SUMMARY
Neoantigens derived from somatic mutations in tumors may provide a critical link between the adaptive immune system and cancer. Here we describe a system to introduce exogenous antigens into genetically engineered mouse lung cancers to mimic tumor neoantigens. We show that endogenous T cells respond to and infiltrate tumors, significantly delaying malignant progression. Despite continued antigen expression, T cell infiltration does not persist and tumors ultimately escape immune attack. Transplantation of cell lines derived from these lung tumors or prophylactic vaccination against the autochthonous tumors, however, results in rapid tumor eradication or selection of tumors that lose antigen expression. These results provide insight into the dynamic nature of the immune response to naturally arising tumors.
INTRODUCTION
The potential for functionally important interactions between a developing tumor and the immune system has been appreciated for over a century. The association of tumor cells and lymphocytes has led researchers to postulate that the immune system actively inhibits the formation and progression of transformed cells and ultimately “shapes” nascent tumors by forcing the selective evolution of tumor cells that can evade the immune response, a phenomenon called tumor immunoediting (Dunn et al., 2002). Significant numbers of T cells specific to mutated tumor proteins have been identified in cancer patients (Finn, 2008; Novellino et al., 2005; Parmiani et al., 2007; Rubio et al., 2003) and several studies have correlated immune infiltration in tumors with improved prognosis (Buckanovich et al., 2008; Budhu et al., 2006; Finak et al., 2008; Galon et al., 2006; Molldrem et al., 2000; Pages et al., 2005; Piersma et al., 2007). However, the persistence of malignant disease despite immune recognition presents an important medical and therapeutic question: how do tumors escape immune surveillance?
Several mouse models have been used to gain insights into the mechanisms by which tumors may subvert immune responses, but each of these has critical limitations. Transplantation of primary or cultured tumor cells is commonly used, but these models are limited because they ectopically introduce large numbers of fully-developed tumor cells that grow rapidly, and transplantation can initiate proinflammatory responses or traffic tumor cells directly to lymphoid organs (Khong and Restifo, 2002; Ochsenbein et al., 2001; Spiotto et al., 2002). Carcinogen-induced models have focused predominantly on sarcomas rather than cancers of epithelial origin and these tumors are expected to harbor large numbers of mutations that may lead to artificially robust immune responses (Dunn et al., 2002; Khong and Restifo, 2002). Transgenic mouse models of cancer that develop tumors spontaneously and express model tumor antigens throughout targeted organs fail to fully recapitulate immune responses against the human disease because antigen expression in normal tissues likely alters the T cell response, and thymic deletion of antigen-specific T cells typically prevents the study of endogenous T cell responses to tumors (Bai et al., 2008; Drake et al., 2005; Getnet et al., 2009; Lyman et al., 2004; Muller-Hermelink et al., 2008; Nguyen et al., 2002; Savage et al., 2008; Smith et al., 1997; Speiser et al., 1997). Furthermore, as transgenic models rely on sporadic tumor-initiating events that can vary widely from tumor to tumor and mouse to mouse (Frese and Tuveson, 2007), it is difficult to follow the dynamics of the T cell response over time.
In contrast, genetically engineered mouse models of many human cancers accurately recapitulate both the genetic and histopathologic progression of the human disease from its earliest lesions to metastasis and can provide spatiotemporal control of tumor onset (Frese and Tuveson, 2007). However, few studies have employed these models to elucidate the interplay between tumors and the immune system (Cheung et al., 2008; Clark et al., 2007; Huijbers et al., 2006; Willimsky and Blankenstein, 2005). This is especially true in the context of lung cancer, which is responsible for more than one million deaths each year worldwide. In an effort to investigate the effect of T cell responses against tumor-specific antigens on tumor progression, we developed a system to induce potentially immunogenic autochthonous lung adenocarcinomas in a genetically engineered mouse model of the disease.
