Abstract
Vascular disease, a significant cause of morbidity and mortality in the developed world, results from vascular injury. Following vascular injury, damaged or dysfunctional endothelial cells and activated SMCs engage in vasoproliferative remodeling and the formation of flow-limiting intimal hyperplasia (IH). We hypothesized that vascular injury results in decreased bioavailability of NO secondary to dysregulated arginine-dependent NO generation. Furthermore, we postulated that nitrite-dependent NO generation is augmented as an adaptive response to limit vascular injury/proliferation and can be harnessed for its protective effects. Here we report that sodium nitrite (intraperitoneal, inhaled, or oral) limited the development of IH in a rat model of vascular injury. Additionally, nitrite led to the generation of NO in vessels and SMCs, as well as limited SMC proliferation via p21Waf1/Cip1 signaling. These data demonstrate that IH is associated with increased arginase-1 levels, which leads to decreased NO production and bioavailability. Vascular injury also was associated with increased levels of xanthine oxidoreductase (XOR), a known nitrite reductase. Chronic inhibition of XOR and a diet deficient in nitrate/nitrite each exacerbated vascular injury. Moreover, established IH was reversed by dietary supplementation of nitrite. The vasoprotective effects of nitrite were counteracted by inhibition of XOR. These data illustrate the importance of nitrite-generated NO as an endogenous adaptive response and as a pathway that can be harnessed for therapeutic benefit.
Introduction
Vascular disease contributes significantly to morbidity and mortality in the developed world (1). Current treatments for this disease process, including surgical bypass and percutaneous interventions, are limited by the formation of intimal hyperplasia (IH) and restenosis (2). IH is an exaggerated healing process initiated by injury and characterized by platelet aggregation, leukocyte chemotaxis, extracellular matrix changes, endothelial cell apoptosis, and vascular SMC proliferation and migration (3). Investigations into vascular biology have led to the association of vascular pathology with decreased bioavailability of NO. NO is endogenously formed in the vascular endothelium by NOS using l-arginine as a substrate (4). The decreased bioavailability may occur secondary to increased consumption of NO by reactive oxygen species within the injured vessel wall or impaired synthesis of NO, possibly via decreased endothelial NO synthase, eNOS uncoupling, or dysregulation of l-arginine metabolism.
l-Arginine is an important substrate for both NOS and arginase-1 enzymes, and increased arginase activity can deplete substrate availability for NO production. Interestingly, arginase-1 produces l-ornithine and activates the ornithine decarboxylase pathway that produces polyamines. These polyamines and arginase have been shown to increase vascular SMC hyperplasia and migration in vitro (5, 6). In addition, because system y+, the principal cationic amino acid transport system in NO-producing cells, has similar Km values for arginine and ornithine, these amino acids can compete for cellular uptake (7). A decreased arginine to ornithine ratio resulting from increased arginase activity may also contribute to impaired arginine bioavailability for NOS. Furthermore, it has been shown that increased arginase expression promotes neointima formation within injured vessels (8).
Further investigation into NO led to the discovery of multiple vasoprotective characteristics, including vasorelaxation, inhibition of endothelial cell apoptosis, inhibition of platelet aggregation and adhesion, inhibition of leukocyte chemotaxis, and inhibition of SMC proliferation and migration (3). These properties have led to investigations to develop therapies that enhance NO signaling. NO-based therapeutics under investigation include dietary l-arginine (9–13), drug-eluting stents (14), inhalational NO gas (15, 16), and NOS gene therapy (17, 18). Unfortunately gene therapy remains limited by vectors, delivery systems, and mutational concerns. Inhalational NO gas is primarily utilized in the intensive care unit setting and has a narrow therapeutic window in terms of dose and exposure. In addition, it is limited by systemic toxicity, as hemodynamic instability can occur when its use is interrupted (19, 20). Local delivery of pharmacological donors requires surgical manipulation and may contribute to vascular injury prior to initiation of therapy. In addition, clinical studies in oral l-arginine therapy in vascular disease have led to controversial results, and the three long-term studies (10–12) generated conflicting data.
Nitrate (NO3–) and nitrite (NO2–), once thought to be waste products of NO metabolism, are now known to contribute substantially to NO biology by functioning as an endocrine reservoir capable of being reduced to NO within hypoxic, ischemic, or injured tissues (21, 22). Nitrite is reduced to bioactive NO along an oxygen and physiological pH gradient by a variety of mechanisms, including enzymatic reduction by deoxyhemoglobin and deoxymyoglobin, components of the electron transport system, and xanthine oxidoreductase (XOR) (21, 23–29). Nitrite has been shown to limit ischemia/reperfusion-induced (I/R-induced) apoptosis and cytotoxicity in heart, liver, and brain (30, 30–33). Furthermore, nitrite has demonstrated the ability to mediate hypoxic pulmonary vasodilatation in a deoxyhemoglobin- and pH-dependent fashion (34).
Nitrite forms from the reduction of dietary nitrate by oral bacterial flora and is potentially the cardioprotective agent in the nitrate-rich Mediterranean diet (35). The cytoprotective effects of nitrite in the heart and liver are measurable at nitrite doses of less than 1.2 nmol in murine models of myocardial infarction and hepatic IR (33). These doses increase plasma nitrite levels by less than 10% and are consistent with the increases observed after the ingestion of a standard leafy green salad (35) or after moderate exercise (36). Furthermore, mice with diminished basal plasma nitrite concentrations are more susceptible to IR injury, an effect that is attenuated by administration of exogenous nitrite (37). These data suggest that a diet rich in nitrate and nitrite may have profound cytoprotective effects and could constitute the “active” ingredient of the cardioprotective Mediterranean diet. In addition, humans given dietary nitrate supplementation for 3 days demonstrated a significant decrease in blood pressure, suggesting the feasibility and efficacy of a dietary supplement as a potential therapy (38).
Given the multiple vasoprotective characteristics of NO combined with the known effects of nitrite, we hypothesize that (a) vascular injury results in dysregulated arginine/NOS signaling within the vascular wall and the nitrite/XOR pathway serves as an adaptive response to produce vasoprotective NO in this setting; and that (b) nitrite supplementation protects against or reverses vascular injury via XOR.
Results
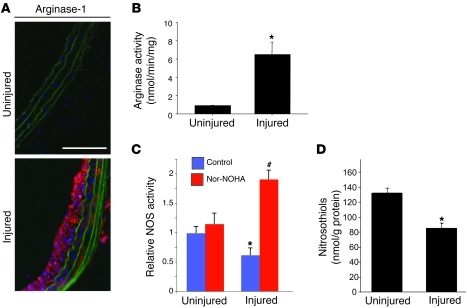
Vascular injury increased arginase-1 and decreased NO production.
Vascular injury results in neointimal hyperplasia, which is marked by uncontrolled SMC proliferation. We hypothesize that neointimal hyperplasia is potentiated secondary to limited availability of the NO synthase substrate arginine, leading to diminished production of vasoprotective NO. The influence of vascular injury on levels and activity of the enzyme arginase-1, which competes with NOS enzymes for arginine, was determined. Both injured and contralateral uninjured carotid arteries were harvested from rats 1 week after balloon injury, and arginase-1 levels and activity were determined. Vascular injury resulted in increased levels of arginase-1 within the vessel wall as determined by immunohistochemistry and increased arginase activity (Figure 1, A and B). NOS activity was measured in homogenates from freshly harvested control or 7-day post-injury carotids. In initial assays NOS activity in tissue homogenates from uninjured vessels appeared to be significantly greater compared with that in homogenates from injured vessels (Figure 1C). This decreased NOS activity was found despite increased NOS mRNA and protein levels (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI44079DS1). Because arginase can interfere with accurate determinations of NOS activity, the experiments were also performed in the presence of the arginase inhibitor N-hydroxy-nor-l-arginine (nor-NOHA; 100 μM). Inhibition of arginase resulted in significantly increased NOS activity from the homogenates of injured vessels. We also determined the influence of vascular injury and IH on NO bioavailability by measuring S-nitrosothiol levels within the vessel wall. Relative S-nitrosothiol levels were decreased in injured vessels compared with control uninjured vessels 4 weeks after injury (Figure 1D). Together these results suggest that following vascular injury, arginase limits that availability of arginine as a NOS substrate and decreases NO production. This decreased production of NO occurs despite increased NOS enzyme levels.
