Abstract
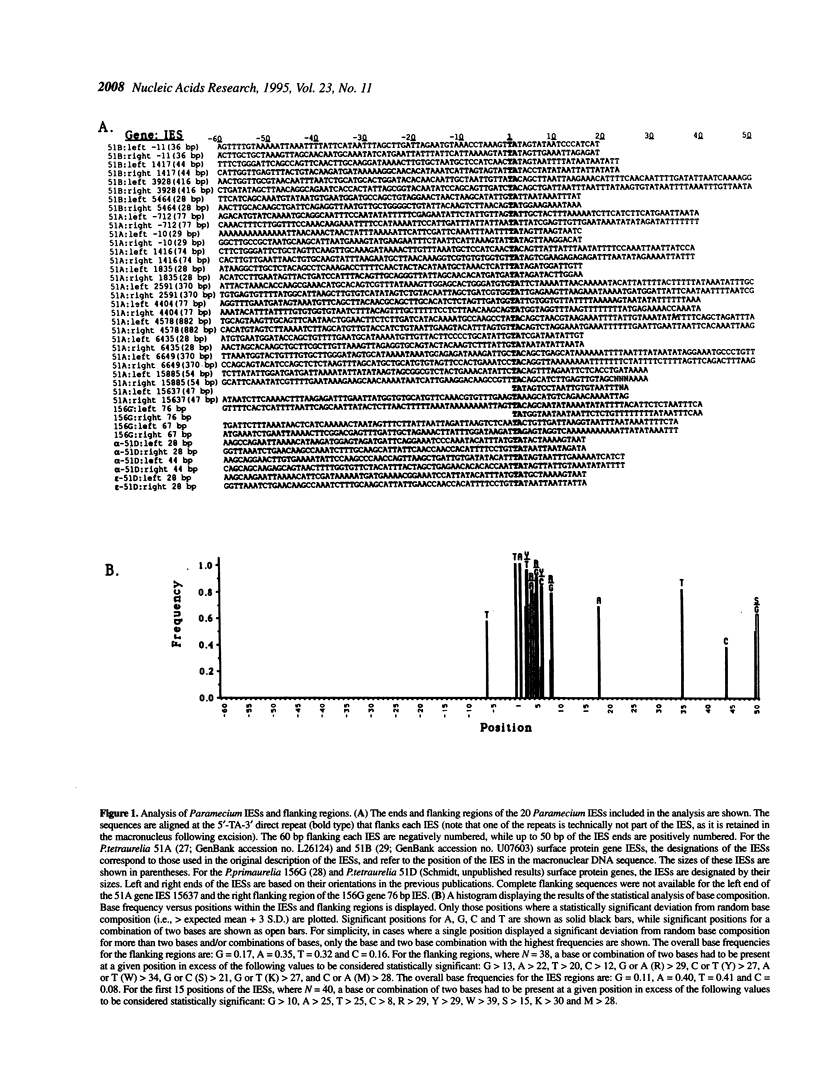
During the formation of a transcriptionally active macronucleus, ciliated protozoa excise large numbers of interstitial segments of DNA (internal eliminated sequences; IESs) from their chromosomes. In this study we analyze the published sequences of 20 IESs that interrupt surface protein genes of Paramecium and identify a consensus inverted terminal repeat. This sequence is similar to the ends of the Tc1-related transposons found in nematodes and other metazoans, as well as to both the ends of the Tec transposons and at least some of the IESs in the distantly related ciliate Euplotes crassus. The results of these analyses bolster previous proposals that IESs were created by transposition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L. Chromosome end formation and internal sequence elimination as alternative genomic rearrangements in the ciliate Paramecium. J Mol Biol. 1994 Feb 18;236(2):421–426. doi: 10.1006/jmbi.1994.1154. [DOI] [PubMed] [Google Scholar]
- Austerberry C. F., Yao M. C. Sequence structures of two developmentally regulated, alternative DNA deletion junctions in Tetrahymena thermophila. Mol Cell Biol. 1988 Sep;8(9):3947–3950. doi: 10.1128/mcb.8.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird S. E., Fino G. M., Tausta S. L., Klobutcher L. A. Micronuclear genome organization in Euplotes crassus: a transposonlike element is removed during macronuclear development. Mol Cell Biol. 1989 Sep;9(9):3793–3807. doi: 10.1128/mcb.9.9.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbaum P., Dönhoff T., Klein A. Macronuclear and micronuclear configurations of a gene encoding the protein synthesis elongation factor EF 1 alpha in Stylonychia lemnae. Mol Microbiol. 1991 Jun;5(6):1567–1575. doi: 10.1111/j.1365-2958.1991.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Collins J., Forbes E., Anderson P. The Tc3 family of transposable genetic elements in Caenorhabditis elegans. Genetics. 1989 Jan;121(1):47–55. doi: 10.1093/genetics/121.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doak T. G., Doerder F. P., Jahn C. L., Herrick G. A proposed superfamily of transposase genes: transposon-like elements in ciliated protozoa and a common "D35E" motif. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):942–946. doi: 10.1073/pnas.91.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus D. H. Evidence suggesting an evolutionary relationship between transposable elements and immune system recombination sequences. Mol Immunol. 1992 Jun;29(6):807–810. doi: 10.1016/0161-5890(92)90191-y. [DOI] [PubMed] [Google Scholar]
- Eder C., Maercker C., Meyer J., Lipps H. J. The processing of macronuclear DNA sequences during macronuclear development of the hypotrichous ciliate Stylonychia lemnae. Int J Dev Biol. 1993 Sep;37(3):473–477. [PubMed] [Google Scholar]
- Ghosh S., Jaraczewski J. W., Klobutcher L. A., Jahn C. L. Characterization of transcription initiation, translation initiation, and poly(A) addition sites in the gene-sized macronuclear DNA molecules of Euplotes. Nucleic Acids Res. 1994 Jan 25;22(2):214–221. doi: 10.1093/nar/22.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R., James C., Yao M. C. A distant 10-bp sequence specifies the boundaries of a programmed DNA deletion in Tetrahymena. Genes Dev. 1993 Dec;7(12A):2357–2365. doi: 10.1101/gad.7.12a.2357. [DOI] [PubMed] [Google Scholar]
