Abstract
Axonal regeneration in C. elegans was first reported five years ago. Individual GFP-labeled axons can be severed using laser microsurgery and their regrowth followed in vivo. Several neuron types display robust regrowth after injury, including motor and sensory neurons. The small size and transparency of C. elegans make possible large-scale genetic and pharmacological screens for regeneration phenotypes.
Introduction
Since the work of Ramon y Cajal it has been known that axons have the ability to regrow after damage (Ramon y Cajal, 1913–14). Axon regeneration has been long studied in a variety of vertebrate and invertebrate organisms, but until recently had not been demonstrated in the nematode C. elegans. The small size, anatomical simplicity, and transparency of C. elegans allows genetically labeled axons to be severed in vivo using a laser and their regrowth followed with single-axon resolution. In 2004 C. elegans motor neurons were shown to regrow after femtosecond laser surgery and to re-establish functional connections (Yanik et al., 2004). This work revealed the potential of C. elegans for large scale genetic screening to discover new regulators of axon regrowth, a promise that has recently been fulfilled with the recent discovery of the central role of the DLK kinase pathway in axon regrowth (Hammarlund et al., 2009; Yan et al., 2009). Here we first review some basic biology of axon regeneration, then how axons can be severed in C. elegans and the insights into regeneration that have emerged from C. elegans studies.
Axon regeneration is regulated by extrinsic and intrinsic factors
Axons in many organisms are capable of extensive regrowth after injury, suggesting an intrinsic capacity for regenerative growth is a conserved feature of differentiated neurons (Chen et al., 2007). However not all axons regrow after injury, due to variation either in intrinsic ability to regrow, or the presence of extrinsic inhibitory environment. The most notable exception to the rule of regeneration competence is in the adult mammalian central nervous system (CNS). The inability of most adult CNS axons to regrow results in part from the inhibitory microenvironment of the CNS, specifically myelin and chondroitin sulfate proteoglycans (Yiu and He, 2006). Over the past decade an intensive effort has identified several of the inhibitory components of CNS myelin as well their neuronal receptors (McKerracher et al., 1994; Atwal et al., 2008). Importantly, the inhibitory effects of myelin can be overcome in part by manipulating neuronal signaling pathways such as the cAMP pathway (Qiu et al., 2002). Other ‘intrinsic regulators’ of regrowth ability include the mTor pathway (Park et al., 2008). Numerous studies have identified ‘regeneration associated genes’ whose mRNAs are upregulated after injury; some, such as the Krüppel-like transcription factors, also play critical roles in determining intrinsic regrowth potential (Moore et al., 2009).
Femtosecond laser surgery of C. elegans axons
The C. elegans nervous system contains 302 neurons, each with a simple and largely invariant morphology. Neurons can be labeled with genetically encoded fluorescent proteins and imaged with single axon resolution. Since the early days of C. elegans work, ultraviolet lasers have been widely used to ablate cell bodies (Sulston and White, 1980). During the 1990s, the development of femtosecond laser technology opened up the possibility of more spatially controlled surgical manipulations. The physical basis of femtosecond laser surgery has been reviewed recently (Chung and Mazur, 2009; Tsai et al., 2009). Femtosecond lasers use extremely short pulses (~100 fs) to deliver infrared laser light with high peak power. Femtosecond lasers usually can be operated with a high pulse repetition rate (“MHz mode”) or a lower repetition rate (“kHz mode”). The higher repetition rate of MHz mode laser surgery can create a larger area of damage, whereas kHz mode surgery is more precise and can dissect subcellular structures (Wu et al., 2007; Bourgeois and Ben-Yakar, 2008). UV lasers can also be used to sever C. elegans axons, but with less fine control (Wu et al., 2007). As well as allowing studies of regenerative growth, femtosecond laser surgery can define the role of specific neuronal processes in behavior, focusing on the acute effects of surgery prior to any regenerative response (Chung et al., 2006; Zhang et al., 2008). Several groups have now demonstrated the feasibility of laser surgery on worms immobilized in microfluidic devices (Rohde et al., 2007; Guo et al., 2008; Zeng et al., 2008), permitting high-throughput surgery and screens for regrowth phenotypes.
