Abstract
Elite controllers represent a unique group of HIV-1–infected persons with undetectable HIV-1 replication in the absence of antiretroviral therapy. However, the mechanisms contributing to effective viral immune defense in these patients remain unclear. Here, we show that compared with HIV-1 progressors and HIV-1–negative persons, CD4+ T cells from elite controllers are less susceptible to HIV-1 infection. This partial resistance to HIV-1 infection involved less effective reverse transcription and mRNA transcription from proviral DNA and was associated with strong and selective upregulation of the cyclin-dependent kinase inhibitor p21 (also known as cip-1 and waf-1). Experimental blockade of p21 in CD4+ T cells from elite controllers resulted in a marked increase of viral reverse transcripts and mRNA production and led to higher enzymatic activities of cyclin-dependent kinase 9 (CDK9), which serves as a transcriptional coactivator of HIV-1 gene expression. This suggests that p21 acts as a barrier against HIV-1 infection in CD4+ T cells from elite controllers by inhibiting a cyclin-dependent kinase required for effective HIV-1 replication. These data demonstrate a mechanism of host resistance to HIV-1 in elite controllers and may open novel perspectives for clinical strategies to prevent or treat HIV-1 infection.
Introduction
HIV-1 infection leads to progressively rising viremia, loss of CD4+ T cell counts, and clinical symptoms of immunodeficiency in the vast majority of untreated individuals; however, a small proportion of patients maintain undetectable levels of viral replication in the absence of antiretroviral therapy (1, 2). These individuals, termed elite controllers, have moved into the center of current efforts to identify correlates of immune protection against HIV-1, but the mechanisms responsible for undetectable HIV-1 viremia in these patients remain unresolved. HIV-1–specific CD8+ T cells from elite controllers have specific cytotoxic (3), proliferative (4, 5), and cytokine secretion (6) properties and are effective in restricting HIV-1 replication in in vitro tissue culture experiments (7, 8), but they are not a sufficient, and sometimes not even a necessary, component of effective immune activity against HIV-1 in vivo (9). Although the major genetic determinants of HIV-1 control have recently been shown to relate to specific amino acid polymorphisms in the HLA-B binding cleft (10), these account for less than 25% of the variability in viral load (VL), further suggesting that mechanisms other than T cell–mediated immune activity play a role in HIV-1 immune defense in elite controllers. Broadly neutralizing antibodies seem to have limited activities in elite controllers (11), and the continuous selection of escape mutations in targeted epitopes is likely to further limit their antiviral effects.
In addition to adaptive immune responses against HIV-1, intrinsic mechanisms that restrict HIV-1 replication in CD4+ target cells might play an important role in mediating resistance to HIV-1 infection in elite controllers. Several prior studies have demonstrated that CD4+ T cells from elite controllers are generally permissive to HIV-1 infection and that they can harbor pathogenic, replication-competent virus in vivo (12–14). Moreover, after in vitro activation, CD4+ T cells from elite controllers are capable of supporting productive HIV-1 infection (13, 15), but prior experimental procedures, particularly when involving vigorous in vitro activation of cells, may have been insufficiently sensitive to detect intrinsic resistance mechanisms that restrict HIV-1 replication by altered expression patterns of host genes.
Notably, a panel of different human proteins has been identified that can modulate the cellular susceptibility to HIV-1 infection by interfering with different steps of the viral replication cycle (16). p21 (encoded by CDKN1A; also known as wif-1 and cip-1) belongs to this class of proteins and functions as a potent inhibitor of cyclin dependent kinases, a group of host enzymes required for effective replication of HIV-1 and other viruses (17, 18). Recently, p21 has been shown to be able to modulate the efficacy of HIV-1 replication in human macrophages (19) and hematopoietic stem cells (20). In this study, we demonstrated an intrinsic partial resistance of CD4+ T cells from elite controllers to HIV-1 infection that is mediated, at least in part, by strong and selective upregulation of p21 in CD4+ T cells from these patients. These results identified what we believe to be a novel cell-intrinsic mechanism of immune protection to HIV-1 infection in elite controllers that might offer new approaches for enhancing host resistance to HIV-1 infection.
Results
Reduced susceptibility of CD4+ T cells from elite controllers to HIV-1 infection.
Prior studies have shown that in vitro activation of CD4+ T cells from HIV-1 elite controllers can support replication of HIV-1 isolates; however, many of these studies used high viral inoculums and robust, PHA-mediated activation of CD4+ T cells, which may not appropriately reflect physiological HIV-1 infection conditions (12, 13). To further define the relative susceptibility of CD4+ T cells from elite controllers to HIV-1 infection, we performed ex vivo infection assays using CD4+ T cells from elite controllers (viremia undetectable by commercial assays; median 843 CD4+ T cells/μl, range 188–1,437 cells/μl) and reference cohorts of individuals with viremic control (median VL 259 copies/ml, range 49–2,170 copies/ml; median 775 CD4+ T cells/μl, range 465–1,571 cells/μl), progressive HIV-1 infection (median VL 145,000 copies/ml, range 14,000–750,000 copies/ml; median 304 CD4+ T cells/μl, range 6–701 cells/μl), or no HIV-1 infection at all. Activated populations of CD4+ T cells were generated by 5-day in vitro culture with exogenous IL-2 and anti-CD3/CD8 bispecific antibodies, which stimulate CD4+ T cells by TCR triggering (21) while simultaneously deleting CD8+ T cells; this procedure resulted in a homogenous population of CD3+CD4+ cells with an activated effector memory phenotype (HLA-DR+CCR7–CD45RA–; >97.5%) with essentially no detectable CD3+CD8+ cytotoxic T cells (<0.1%), regardless of the patient cohort. Subsequently, cells were infected with primary X4-tropic isolate (92HT599; MOI 0.1) or R5-tropic HIV-1 isolate (91US056; MOI 0.001); viral replication was subsequently analyzed by p24 ELISA assays on day 7 after infection.
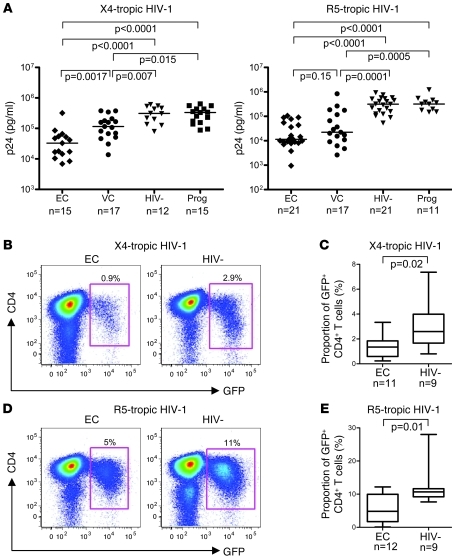
Using these activation and culture conditions, we consistently observed substantially less p24 production in CD4+ T cells from elite controllers compared with HIV-1–negative persons and HIV-1 progressors (Figure 1A). Infection of CD4+ T cells from viremic controllers also resulted in lower levels of p24 production compared with HIV-1–negative persons or HIV-1 progressors, although differences were more modest. Significantly lower p24 antigen production in elite and viremic controllers compared with HIV-1–negative persons and HIV-1 progressors was similarly observed on days 3 and 5 after infection (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI44539DS1).
Figure 1. Reduced susceptibility of CD4+ T cells from elite controllers to HIV-1 infection.
