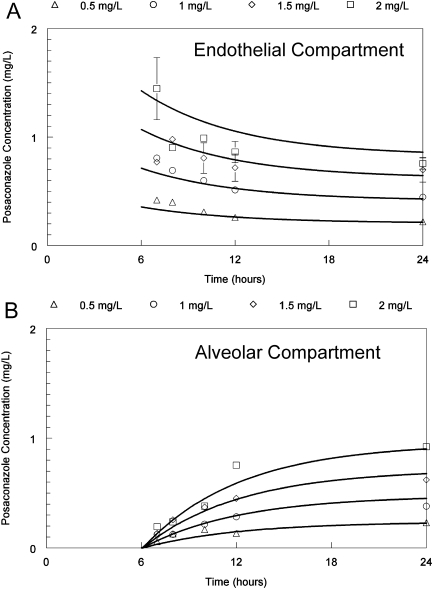
The concentration time profile of posaconazole in the endothelial compartment was relatively flat, with a slow decline over time as drug distributed in to the cellular bilayer and contiguous alveolar compartment. Posaconazole concentrations in the alveolar compartment steadily increased throughout the experimental period (Figure 1).
Figure 1.
Pharmacokinetics of posaconazole in the in vitro model of the human alveolus following the administration of drug to the endothelial compartment 6-hours post-inoculation of conidia. The top and bottom panels show the concentration-time profile in the endothelial and alveolar compartments, respectively.
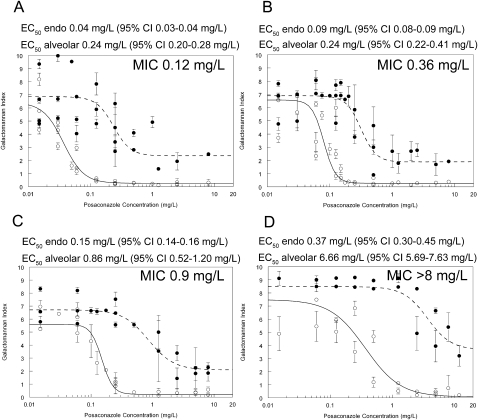
The exposure-response relationships for each strain are shown in Figure 2. Consistent with previous pharmacological studies in this model [11], the concentration required to reach near-maximal antifungal effect was higher in the alveolar compartment compared with the endothelial compartment. The MIC affected the shape of the exposure-response relationships, and the estimates and corresponding 95% confidence intervals for the E50: those strains with a higher MIC had higher E50. Furthermore, because the 95% confidence interval for these estimates did not overlap, these differences in E50 were statistically significant.
Figure 2.
Exposure-response relationships for posaconazole against various strains of Aspergillus fumigatus with differing MICs in the in vitro model of the human alveolus. The solid and broken lines are the exposure-response relationships in the endothelial and alveolar compartments, respectively. (A) GFP-producing strain; (B) F/14532; (C) F/16216; (D) F/11628
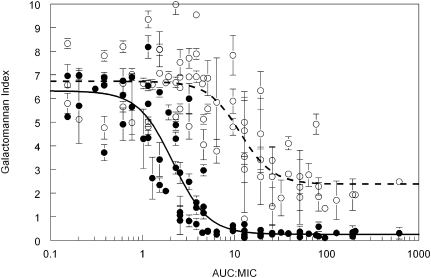
The MIC accounted for at least a portion of the observed variability for the exposure-response relationships between the various strains. For example, the estimates for E50 in the endothelial compartment varied 3.75-fold, but the E50/MIC only varied 0.67-fold (Figure 2). An AUC:MIC of ∼100 resulted in near maximal suppression of galactomannan concentrations in both the endothelial and alveolar compartments of the in vitro model (Figure 3).
Figure 3.
Relationship between the total posaconazole AUC:MIC in the endothelial compartment and the decline in galactomannan for the GFP-producing strain, F/14532 and F/16216. The solid black line is the fit of an inhibitory sigmoid Emax model to data from the endothelial compartment (solid circles) given by the equation: Galactomannan Index = 6.32−(6.06*(AUC:MIC)2.31/2.152.31 +(AUC:MIC)2.31; r2 = 85.2%. The broken line is the fit of the same model to data from the alveolar compartment (open circles) and is given by: Galactomannan Index = 6.73−(4.35*(AUC:MIC)2.48/11.552.48 +(AUC:MIC)2.48; r2 = 64.2%.