Figure 1. Vascular injury increases arginase-1 and decreases NO production.
(A) Arginase-1 immunohistochemistry (red) within uninjured control vessels and increased expression within vessels 7 days after injury. Scale bar: 100 μm. (B) Arginase activity is increased 7 days after vascular injury compared with uninjured vessels (*P < 0.05; results are mean ± SEM of 2 separate carotid lysates, each lysate pooled from 2 rats). (C) Ex vivo NOS activity is decreased in vessels 7 days after injury compared with uninjured vessels (*P < 0.05); however, the arginase inhibitor nor-NOHA increases NOS activity in injured vessels (#P < 0.01; 4 vessels per condition, each measured in duplicate). (D) Vascular injury results in decreased S-nitrosothiol–modified protein concentrations within the vessel wall (*P < 0.05).
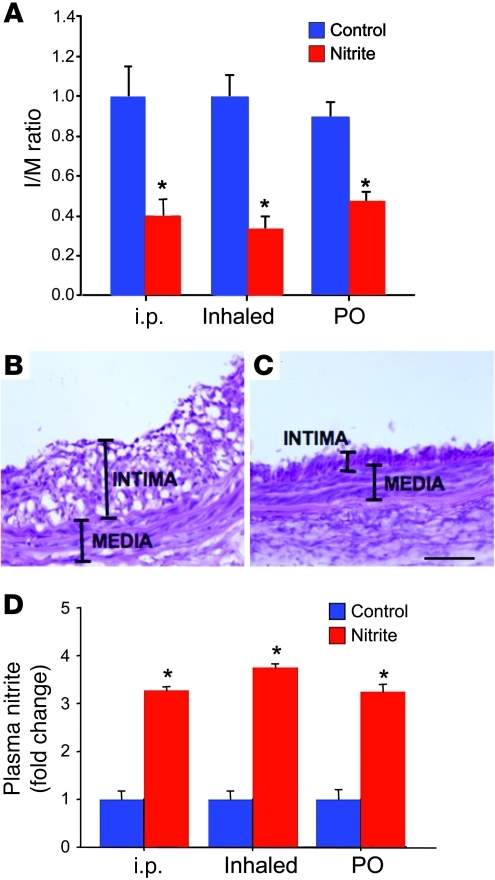
Sodium nitrite protects against the formation of IH.
The ability of sodium nitrite to protect against the formation of IH prior to vascular injury was determined. Rats were treated with sodium nitrite via a single intraperitoneal injection or inhalation of the nebulized form, or 24 hours of oral supplementation prior to carotid artery injury. Control rats were treated with the same volume of vehicle via the same delivery strategy. IH was determined 14 days after balloon injury. Sodium nitrite treatment via each delivery method (intraperitoneal injection, inhaled nebulization, or oral supplementation) decreased IH compared with controls (Figure 2, A–C). Intraperitoneal injection decreased the intima to media (I/M) ratio by 60% ± 9% compared with controls (P < 0.01, n = 8 rats per group). Nebulized sodium nitrite decreased IH by 77% ± 7% compared with controls (P < 0.01, n = 8 rats per group). Finally, oral supplementation resulted in a 47% ± 5% reduction versus controls (P < 0.01, n = 6 rats per group). Furthermore, increased serum nitrite levels were confirmed in the rats following delivery by each strategy (Figure 2D). With each method of delivery, these increases in serum nitrite were relatively low and within levels that can be achieved under varied physiological conditions (22, 35). These data suggest that sodium nitrite can be utilized as an effective therapy to limit IH.
Figure 2. Sodium nitrite limits the formation of IH following vascular injury.
(A) Intraperitoneal injection of sodium nitrite decreased the I/M ratio by 60% ± 9% compared with controls (n = 6/group; *P < 0.01). Nebulized sodium nitrite decreased IH by 77% ± 7% compared with controls (n = 8/group). Oral supplementation of sodium nitrite resulted in a 47% ± 5% reduction versus controls (n = 6/group). *P < 0.01. (B and C) H&E staining of representative carotid arteries. (B) Control injured artery; original magnification, ×40. (C) Artery pretreated with oral nitrite supplementation prior to injury; original magnification, ×40. Scale bar: 50 μm. (D) Serum nitrite levels were increased 3.5- to 4-fold in the rats following delivery by all 3 strategies (n = 6/group; *P < 0.01).
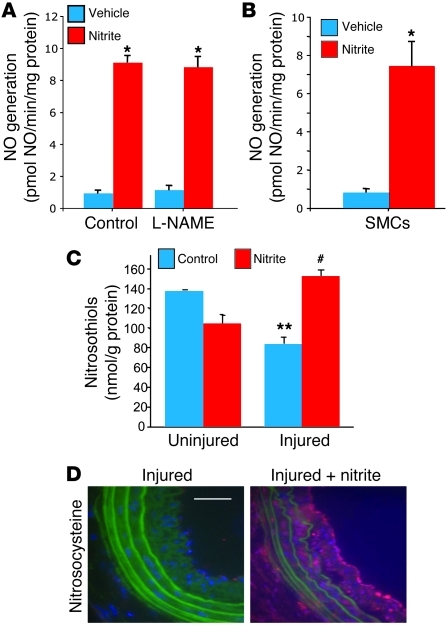
Sodium nitrite increases the generation of NO within the vessel wall and in cultured SMCs.
To determine whether sodium nitrite treatment leads to the production of biologically active NO, we added sodium nitrite (250 μM) to freshly harvested carotid artery homogenates and measured NO generation via a NO analyzer. Nitrite significantly increased NO generation (9.1 ± 0.44 pmol NO/min/mg protein) compared with vehicle (0.9 ± 0.2 pmol NO/min/mg protein; P < 0.01). Furthermore, this increase in NO was not influenced by the NOS inhibitor N-nitro-l-arginine methyl ester (L-NAME, 1 mM; Figure 3A). Control vessels without the addition of nitrite demonstrated minimal changes in NO production. These data suggest that sodium nitrite is reduced to biologically active NO within the vessel wall in a NOS-independent fashion. Similarly, primary SMCs in vitro also generated NO from sodium nitrite. Nitrite (100 μM) was added to rat aortic SMCs, and NO generation was determined. NO generation was significantly higher in nitrite-exposed SMCs (7.2 ± 1.4 pmol NO/min/mg protein) compared with vehicle-treated SMCs (0.87 ± 0.14 pmol NO/min/mg protein; P < 0.01) (Figure 3B). These data demonstrate that the primary SMCs can reduce nitrite to biologically active NO.
Figure 3. Sodium nitrite increases NO generation.