Patterns of regeneration of C. elegans neurons
Several kinds of C. elegans neuron display regrowth responses after surgery, although the exact pattern and extent of regeneration varies depending on the type of neuron (see Table 1), developmental stage, and subcellular location of the position of axotomy. Here we describe some aspects of the regrowth responses of motor and sensory axons severed in late larval or young adult stages. Femtosecond laser surgery creates a 1–5 µm break in the axon, followed by a retraction of the cut ends of the axonal process (Wu et al., 2007). Within a few hours, filopodia form from the stump of the proximal fragment (i.e., proximal to the cell body); distal axonal fragments have also been observed to extend small processes and growth cones in some circumstances (Guo et al., 2008). Between 6 and 10 hours post surgery a growth cone-like structure typically forms from the proximal stump and begins to extend (Figure 1) (Wu et al., 2007; Gabel et al., 2008). The delay between axotomy and growth cone formation is variable and may be affected by the presence of anesthetic used for immobilization (Guo et al., 2008). The rate of axon regrowth is typically slower than the highest rates of growth cone extension observed during development (Wu et al., 2007). In PLM neurons we have observed regrowing axons extend up to ~200 µm over the 24 h post axotomy, although the average regrowth is ~100 µm. Although normally distributed, the growth is highly variable. It is not currently understood why there is such stochastic variation in regrowth; it could reflect both biological variability and variation in experimental conditions. Growth cone extension and branching can continue for 2–3 days after axotomy.
Table 1.
Regenerative abilities of different types of C. elegans neuron
| Neuron Type | Name of the neuronal process | Pattern of regrowth after axotomy in L4/adult stage |
|---|---|---|
| GABAergic Motor Neurons | DD and VD commissures | Regrow when cut laterally or at ventral cord. 70% of the commissures reaches dorsal cord at 24h (Yanik et al., 2004; Wu et al., 2007; Hammarlund et al., 2009) |
| Cholinergic Motor Neurons | DA, DB commissures | Regrowth to dorsal cord (Gabel et al., 2008) |
| Mechanosensory touch neuron | PLM axon | Regrows ~100 µm at 24 h after axotomy when cut 50 µm from cell body. 25% of regrowing axons reach ventral cord either by making branch or by growing directly to cord. |
| ALM axon | Regrows ~100 µm (one-third of original length of ALM) at 24h after axotomy | |
| AVM axon | Regrows ~60 µm at 24 h after axotomy 65% of regrowing AVM reach ventral cord (Gabel et al., 2008) | |
| Serotonergic egg laying Motor neuron | HSN axon | Some regrowth; extent not quantitated (Gabel et al., 2008) |
| Chemosensory amphid neurons | ASH axon | No regrowth (Gabel et al., 2008) |
| AWC axon | No regrowth (Gabel et al., 2008) | |
| AWB dendrite | Partial regrowth (Wu et al., 2007) | |
| ASH dendrite | No recovery of function (Chung et al., 2006) | |
| Thermosensory amphid neuron | AFD dendrite | No recovery of function (Chung et al., 2006) |
| Phasmid neuron | PHA, PHB dendrites | No regrowth (Chung et al., 2006) |
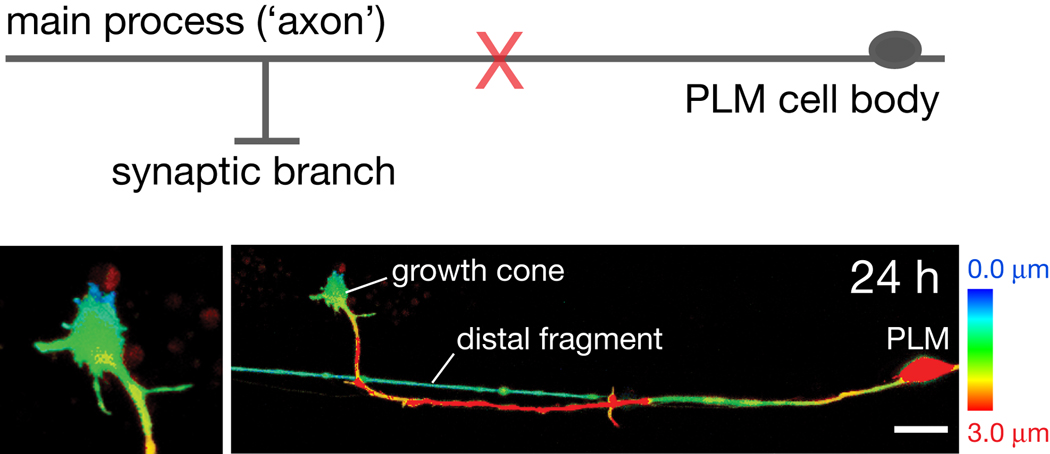
Figure 1. Axotomy and regrowth of PLM mechanosensory neuron.