(A) p24 antigen production in activated CD4+ T cells from elite controllers (EC), viremic controllers (VC), HIV-1–negative persons (HIV-), and HIV-1 progressors (Prog) after exogenous HIV-1 infection with the X4-tropic primary isolate 92HT599 and the primary R5-utilizing strain 91US056. Data were obtained on day 7 after infection; similar patterns of p24 antigen production were observed on days 3 and 5 after infection (Supplemental Figure 1). Baseline p24 levels in autologous cells without exogenous HIV-1 infection were subtracted as background. Significance was tested using Mann-Whitney U test. (B–E) Flow cytometric assessment of HIV-1 replication in activated CD4+ T cells from elite controllers and HIV-1–negative persons on day 4 after infection with GFP-encoding X4- and R5-tropic HIV-1 isolates. (B and D) Representative flow cytometry dot plots from 1 elite controller and 1 HIV-1–negative person. Percentages indicate the respective proportions of gated GFP+ CD4+ cells. (C and E) Proportion of GFP+ CD4+ T cells from the indicated subjects. Bounds of boxes denote interquartile range; lines within boxes denote median; whiskers indicate range.
To further investigate the susceptibility of CD4+ T cells from elite controllers to HIV-1, we performed ex vivo infection experiments using an alternative set of X4 (NL4-3)- and R5 (Ba-L)- tropic HIV-1 isolates encoding GFP, which allowed for monitoring of HIV-1 replication on a single-cell level by flow cytometry. After ex vivo activation of CD4+ T cells using the previously described technique, we observed that infection with these isolates at MOI 0.01 resulted in significantly higher proportions of GFP+ CD4+ T cells in HIV-1–negative persons compared with elite controllers (Figure 1, B–E), consistent with our initial findings of reduced p24 antigen production in CD4+ T cells from these patients.
In line with other studies (22), we found that in direct ex vivo assessments, elite controllers and viremic controllers had higher baseline proportions of activated HLA-DR+ CD4+ T cells than did HIV-1–negative persons (P = 0.04 and P = 0.01, respectively), which indicates that reduced susceptibilities to viral infection in controllers were not related to lower baseline levels of CD4+ T cell immune activation. Moreover, no significant differences were observed among the surface expression of CD4 (P > 0.4), CCR5 (P > 0.36), or CXCR4 (P > 0.4), measured either directly ex vivo or after in vitro activation, between the different study cohorts, which suggests that coreceptor-mediated entry mechanisms were not responsible for altered susceptibilities to HIV-1 in these patient cohorts. Overall, these results demonstrate partial resistance of in vitro–stimulated CD4+ T cells from elite controllers to HIV-1 infection.
Inhibition of early viral replication steps in CD4+ T cells from elite controllers.
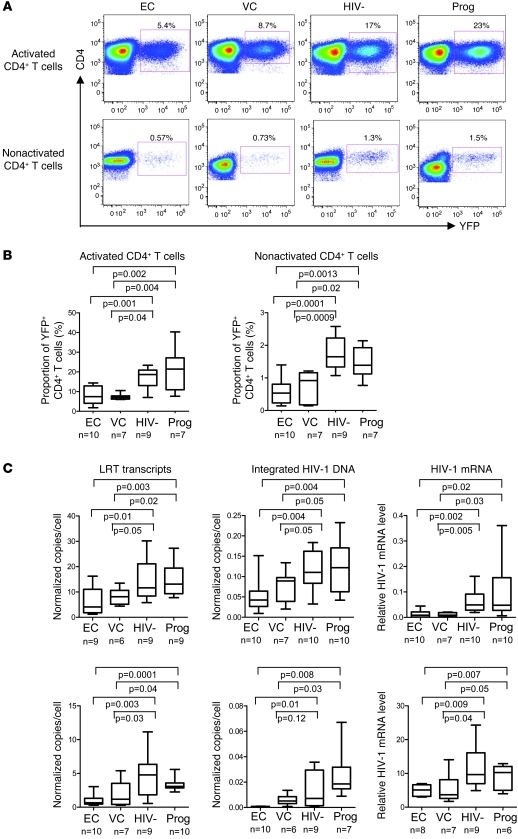
To identify steps of the viral replication cycle that may be inhibited in CD4+ T cells from elite controllers, we subsequently conducted ex vivo infection experiments with a yellow fluorescence protein–encoding (YFP-encoding), VSV-G–pseudotyped HIV-1 vector that bypasses viral coreceptor-mediated entry steps and causes single cycles of HIV-1 infection without supporting production of new viral progeny during the viral postintegration phase. Using ex vivo activated or nonactivated CD4+ T cells, we observed that the proportion of YFP+ CD4+ T cells from elite controllers and viremic controllers was significantly smaller than that of cells from HIV-1–uninfected persons and HIV-1 progressors (Figure 2, A and B). This suggests that the reduced permissiveness of CD4+ T cells from elite controllers may be mediated during the early phase of the viral replication cycle.
Figure 2. Analysis of early HIV-1 replication steps in CD4+ T cells after infection with a single-cycle YFP-encoding, VSV-G–pseudotyped HIV-1 vector.
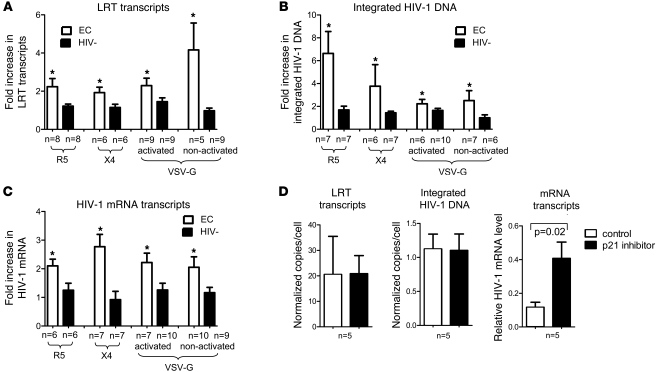
(A) Representative flow cytometry dot plots reflecting the proportion of YFP+ CD4+ T cells from the indicated cohorts with or without prior ex vivo activation. Percentages indicate the respective proportions of gated YFP+ CD4+ cells. (B) Proportion of YFP+ CD4+ cells in activated and nonactivated CD4+ T cells in the indicated cohorts. (C) Quantitative analysis of LRT transcripts, integrated HIV-1 DNA, and HIV-1 mRNA transcripts in activated or nonactivated CD4+ T cells from the 4 study cohorts. HIV-1 transcript numbers from autologous virus measured in cells without exogenous infection were subtracted as background. Bounds of boxes denote interquartile range; lines within boxes denote median; whiskers indicate range. Statistical comparison was performed using Student’s t tests.
To confirm this finding, we quantitatively assessed early viral replication steps after primary infection with the VSV-G–pseudotyped HIV-1 vector. These experiments revealed that levels of HIV-1 late reverse transcripts (LRTs), integrated HIV-1 DNA, and HIV-1 mRNA transcripts were diminished in activated CD4+ T cells from elite controllers and viremic controllers compared with corresponding cells from HIV-1–negative persons and HIV-1 progressors (Figure 2C). Similar observations were also made when CD4+ T cells isolated by negative selection were infected directly ex vivo without prior in vitro activation. Overall, these results are indicative of reduced efficacy of early viral replication steps in CD4+ T cells from elite controllers and viremic controllers.
Upregulation of p21 in CD4+ T cells from elite controllers.