RESULTS
Autochthonous lung tumors expressing tumor antigens are infiltrated by lymphocytes early during tumor development
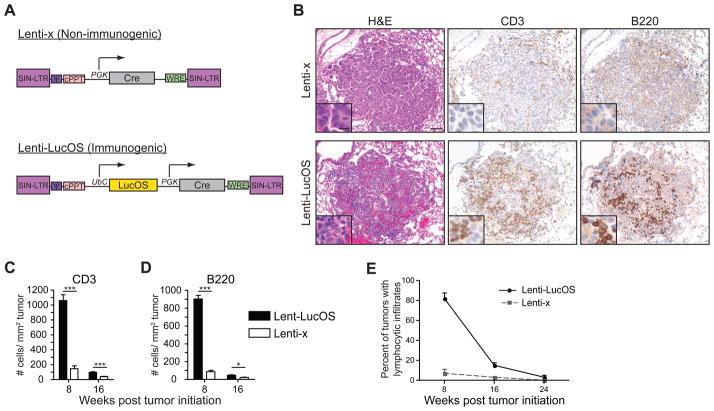
To generate autochthonous lung tumors in a spatiotemporally controlled fashion, we utilized a model of human lung adenocarcinoma driven by the conditional expression of oncogenic K-rasG12D in combination with the loss of p53 (DuPage et al., 2009; Jackson et al., 2005; Jackson et al., 2001). Tumor formation was initiated in K-rasLSL-G12D/+;p53fl/fl mice by inhalation of lentiviral vectors expressing Cre recombinase. To induce tumors that lack expression of model antigens, we used a lentiviral vector expressing Cre alone (Lenti-x, Figure 1A). These tumors exhibited little to no lymphocyte infiltration throughout their development as assessed by hematoxylin and eosin (H&E) staining and immunohistochemistry (Figure 1B–1E). To induce tumors that express model tumor antigens, we engineered lentiviral vectors that, in addition to Cre, express the T cell antigens SIYRYYGL (SIY) and two antigens from ovalbumin – SIINFEKL (SIN, OVA257–264) and OVA323–339 – fused to the C-terminus of luciferase, enabling us to monitor antigen expression in tumors (Lenti-LucOS, Figure 1A). As shown in Figure 1, Lenti-LucOS-induced tumors had large numbers of infiltrating lymphocytes including both T and B cells at 8 weeks after tumor initiation (Figure 1B–1E). There were no differences in innate immune cell infiltrates between tumor types (Figure S1).
Figure 1. Induction of lung tumors using lentiviral vectors.
(A) Design of Lenti-x and Lenti-LucOS (SIN-LTR: self-inactivating long terminal repeat, ψ: HIV packaging signal, cPPT: central polypurine tract, PGK: phosphoglycerate kinase promoter, WRE: woodchuck post-transcriptional regulatory element, UbC: ubiquitin C promoter).
(B) Lung tumors induced with Lenti-x or Lenti-LucOS were stained with H&E, anti-CD3, or anti-B220, 8 weeks after tumor initiation. Scale = 50μm (inset 10μm).
(C and D) Quantification of immune infiltrates by IHC for CD3 and B220 8 and 16 weeks post tumor initiation. p-values are ~10−8, and ~10−3 for CD3 and ~10−15 and 0.02 for B220 at 8 and 16 weeks, respectively. n= 2–5 mice, 16–100 tumors, per group.
(E) Percent of lung tumors/mouse containing infiltrating lymphocytes at 8, 16, and 24 weeks after tumor initiation. n= 9–22 mice, 49–1594 tumors, per group.
Data are mean ± SEM. See also Figure S1.
T cell responses against tumor antigens are generated but not maintained
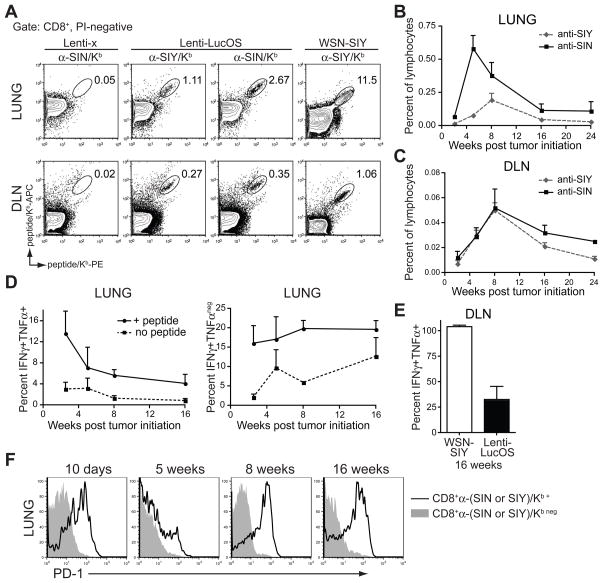
To examine the T cell response to LucOS expression in tumors over time, we assessed the degree of T cell infiltration at 16 and 24 weeks after tumor initiation. In contrast to the robust lymphocytic infiltration of Lenti-LucOS tumors 8 weeks after tumor initiation, there was a dramatic reduction in tumor-infiltrating lymphocytes at later time-points, indicating that the immune response was not sustained or tumors had escaped (Figure 1C–1E). We performed flow cytometry with MHCI/Kb DimerX reagents loaded with SIY or SIN peptides to determine whether T cell infiltrates were specific for the model antigens. At several time points after tumor initiation with Lenti-LucOS, we detected CD8+ T cells reactive to SIY/Kb and SIN/Kb in the lungs and the mediastinal lymph nodes draining the lungs (DLN) (Figure 2A). As a control for robust T cell responses to the same antigens in the same organ, we infected mice with a recombinant influenza virus engineered to express SIY (WSN-SIY, Figure 2A). The antigen-specific T cell response to Lenti-LucOS tumors peaked between 4 and 8 weeks after tumor initiation but declined thereafter in the lungs and DLN, whereas T cells responding to SIY expressed by influenza expanded and contracted rapidly in the lungs (within 2–4 weeks) and persisted in the DLN (Figure 2B, 2C, and Figure S2A–B). We hypothesize that the delayed T cell response to tumors was due to relatively low levels of antigen produced and presented in the lung DLNs early during