Diagram of the PLM mechanosensory neuron showing position of laser axotomy relative to the synaptic branch and cell body. Lower panels, confocal projection of PLM regrowth 24 h post axotomy, showing a growth cone at the tip of the regrowing axon (enlarged in left panel) and the distal fragment of the axon. Different focal planes in the z axis are color coded from surface (blue) to deep (red). Transgenic marker, Pmec-4-GFP (zdIs5). Scale, 10 µm.
Different neuron types display different regenerative abilities, summarized in Table 1. The initial studies of Yanik et al. (2004) focused on inhibitory GABAergic motor neurons, which can regrow and re-establish motor function in adults. Mechanosensory neurons (PLM, ALM, AVM) display robust regrowth and have been studied in several labs (Wu et al., 2007; Gabel et al., 2008). The HSN neurons also show robust regrowth (Gabel et al., 2008). In contrast, several amphid or phasmid chemosensory neurons display little or no regrowth in L4 or adult stages (Yanik et al., 2006; Wu et al., 2007; Gabel et al., 2008). Such neurons have a bipolar morphology, with a sensory process (‘dendrite’) and an axon with presynaptic specializations. The dendrites of AWB display a small regrowth response (Wu et al., 2007), whereas dendrites of the thermosensory AFD neurons do not appear to regrow (Chung et al., 2006). The axonal processes of AWC and ASH also fail to regrow after axotomy, although amphid axons severed in earlier larval stages have been reported to regrow (Chung and Mazur, 2009). It is not yet clear whether the low regrowth of these neurons reflects a decline in intrinsic ability or the development of an inhibitory microenvironment.
The position of axotomy within the cell is often a major determinant of regrowth. As a rule, the regrowth response changes with distance of axotomy from the cell body (Wu et al., 2007; Gabel et al., 2008). In addition, in branched PLM neurons the position of axotomy relative to the synaptic branch affects the degree of regrowth (Wu et al., 2007). After axotomy distal to the branch point, almost no regrowth occurs, in contrast to axotomy at any point proximal to the branch. However when the branch and main process are severed simultaneously, regeneration of the main process is observed. These observations suggest that the synaptic branch can actively repress regrowth from the distal part of the main process. The mechanism of such ‘branch inhibition’ could be of general interest, as many spinal cord neurons have collateral branches.
After axotomy the isolated distal fragment of a C. elegans axon appears to undergo degeneration and fragmentation, as judged by the beading and disappearance of GFP labels from distal fragments (Yanik et al., 2006; Wu et al., 2007). This degeneration appears reminiscent of Wallerian degeneration, long studied in vertebrates and Drosophila. Wallerian degeneration is an active process whose mechanism has been the subject of intense scrutiny (Raff et al., 2002; Vargas and Barres, 2007). At present it is not known whether axon degeneration in C. elegans shares any molecular similarity with Wallerian degeneration in other organisms. Notably, the degeneration can be prevented by the apparent fusion of the regrowing axon with the distal axon fragment (Yanik et al., 2006). Reconnection or fusion events are frequently observed after the more localized damage caused by kHz mode axotomy (Guo et al., 2008). Fusion of axonal fragments has been reported in other organisms (Deriemer et al., 1983), but its molecular basis remains unknown.
The DLK-1 pathway is essential for axon regeneration
Insight into the regulation of regrowth came from an ingenious screen exploiting an axon fragility mutant. Loss of function in the neuronal β-spectrin UNC-70 results in fragile axons; during larval development, movement of the animal triggers progressive axon fragmentation, which then provokes spontaneous regrowth from damaged fragments (Hammarlund et al., 2007). By screening for genes required for spontaneous regrowth Hammarlund et al. found that a MAP kinase cascade involving the MAPKKK DLK-1, MAPKK MKK-4 and the p38 MAPK PMK-3 was essential for regrowth of GABAergic motor neurons (Hammarlund et al., 2009). Moreover, overexpression of DLK-1 causes improved regeneration of motor neurons following axotomy, suggesting activity of the DLK-1 pathway is rate limiting.