The reduced HIV-1 susceptibility in CD4+ T cells from elite controllers might be caused by specific host proteins that restrict HIV-1 replication steps; however, upregulation of classical host restriction factors — such as APOBEC3G, Trim5α, or Tetherin — in CD4+ T cells from elite controllers has not been demonstrated in prior work (15, 23). More recently, the cyclin-dependent kinase inhibitor p21 has been shown to modulate HIV-1 reverse transcription and integration in macrophages and hematopoietic stem cells (19, 20). To analyze whether p21 is involved in the reduced HIV-1 susceptibility of CD4+ T cells from elite controllers, we determined CDKN1A mRNA expression in CD4+ T cells by quantitative RT-PCR. To avoid biases from differential representation of HLA-DR+ and HLA-DR– CD4+ T cells in elite controllers, HIV-1–negative persons, and HIV-1 progressors (22), we sorted HLA-DR+ and HLA-DR– CD4+ T cells and assessed CDKN1A mRNA expression separately in these cell subsets.
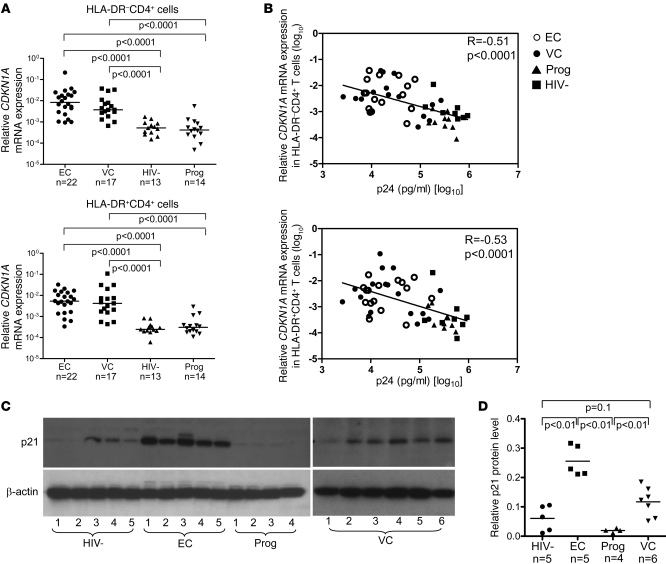
As shown in Figure 3A, we observed a highly significant upregulation of CDKN1A mRNA in HLA-DR– CD4+ T cells from elite controllers compared with cells from HIV-1–negative persons and HIV-1 progressors. Expression of CDKN1A mRNA in HLA-DR– CD4+ T cells from viremic controllers was also increased, although differences compared with the reference cohorts were slightly less pronounced. A strong upregulation of CDKN1A mRNA expression was also seen in more activated, HLA-DR+ CD4+ T cells from elite controllers, and to a lesser extent from viremic controllers; however, overall levels of CDKN1A mRNA expression were slightly smaller in these cells compared with HLA-DR– CD4+ T cells. Interestingly, a highly significant inverse correlation was found between CDKN1A mRNA expression levels in CD4+ T cells and susceptibility to HIV-1 infection, as determined by levels of p24 production after infection of ex vivo activated cells with an R5-tropic HIV-1 isolate (Figure 3B). Moreover, the elevated CDKN1A mRNA expression levels in CD4+ T cells from elite controllers and viremic controllers corresponded to increased p21 protein expression intensities, as determined by quantitative Western blot analysis (Figure 3, C and D).
Figure 3. Upregulation of p21 expression in CD4+ T cells from elite controllers.
(A) Relative CDKN1A mRNA expression in sorted HLA-DR– and HLA-DR+ CD4+ T cells from the indicated groups. (B) Correlation between relative CDKN1A mRNA expression in HLA-DR– and HLA-DR+ CD4+ T cells and susceptibility of CD4+ T cells to HIV-1 infection, as determined by p24 production 7 days after infection of in vitro activated cells with R5-tropic HIV-1. Data from elite controllers, viremic controllers, HIV-1–negative persons, and HIV-1 progressors were included. Pearson’s correlation coefficient is shown. (C) Western blots reflecting p21 and β-actin protein expression in CD4+ T cells from the indicated cohorts. (D) Quantitative p21 protein expression in CD4+ T cells from indicated study patients. (A, B, and D) Statistical comparison was performed using Student’s t tests.
We also observed that the levels of CDKN1A mRNA expression remained elevated in elite controllers after ex vivo stimulation using anti-CD3/CD8 bispecific antibodies and that CDKN1A mRNA and protein expression intensities in CD4+ T cells remained largely unaffected by short-term infection with HIV-1 (Supplemental Figure 2). There was no significant difference between CDKN1A mRNA expression intensities in elite and viremic controllers expressing HLA-B27 or HLA-B57 compared with those lacking protective MHC class I alleles (Supplemental Figure 3). Other members of the family of cyclin-dependent kinase inhibitors, such as p27 or p57, were not differentially expressed in CD4+ T cells from elite controllers and reference cohorts (Supplemental Figure 4). Taken together, these data demonstrated that CD4+ T cells from elite controllers and viremic controllers expressed higher levels of p21 than did HIV-1–negative persons and HIV-1 progressors and that levels of p21 expression were inversely correlated to CD4+ T cell susceptibility to HIV-1 infection.
p21 reduces the susceptibility of CD4+ T cells to HIV-1 infection.
To test whether the elevated p21 levels in CD4+ T cells from elite controllers functionally contribute to resistance to HIV-1 infection, we performed ex vivo infection assays of CD4+ T cells transfected with siRNA, inducing effective downregulation of p21 expression (Supplemental Figure 5), or with control siRNA that does not affect CDKN1A gene expression. Alternatively, a highly selective small-molecule inhibitor of p21 (24) that leads to ubiquitin-mediated proteasomal degradation of p21, was used for pharmacological inhibition of p21 (Supplemental Figure 5); respective control cells were treated with the carrier DMSO only.
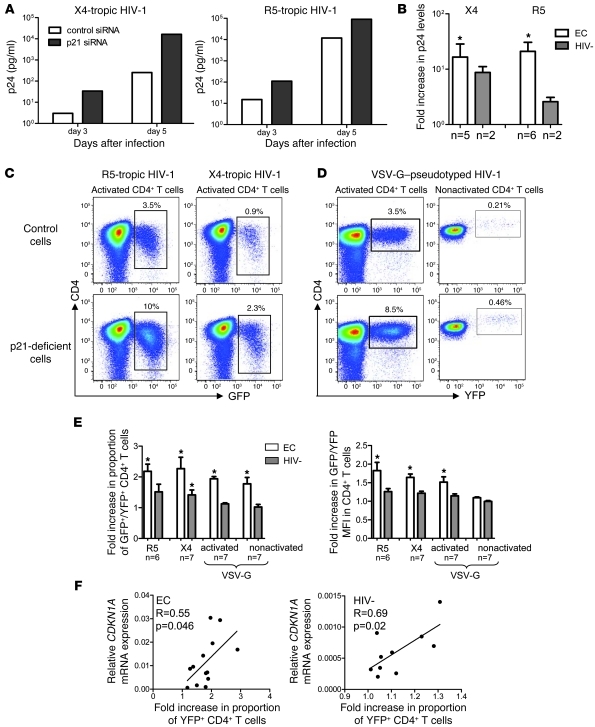
siRNA-mediated p21 inhibition in ex vivo activated CD4+ T cells resulted in a substantial increase of p24 levels measured in the supernatant after infection with X4- or R5-tropic primary HIV-1 isolates (Figure 4, A and B). Moreover, after infection of activated CD4+ T cells with GFP-encoding X4- or R5-tropic HIV-1 isolates, siRNA-mediated silencing of p21 led to a 2- to 3-fold augmentation of the proportion of GFP+ CD4+ T cells (Figure 4, C and E). Significantly elevated numbers of infected cells were also observed when activated or directly ex vivo isolated CD4+ T cells were infected with a single-cycle VSV-G–pseudotyped, YFP-encoding HIV-1 vector in the presence of the pharmaceutical inhibitor of p21 (Figure 4, D and E). In all these experiments, effects of p21 inhibition on viral replication were more pronounced in CD4+ T cells from elite controllers compared with cells from HIV-1–negative persons, consistent with higher expression levels of p21 in elite controllers. Moreover, there was a positive correlation between baseline CDKN1A mRNA expression in CD4+ T cells and fold increase in HIV-1 replication after p21 inhibition (Figure 4F).