(A) NO generation from injured carotid arteries ex vivo demonstrated a significant increase with the addition of sodium nitrite (9.1 ± 0.44 pmol NO/min/mg protein) compared with vehicle-exposed vessels (0.9 ± 0.2 pmol NO/min/mg protein; *P < 0.01) even in the presence of the NOS inhibitor L-NAME. n = 4 arteries per group, each measured in triplicate. (B) NO generation from cultured SMCs demonstrated a significant increase with the addition of sodium nitrite (7.2 ± 1.4 pmol NO/min/mg protein) compared with vehicle (0.87 ± 0.14 pmol NO/min/mg protein; *P < 0.01). The results are the mean ± SEM for 3 independent experiments, with experiments performed in triplicate for each condition. (C) S-nitrosothiol–modified protein concentration in carotid arteries 4 weeks after injury demonstrated decreased S-nitrosothiol concentration compared with uninjured vessels (**P < 0.05). Oral sodium nitrite supplementation during days 15–28 increased S-nitrosothiol content in injured vessels (#P < 0.05 compared with non-nitrite injured vessels). n = 4 vessels per group. (D) Immunohistochemistry for S-nitrosocysteine (red) demonstrated minimal expression within the injured vessels of control rats versus the injured vessels of rats supplemented with nitrite. Scale bar: 50 μm.
As a “signature” of NO biological chemistry, S-nitrosothiol–modified protein concentrations were determined in vessel homogenates from uninjured and injured carotid arteries of rats that received no additional treatment or rats that received oral sodium nitrite on days 15–28. S-nitrosothiol measurements demonstrated that control, injured vessels had decreased S-nitrosothiol concentration compared with uninjured vessels, and sodium nitrite treatment led to significantly increased S-nitrosothiol concentrations in injured vessels (Figure 3C). Similar results were illustrated with delivery of intraperitoneal inhalation of the nebulized form of sodium nitrite (Supplemental Figure 3). Relative S-nitrosocysteine levels were also determined in untreated or sodium nitrite–treated injured vessels by immunohistochemistry (Figure 3D). Sodium nitrite similarly increased NO generation in human arterial segments (Supplemental Figure 5).
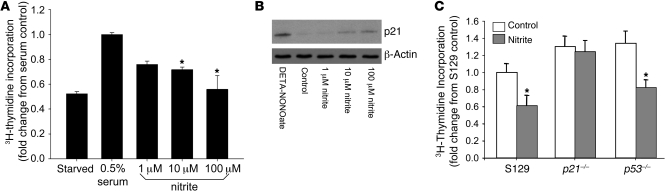
Sodium nitrite inhibits SMC proliferation.
Based upon the ability of sodium nitrite to inhibit the formation of IH and the known antiproliferative effects of NO on SMCs, the influence of sodium nitrite on SMC proliferation was investigated. Cells were incubated with sodium nitrite (0–100 μM), and proliferation was determined by measuring 3H-thymidine incorporation after 24 hours. Of note, baseline nitrite levels in newly filtered sterile media were determined and were 131 ± 31 nM. Nitrite decreased SMC proliferation in a dose-dependent fashion (P < 0.01) (Figure 4A). These effects of nitrite were not diminished by NOS inhibition (data not shown). These findings are consistent with the effects of NO donors or NOS enzyme overexpression in SMCs (39). Thus, sodium nitrite’s protection against the development of IH may be mediated in part through the inhibition of SMC proliferation.
Figure 4. Sodium nitrite inhibited SMC proliferation and was dependent upon p21Waf1/Cip1.
(A) Proliferation detected from 3H-thymidine in cultured SMCs demonstrated inhibition with sodium nitrite in a dose-dependent fashion (*P < 0.01). The results are the mean ± SEM of 4 independent experiments, with experiments performed in triplicate for each condition. (B) Western blot analysis demonstrated increased expression of p21 in the presence of the NO donor DETA-NONOate (50 μM) and sodium nitrite (0–100 μM). Blot is representative of 3 independent experiments. (C) Sodium nitrite inhibited wild-type and rapidly proliferating p53-knockout mouse SMC proliferation (*P < 0.05). However, sodium nitrite did not inhibit p21-knockout mouse SMC proliferation. The results are the mean ± SEM of 3 independent experiments, with experiments performed in triplicate for each condition.
Inhibition of SMC proliferation is dependent on the cyclin-dependent kinase inhibitor p21Waf1/Cip1.
Others and we have previously illustrated (40–42) that NO-induced inhibition of proliferation in SMCs is dependent on the upregulation of the cyclin-dependent kinase inhibitor p21Waf1/Cip1. In order to determine whether sodium nitrite increases p21 expression, we treated SMCs with or without sodium nitrite (0–100 μM) or DETA-NONOate as a positive control (50 μM) and determined p21 protein levels by Western blotting after 24 hours. Sodium nitrite increased p21 protein levels in a dose-dependent fashion (Figure 4B). The dependence of sodium nitrite on p21 as a signaling mechanism downstream of NO production to inhibit proliferation was studied using primary mouse aortic SMCs from wild-type (S129) or p21-knockout (Cdkn1–/–) mice. Sodium nitrite inhibited wild-type but not p21-knockout SMC proliferation (Figure 4C). Because SMCs from p21-knockout mice demonstrate an increased proliferative rate compared with wild-type cells, the influence of sodium nitrite on SMCs harvested from p53-knockout mice, which also demonstrate an increased basal proliferative rate compared with wild-type cells, was determined. Nitrite exposure resulted in a comparable and significant decrease in proliferation in p53–/– cells and wild-type cells.
The protective effects of sodium nitrite are dependent on XOR.
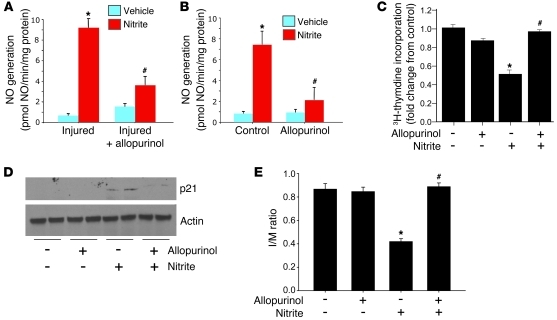
The mechanism of reduction of nitrite to biologically active NO was next investigated. Studies have identified multiple enzymes that possess nitrite reductase activity, including XOR. This potential nitrite reductase has been suggested to be one of the predominant reductases within the vasculature (43). Based upon this knowledge, the role of XOR in mediating the effects of sodium nitrite was investigated. NO generation was measured in both homogenates from carotid arteries 1 week after vascular injury and cultured SMCs with and without nitrite or the XOR inhibitor allopurinol. Allopurinol significantly decreased NO generation in both carotid artery homogenates and SMCs (Figure 5, A and B). To determine whether XOR activity was necessary for the effects of nitrite on SMC proliferation and p21 expression, we treated SMCs with sodium nitrite with and without allopurinol. As shown previously, sodium nitrite inhibited SMC proliferation and increased p21 protein levels. The addition of allopurinol, however, inhibited these effects (Figure 5, C and D).
Figure 5. Sodium nitrite–induced NO generation, inhibition of SMC proliferation, and p21 induction are dependent on XOR.
Allopurinol (100 μM) inhibited nitrite-induced (250 μM) NO generation within ex vivo carotid arteries (A; n = 4 independent arteries, each condition measured in triplicate; *P < 0.01 compared with vehicle, #P < 0.05 compared with nitrite-treated, injured vessels) and cultured SMCs (B; each condition measured in triplicate in 3 independent experiments; *P < 0.01 compared with vehicle, control SMCs, #P < 0.05 compared with nitrite-treated, control SMCs). (C) Allopurinol prevented nitrite-induced inhibition of SMC proliferation (*P < 0.01 compared with non-nitrite controls; #P < 0.05 compared with nitrite-treated SMCs). The results are the mean ± SEM of 3 independent experiments, with experiments performed in triplicate for each condition. (D) Western blot analysis demonstrated increased p21 protein levels following nitrite treatment, an effect that was inhibited by the addition of allopurinol (representative blot of 3 independent experiments). (E) Protection against IH formation by oral sodium nitrite pretreatment was inhibited in the presence of allopurinol (100 μM/kg/d; 48 hours prior to injury and 24 hours after injury) (n = 6/group; *P < 0.01 compared with non-nitrite-treated rats; #P < 0.01 compared with nitrite-treated rats). Brief allopurinol treatment alone had no effect on IH in non-nitrite-treated, injured vessels.