- Godiska R., Yao M. C. A programmed site-specific DNA rearrangement in Tetrahymena thermophila requires flanking polypurine tracts. Cell. 1990 Jun 29;61(7):1237–1246. doi: 10.1016/0092-8674(90)90688-b. [DOI] [PubMed] [Google Scholar]
- Goodrich J. A., Schwartz M. L., McClure W. R. Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF). Nucleic Acids Res. 1990 Sep 11;18(17):4993–5000. doi: 10.1093/nar/18.17.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Lüthy R., Eisenberg D. Profile analysis. Methods Enzymol. 1990;183:146–159. doi: 10.1016/0076-6879(90)83011-w. [DOI] [PubMed] [Google Scholar]
- Heinonen T. Y., Pearlman R. E. A germ line-specific sequence element in an intron in Tetrahymena thermophila. J Biol Chem. 1994 Jul 1;269(26):17428–17433. [PubMed] [Google Scholar]
- Henikoff S. Detection of Caenorhabditis transposon homologs in diverse organisms. New Biol. 1992 Apr;4(4):382–388. [PubMed] [Google Scholar]
- Henikoff S. Playing with blocks: some pitfalls of forcing multiple alignments. New Biol. 1991 Dec;3(12):1148–1154. [PubMed] [Google Scholar]
- Herrick G., Cartinhour S. W., Williams K. R., Kotter K. P. Multiple sequence versions of the Oxytricha fallax 81-MAC alternate processing family. J Protozool. 1987 Nov;34(4):429–434. doi: 10.1111/j.1550-7408.1987.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Herrick G., Cartinhour S., Dawson D., Ang D., Sheets R., Lee A., Williams K. Mobile elements bounded by C4A4 telomeric repeats in Oxytricha fallax. Cell. 1985 Dec;43(3 Pt 2):759–768. doi: 10.1016/0092-8674(85)90249-1. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Doktor S. Z., Frels J. S., Jaraczewski J. W., Krikau M. F. Structures of the Euplotes crassus Tec1 and Tec2 elements: identification of putative transposase coding regions. Gene. 1993 Oct 29;133(1):71–78. doi: 10.1016/0378-1119(93)90226-s. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Krikau M. F., Shyman S. Developmentally coordinated en masse excision of a highly repetitive element in E. crassus. Cell. 1989 Dec 22;59(6):1009–1018. doi: 10.1016/0092-8674(89)90757-5. [DOI] [PubMed] [Google Scholar]
- Jaraczewski J. W., Jahn C. L. Elimination of Tec elements involves a novel excision process. Genes Dev. 1993 Jan;7(1):95–105. doi: 10.1101/gad.7.1.95. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Simon M. I. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell. 1985 Jul;41(3):781–791. doi: 10.1016/s0092-8674(85)80059-3. [DOI] [PubMed] [Google Scholar]
- Katoh M., Hirono M., Takemasa T., Kimura M., Watanabe Y. A micronucleus-specific sequence exists in the 5'-upstream region of calmodulin gene in Tetrahymena thermophila. Nucleic Acids Res. 1993 May 25;21(10):2409–2414. doi: 10.1093/nar/21.10.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A. Developmentally excised DNA sequences in Euplotes crassus capable of forming G quartets. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1979–1983. doi: 10.1073/pnas.92.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L. Developmentally controlled genomic rearrangements in ciliated protozoa. Curr Opin Genet Dev. 1991 Oct;1(3):397–403. doi: 10.1016/s0959-437x(05)80306-5. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Turner L. R., LaPlante J. Circular forms of developmentally excised DNA in Euplotes crassus have a heteroduplex junction. Genes Dev. 1993 Jan;7(1):84–94. doi: 10.1101/gad.7.1.84. [DOI] [PubMed] [Google Scholar]