tumor development. To further investigate the early response, we analyzed the T cell response after Lenti-LucOS introduction into the lungs of mice that lacked the K-rasLSL-G12D allele, and could not form tumors (Figure S2C–H). We found that SIY and SIN-specific T cells were generated in the absence of tumor formation, presumably due to antigen expression in normal cells of the lung (Figure S2I–K). This indicates that while antigen expression is largely restricted to tumors in this system, the lentiviral infection can stimulate antigen-specific T cells that may contribute to the ensuing anti-tumor T cell response. Importantly, however, the T cell response in the absence of transformation was weak and short-lived when compared to mice that generated tumors (Figure S2C–H). This may indicate that the immune system responds differently to transformed cells or that tumor proliferation within the first several days following K-rasG12D activation leads to greater antigen production and, hence, more T cell activation.
Figure 2. T cell responses to tumor antigens are generated but not sustained.
(A) Representative analysis of SIY and SIN specific CD8+ T cells in the lung and the draining mediastinal lymph node (DLN) of Lenti-x or Lenti-LucOS tumor-bearing mice or WSN-SIY infected mice using peptide-loaded Kb reagents. FACS plots are gated on PI-negative, CD8+ cells.
(B and C) Percent of lymphocytes specific for SIN or SIY during Lenti-LucOS tumor progression in the lung or DLN. The percent of anti-SIN or anti-SIY reactive cells (of total lymphocytes) was determined as shown in Figure S2A. n= 2–5 mice per time-point.
(D) IFNγ and TNFα cytokine production in SIY and SIN-reactive T cells from the lungs of Lenti-LucOS tumor-bearing mice at several time points after tumor initiation. The percentage of SIY and SIN-specific T cells from the lungs that were IFN-γ+TNF-α+ or IFN-γ+TNF-αneg in the absence (no peptide) or in the presence of SIY and SIN peptides (+ peptide) is shown. Percentages were determined by staining duplicate samples for either DimerX or cytokine production as described in Figure S2L. n= 2–7 mice per time-point.
(E) The percentage of SIY and SIN-specific T cells in the DLN that were IFN-γ+TNF-α+ 16 weeks after tumor initiation with Lenti-LucOS or WSN-SIY infection. n= 4 mice per group.
(F) PD-1 surface expression on anti-SIY/Kb+ and anti-SIN/Kb+ CD8+ cells (open histograms) or non-specific CD8+ cells (filled histograms) from the lungs of Lenti-LucOS tumor-bearing mice at several time-points after tumor initiation. n= 2–3 mice per group.
Data are mean ± SEM. See also Figure S2.
Endogenous tumor-reactive T cells are not fully functional and display traits of terminally-differentiated effector cells
To determine whether T cells specific to the tumor antigens were functional, we measured their capacity to produce IFN-γ and TNF-α at multiple time-points after tumor initiation (Figure 2D, 2E and Figure S2L–M). Very few tumor-reactive T cells in the lungs had the capacity to produce both IFN-γ and TNF-α, and this activity was almost completely lost within the first five weeks of tumor development, whereas T cells maintained production of IFN-γ alone (Figure 2D). This pattern of cytokine production during tumor progression contrasted with the productive T cell response against WSN-SIY and may indicate an increase in terminally-differentiated effector T cells (IFN-γ+TNF-αneg) that is accompanied by a progressive loss of high-quality, memory-like T cells (IFN-γ+TNF-α+) (Figure 2D, 2E, and Figure S2N) (Seder et al., 2008; Wherry et al., 2007). It is possible that persistent, non-productive, T cell engagement with tumor antigens drives T cells into an exhausted state (Bucks et al., 2009; Mueller and Ahmed, 2009; Redmond and Sherman, 2005). Consistent with this, as tumors progressed, tumor-reactive T cells showed increased expression of PD-1, a marker of T cell exhaustion, and low expression of CD127 (the IL-7Rα chain), a marker of long-lived memory cells (Figure 2F and not shown).