Inhibition of IH by sodium nitrite is dependent on XOR activity.
The role of XOR as a nitrite reductase that mediates the protective effects of sodium nitrite on IH was investigated. Rats were treated with allopurinol for 48 hours before and 24 hours after carotid artery injury. As above, sodium nitrite was delivered in the drinking water for 24 hours prior to carotid injury. As expected, oral sodium nitrite prevented IH (P < 0.01 versus injured controls); however, allopurinol reversed the protective effects of nitrite (Figure 5E; P < 0.001 versus injured nitrite-treated rats; n = 6/group). Allopurinol alone had no significant effect on IH. Alternatively, studies utilizing a single delivery of sodium nitrite by oral gavage dosing had similar effects (Supplemental Figure 2).
Vascular injury results in increased XOR.
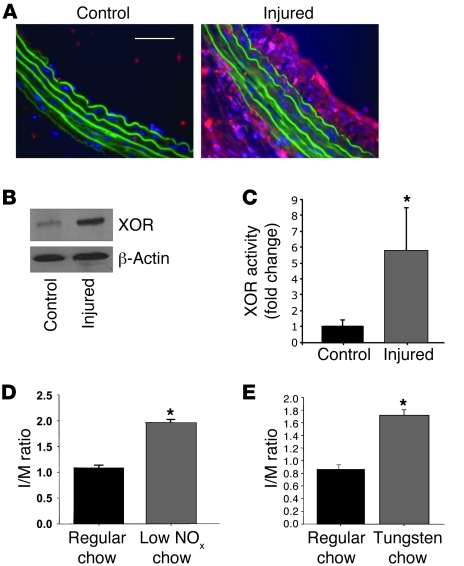
Because XOR acts as a potent and relevant nitrite reductase within the vascular wall and reverses the protective effects of sodium nitrite, the influence of vascular injury on the expression and activity of XOR was determined. Both immunohistochemistry and Western blot analysis demonstrated that XOR expression was increased within injured vessels compared with uninjured vessels (Figure 6, A and B). Additionally, XOR enzymatic activity was increased within injured vessels versus uninjured vessels (Figure 6C). XOR may be upregulated as a compensatory vascular response to increase NO production within the vasculature independent of arginine/NOS signaling. In order to investigate the role this pathway as an adaptive response to limit vascular injury, we fed and maintained rats on a diet with minimal nitrates/nitrites (NOx– diet). Compared with rats fed standard chow, those maintained on the NOx– diet demonstrated exacerbated IH (I/M ratios of 1.08 ± 0.04 vs. 1.97 ± 0.16; P < 0.01, n = 6 rats per group; Figure 6D). In an additional experiment, rats were fed a tungsten-supplemented diet, which has previously been shown (43) to inhibit XOR activity compared with that in control animals on standard chow. This diet was initiated 3 weeks prior to vascular injury and maintained throughout the duration of the experiment. The inhibition of XOR activity by dietary tungsten supplementation was confirmed (data not shown). Arteries were harvested 2 weeks after injury, and the formation of IH was determined. Rats fed a tungsten-rich diet had a more pronounced vascular injury compared with rats fed standard chow (I/M ratios of 0.86 ± 0.07 vs. 1.72 ± 0.10; P < 0.01, n = 6 rats per group; Figure 6E). These data suggest that dietary nitrates/nitrites, as well as XOR, act as part of an endogenous protective response to limit IH following vascular injury.
Figure 6. Arterial injury increased XOR expression and activity.
(A) XOR immunohistochemistry (red) within uninjured control vessels and injured vessels. Scale bar: 50 μm. (B) Western blot analysis demonstrated increased XOR within injured versus uninjured vessels (representative results of 4 independent experiments). (C) XOR activity is increased within injured versus uninjured control vessels (*P < 0.05; n = 7–8 vessels per group). (D) A NOx– diet resulted in significantly greater I/M ratios compared with rats kept on regular chow (n = 6/group, *P < 0.01). (E) A tungsten-rich diet, which inhibits XOR activity, resulted in significantly increased I/M compared with standard chow (n = 6/group, *P < 0.01).
Sodium nitrite reverses established IH.
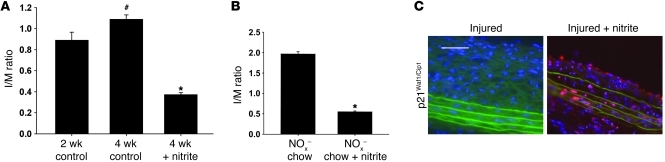
Because vascular injury results in increased XOR activity, we hypothesized that sodium nitrite could be utilized as a therapeutic to reverse established IH. One group of rats was sacrificed 2 weeks after injury to quantify I/M ratios that were present at the time sodium nitrite therapy was initiated. A second group of rats was housed an additional 2 weeks prior to sacrifice to quantify IH at the 4-week time point. A final group of rats received drinking water supplemented with sodium nitrite starting 2 weeks after injury, and IH was determined 4 weeks after injury. Average daily sodium nitrite delivery was estimated to be 9.6 μmol/kg/d based upon assumed average water intake. Nitrite-treated rats had a 66% ± 6% decrease in I/M ratio compared with 4-week injured controls (P < 0.01, n = 6/group) and a 59% ± 4% reduction compared with 2-week injured controls (P < 0.01, n = 6/group) (Figure 7A). Furthermore, supplemental oral sodium nitrite also reversed IH in rats maintained on a NOx– diet. Rats were fed a NOx– diet, and carotid injury was induced. All rats were maintained on this chow throughout the experiment. At 2 weeks after injury, rats were randomized to receive water supplemented with sodium nitrite or continue on de-ionized water, and all animals were sacrificed 4 weeks after vascular injury. Nitrite-treated rats on the NOx– diet had a 72% ± 4% reduction in IH formation compared with non-nitrite-rescued rats (P < 0.01, n = 6 rats per group) (Figure 7B). Sodium nitrite delivered by alternative dosing strategies was also able to reverse IH (Supplemental Figure 2). Sodium nitrite supplementation effectively reversed IH in a mouse carotid injury model (Supplemental Figure 4). Additionally, p21Waf1/Cip1 expression within the neointima was increased in vessels from sodium nitrite–treated rats compared with injured vessels from control rats (Figure 7C). These data demonstrate the ability of nitrite to reverse established IH and contribute to negative remodeling within the injured vasculature.
Figure 7. Sodium nitrite reversed established IH following vascular injury.
(A) IH continues to progress from 2 to 4 weeks after injury (#P < 0.05). Sodium nitrite treatment on days 15–28 after vascular injury decreased I/M ratios by 66% ± 6% compared with untreated injured controls as measured 4 weeks after injury (*P < 0.01, n = 6/group). (B) Rats kept on a NOx– diet, which had an exaggerated injury response, were also rescued by sodium nitrite supplementation in drinking water from days 15 to 28 after injury. Sodium nitrite resulted in a 72% ± 8% reduction in I/M ratios compared with rats kept on a NOx– diet without nitrite supplementation (*P < 0.01, n = 6/group). (C) Immunohistochemistry for p21 (red) demonstrated minimal expression within injured vessels versus nitrite-treated injured vessels. Scale bar: 50 μm.