Murine Pharmacokinetics and Pharmacodynamics
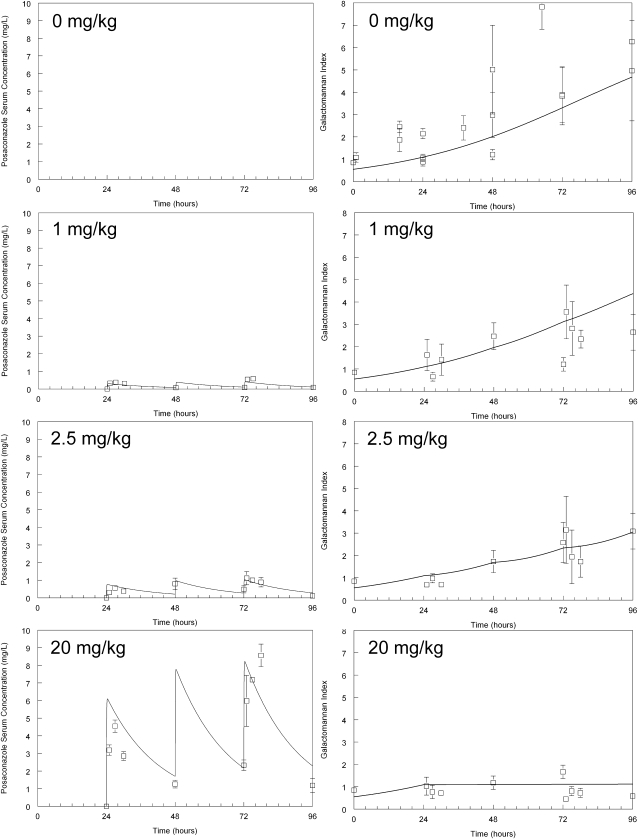
The murine pharmacokinetics for dosages of posaconazole used in this study were linear. Following oral administration, concentrations rapidly increased and then declined with a terminal half life of 13.87 h. The galactomannan concentrations progressively increased in the serum of untreated mice infected with Aspergillus. The model estimates for the baseline galactomannan in uninfected mice at the time of inoculation was 0.55 (t = 0), which then increased to 1.09 (t = 24) at the time of treatment initiation. The administration of posaconazole caused a dose-dependent decline in galactomannan concentrations. The serum concentration of posaconazole suppressed (but did not kill) the growth of Aspergillus (because a rise followed by a fall in galactomannan concentrations was not observed). Near-maximal effect (suppression) was observed with dosages of 20 mg/kg. The variance around the point estimates of the mean dramatically diminished with this dosage, further suggesting the attainment of near-maximal effect (Figure 4).
Figure 4.
Pharmacokinetics and pharmacodynamics of posaconazole in the inhalational murine model of invasive pulmonary aspergillosis. The raw data (mean ± SEM) and the fit of the mathematical model (derived from the entire dataset) to the data (solid line) is shown for each posaconazole regimen.
The fit of the mathematical model to the data was highly acceptable with a coefficient of determination for the observed- predicted values for the serum pharmacokinetics and pharmacodynamics after the Bayesian step of 0.95 and 0.72, respectively, with acceptable measures of precision and bias. The parameter means, medians, and standard deviations for the mathematical model are summarized in Table 1.
Table 1.