Figure 4. Inhibition of p21 enhances HIV-1 replication in CD4+ T cells.
(A and B) Activated CD4+ T cells from elite controllers or HIV-1–negative persons were infected with X4- or R5-tropic primary HIV-1 isolates in the presence of p21-specific or control siRNA; HIV-1 replication was assessed by p24 antigen levels in culture supernatants. (A) Representative example from an HIV-1 elite controller. (B) Fold increase (mean and SD) of p24 levels in p21-deficient versus control cells in indicated persons. (C–F) Flow cytometric assessment of HIV-1 replication in CD4+ T cells after p21 inhibition. CD4+ T cells were infected with GFP-encoding X4- or R5-tropic HIV-1 strains in the presence of p21-siRNA or control siRNA or with a YFP-encoding VSV-G–pseudotyped HIV-1 vector in the presence of a pharmacological p21 inhibitor or the carrier DMSO. (C and D) Representative dot plots from an elite controller. Percentages indicate the proportion of gated CD4+ T cells. (E) Fold increase (mean and SD) in the proportion of GFP+/YFP+ cells or in GFP/YFP mean fluorescence intensity in p21-deficient cells compared with controls. White bar, EC; Grey bar, HIV-1. (F) Correlation between CDKN1A mRNA expression in HLA-DR– CD4+ T cells from elite controllers and HIV-1–negative persons and corresponding fold increases of YFP+ cells after p21 inhibition. *P < 0.05; p21-deficient cells versus control cells treated with unspecific siRNA or SMSO; paired Wilcoxon test. Statistical comparison was performed using Student’s t test.
To test whether p21 inhibition enhances susceptibility to HIV-1 infection by inducing higher levels of immune activation, we simultaneously assessed the activation phenotype of CD4+ T cells in our infection experiments by determining the surface expression of CD25, HLA-DR, and CD38. We found that all of the ex vivo activated cells demonstrated high expression of these markers, regardless of p21 inhibition (Supplemental Figure 6), which suggests that, at least during in vitro culture with exogenous IL-2 and anti-CD3/CD8 antibodies, p21 knockdown does not have a major effect on T cell activation. Using these specific culture conditions, we also did not observe any detectable effect of p21 inhibition on CD4+ T cell viability or proliferation, as determined by flow cytometric analysis after staining with a cell viability dye or CFSE (Supplemental Figure 6). Taken together, these data indicate that p21 inhibition in CD4+ T cells from elite controllers enhances HIV-1 replication without detectably affecting their activation or viability.
p21 independently inhibits HIV-1 reverse transcription and HIV-1 mRNA transcription from proviral DNA.
To investigate how p21 interferes with viral replication steps in CD4+ T cells from elite controllers, we subsequently analyzed intra- and extrachromosomal HIV-1 DNA and viral mRNA in CD4+ T cells infected with X4- and R5-tropic viral isolates in the presence of p21 siRNA or control siRNA. As demonstrated in Figure 5, A–C, we observed a significant augmentation of HIV-1 LRT transcripts, integrated HIV-1 DNA, and HIV-1 mRNA transcripts after silencing of p21. Notably, the strongest increase was seen for chromosomally integrated HIV-1 DNA, most likely as a result of the accumulation of these stable, long-lived forms of HIV-1 DNA during several HIV-1 replication cycles, as opposed to the more short-lived LRT and mRNA transcripts. p21 inhibition also resulted in marked increases of HIV-1 LRT transcripts, integrated HIV-1 DNA, and HIV-1 mRNA after infection with the single-cycle VSV-G–pseudotyped HIV-1 vector, in both in vitro activated and nonactivated CD4+ T cells. Importantly, using X4-tropic, R5-tropic, and VSV-G–pseudotyped HIV-1, the effects of p21 inhibition on these viral replication steps were most pronounced in CD4+ T cells from elite controllers, consistent with higher levels of p21 expression in cells from these patients.
Figure 5. p21 inhibition increases multiple early HIV-1 replication steps.
(A–C) Quantitative analysis of HIV-1 LRT transcripts (A), integrated HIV-1 DNA (B), and HIV-1 mRNA transcripts (C) in CD4+ T cells from elite controllers or HIV-1–negative persons after inhibition of p21. Activated CD4+ T cells were infected with X4- or R5-tropic HIV-1 isolates after electroporation with p21-specific or control siRNA. Alternatively, activated or nonactivated CD4+ T cells were infected with a single-cycle VSV-G–pseudotyped HIV-1 vector in the presence of a small molecule inhibitor of p21 or DMSO as control. Data show mean and SD fold increase of respective copy numbers in p21-deficient cells compared with corresponding control cells from the indicated number of subjects. *P < 0.05; p21 deficient cells versus control cells treated with unspecific siRNA or SMSO; paired Wilcoxon test. (D) Effect of p21 inhibition on HIV-1 mRNA transcription from proviral HIV-1 DNA. Ex vivo activated CD4+ T cells from elite controllers were infected with a VSV-G–pseudotyped HIV-1 vector; YFP+ cells were sorted after 36 hours and exposed to p21 inhibitor. Data are mean and SD of LRT transcripts, integrated HIV-1 DNA, and viral mRNA from sorted YFP+ CD4+ T cells 84 hours after infection. Statistical comparison was performed using paired Wilcoxon test.
Interestingly, we observed that the ratio of integrated HIV-1 DNA to reverse transcripts in activated CD4+ T cells did not significantly differ upon single-cycle infection of CD4+ T cells from HIV-1–negative persons and elite controllers, either in the absence (0.01 vs. 0.01, P = 0.62) or in the presence (0.01 vs. 0.01, P = 0.38) of p21 inhibition; this suggests that p21 does not affect HIV-1 integration independently of reverse transcription. In contrast, ratios of HIV-1 mRNA transcripts to LRTs and to integrated DNA levels were significantly different between HIV-1–negative persons and elite controllers (mRNA/LRT, 0.0054 vs. 0.0009, P = 0.01; mRNA/integrated DNA, 0.71 vs. 0.12, P = 0.04), but this difference was no longer observed after cells were exposed to the p21 inhibitor (mRNA/LRT, 0.004 vs. 0.005, P = 0.79; mRNA/integrated DNA, 0.37 vs. 0.38, P = 0.93). This is consistent with an effect of p21 on viral mRNA expression that is independent of viral replication steps during the preintegration phase.
To experimentally confirm this, we infected activated CD4+ T cells from elite controllers with the YFP-encoding VSV-G–pseudotyped HIV-1 vector and sorted YFP+ CD4+ T cells after 36 hours, when HIV-1 reverse transcription and HIV-1 integration were largely completed in this single-cycle infection system. Addition of the pharmaceutical p21 inhibitor to these sorted cells for the subsequent 48 hours substantially increased HIV-1 mRNA transcription from integrated proviruses, while leaving HIV-1 reverse transcription and integration unaffected (Figure 5D). Overall, these data showed that p21 can independently inhibit multiple steps in the HIV-1 replication cycle, including reverse transcription and viral mRNA transcription.
p21 inhibits CDK9 and transcriptional elongation of HIV-1 mRNA in CD4+ T cells from elite controllers.