Discussion
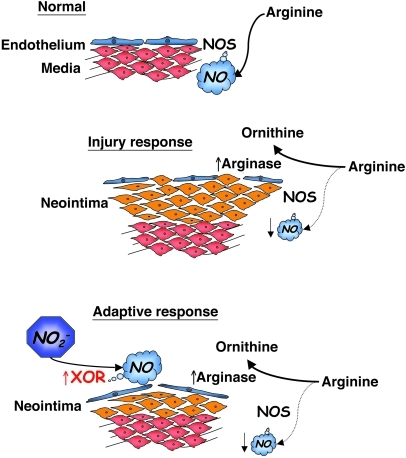
This study demonstrates that vascular injury is associated with increased arginase-1 within the vascular wall, likely resulting in decreased NOS activity and NO production. Low-dose sodium nitrite prevents or reverses vascular IH in a rat carotid injury model. Furthermore, sodium nitrite is metabolized to biologically active NO by XOR to limit SMC proliferation via a p21Waf1/Cip1–dependent mechanism. Rats fed a diet deficient in nitrate/nitrite or a tungsten-rich diet that inhibits XOR activity demonstrated significantly more severe IH. Moreover, XOR is increased in the vessel wall following injury, suggesting that nitrite/XOR is an adaptive signaling response to increase vasoprotective NO in an arginine/NOS-independent fashion. A schema for this is presented in Figure 8.
Figure 8. Schematic representation of vascular injury–induced arginase and XOR and generation of NOS-independent NO.
Under normal conditions, eNOS utilizes l-arginine to produce vasoregulatory NO. Following vascular injury, an increase in arginase competes for l-arginine as a substrate and decreases NOS-generated NO production. As part of an adaptive response, XOR reduces circulating nitrite (NO2–) to NO to limit injury. Supplementing nitrite can augment this response to serve as a therapeutic.
NO has been shown to have many vasoprotective properties, which has led to investigation into NO-based therapies to prevent vaso-occlusive complications of vascular injury (44, 45). However, NO-based therapies have had limited clinical success in protecting against IH. Nitrite has been identified as an important endocrine reservoir of NO activity and has been well recognized as being cytoprotective in multiple mammalian I/R models (29, 31, 33). In these published models, nitrite was delivered immediately before reperfusion or as a preconditioning agent, and its protective ability was demonstrated to be dependent upon its reduction to NO. The potential therapeutic use of nitrite to limit or reverse IH has not been investigated. Several studies have reported the cardiovascular effects of nitrate and/or nitrite, including influences on vasodilation and blood pressure (26, 46). Organic nitrates have been used therapeutically as effective vasodilators but are limited as long-term drugs to prevent vascular remodeling because of the development of tolerance. It is known that the Mediterranean diet is inversely related to vascular disease (47, 48). It is thought that many different dietary micro- and macronutrients act synergistically to reduce vascular disease and other chronic diseases. The Mediterranean diet includes relatively high doses of dietary nitrates, which are then converted to nitrite within the human body via commensal bacteria in the human pharynx. Of note, the dosage of dietary nitrite utilized in this study on a mg/kg basis is consistent with the daily nitrite intake/production of that in the average Mediterranean diet (21). The findings in this article support the hypothesis that dietary nitrates/nitrite contribute significantly to cardiovascular health and serve to limit vascular pathology. This is underscored by the finding that IH was nearly doubled in rats fed a nitrate/nitrite-reduced diet (NOx– diet) compared with rats fed standard chow. Additionally, these data demonstrate that nitrite delivery by several routes can increase plasma nitrite levels, but do not show what percentage of nitrite is metabolized.
The metabolism of nitrite to NO can occur by several mechanisms, including acidic disproportionation along oxygen and pH gradients, as well as by enzymatic reduction (22). Proteins that have shown the ability to reduce nitrite to NO include deoxyhemoglobin, deoxymyoglobin, components of the electron transport system, and XOR (26–29). XOR is a highly conserved member of the molybdoenzyme family and is best known for its catalytic role in purine degradation, metabolizing hypoxanthine and xanthine to uric acid with associated generation of superoxide (49). The reduction of nitrite to NO has been shown to occur at the molybdenum location, which also happens to be the binding site for the XOR inhibitors allopurinol and oxypurinol (28). Our data reveal that XOR reduces nitrite to NO within vessels ex vivo and within cultured SMCs in vitro. In addition, rats briefly treated with allopurinol for 48 hours prior to and for 24 hours after sodium nitrite treatment lost the protective benefit of this therapeutic. Moreover, these data show increased XOR expression and activity within injured vessels. When rats were fed and maintained on a diet rich in tungsten, which inhibits XOR by replacing the molybdenum in the active site of this enzyme, vascular injury was exacerbated. Taken together with data demonstrating the contribution of dietary nitrates/nitrite, these data suggest that XOR accounts for a significant proportion of the nitrite reductase activity within the vasculature and functions as part of an adaptive response within the vasculature to produce NO in an arginine/NOS-independent manner. The ex vivo data are interpreted with some caution, as these experiments were done in the absence of hemoglobin. However, allopurinol reverses the effects of sodium nitrite to limit IH formation, suggesting that the XOR pathway is biologically relevant in vivo. Additionally, XOR has been shown to act as the predominant nitrite reductase within the lung in models of pulmonary arterial hypertension (43). Further studies are needed to determine the role of other potential nitrite reductases.
Tungsten was utilized for these in vivo investigations based upon its efficacy as an inhibitor of XOR activity. Although dietary tungsten supplementation effectively inhibits XOR activity, it is not a specific inhibitor of this enzyme and also affects other enzymes containing molybdenum, and such considerations must be kept in mind when drawing conclusions regarding the dependency of nitrite on XOR. However, genetic approaches to manipulate this enzyme are challenging due to the lethality of XOR-null mice and the difficulty in effectively and/or specifically knocking down XOR in the vasculature.
Allopurinol was utilized as a short-term inhibitor of XOR in vivo when the effects of 24 hours of oral sodium nitrite pretreatment were investigated. Because uric acid has been shown to activate arginase activity in cultured endothelial cells (50), the administration of allopurinol alone might have been anticipated to have a beneficial effect. However, the effect of allopurinol alone on NO generation by injured vessels was modest at best in comparison to the effect of allopurinol plus nitrite (Figure 5A), likely because activation of arginase by uric acid occurs at concentrations typical of hyperuricemia in humans, which are significantly higher than those found in rodents. Unlike rodents, humans lack uricase and thus cannot degrade uric acid. Thus, administration of allopurinol even in the absence of added nitrite may have a significantly greater beneficial effect on NO generation in injured vessels of humans than in rodents.
These data demonstrate that sodium nitrite is reduced to NO, which then limits SMC proliferation via a p21-dependent pathway. This relationship between nitrite and p21 is suggested in vivo, where p21 was shown to be upregulated within the limited neointima following nitrite treatment. Previously published studies focusing on the inhibition of SMC proliferation by NO have also shown it to be dependent upon p21 (40–42). Our findings support the hypothesis that the effect of nitrite on SMC proliferation is exerted via NO-mediated downstream signaling. However, other direct signaling effects may also be involved that have yet to be elucidated. Furthermore, although nitrite can limit SMC proliferation, the effects of nitrite in limiting or reversing IH likely involve many other mechanisms. These might include reduction of inflammation at the time of vascular injury, which occurs when nitrite is delivered as a pretreatment; and enhancement of negative vascular remodeling via other cellular processes such as autophagy, which occurs when nitrite is delivered after the establishment of IH.