The Estimates for the Parameters from the Population Pharmacokinetic Model Fitted to the Murine Pharmacokinetic and Pharmacodynamic Data
| Parametera | Mean | Median | SDb |
| Ka, h−1 | 14.79 | 12.67 | 6.63 |
| Vc/F, L | 0.059 | 0.049 | 0.015 |
| SCL/F, L/h | 0.004 | 0.004 | 0.001 |
| Kcp, h−1 | 7.17 | 8.99 | 4.98 |
| Kpc, h−1 | 20.13 | 19.06 | 3.27 |
| Kgmax, GMI/h | 0.032 | 0.029 | 0.015 |
| C50g, mg/L | 0.598 | 0.382 | 0.344 |
| Hg | 2.592 | 2.382 | 1.309 |
| POPMAX, GMI | 7.29 | 7.61 | 1.07 |
| Initial Condition, GMI | 0.55 | 0.59 | 0.27 |
NOTE. aKa is the first-order rate constant that describes the flux of drug from the gut to the central compartment; Vc is the volume of the central compartment; F is the bioavailability; SCL is the clearance from the central compartment; Kcp and Kpc are the first order rate constants connecting the central and peripheral compartments; Kgmax is the growth constant for Aspergillus (measured in terms of the rate of release of galactomannan); C50g is the concentration of posaconazole that results in half-maximal suppression of growth; Hg is the slope; POPMAX is the maximum theoretical achievable galactomannan concentration and the initial condition is the baseline galactomannan reading prior to exposure.
bSD: standard deviation.
Relationship between AUC: MIC and the Anti-Aspergillus Effect
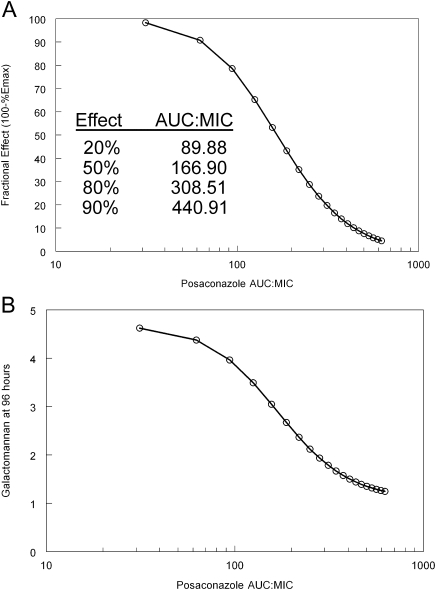
The relationship between the total drug AUC:MIC in the serum and the decline in galactomannan in mice is shown in Figure 5. Progressively higher drug exposures resulted in a progressively greater decline in galactomannan. An AUC:MIC of 89.88, 166.90, 308.51, and 440.91 resulted in 20%, 50%, 80%, and 90% of the maximal antifungal effect, respectively (Figure 5A). Progressively higher AUC:MIC values also resulted in a decrease in the concentration of galactomannan at the end of the experimental period (Figure 5B). Higher drug exposures only resulted in a relatively small additional decline in the biomarker.
Figure 5.
Relationship between the area under the concentration-time curve and the antifungal effect. These predictions have been derived from the mathematical model: (A) The relationship between AUC:MIC and the antifungal effect as measured by the proportion of Emax; (B) the relationship between the AUC:MIC and the galactomannan concentrations in mice at the end of the experiment
DISCUSSION
Invasive aspergillosis is a syndrome that is still associated with significant morbidity and mortality [1]. Posaconazole is a triazole with broad spectrum activity against Aspergillus spp. This compound is licensed for the prevention of invasive fungal infections and for the treatment of patients who are failing or intolerant to other licensed antifungal agents [6]. Little is known, however, about the use of posaconazole for the primary treatment of invasive aspergillosis. To our knowledge, this is the first study to use an inhalational laboratory animal model of invasive pulmonary aspergillosis combined with mathematical modeling to link drug exposure with the antifungal effect.
We used galactomannan as a biomarker with which to assess unrestrained fungal growth and the antifungal effect of posaconazole. This strategy is supported by numerous laboratory and clinical data that suggest this large molecular weight antigen is intricately tied to pathogenesis of invasive pulmonary aspergillosis [11]. Furthermore, galactomannan has prognostic value that allows the effect of antifungal therapy to be assessed and may potentially be a useful biomarker for assessing the response to antifungal therapy in clinical trials [5, 18, 19, 20]. However, one of its limitations for pharmacodynamic studies is its relatively limited dynamic range. This is further compounded by relatively large variability that is inherent to laboratory animal models of invasive pulmonary aspergillosis. Consequently, we did not attempt a classical dose fractionation study to explore the pharmacodynamics of posaconazole against A. fumigatus The extent of variability would likely have impeded the ability to resolve any differences in antifungal effect from various schedules of drug administration. Rather, we mathematically linked serum concentrations of posaconazole to the anti-Aspergillus activity as quantified by circulating galactomannan concentrations. To enable the clinical consequences of the experimental data to be further explored, we quantified drug exposure in terms of AUC:MIC.