Loss of function in the DLK-1 pathway has no detectable effect on developmental axon outgrowth in the wild type. However the DLK-1 pathway had been previously identified in the context of synaptogenesis, where it is regulated by the presynaptic protein RPM-1 (Nakata et al., 2005). What are the downstream targets of this pathway? Recent work has shown that a key target of DLK-1 is the CCAAT/Enhancer Binding Protein related bZip protein CEBP-1 (Yan et al., 2009). Like DLK-1, CEBP-1 is not required for developmental axon outgrowth but is essential for axotomy-triggered regrowth. Intriguingly, both DLK-1 and CEBP-1 are localized to synapses and axons, suggesting they could function locally in response to axonal injury. Indeed, cebp-1 mRNA is axonally localized and axotomy triggers local translation of CEBP-1 in proximal and distal axon fragments (Yan et al., 2009). The role of axonally translated CEBP-1 is not yet clear; it may be retrogradely transported to the cell body, or might have an as yet unknown function in the axon.
How does injury trigger the DLK-1 cascade? In synaptogenesis, DLK-1 is downregulated by the E3 ubiquitin ligase RPM-1. An attractive model might be that axon injury either inhibits RPM-1 or disrupts the RPM-1/DLK-1 interaction, perhaps by physically separating synaptic RPM-1 from axonally transported DLK-1. However although rpm-1 mutants display slightly increased motor neuron regrowth (Hammarlund et al., 2009) they have apparently normal mechanosensory neuron regrowth after axotomy (Yan et al., 2009). Additional pathways may also be involved in activation of DLK-1 in response to axonal damage. Recent work shows that laser axotomy triggers a local calcium transient in the severed axon, and that increasing calcium or cAMP levels can enhance regeneration in PLM neurons (Ghosh-Roy et al., in press). The effects of elevated calcium or cAMP require DLK-1, consistent with these second messenger cascades acting upstream of DLK-1 in an axonal response to injury.
Regrowth of adult axons does not simply recapitulate developmental outgrowth
A regrowing axon must in some ways recapitulate its own outgrowth in embryonic development, extending and navigating in response to local and long-range cues to reach its correct target area. The environment navigated by an axon in adult stages is very different from that of embryogenesis: developmental guidance cues may no longer be expressed, may be expressed in different patterns from that in development, or may be diluted by the increased dimensions of the adult body. Adult cells and tissues may physically block movements of regrowing axons.
GABAergic motor neurons reliably regrow dorsally towards their muscle targets in late larval and adult stages (Wu et al., 2007). During development the motor neuron growth cones navigate away from ventral sources of netrin/UNC-6. Presumably, a gradient of netrin/UNC-6 gradient remains in later stages and is responsible for the dorsal guidance of regrowing motor commissures. Direct tests of the roles of netrin/UNC-6 in motor axon regrowth remain challenging as the mutants have drastic defects in developmental guidance.
The situation is slightly clearer in the AVM touch neuron (Gabel et al., 2008). Developmental AVM ventral guidance is driven by a combination of attraction to ventral netrin and repulsion from dorsal cues such as Slit and the TGFβ family member UNC-129. In adult animals AVM targeting to the ventral midline remains accurate and requires both netrin/UNC-6 and Slit/SLT-1. Surprisingly, however, their receptors (DCC/UNC-40 and Robo/SAX-3, respectively) do not appear to be required for AVM regrowth or ventral guidance, suggesting other receptors may participate in netrin or Slit signal transduction during regenerative growth. The identity of such receptors is not yet known.
The cues guiding the anterior growth of the longitudinal processes of touch neurons during embryogenesis remain less well characterized. ALM and PLM can regrow accurately when severed in the L1 stage, but their anterior guidance slowly declines during larval development (Wu et al., 2007). Signaling via the Eph receptor VAB-1 is known to position the termination point of PLM in development. In contrast, during regeneration VAB-1 activity appears to interfere with guidance of the regrowing PLM axon (Wu et al., 2007). This is reminiscent of several findings in vertebrates, in which guidance cues exert aberrant inhibitory effects on regrowing axons (Low et al., 2008).
Perspectives
C. elegans is rapidly becoming established as a tractable model for studying the genetic basis of axon repair. The pace of technological improvements suggests that more automated axotomy procedures could be feasible in the near future, permitting large scale screening for genes or chemicals that affect regenerative growth. Such screening is clearly essential to exploit the unique virtues of the C. elegans model.