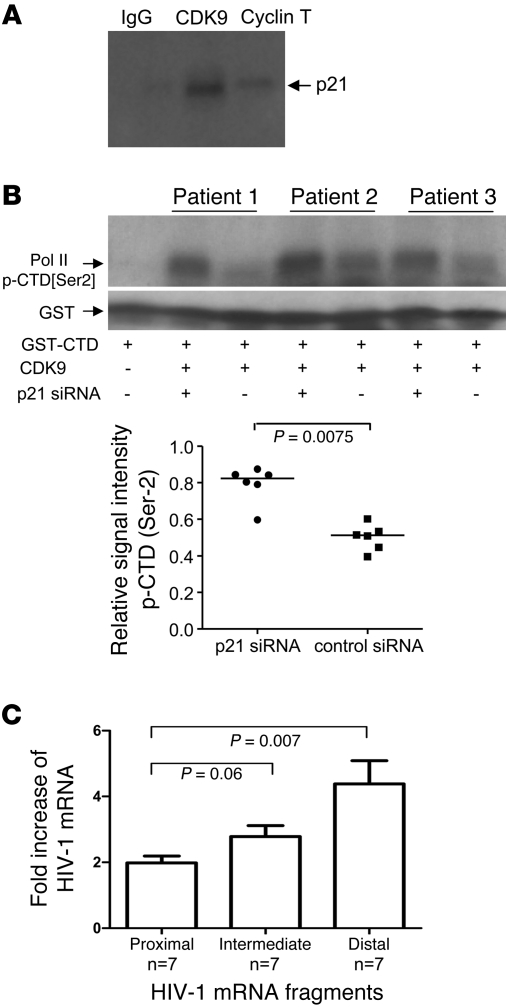
To further explore mechanisms of p21-mediated inhibition of HIV-1 replication in CD4+ T cells, we focused on how the cyclin-dependent kinase inhibitor p21 can block HIV-1 replication by interfering with cyclin-dependent kinase 9 (CDK9), which represents the active enzymatic component of positive transcription elongation factor b (p-TEFb; ref. 25). This host protein complex can phosphorylate the C-terminal domain (CTD) of human RNA polymerase II, which is required for effective elongation of primary HIV-1 mRNA transcripts (26, 27). To investigate whether p21 can interact with CDK9 in CD4+ T cells, we performed coimmunoprecipitation experiments and kinase assays using protein extracts from primary CD4+ T cells from elite controllers. As shown in Figure 6A, we observed that p21 coprecipitated with CDK9 and, to a lesser degree, with cyclin T, the 2 major components of p-TEFb. Moreover, we found that CDK9 isolated from siRNA-treated, p21-deficient CD4+ T cells had a stronger ability to phosphorylate the CTD of polymerase II, as opposed to CDK9 isolated from p21-proficient CD4+ T cells treated with control siRNA (Figure 6B); this is consistent with an inhibitory effect of p21 on the enzymatic activity of CDK9.
Figure 6. p21 inhibits enzymatic activity of CDK9 and affects HIV-1 transcriptional elongation.
(A) Whole protein lysates of CD4+ T cells from elite controllers were precipitated with anti-CDK9, anti–cyclin T, or unspecific anti-IgG control antibodies and subsequently interrogated with p21-specific antibodies using Western blotting. Shown is 1 representative experiment of 2. (B) Assessment of the enzymatic activity of CDK9 in CD4+ T cells from elite controllers electroporated with p21-specific or control siRNA. CDK9 isolated from electroporated CD4+ T cells was mixed with a recombinant, GST-tagged protein representing the CTD of human RNA polymerase II serving as a substrate for CDK9. Phosphorylation of CTD polymerase II was detected by phospho-Ser2–specific antibodies in 3 representative patients. Cumulative data from 6 patients per group are also shown. Statistical comparison was performed using paired Wilcoxon test. (C) Influence of p21 on transcription of proximal and distal HIV-1 mRNA transcripts. CD4+ T cells from elite controllers were infected with VSV-G–pseudotyped HIV-1 after treatment with p21 inhibitor or DMSO as control. Data are mean and SD fold increase in expression of proximal, intermediate, and distal HIV-1 mRNA transcripts in p21-deficient relative to control cells. Statistical comparison between expression intensity of different mRNA transcripts was performed using Mann-Whitney U test.
To further analyze whether p21-mediated inhibition of CDK9 affects transcriptional elongation of primary HIV-1 mRNA transcripts, we compared the expression of HIV-1 mRNA transcripts located proximal or distal to the transcription initiation site in p21-deficient and -proficient CD4+ T cells, as described in a previous study (28). These experiments indicated that p21 inhibition caused approximately 2-fold increases in the expression of proximal HIV-1 mRNA transcripts, whereas a significantly more pronounced augmentation was observed for the expression of distal HIV-1 mRNA transcripts (Figure 6C); this suggests that p21 affects HIV-1 mRNA transcriptional elongation more strongly than does transcriptional initiation, consistent with an indirect antiretroviral effect of p21 that is mediated through blockade of CDK9/p-TEFb (27). Overall, these results indicate that p21 can reduce HIV-1 mRNA transcription through inhibition of CDK9, the enzymatic component of p-TEFb.
Discussion
Elite controllers represent a model for successful immune activity against HIV-1, and a widespread consensus has emerged that the identification of specific host defense mechanisms in these patients may provide critical information for the design of HIV-1 vaccines and for the development of clinical strategies to induce a functional cure of this disease. Here, we shed light on these mechanisms by demonstrating that p21, a cyclin-dependent kinase inhibitor that has previously been recognized mainly for its role as a tumor suppressor (29), was strongly upregulated in CD4+ T cells from elite controllers and reduced the susceptibility of these cells to HIV-1 by inhibiting viral reverse transcription and mRNA transcription. Moreover, we showed that the antiretroviral effects of p21 on HIV-1 mRNA transcription were, at least in part, indirectly mediated by the ability of p21 to inhibit the enzymatic activity of CDK9, a host protein essential for proper elongation of HIV-1 mRNA (27). Overall, these results suggest that p21 represents a previously unrecognized, CD4+ T cell–intrinsic HIV-1 immune defense mechanism that contributes to the ability of elite controllers to maintain undetectable VLs and resist HIV-1–associated disease manifestations.
To our knowledge, the studies presented here are the first to show that a specific host protein is directly involved in reducing the susceptibility of CD4+ T cells to HIV-1 in vivo in elite controllers. Whether upregulation of p21 expression in T cells is a constitutive characteristic of elite controllers or evolves as part of a specific immune response to HIV-1 infection in these patients is currently unclear, but studies involving genetic relatives of elite controllers and preinfection analysis of HIV-1 patients who develop an elite controller phenotype will be important in this context. Furthermore, it is noteworthy that the CDKN1A gene is located on chromosome 6 in relative proximity to MHC class I genes, some of which are significantly associated with the elite controller phenotype (10, 30). Although we did not observe any relationship between p21 expression intensity in CD4+ T cells and protective HLA alleles, such as HLA-B57 and HLA-B27, it remains to be determined in larger studies whether MHC class I gene polymorphisms associated with better HIV-1 disease outcomes are linked to specific mutations in the CDKN1A gene (31, 32) that may affect its expression. Moreover, the transcription factor Foxo3A, which may be involved in the regulation of CDKN1A gene expression, is uniquely expressed in CD4+ T cells from elite controllers (33), and it will be important to analyze whether it participates in the epigenetic control of high-level p21 expression in such cells.