A recent study by Mavridou et al [21] also examined the pharmacodynamics of posaconazole against Aspergillus fumigatus. There are a number of important distinctions between the 2 studies that warrant further discussion. First, we used an inhalational pulmonary model rather than a disseminated model; the former is a more faithful mimic of human pathogenesis and disease and may enable organ-specific idiosyncrasies in drug effect to be elucidated [3]; second, we used a biomarker (galactomannan) rather than survival as a measure of drug effect; it may be argued that the former represents a more direct measure of the drug-pathogen interaction than survival, which is affected by a multitude of factors that are extraneous to the antifungal activity of posaconazole. Additional studies and cross-validation of various experimental models and biomarkers for invasive pulmonary aspergillosis are still required.
The pharmacodynamic endpoint in experimental models that best predicts the outcome of patients with invasive pulmonary aspergillosis is not known (eg, E20, E50, E80, 50% survival, 100% survival, etc.). Estimates for the AUC0-24 for patients receiving posaconazole 800 mg/day are ∼13–17 mg.h/L [22, 23]. Therefore, the average patient infected with an Aspergillus strain with an MIC at the edge of the wild-type population (eg, 0.125 mg/L; defined as the mode plus 2 dilutions) and receiving 800 mg/day will achieve an AUC:MIC of ∼100–150. Mavridou et al [21] observed 50% survival in mice with an AUC:MIC 321.3 and near-maximal survival with an AUC:MIC of ∼1000. In our study, we observed 50% antifungal effect with an AUC:MIC of 166.9. Even the use of this lower value as a pharmacodynamic target implies that non–wild-type isolates with elevated MICs (eg, MIC 0.25–0.5 mg/L) may be difficult to treat in persistently neutropenic hosts with acute invasive pulmonary aspergillosis, and therefore may have implications for the identification of in vitro susceptibility breakpoints.
The findings of our study are qualified by the following: (1) we only used a single strain of A. fumigatus in the in vivo study; (2) a persistently neutropenic model was used; while this is the most rigorous test for antifungal effect, it is possible that lower drug exposures (AUC:MIC) may be adequate for non-neutropenic patients with invasive pulmonary aspergillosis and this deserves further study; (3) we made an explicit assumption that the rate and extent of trafficking of posaconazole from the bloodstream to the site where it exerts its antifungal effect is the same in mice and humans.
Despite these potential limitations, this study provides an experimental basis for the selection of posaconazole regimens suitable for the primary treatment of invasive pulmonary aspergillosis. This is especially pertinent given the development of new oral and intravenous formulations that may be associated with greater systemic drug exposure than is possible using the currently licensed compound. Furthermore, this study provides a paradigm by which many remaining clinically relevant issues for the treatment of patients with invasive pulmonary aspergillosis can be addressed.
Funding
This study was supported, in part, by Schering Plough, the Fungal Research Trust, and NIH contract N01-AI-30041. William Hope is supported by a National Institute of Health (NIHR) Clinician Scientist Fellowship.
Appendix
The murine pharmacokinetics of posaconazole and the antifungal effect induced by its administration to persistently neutropenic mice were described using the following four simultaneous inhomogeneous differential equations:
| (1) |
| (2) |
| (3) |
| (4a) |
| (4b) |
Equations (1), (2), and (3) describe the pharmacokinetics of posaconazole. Compartments 1, 2, and 3 represent the gut, central, and peripheral compartments respectively. X(1), X(2), X(3) represent the amount of the drug (in milligrams) in these respective compartments. Posaconazole was administered orally as a bolus (B(1)). In the gut, it equilibrates with the central compartment (compartment 2), which in turn equilibrates with the rest of the body (compartment 3). There was first-order elimination from the central compartment (SCL, liters/h), which has volume Vc (liters). K represents the various first-order inter-compartmental rate constants.