Adoptively transferred T cells primed by tumors rapidly lose activity
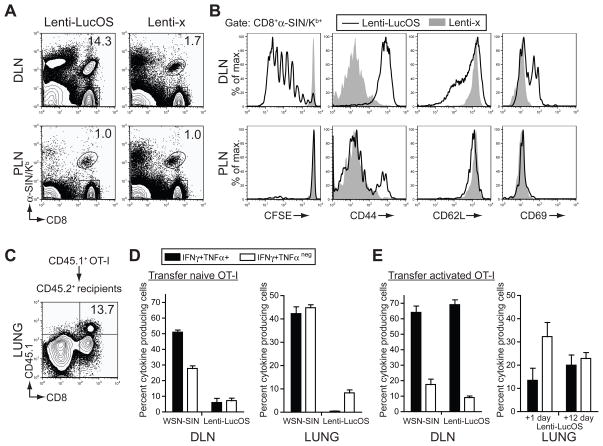
To develop a more detailed view of the dynamics of the T cell response to established tumors from activation to effector function, we transferred naïve 2C or OT-I TCR transgenic T cells (which recognize SIY or SIN, respectively) into mice bearing Lenti-x or Lenti-LucOS tumors. Three days after transfer, 2C and OT-I T cells were activated and proliferated in the lung-draining lymph nodes and they could be detected in the lungs 12–14 days after transfer (Figure 3A–C). However, the activity of these T cells was significantly impaired in the DLN and lungs compared to T cells responding to the same antigens expressed in the context of influenza infection (Figure 3D, Figure S3). Therefore, although T cells can be stimulated to proliferate and migrate to tumors, few T cells retain the capacity to produce effector cytokines in response to tumors.
Figure 3. Adoptively transferred T cells are not fully functional in response to established tumors.
(A) FACS analysis comparing the accumulation of SIN-reactive, OT-I T cells in the mediastinal DLN and the inguinal peripheral lymph nodes (PLN) three days after adoptive transfer of naïve OT-I cells into Lenti-x or Lenti-LucOS tumor-bearing mice 10 weeks after tumor initiation (similar results observed four weeks after tumor initiation). Percent of total CD8+ cells that are anti-SIN/Kb+ is indicated. n≥ 3 mice for Lenti-LucOS or ≥1 mouse for Lent-x tumor-bearing mice in each of two independent experiments.
(B) Histogram plots of CD8+anti-SIN/Kb+ gated cells for CFSE dilution or surface expression of CD44, CD62L, or CD69 from the DLN or PLN of Lenti-LucOS (open histogram) or Lenti-x (filled histogram) tumor-bearing mice. Undivided (CFSEhi) CD8+anti-SIN/Kb+ cells were examined in CD69 plots to detect initial T cell activation. n≥ 3 mice for Lenti-LucOS or ≥1 mouse for Lent-x tumor-bearing mice in each of two independent experiments.
(C) FACS analysis for the presence of OT-I T cells in the lungs of Lenti-LucOS tumor-bearing mice 12 days after adoptive transfer of naïve CD45.1+ OT-I T cells (tumor-bearing mice were CD45.2+). n≥ 3 mice in at least three independent experiments.
(D) Percent of CD8+CD45.1+ gated OT-I T cells producing IFNγ+TNFα+ or IFNγ+TNFαneg from the DLN or lung after transfer of naïve OT-I T cells. WSN-SIN represents analysis of OT-I T cells 7 days after WSN-SIN infection. Lenti-LucOS represents analysis of OT-I T cells 12 days after transfer into Lenti-LucOS tumor-bearing mice between 12 and 16 weeks after tumor initiation. n= 6–7 mice per group.
(E) Percent of CD8+CD45.1+ gated OT-I T cells producing IFNγ+TNFα+ or IFNγ+TNFαneg from the DLN or lung after transfer of in vitro-activated OT-I T cells. Lenti-LucOS represents analysis one day (lung only, n= 6 mice) and 12 days after transfer (n= 3 mice) into Lenti-LucOS tumor-bearing mice between 10 and 14 weeks after tumor initiation. WSN-SIN in the DLN represents analysis of naïve OT-I T cells 14 days after WSN-SIN infection (n= 3 mice).