The ability of p21 to modulate the susceptibility of human cells to HIV-1 infection by interfering with individual viral replication steps is increasingly being recognized. In hematopoietic stem cells, p21 was shown to selectively inhibit HIV-1 integration, while leaving reverse transcription unaffected (20); this might represent a stem cell–specific mode of protection against HIV-1 infection (34). In macrophages, p21 can reduce the efficacy of reverse transcription and integration (19), and prior studies in lymphocytic cell lines suggested that pharmaceutical cyclin-dependent kinase inhibitors can suppress HIV-1 gene expression from proviral HIV-1 DNA (35). Our data in activated primary CD4+ T cells suggest that p21 can independently inhibit HIV-1 reverse transcription and mRNA transcription; an independent effect on HIV-1 integration was less evident, given that ratios between LRT transcripts and integrated HIV-1 copy levels were almost identical between elite controllers and HIV-1–negative persons and were unaffected by p21 inhibition. Overall, these data suggest that p21 can affect multiple early viral replication steps and that the specific influence of p21 on the fate of viral transcripts critically depends on the infected cell type.
As a molecular mechanism underlying the ability of p21 to inhibit viral replication, we show here that p21 blocked the enzymatic activity of CDK9 and its known function to enhance transcriptional elongation of HIV-1 mRNA transcripts through phosphorylation of RNA polymerase II (27, 36). This suggests that p21 affects HIV-1 mRNA transcription by a targeted blockade of a host protein that is essential for effective HIV-1 gene expression. A similar inhibitory effect on CDK9 was recently demonstrated for p57, a cyclin-dependent kinase inhibitor closely related to p21 (37); however, p57 expression in CD4+ T cells did not differ significantly between elite controllers and HIV-1–negative persons and is therefore unlikely to contribute substantially to restriction of HIV-1 infection in elite controllers. It is also important to recognize that pharmaceutical inhibitors of cyclin-dependent kinases can potently suppress HIV-1 replication in vitro by mechanisms that involve CDK9 inhibition (17). The ability of p21 to block the host protein CDK9, instead of interfering directly with viral replication enzymes, may also explain why HIV-1 does not appear to be capable to escape from p21-mediated restriction of HIV-1 replication in a similar manner as it evades alternative host restriction factors. In line with this, no selection of viral escape variants by pharmaceutical cyclin-dependent kinase inhibitors was observed, even when generation of viral escape variants was aggressively attempted by long-term viral cultures in the presence of cyclin-dependent kinase inhibitor monotherapy in suboptimal concentrations (35).
An unresolved issue here is how p21 can affect HIV-1 reverse transcription during the viral preintegration phase. It is possible that p21 indirectly affects reverse transcription through interactions with host proteins that activate different steps of the reverse transcription process, although to our knowledge no such interactions have been documented in prior work. Alternatively, p21 may influence reverse transcription by nonspecifically altering the activation status of the cells. Indeed, p21 has been shown to play a role in regulating activation and proliferation of T lymphocytes in vivo (38). Although we were unable to detect a direct effect of p21 inhibition on CD4+ T cell activation in our short-term culture system of ex vivo activated CD4+ T cells, our experiments do not exclude the possibility that such unspecific effects on cellular physiology may indirectly affect the susceptibility of CD4+ T cells to HIV-1.
Increasing evidence suggests that control of HIV-1 infection in elite controllers is likely to involve multiple different components of the immune system. For instance, a series of prior studies has emphasized the strong cytolytic properties of HIV-1–specific CD8+ T cells in elite controllers (3), which at least in in vitro assays are capable of effectively restricting HIV-1 replication (7). Furthermore, T cells from elite controllers also appear to have unique abilities to proliferate and survive, which may be mediated by a distinct phosphorylation profile of the transcription factor Foxo3a (33). More recently, specific functional properties of myeloid dendritic cells in elite controllers were identified, which were mediated and maintained by a selective expression of immunomodulatory receptors from the leukocyte immunoglobulin-like receptor group (39). It is possible that these different mechanisms all synergize in providing effective HIV-1 immune defense in elite controllers and that, for example, a highly functional CD8+ T cell response against HIV-1 may only develop in the setting of a CD4+ T cell compartment that is less capable of supporting highly replicative HIV-1 infection. Overall, our finding of a cell-intrinsic restriction of HIV-1 replication in CD4+ T cells by upregulation of p21 adds what we believe to be a previously unrecognized component to the understanding of mechanisms contributing to undetectable viremia in elite controllers and might be helpful for future clinical approaches to enhance host resistance to HIV-1 infection.
Methods
Patients.
PBMC samples from HIV-infected individuals and HIV-1–negative persons were used for this study, according to a protocol approved by the Institutional Review Board of Massachusetts General Hospital. All study participants gave written informed consent to participate.
HIV-1 viruses and constructs.
The CXCR4-utilizing primary isolate 92HT599 and the primary CCR5-utilizing HIV-1 strain 91US056 were obtained from the AIDS Research and Reagent Program at the NIH. The Δenv HIV-1 plasmid encoding YFP was derived from the NL4-3 molecular clone (accession no. M19921) and described previously (40, 41). GFP-encoding X4-tropic NL4-3 and R5-tropic Ba-L HIV-1 viruses, as described previously (42), were provided by N. Manel and D. Littman (New York University, New York, New York, USA). Viral particles were produced by transfecting 293T cells with the respective HIV-1 plasmids and, if applicable, with pCG-VSV-G, using TransIT-293 (Mirus) in OptiMEM per the manufacturer’s instructions. Supernatants containing infectious retroviruses were harvested 48 hours after transfection, centrifuged, treated with DNase I (20 U/ml) at room temperature for 1 hour, and stored at –80°C.
Ex vivo infection assays.
CD4+ T cells were stimulated in RPMI medium supplemented with 10% fetal calf serum, recombinant IL-2 (50 U/ml), and an anti-CD3/CD8 bispecific antibody (0.5 μg/ml; ref. 21). After 5 days in culture, a homogenous population of activated HLA-DR+ CD4+ CD3+ T cells with an effector-memory CCR7–CD45RA– phenotype (97.5%) was detected by flow cytometry; the proportion of CD3+CD8+ cytotoxic T cells was less than 0.1%. Cells were infected on day 5 with the designated HIV-1 isolates at the indicated MOIs for 4 hours at 37°C. After 2 washes, cells were resuspended in medium and plated at 5 × 105 cells/well in a 24-well plate. At regular intervals, the cultures were fed by removing and replacing one-half of the culture supernatant with fresh medium. The removed supernatant was cryopreserved for later p24 antigen quantification by ELISA (Dupont); control p24 levels from autologous cells without exogenous viral infection were subtracted from sample p24 levels. Moreover, CD4+ T cells were subjected to flow cytometric analysis of GFP+ CD4+ T cells 96 hours after infection with GFP-encoding X4- or R5-tropic isolates.
In addition to X4- and R5-tropic HIV-1 isolates, activated cells were infected with a VSV-G–pseudotyped HIV-1 vector at a tissue culture infective dose (TCID50) of 1,000 for 2 hours, washed, plated in 24-well plates, harvested after 48 hours of incubation, and processed for flow cytometric analysis of YFP expression. For infection of nonactivated cells, negatively isolated CD4+ T cells with purity greater than 98% of all viable lymphocytes were directly infected with HIV-1 vectors at a TCID50 of 5,000; after cultivation on 24-well plates without exogenous IL-2, cells were analyzed after 96 hours by flow cytometric assessment of YFP expression. When indicated, the p21 inhibitor no. 15 (diluted in DMSO; ref. 24) was added at a concentration of 2 μM 48 hours prior to infection; control samples were treated with DMSO alone.
siRNA-mediated gene knockout.