Within the current study, nitrite was used as a therapeutic treatment in a well-established IH model known to involve cellular proliferation and tissue modification. Interestingly, a single nitrite treatment prior to injury (intraperitoneal and inhalation dosing) or 24 hours of oral nitrite supplementation before and after injury was sufficient to limit the development of IH. Based upon the ability of single or limited treatments to significantly prevent the development of IH, this is potentially of great clinical interest for therapeutic development. Additionally, oral sodium nitrite delivered after the establishment of IH led to vascular remodeling and significantly diminished I/M ratios. Dietary sodium nitrite is straightforward and can be easily administered in any setting.
Another interesting finding is the increased expression and activity of arginase-1 within the neointima, which is associated with diminished NOS activity ex vivo and a decreased “footprint” of NO as measured by S-nitrosothiol–modified protein concentrations and nitrosocysteines. These data suggest that arginase-1 would be at least in part responsible for decreased NO production, possibly by limiting the availability of arginine as a substrate for NOS. Peyton et al. demonstrated increased arginase levels in vivo and protection against the formation of IH utilizing an arginase inhibitor (8). The increased arginase levels may contribute to the lack of effectiveness of dietary l-arginine supplementation in protecting against IH. Other contributing factors that may limit NO bioavailability were not investigated. These factors could include decreased or dysfunctional NOS enzymes or scavenging/consumption of NO, as would be seen with increased reactive oxygen species that can immediately react with NO to form toxic reactive nitrogen species.
Others and we have previously demonstrated and we have confirmed that inducible NOS is increased within the lesion of IH (51), suggesting that this is an additional compensatory mechanism in response to an injured endothelium and decreased endothelial NOS. Additionally, the findings that injured vessels demonstrate decreased NOS activity and the addition of arginase inhibitor ex vivo results in increases NOS activity in injured carotid arteries ex vivo suggest that NOS enzymes are functional, yet limited by substrate availability secondary to competition for substrate. Increased XOR in this setting interacts with circulating nitrite to circumvent this dysregulated “classical” NO pathway. Increased NOS enzyme expression may also have potential deleterious consequences in the setting of vascular injury if the enzyme is uncoupled, leading to reactive oxygen species production.
The two major health issues regarding nitrite toxicity concern the formation of methemoglobinemia and potential carcinogenic effects (52). Methemoglobin arises when the oxygen-carrying ferrous ion (Fe2+) is oxidized by nitrite to the ferric ion (Fe3+), forming a molecule that cannot bind oxygen. Clinically significant cyanosis due to methemoglobinemia occurs with levels approaching 5%. In animal studies examining intravenous nitrite, the increase in methemoglobin remains undetectable even after prolonged nitrite delivery (53). In addition, the US Department of Health and Human Services National Toxicology Program published sodium nitrite toxicology and carcinogenesis studies in 2001 on rats and mice that showed no significant carcinogenic activity despite dose escalations necessary to generation profound methemoglobinemia and weight loss over a 2-year period (54). Our data indicate that a low-dose one-time treatment with nitrite may be sufficient to reduce the formation of IH following arterial injury and minimize the concerns about toxicity.
In summary, we have shown that low-dose sodium nitrite delivered via multiple strategies protects against the development of and reverses existing IH in a rat carotid injury model. These data include orally delivered nitrite at a dose equivalent to what is produced after consumption of a normal serving of green leafy vegetables. At least part of the mechanism of nitrite’s vasoprotective ability is due to its conversion to NO via XOR and inhibition of SMC proliferation. In addition, vascular injury results in local NO deficiency marked by increased arginase-1, and nitrite works via an arginine/NOS-independent pathway. Nitrite-based therapies may prove to be clinically useful adjuncts in the treatment of IH and vascular disease. Further dosing, safety, and mechanistic studies are warranted.
Methods
Cell culture.
SMCs were cultured from explanted thoracic aortas as previously described (55) from male Sprague-Dawley rats (Harlan). Cells were grown in DMEM (low glucose)/Ham’s F12 (1:1 vol/vol) (BioWhittaker); supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 4 mmol/l l-glutamine; and maintained in a 37°C, 95% air/5% carbon dioxide incubator. These cultured cells were only used at passages 3–6.
Cellular proliferation.
Growth-arrested SMCs (serum starvation for 24 hours) were subject to experimental conditions in the presence of 5 μCi/ml 3H-thymidine (NEN) for 24 hours. 3H-thymidine incorporation into trichloroacetic acid–precipitated DNA was quantified by scintillation spectroscopy.
Rat carotid artery balloon injury.
All animal procedures were performed with aseptic techniques in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh. Adult male Sprague-Dawley rats (Harlan) weighing 350–450 g were anesthetized with intraperitoneal sodium pentobarbital (Nembutal) (45 mg/kg; Abbott Laboratories) and supplemental inhalational isoflurane (Forane). The left carotid artery was injured in each animal as previously described (44, 56, 57). Briefly, a 2-French embolectomy catheter (Applied Vascular Engineering) was inserted into the common carotid artery through the external carotid branch, and injury was induced by inflation of the balloon to 5 atmospheres of pressure for 5 minutes. Afterward, the external carotid artery was ligated, and blood flow through the common carotid artery was reestablished.
NO generation.
NO generation was measured in a sealed vessel in which the solution and head space were purged with helium. The vessel was connected in line with a Nitric Oxide Analyzer (Sievers Co.) as previously described (29). In some conditions, homogenates were pretreated with allopurinol (100 μM) or L-NAME (1 mM). NO generation was normalized to tissue or cell protein concentration as measured by the BCA protein assay kit (Pierce). Homogenates were collected in Tris-EDTA buffer.
NOS activity assay.
NOS activity assay was performed with carotid artery tissue homogenates (50 μg) utilizing the NOS activity assay kit by Cayman Chemical as per the manufacturer’s instructions. Briefly, this assay is based upon the conversion of radiolabeled arginine to citrulline in the presence of required NOS substrates (reduced NADPH, molecular oxygen, calcium, calmodulin, and tetrahydrobiopterin [BH4]). To inhibit arginase activity, we performed some reactions in the presence of the arginase inhibitor nor-NOHA (100 μM). The NOS inhibitor NG-nitro-l-arginine (L-NNA; 1 mM) was utilized as a control as recommended.
Animal studies.
All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Each study group utilized 6–8 male Sprague-Dawley rats. Unless indicated, rats were fed a standard chow and allowed to drink tap water. Nitrate and nitrite content from standard chow is measured to be 1,480.4 ± 87.3 nmol/g nitrate and 2.8 ± 0.1 nmol/g nitrite. When specified, some rats were fed a purified low-nitrate and -nitrite (NOx–) diet, TD99366 (Harlan Teklad), and sterilized deionized water prior to and following carotid injury. This diet contains 26.6 ± 2.5 nmol/g nitrate and 2.6 ± 0.2 nmol/g nitrite (58).
Where indicated, rats were treated with sodium nitrite. Oral sodium nitrite supplementation was delivered in the drinking water by addition of 10.5 mg sodium nitrite to each liter of water. Based upon average daily water consumption, this gives an approximate consumption of 9.6 μmol/kg/d (the equivalent nitrite load that is produced from a serving of spinach in a human) (21, 30). In the IH prevention studies, sodium nitrite–supplemented water was given to the animals for 24 hours prior to carotid injury. In the IH reversal studies, sodium nitrite–supplemented water was given to the animals starting 15 days after carotid injury and then continued until day 28. Some rats received a single intraperitoneal delivery of sodium nitrite (500 nmol) 30 minutes prior to carotid injury, while others received a single nebulized dose prior to carotid injury as follows. Rats were placed in a nebulization chamber, and 30 mg sodium nitrite in phosphate buffered saline (pH 7.4; 5 ml) was nebulized into a Plexiglas living environment measuring 24 × 10 × 9 in. over 20 minutes. A diffuser was placed in front of the inlet to allow equal distribution of nebulized droplets, and a controlled fan pulled air through the environment.