What might be the selective advantage of a robust regeneration pathway to C. elegans? Under normal laboratory conditions C. elegans neurons do not undergo spontaneous breakage, however in ‘the wild’ it is likely that C. elegans encounters mechanical or environmental traumas that could potentially damage axons. Unlike some other organisms C. elegans does not appear to retain neuronal stem cells after early larval stages, and so cannot replace damaged neurons. Axonal repair pathways such as DLK-1 may have evolved from homeostatic mechanisms that fine tune axon growth in response to synaptic cues. Such pathways could be co-opted to protect the existing neuronal circuit from environmental damage throughout life.
Most C. elegans studies so far have focused on regeneration-competent neurons in the periphery. In the future it will be important to determine why some axons are not able to regrow, and whether this is related to their position in process bundles. Functional recovery after motor neuron axotomy has been attributed to the regrowth of axons to their target muscles. However C. elegans muscles are unusual in that they extend muscle arms to neuropil regions where they form neuromuscular junctions. It would be interesting to examine whether muscle arms also become reoriented after axotomy. The transparency of C. elegans allows live imaging of calcium dynamics and intracellular transport in single axons; it will be interesting to see how these processes operate during regenerative growth. Finally, the remarkable ability of axon fragments to undergo fusion implies specific mechanisms for recognition and fusion of damaged axons. Analysis in C. elegans could uncover the molecular basis of this ‘self-repair’ process.
Acknowledgements
We thank Zhiping Wang for comments. Our work on axon regeneration is funded by the U.S. National Institutes of Health (R01 NS057317).
References
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Bourgeois F, Ben-Yakar A. Femtosecond laser nanoaxotomy properties and their effect on axonal recovery in C. elegans. Opt Express. 2008;16:5963. doi: 10.1364/oe.16.005963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Chung SH, Clark DA, Gabel CV, Mazur E, Samuel AD. The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation. BMC Neurosci. 2006;7:30. doi: 10.1186/1471-2202-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Mazur E. Femtosecond laser ablation of neurons in C. elegans for behavioral studies. Applied Physics A. 2009;96:335–341. [Google Scholar]
- Deriemer SA, Elliott EJ, Macagno ER, Muller KJ. Morphological evidence that regenerating axons can fuse with severed axon segments. Brain Res. 1983;272:157–161. doi: 10.1016/0006-8993(83)90373-6. [DOI] [PubMed] [Google Scholar]
- Gabel CV, Antoine F, Chuang CF, Samuel AD, Chang C. Distinct cellular and molecular mechanisms mediate initial axon development and adult-stage axon regeneration in C. elegans. Development. 2008;135:1129–1136. doi: 10.1242/dev.013995. [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in C. elegans and require DLK 1 kinase. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.5464-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SX, Bourgeois F, Chokshi T, Durr NJ, Hilliard MA, Chronis N, Ben-Yakar A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat Methods. 2008;5:531–533. doi: 10.1038/nmeth.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking beta-spectrin. J Cell Biol. 2007;176:269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Cajal's degeneration and regeneration of the nervous system. New York, N.Y.: Oxford University Press; 1913–14. [Google Scholar]
- Rohde CB, Zeng F, Gonzalez-Rubio R, Angel M, Yanik MF. Microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc Natl Acad Sci U S A. 2007;104:13891–13895. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, White JG. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 1980;78:577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Blinder P, Migliori BJ, Neev J, Jin Y, Squier JA, Kleinfeld D. Plasma-mediated ablation: an optical tool for submicrometer surgery on neuronal and vascular systems. Curr Opin Biotechnol. 2009;20:90–99. doi: 10.1016/j.copbio.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci U S A. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- Yanik MF, Cinar H, Cinar HN, Gibby A, Chisholm AD, Jin Y, Ben-Yakar A. Nerve regeneration in Caenorhabditis elegans after femtosecond laser axotomy. IEEE Journal of Selected Topics in Quantum Electronics. 2006;12:1283–1291. [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Rohde CB, Yanik MF. Sub-cellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond-laser manipulation. Lab Chip. 2008;8:653–656. doi: 10.1039/b804808h. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chung SH, Fang-Yen C, Craig C, Kerr RA, Suzuki H, Samuel AD, Mazur E, Schafer WR. A self-regulating feed-forward circuit controlling C. elegans egg-laying behavior. Curr Biol. 2008;18:1445–1455. doi: 10.1016/j.cub.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]