- Krikau M. F., Jahn C. L. Tec2, a second transposon-like element demonstrating developmentally programmed excision in Euplotes crassus. Mol Cell Biol. 1991 Sep;11(9):4751–4759. doi: 10.1128/mcb.11.9.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitcham J. L., Lynn A. J., Prescott D. M. Analysis of a scrambled gene: the gene encoding alpha-telomere-binding protein in Oxytricha nova. Genes Dev. 1992 May;6(5):788–800. doi: 10.1101/gad.6.5.788. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The DNA of ciliated protozoa. Microbiol Rev. 1994 Jun;58(2):233–267. doi: 10.1128/mr.58.2.233-267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice A. D., Bugaj B., Fitch D. H., Emmons S. W. Widespread occurrence of the Tc1 transposon family: Tc1-like transposons from teleost fish. Mol Gen Genet. 1994 Sep 28;244(6):606–612. doi: 10.1007/BF00282750. [DOI] [PubMed] [Google Scholar]
- Ribas-Aparicio R. M., Sparkowski J. J., Proulx A. E., Mitchell J. D., Klobutcher L. A. Nucleic acid splicing events occur frequently during macronuclear development in the protozoan Oxytricha nova and involve the elimination of unique DNA. Genes Dev. 1987 Jun;1(4):323–336. doi: 10.1101/gad.1.4.323. [DOI] [PubMed] [Google Scholar]
- Robertson H. M. The mariner transposable element is widespread in insects. Nature. 1993 Mar 18;362(6417):241–245. doi: 10.1038/362241a0. [DOI] [PubMed] [Google Scholar]
- Scott J., Leeck C., Forney J. Analysis of the micronuclear B type surface protein gene in Paramecium tetraurelia. Nucleic Acids Res. 1994 Nov 25;22(23):5079–5084. doi: 10.1093/nar/22.23.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. J., Barkocy-Gallagher G. A., Preer L. B., Preer J. R., Jr Developmentally excised sequences in micronuclear DNA of Paramecium. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2255–2259. doi: 10.1073/pnas.91.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus A., Spencer D. F., Zuker M., Logsdon J. M., Jr, Doolittle W. F. Testing the exon theory of genes: the evidence from protein structure. Science. 1994 Jul 8;265(5169):202–207. doi: 10.1126/science.8023140. [DOI] [PubMed] [Google Scholar]
- Tausta S. L., Turner L. R., Buckley L. K., Klobutcher L. A. High fidelity developmental excision of Tec1 transposons and internal eliminated sequences in Euplotes crassus. Nucleic Acids Res. 1991 Jun 25;19(12):3229–3236. doi: 10.1093/nar/19.12.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. M., Ellingson J. L., Catt D. M., Berger P. J., Karrer K. M. A small family of elements with long inverted repeats is located near sites of developmentally regulated DNA rearrangement in Tetrahymena thermophila. Mol Cell Biol. 1994 Sep;14(9):5939–5949. doi: 10.1128/mcb.14.9.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Doak T. G., Herrick G. Developmental precise excision of Oxytricha trifallax telomere-bearing elements and formation of circles closed by a copy of the flanking target duplication. EMBO J. 1993 Dec;12(12):4593–4601. doi: 10.1002/j.1460-2075.1993.tb06148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luenen H. G., Colloms S. D., Plasterk R. H. The mechanism of transposition of Tc3 in C. elegans. Cell. 1994 Oct 21;79(2):293–301. doi: 10.1016/0092-8674(94)90198-8. [DOI] [PubMed] [Google Scholar]