In one experiment, rats received water supplemented with allopurinol (Sigma-Aldrich) to give an approximate consumption of 100 mg/kg/d for 48 hours prior to injury and then removed 24 hours after carotid injury. See Supplemental Methods for description of mouse carotid injury model.
Tissue processing and analysis.
Rat carotid arteries were perfused and fixed in situ with phosphate-buffered saline (pH 7.4) and 2% paraformaldehyde 14 or 28 days after injury. Vessels were fixed for 1 hour at 4°C in 2% paraformaldehyde and cryoprotected in 30% sucrose at 4°C overnight. Vessels were quick-frozen with 2-methylbutane. Seven-micrometer-thick cryosections were cut at equally spaced intervals along the length of the vessels. Sections were then stained with H&E. The center of injury was identified, and luminal, intimal, medial, and total vessel areas were measured in 8 sections, spaced approximately 200 μm apart within the region of injury. Images were obtained with an Olympus Provis microscope, and areas were measured using Scion Image.
Western blot analysis.
SMCs were collected after 24 hours of exposure to the different treatments, and whole cell lysates were sonicated and protein quantified with the BCA protein assay. Rat carotid arteries used for Western blot analysis were perfused in situ with phosphate-buffered saline (pH 7.4) and then quick frozen with 2-methylbutane. These frozen arteries were then crushed and sonicated, and protein was quantified with the BCA protein assay. Gel samples (40 μg) were subjected to SDS-PAGE on 15% gels. After electrophoresis, proteins were transferred to nitrocellulose membranes (Schleicher & Schuell) as described previously (59). These membranes were blocked with 5% milk and incubated with a mouse polyclonal anti-p21 antibody (1:500; BD) and a rabbit polyclonal anti-XOR antibody (XOR 1:5,000; Santa Cruz Biotechnology Inc.), followed by a horseradish peroxidase–linked goat anti-mouse or goat anti-rabbit immunoglobulin (1:20,000; Pierce) as previously described (60). These proteins were visualized using chemoluminescent reagents (Supersignal Substrate; Pierce).
Immunohistochemistry.
For immunohistochemical staining, sections were blocked with 2% BSA, incubated with primary antibody (XOR 1:5,000 for 1 hour, Santa Cruz Biotechnology Inc.; Arginase I 1:500 for 1 hour, BD; S-nitrosocysteine 1:500 overnight, AG Scientific; and p21 1:250 overnight, BD), then incubated with the secondary antibody for 1 hour (goat anti–rabbit or goat anti–mouse Cy3, 1:3,000; Amersham). The nuclei were stained with Hoechst 33325 (2 μg/ml in water) (Sigma-Aldrich) for 10 seconds. Slides were covered in Gelvatol mounting media (made from PVA [Sigma-Aldrich], glycerol [Sigma-Aldrich], and sodium azide [Fisher]) and a coverslip and allowed to dry overnight at 4°C. Images were collected with a ×10 and ×40 objective together with multipass fluorescence cubes to ensure perfect image registration. The image collection system included an Olympus Provis microscope, a high-sensitivity, integrating 3-chip color camera (Sony Corp.), a frame grabber board (Coreco Inc.), and Optimus software (Media Cybernetics). All images were collected with the same camera and microscope settings (3 frames of integration), with no neutral density fields.
XOR activity.
XOR activity was determined in ex vivo carotid arteries both with and without balloon injury (n = 8 vessels per group) via HPLC with electrochemical detection. Prior to measurement of enzymatic activity, endogenous urate was removed by eluting the sample on a Sephadex G-25 column (GE Healthcare). Samples were then treated with oxonic acid (2 mM) to inhibit plasma uricase activity. Xanthine (75 μM) was then added at 37°C for 60 minutes and XOR activity assessed by monitoring the production of urate. These reactions were performed in the absence and presence of NAD+ (0.5 mM) and pyruvic acid (5 mM) in order to assess xanthine oxidase (XO) and total XOR activity, respectively. The specificity of this detection method for urate production by XOR was verified by inhibition of urate formation following allopurinol addition.
Arginase activity.
Arginase activity was determined in ex vivo carotid arteries both with and without balloon injury (n = 2 measurements, each measurement done on lysates combined from 2 vessels). Arginase activity was determined as the conversion of [14C]guanidino-arginine to [14C]urea, which was converted to 14CO2 by urease and trapped as Na2 14CO3 for scintillation counting, as described previously (61). Briefly, carotid lysates were incubated for 10 minutes at 55°C in complete assay mixtures lacking arginine. The reaction was initiated by addition of labeled arginine, and incubation was continued at 37°C for 2.5 hours. The reaction was terminated by heating at 100°C for 3 minutes. Samples were incubated with urease at 37°C for 45 minutes, and Na2 14CO3 was trapped on NaOH-soaked filters following acidification of the samples with HCl to volatilize the 14CO2. Results are expressed as nmol/min/mg.
Collection and measurement of plasma nitrite and vessel nitrosothiol levels.
Nitrite plasma levels were measured in control rats, rats treated with 24 hours of oral nitrite supplementation (9.6 μmol/kg/d), rats receiving a single nebulized nitrite treatment (30 mg over 20 minutes), and rats receiving a single intraperitoneal injection of 500 nmol of sodium nitrite. Blood samples were drawn into heparin-coated syringes and centrifuged (5,000 g for 5 minutes) immediately to avoid nitrite consumption by hemoglobin. Plasma was immediately collected and frozen for later analysis. Carotid artery S-nitrosothiol levels were determined in control vessels, injured vessels 2 weeks after injury, and injured vessels after rats received 2 weeks of oral nitrite supplementation (9.6 μmol/kg/d). Rats were perfused with cold phosphate-buffered saline (pH 7.4), and carotid arteries were removed and flash frozen using liquid nitrogen. Nitrite levels in plasma and vessels were measured using triiodide-based reductive chemiluminescence as described and validated previously (62, 63).
Viability.
Trypan blue exclusion was analyzed by trypsinizing (0.25%; Gibco, Invitrogen) the cells and diluting the cells at a 1:4 ratio with trypan blue (Gibco, Invitrogen) to determine whether high levels of nitrite were cytotoxic to SMCs. All contents of the tissue culture dish wells were analyzed so that any floating cells or debris were not missed. Cells were counted with a hemocytometer, and percentage viability was calculated by dividing the number of cells excluding trypan blue by the total number of cells.
Statistics.
Results are expressed as mean ± SEM. Differences among groups were analyzed with 1-way ANOVA with the Student-Newman-Keuls post-hoc test for all pairwise comparisons (SigmaStat; SPSS). Statistical significance was assumed when P was less than 0.05.
Supplementary Material
Acknowledgments
We are appreciative of the excellent technical assistance of Diane Kepka-Lenhart. This work was supported in part by the American Heart Association, Great Rivers Affiliate grant 09GRNT2050220 (to B.S. Zuckerbraun); NIH grants R01HL098032, RO1HL096973, and RC1DK085852 (to M.T. Gladwin) and R01GM57384 (to S.M. Morris); and the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (to M.T. Gladwin).
Footnotes
Conflict of interest: Mark T. Gladwin is listed as a co-inventor on an NIH government patent on the use of sodium nitrite for cardiovascular indications.
Citation for this article: J Clin Invest. 2011;121(4):1646–1656. doi:10.1172/JCI44079.