CD4+ T cells were nucleofected using the Nucleofector device according to the manufacturer’s protocol (Lonza). Briefly, CD4+ T cells were suspended in 100 μl transfection solution (Lonza), and p21-specific or control siRNA (Dharmacon) was added at a concentration of 4 nmol/ml. Samples were then transferred into nucleofection cuvettes and transfected using program T-023. Next, cells were resuspended in culture medium supplemented with 20% fetal calf serum; IL-2 (50 U/ml) was added 2 hours after transfection. 24 hours after infection, expression of p21 was assessed using quantitative RT-PCR and Western blots, and cells were infected with indicated HIV-1 strains.
p21 expression assay.
CDKN1A mRNA expression was analyzed by quantitative RT-PCR using the standard Taqman expression assay with primers and probes synthesized by the instrument manufacturer (Applied Biosystems). Actb (encoding β-actin) was used as a housekeeping gene. For analysis of p21 protein expression, whole protein was extracted from sorted T cells using the Protein and RNA Isolation System (PARIS; Ambion). Following denaturation at 95°C for 10 minutes, samples were resolved on NuPAGE Bis-Tris 10% gels (Invitrogen) and electroblotted. Blots were then hybridized with biotinylated monoclonal antibodies against p21 (clone 187; Santa Cruz Biotechnology) and visualized with a streptavidin-HRP conjugate, followed by ECL detection (Amersham Biosciences) and quantification using a LumiImager. Control hybridizations were performed with a β-actin–specific antibody.
Immunoprecipitation experiments.
CD4+ T cells were suspended in lysis buffer containing 10 mM Tris-HCl, pH 7.4; 100 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM NaF; 20 mM Na3P2O7; 1 mM Na3VO4; 1% Triton X-100; 10% glycerol; 0.1% SDS; 0.5% deoxycholate supplemented with protease inhibitors (containing 4-[2-aminoethyl]benzenesulfonyl fluoride [AEBSF], E-64, bestatin, leupeptin, and aprotinin); and 1 mM phenylmethylsulfonyl fluoride for 2 hours on ice. Equal amounts of cell lysates were incubated with 2 μg of anti-CDK9 antibody (Thermo Scientific), anti-cyclin T1 antibody (Abcam), or rabbit IgG (Santa Cruz Biotechnology) for 2 hours at 4°C. 15 μl of protein A magnetic beads (New England Biolabs) were washed with cell lysis buffer and subsequently incubated with the cell lysates for 1 hour at 4°C. Subsequently, beads were washed 4 times with PBS plus 0.25% Tween. Proteins bound to the beads were dissolved in loading buffer, boiled, and subjected to SDS-PAGE followed by Western blotting using antibodies against p21 (Santa Cruz Biotechnology).
In vitro kinase assay.
CD4+ T cell extracts were prepared in cell lysis buffer as described above. Cleared supernatants were immunoprecipitated with anti-CDK9 antibody (Cell Signaling Technology) and protein A beads. The beads were sequentially washed with lysis buffer, lysis buffer plus 0.5 M LiCl, and kinase buffer (20 mM HEPES, pH 7.5; 50 mM NaCl; 10 mM MgCl2; and 1 mM dithiothreitol). The immunoprecipitate was resuspended in 25 μl kinase buffer containing 5 mM ATP and 0.5 μg purified GST-CTD polymerase II substrate (37). The kinase reaction was conducted at 30°C for 20 minutes and stopped by addition of SDS-PAGE loading buffer. Proteins were separated on SDS-PAGE and transferred to nitrocellulose. The phosphorylation of GST-CTD was determined by RNA polymerase II Ser2 phosphorylation–specific antibodies (Bethyl Laboratories).
Cell sorting and flow cytometry.
PBMCs were stained with a viability dye and indicated monoclonal antibodies for 20 minutes. After 2 washes, viable cells were live-sorted on a FACS Aria cell sorter (BD Biosciences) at 70 pounds per square inch. Live sorting was carried out in an appropriate and specifically designated biosafety cabinet (Baker Hood) according to a NIH–approved protocol. For phenotypic characterization of CD4+ T cells, cells were stained with viability dye and antibodies directed against CD4, HLA-DR, CD25, CD38, CCR5, CXCR4, CCR7, and/or CD45RA, as indicated. After 20 minutes, cells were washed, fixed in paraformaldehyde, and acquired on a LSRII flow cytometer (BD). Data were analyzed using FlowJo software.
Detection of HIV-1 DNA.
For detection of HIV-1 LRTs, cell lysates were harvested 18 hours after infection of activated cells with VSV-G–pseudotyped HIV-1 and 48 hours after infection of activated cells with X4- or R5-tropic HIV-1 or infection of nonactivated cells. Amplification was performed with primers MH531 and MH532 and probe LRT-P, as previously described (43). Cell lysates collected 48 hours (VSV-G–pseudotyped activated CD4+ T cells) or 96 hours (activated cells infected with X4- or R5-tropic HIV-1 or nonactivated cells infected with VSV-G–pseudotyped virus) were used for detection of integrated HIV-1 DNA, using nested PCR with Alu-1/Alu-2 primers and HIV-1 LTR primer L-M667 for the first-round PCR and LTR primer AA55M, Lambda T primers, and MH603 probe for the second-round quantitative PCR (43–45). Primers (CCR5 forward, 5′-GCTGTGTTTGCGTCTCTCCCAGGA-3′; CCR5 reverse, 5′-CTCACAGCCCTGTGCCTCTTCTTC-3′) and probe (5′-FAM-AGCAGCGGCAGGACCAGCCCCAAG-TAMRA-3′) amplifying CCR5 as a housekeeping gene were used for the quantification of input cell numbers. Serial dilutions of DNA from cell lysates of the HIV-1–infected cell line 293T (provided by F. Bushman, University of Pennsylvania, Philadelphia, Pennsylvania, USA) were used for reference purposes.
HIV-1 mRNA analysis.
mRNA extracted 48 hours (activated cells infected with VSV-G–pseudotyped virus) or 96 hours (activated cells infected with X4- or R5-tropic HIV-1 or nonactivated cells infected with VSV-G–pseudotyped virus) after infection was reverse transcribed according to standard protocols. HIV-1 cDNA was amplified using SYBR Green–based quantitative PCR with forward primer 5-GTAATACCCATGTTTTCAGCATTATC-3 and reverse primer 5-TCTGGCCTGGTGCAATAGG-3 (bp 836–1,015 from transcription initiation site). A proximal HIV-1 mRNA fragment located adjacent to the transcription initiation site (bp 29–180) was amplified with forward primer 5-TGGGAGCTCTCTGGCTAACT-3 and reverse primer 5-TGCTAGAGATTTTCCACACTGA-3. A more distal HIV-1 mRNA fragment was amplified using forward primer 5-GAGAACTCAAGATTTCTGGGAAG-3 and reverse primer 5-AAAATATCGATCGCCCACAT-3 (bp 2,341–2,433 from transcription initiation site). Data were normalized to the housekeeping gene Actb.
Statistics.