Equation (4) describes the rate of change of the galactomannan index in the serum. X(4) represents galactomannan index in the serum. The term 4a describes the capacity-limited growth of Aspergillus and contains the parameters Kgmax, and POPMAX, which represent the maximum growth rate constant and the maximum theoretical galactomannan concentration respectively. As galactomannan concentrations approaches POPMAX, the rate of growth slows and approaches zero. The term 4b in equation 4 provides a way of allowing posaconazole to exert a fungistatic as opposed to a fungicidal effect (ie, growth suppression rather than explicit killing). In the absence of drug, this term defaults to 1 and Aspergillus grows in an unrestrained manner. Following progressively higher concentrations of posaconazole, the growth rate of Aspergillus approaches 0. C50g is the concentration of posaconazole at which the effect on growth is half-maximal, and Hg is the associated slope function.
References
- 1.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–84. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW, Hope WW. Therapy for fungal diseases: opportunities and priorities. Trends Microbiol. 2010;18:195–204. doi: 10.1016/j.tim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Hope WW, Drusano GL. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin Microbiol Infect. 2009;15:602–12. doi: 10.1111/j.1469-0691.2009.02913.x. [DOI] [PubMed] [Google Scholar]
- 4.Torres HA, Hachem RY, Chemaly RF, Kontoyiannis DP, Raad II. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect Dis. 2005;5:775–85. doi: 10.1016/S1473-3099(05)70297-8. [DOI] [PubMed] [Google Scholar]
- 5.Petraitiene R, Petraitis V, Groll AH, et al. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob Agents Chemother. 2001;45:857–69. doi: 10.1128/AAC.45.3.857-869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 7.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 8.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 9.Howard SJ, Cerar D, Anderson MJ, et al. Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009;15:1068–76. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. 2009;9:789–95. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 11.Hope WW, Kruhlak MJ, Lyman CA, et al. Pathogenesis of Aspergillus fumigatus and the kinetics of galactomannan in an in vitro model of early invasive pulmonary aspergillosis: implications for antifungal therapy. J Infect Dis. 2007;195:455–66. doi: 10.1086/510535. [DOI] [PubMed] [Google Scholar]
- 12.Warn PA, Sharp A, Morrissey G, Denning DW. Activity of aminocandin (IP960; HMR3270) compared with amphotericin B, itraconazole, caspofungin and micafungin in neutropenic murine models of disseminated infection caused by itraconazole-susceptible and -resistant strains of Aspergillus fumigatus. Int J Antimicrob Agents. 2010;35:146–51. doi: 10.1016/j.ijantimicag.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Cuenca-Estrella M, Arendrup MC, Chryssanthou E, et al. Multicentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST) Clin Microbiol Infect. 2007;13:1018–22. doi: 10.1111/j.1469-0691.2007.01790.x. [DOI] [PubMed] [Google Scholar]
- 14.Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE, Jr, Ibrahim AS. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2004;48:1908–11. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource; 2009. [Google Scholar]
- 16.Leary R, Jelliffe R, Schumitzky A, van Guilder M. Proceedings, 14th IEEE Computer Society. Bethesda, MD: 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models; pp. 389–94. [Google Scholar]
- 17.Warn PA, Sharp A, Parmar A, Majithiya J, Denning DW, Hope WW. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob Agents Chemother. 2009;53:3453–61. doi: 10.1128/AAC.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo S, Bryar JM, Baden LR, Marty FM. Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J Clin Microbiol. 2010;48:1255–60. doi: 10.1128/JCM.02281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miceli MH, Grazziutti ML, Woods G, et al. Strong correlation between serum Aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin Infect Dis. 2008;46:1412–22. doi: 10.1086/528714. [DOI] [PubMed] [Google Scholar]
- 20.Maertens J, Buve K, Theunissen K, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer. 2009;115:355–62. doi: 10.1002/cncr.24022. [DOI] [PubMed] [Google Scholar]
- 21.Mavridou E, Bruggemann RJ, Melchers WJ, Mouton JW, Verweij PE. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob Agents Chemother. 2010;54:860–5. doi: 10.1128/AAC.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AbuTarif MA, Krishna G, Statkevich P. Population pharmacokinetics of posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Curr Med Res Opin. 2010;26:397–405. doi: 10.1185/03007990903485056. [DOI] [PubMed] [Google Scholar]
- 23.Ullmann AJ, Cornely OA, Burchardt A, et al. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother. 2006;50:658–66. doi: 10.1128/AAC.50.2.658-666.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]