See the related Commentary beginning on page 1258.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Barbato JE, Tzeng E. Nitric oxide and arterial disease. J Vasc Surg. 2004;40(1):187–193. doi: 10.1016/j.jvs.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007;45(6 suppl A):A64–A73. doi: 10.1016/j.jvs.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 5.Wei LH, Wu G, Morris SM, Jr, Ignarro LJ. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc Natl Acad Sci U S A. 2001;98(16):9260–9264. doi: 10.1073/pnas.161294898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero MJ, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. . Circ Res. 2008;102(1):95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Closs EI. Expression, regulation and function of carrier proteins for cationic amino acids. Curr Opin Nephrol Hypertens. 2002;11(1):99–107. doi: 10.1097/00041552-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Peyton KJ, et al. Arginase promotes neointima formation in rat injured carotid arteries. Arterioscler Thromb Vasc Biol. 2009;29(4):488–494. doi: 10.1161/ATVBAHA.108.183392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum A, et al. Oral L-arginine in patients with coronary artery disease on medical management. Circulation. 2000;101(18):2160–2164. doi: 10.1161/01.cir.101.18.2160. [DOI] [PubMed] [Google Scholar]
- 10.Dudek D, et al. L-arginine supplementation does not inhibit neointimal formation after coronary stenting in human beings: an intravascular ultrasound study. Am Heart J. 2004;147(1):E12. doi: 10.1016/j.ahj.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Walker HA, et al. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol. 2001;38(2):499–505. doi: 10.1016/S0735-1097(01)01380-8. [DOI] [PubMed] [Google Scholar]
- 12.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116(2):188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 13.Boger RH. L-Arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr Metab Care. 2008;11(1):55–61. doi: 10.1097/MCO.0b013e3282f2b0c3. [DOI] [PubMed] [Google Scholar]
- 14.Ansel GM, Lumsden AB. Evolving modalities for femoropopliteal interventions. J Endovasc Ther. 2009;16(1):II82–II97. doi: 10.1583/08-2654.1. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353(25):2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 16.Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109(25):3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 17.Barbato JE, Kibbe MR, Tzeng E. The emerging role of gene therapy in the treatment of cardiovascular diseases. Crit Rev Clin Lab Sci. 2003;40(5):499–545. [PubMed] [Google Scholar]
- 18.Kibbe MR, Tzeng E. Nitric oxide synthase gene therapy in vascular pathology. Semin Perinatol. 2000;24(1):51–54. doi: 10.1016/S0146-0005(00)80056-7. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto A, Momomura S, Hirata Y, Aoyagi T, Sugiura S, Omata M. Inhaled nitric oxide and exercise capacity in congestive heart failure. Lancet. 1997;349(9057):999–1000. doi: 10.1016/s0140-6736(05)62897-8. [DOI] [PubMed] [Google Scholar]
- 20.Roger N, Barbera JA, Roca J, Rovira I, Gomez FP, Rodriguez-Roisin R. Nitric oxide inhalation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156(3 pt 1):800–806. doi: 10.1164/ajrccm.156.3.9611051. [DOI] [PubMed] [Google Scholar]
- 21.Gladwin MT, et al. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1(6):308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 23.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1(8):804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 24.Duncan C, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1(6):546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 25.Gladwin MT, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291(5):H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 26.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427(2):225–228. doi: 10.1016/S0014-5793(98)00430-X. [DOI] [PubMed] [Google Scholar]
- 29.Shiva S, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100(5):654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 30.Shiva S, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204(9):2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripatara P, et al. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. . J Am Soc Nephrol. 2007;18(2):570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 32.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75(2):327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duranski MR, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter CJ, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10(10):1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 35.Lundberg JO, Feelisch M, Bjorne H, Jansson EA, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15(4):359–362. doi: 10.1016/j.niox.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Maeda S, et al. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res. 2004;27(12):947–953. doi: 10.1291/hypres.27.947. [DOI] [PubMed] [Google Scholar]
- 37.Shiva S, et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2(9):486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 38.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar R, Gordon D, Stanley JC, Webb RC. Cell cycle effects of nitric oxide on vascular smooth muscle cells. Am J Physiol. 1997;272(4 pt 2):H1810–H1818. doi: 10.1152/ajpheart.1997.272.4.H1810. [DOI] [PubMed] [Google Scholar]
- 40.Bauer PM, Buga GM, Ignarro LJ. Role of p42/p44 mitogen-activated-protein kinase and p21waf1/cip1 in the regulation of vascular smooth muscle cell proliferation by nitric oxide. Proc Natl Acad Sci U S A. 2001;98(22):12802–12807. doi: 10.1073/pnas.211443198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kibbe MR, et al. Inducible nitric oxide synthase (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg. 2000;31(6):1214–1228. doi: 10.1067/mva.2000.105006. [DOI] [PubMed] [Google Scholar]
- 42.Zuckerbraun BS, et al. Nitric oxide-induced inhibition of smooth muscle cell proliferation involves S-nitrosation and inactivation of RhoA. Am J Physiol Cell Physiol. 2007;292(2):C824–C831. doi: 10.1152/ajpcell.00592.2005. [DOI] [PubMed] [Google Scholar]
- 43.Zuckerbraun BS, et al. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121(1):98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 44.Shears LL, et al. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg. 1998;187(3):295–306. doi: 10.1016/S1072-7515(98)00163-X. [DOI] [PubMed] [Google Scholar]
- 45.von der Leyen HE, et al. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci U S A. 1995;92(4):1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb AJ, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de LM, Salen P. The Mediterranean diet: rationale and evidence for its benefit. Curr Atheroscler Rep. 2008;10(6):518–522. doi: 10.1007/s11883-008-0080-5. [DOI] [PubMed] [Google Scholar]
- 48.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119(8):1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555(pt 3):589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zharikov S, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shears LL. Inducible nitric oxide synthase suppresses the development of allograft arteriosclerosis. J Clin Invest. 1997;100(8):2035–2042. doi: 10.1172/JCI119736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mensinga TT, Speijers GJ, Meulenbelt J. Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev. 2003;22(1):41–51. doi: 10.2165/00139709-200322010-00005. [DOI] [PubMed] [Google Scholar]
- 53.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293(12):1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 54. National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of sodium nitrite (cas no. 7632-00-0) in F344/N rats and B6C3F1 mice (drinking water studies). NIH publication no. 01-3954. http://www.ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr495.pdf . Published May 2001. Accessed March 4, 2011. [Google Scholar]
- 55.Yu SM, Tsai SY, Guh JH, Ko FN, Teng CM, Ou JT. Mechanism of catecholamine-induced proliferation of vascular smooth muscle cells. Circulation. 1996;94(3):547–554. doi: 10.1161/01.cir.94.3.547. [DOI] [PubMed] [Google Scholar]
- 56.Granada JF, et al. Single perivascular delivery of mitomycin C stimulates p21 expression and inhibits neointima formation in rat arteries. Arterioscler Thromb Vasc Biol. 2005;25(11):2343–2348. doi: 10.1161/01.ATV.0000184779.01822.9d. [DOI] [PubMed] [Google Scholar]
- 57.Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104(22):2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 58.Raat NJ, et al. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic Biol Med. 2009;47(5):510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzeng E, et al. Vascular gene transfer of the human inducible nitric oxide synthase: characterization of activity and effects on myointimal hyperplasia. Mol Med. 1996;2(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- 60.Zuckerbraun BS, Shapiro RA, Billiar TR, Tzeng E. RhoA influences the nuclear localization of extracellular signal-regulated kinases to modulate p21Waf/Cip1 expression. Circulation. 2003;108(7):876–881. doi: 10.1161/01.CIR.0000081947.00070.07. [DOI] [PubMed] [Google Scholar]
- 61.Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol. 1998;275(5 pt 1):E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- 62.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851(1–2):93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res. 2003;37(1):1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.