Data are summarized as means and SD or using box and whisker plots (indicating the median, interquartile range, and minimum and maximum values). Pearson’s correlation coefficient was calculated to analyze correlations. Differences between nominal data were tested for statistical significance by 2-tailed Student’s t test, Mann-Whitney U test, or paired Wilcoxon test as appropriate. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
X.G. Yu and M. Lichterfeld are both recipients of the Doris Duke Clinical Scientist Development Award. This study was supported by the NIH (grants AI078799 and AI089339 to X.G. Yu and AI093203 to M. Lichterfeld). Work in the laboratory of R.H. Weiss was supported by the NIH (grants CA135401 and DK082690) and by the Medical Service of the US Department of Veterans’ Affairs. W.D. Cress is supported by the National Cancer Institute (grant P50 CA119997) and the Florida Department of Health (grant FDH 08BB-05). Elite controller patient recruitment was supported by the Mark and Lisa Schwartz Foundation and the Bill and Melinda Gates Foundation. The authors would like to thank Frederic Bushman (University of Pennsylvania) for sharing the HIV-1 infected cell line 293T, Nicholas Chomont (VGTI Florida) for sharing a detailed protocol for assessment of integrated HIV-1 DNA, Nicholas Manel and Dan R. Littman (New York University) for sharing GFP-encoding X4- and R5-tropic HIV-1 viruses, and Daniel Kavanagh and Sylvie Le Gall (Ragon Institute) for helpful discussion.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(4):1549–1560. doi:10.1172/JCI44539.
References
- 1.Saez-Cirion A, et al. HIV controllers–How do they tame the virus? Trends Immunol. 2007;28(12):532–540. doi: 10.1016/j.it.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Walker BD. Elite control of HIV Infection: implications for vaccines and treatment. Top HIV Med. 2007;15(4):134–136. [PubMed] [Google Scholar]
- 3.Migueles SA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migueles SA, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 5.Lichterfeld M, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200(6):701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez-Cirion A, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saez-Cirion A, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. . J Immunol. 2009;182(12):7828–7837. doi: 10.4049/jimmunol.0803928. [DOI] [PubMed] [Google Scholar]
- 9.Emu B, et al. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. . J Virol. 2008;82(11):5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereyra F, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria-Rose NA, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84(3):1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blankson JN, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. . J Virol. 2007;81(5):2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamine A, et al. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study). AIDS. 2007;21(8):1043–1045. doi: 10.1097/QAD.0b013e3280d5a7ac. [DOI] [PubMed] [Google Scholar]
- 14.Julg B, et al. Infrequent recovery of HIV from, but robust exogenous infection of activated CD4+ T-cells from HIV elite controllers. Clin Infect Dis. 2010;51(2):233–238. doi: 10.1086/653677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi SK, Siliciano JD, Bailey JR, Siliciano RF, Blankson JN. Role of APOBEC3G/F-mediated hypermutation in the control of human immunodeficiency virus type 1 in elite suppressors. J Virol. 2008;82(6):3125–3130. doi: 10.1128/JVI.01533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strebel K, Luban J, Jeang KT. Human cellular restriction factors that target HIV-1 replication. BMC Med. 2009;7:48. doi: 10.1186/1741-7015-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schang LM, St Vincent MR, Lacasse JJ. Five years of progress on cyclin-dependent kinases and other cellular proteins as potential targets for antiviral drugs. Antivir Chem Chemother. 2006;17(6):293–320. doi: 10.1177/095632020601700601. [DOI] [PubMed] [Google Scholar]
- 18.Schang LM. Cyclin-dependent kinases as cellular targets for antiviral drugs. J Antimicrob Chemother. 2002;50(6):779–792. doi: 10.1093/jac/dkf227. [DOI] [PubMed] [Google Scholar]
- 19.Bergamaschi A, David A, Le Rouzic E, Nisole S, Barre-Sinoussi F, Pancino G. The CDK inhibitor p21Cip1/WAF1 is induced by FcgammaR activation and restricts the replication of human immunodeficiency virus type 1 and related primate lentiviruses in human macrophages. J Virol. 2009;83(23):12253–12265. doi: 10.1128/JVI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Scadden DT, Crumpacker CS. Primitive hematopoietic cells resist HIV-1 infection via p21. . J Clin Invest. 2007;117(2):473–481. doi: 10.1172/JCI28971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spentzou A, et al. Viral inhibition assay: a CD8 T cell neutralization assay for use in clinical trials of HIV-1 vaccine candidates. J Infect Dis. 2010;201(5):720–729. doi: 10.1086/650492. [DOI] [PubMed] [Google Scholar]
- 22.Hunt PW, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotger M, et al. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 2010;6(2):e1000781. doi: 10.1371/journal.ppat.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SH, Wang X, Liu R, Lam KS, Weiss RH. High throughput screening of a small molecule one-bead-one-compound combinatorial library to identify attenuators of p21 as chemotherapy sensitizers. Cancer Biol Ther. 2008;7(12):2015–2022. doi: 10.4161/cbt.7.12.7069. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Yik JH. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev. 2006;70(3):646–659. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancebo HS, et al. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 1997;11(20):2633–2644. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores O, et al. Host-cell positive transcription elongation factor b kinase activity is essential and limiting for HIV type 1 replication. Proc Natl Acad Sci U S A. 1999;96(13):7208–7213. doi: 10.1073/pnas.96.13.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R, Liu M, Zhang K, Zhou Q. Isolation and functional characterization of P-TEFb-associated factors that control general and HIV-1 transcriptional elongation. Methods. 2011;53(1):85–90. doi: 10.1016/j.ymeth.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 31.Gravina S, et al. Identification of single nucleotide polymorphisms in the p21 (CDKN1A) gene and correlations with longevity in the Italian population. Aging (Albany NY). 2009;1(5):470–480. doi: 10.18632/aging.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong EK, et al. p21 gene polymorphisms in systemic lupus erythematosus. Rheumatology (Oxford). 2007;46(2):220–226. doi: 10.1093/rheumatology/kel210. [DOI] [PubMed] [Google Scholar]
- 33.van Grevenynghe J, et al. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med. 2008;14(3):266–274. doi: 10.1038/nm1728. [DOI] [PubMed] [Google Scholar]
- 34.Bieniasz PD. An intrinsic host defense against HIV-1 integration? J Clin Invest. 2007;117(2):302–304. doi: 10.1172/JCI31290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, et al. Inhibition of human immunodeficiency virus type 1 transcription by chemical cyclin-dependent kinase inhibitors. J Virol. 2001;75(16):7266–7279. doi: 10.1128/JVI.75.16.7266-7279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieniasz PD, Grdina TA, Bogerd HP, Cullen BR. Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription. Proc Natl Acad Sci U S A. 1999;96(14):7791–7796. doi: 10.1073/pnas.96.14.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Y, Chen L, Wright GM, Pillai SR, Chellappan SP, Cress WD. CDKN1C negatively regulates RNA polymerase II C-terminal domain phosphorylation in an E2F1-dependent manner. J Biol Chem. 2010;285(13):9813–9822. doi: 10.1074/jbc.M109.091496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balomenos D, et al. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat Med. 2000;6(2):171–176. doi: 10.1038/72272. [DOI] [PubMed] [Google Scholar]
- 39.Huang J, et al. Leukocyte immunoglobulin-like receptors maintain unique antigen-presenting properties of circulating myeloid dendritic cells in HIV-1-infected elite controllers. J Virol. 2010;84(18):9463–9471. doi: 10.1128/JVI.01009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brass AL, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319(5865):921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 41.Sutton RE, Wu HT, Rigg R, Bohnlein E, Brown PO. Human immunodeficiency virus type 1 vectors efficiently transduce human hematopoietic stem cells. J Virol. 1998;72(7):5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189(11):1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7(5):631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- 44.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brussel A, Delelis O, Sonigo P. Alu-LTR real-time nested PCR assay for quantifying integrated HIV-1 DNA. Methods Mol Biol. 2005;304:139–154. doi: 10.1385/1-59259-907-9:139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.