Data are mean ± SEM. See also Figure S3.
Two potential mechanisms may explain the dysfunction of naïve T cells responding to antigens expressed by the Lenti-LucOS tumors: the presence of an immunosuppressive environment in tumor-bearing mice or the suboptimal priming of T cells by antigens expressed in tumors. To test whether the priming of T cells in the DLN is impaired, we assayed whether T cell activity in the lungs could be rescued by transferring effector T cells that were activated in vitro. Indeed, in vitro activated tumor-specific T cells immediately had the capacity to produce both IFN-γ and TNF-α and maintained this activity in the lungs of Lenti-LucOS tumor-bearing mice (Figure 3D and 3E). Furthermore, in vitro activated T cells had comparable function in DLNs to T cells primed in vivo by WSN-SIN (Figure 3E). T cell activity may be exacerbated by interactions with cells or secreted factors in the tumor microenvironment. Foxp3+ T regulatory cells were found to specifically infiltrate Lenti-LucOS tumors (Figure S1). Nevertheless, T cell priming by tumor antigens is clearly suboptimal and contributes to the weak anti-tumor T cell response.
Antigen expression and presentation is maintained in Lenti-LucOS tumors
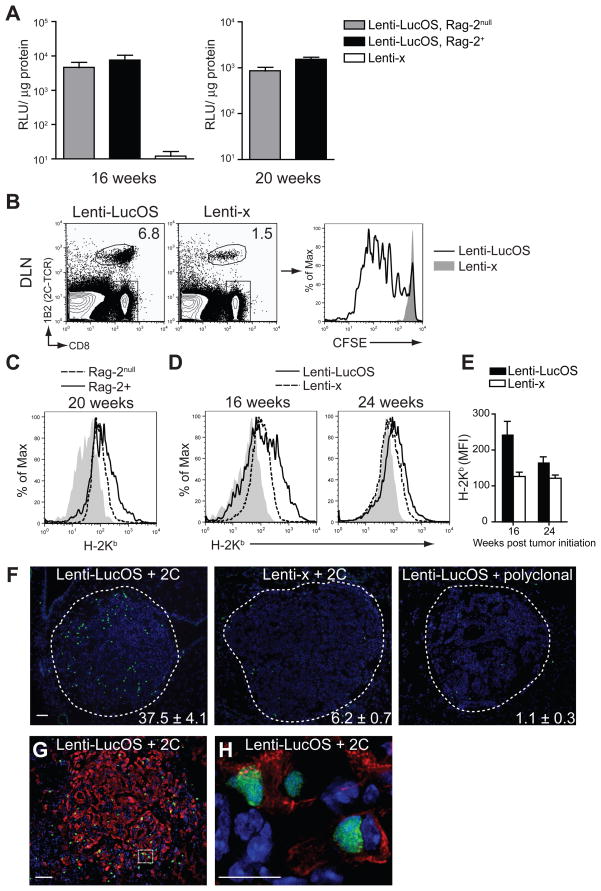
The loss of T cell infiltrates in tumors during tumor progression could also be due to the selection of tumors that lose antigen expression or antigen presentation to escape the immune response (Dunn et al., 2002; Khong and Restifo, 2002; Uyttenhove et al., 1983; Ward et al., 1990; Zhou et al., 2004). To determine whether the T cell response was driving the outgrowth of tumors lacking antigen, we compared luciferase activity in Lenti-LucOS tumors generated in immune-competent and Rag-2−/− mice. At 16 and 20 weeks post tumor initiation, time-points associated with the dissipation of immune infiltrates in tumors, luciferase levels were comparable between normal and immune-compromised mice (Figure 4A). Tumor antigen presentation in the DLN was maintained during the latest stages of disease because tumor-specific 2C T cells still proliferated specifically in the DLN of Lenti-LucOS tumor-bearing mice (Figure 4B). Lenti-LucOS tumors also maintained higher expression of MHC class I compared to Lenti-LucOS tumors that arose in Rag-2−/− mice or Lenti-x tumors (Figure 4C, 4D). However, MHC class I expression decreased on Lenti-LucOS tumors between 16 and 24 weeks after tumor initiation (Figure 4D, 4E). Reduced MHC class I expression could contribute to tumor escape and may result from waning anti-tumor T cell activity and infiltration into tumors. In support of this hypothesis, we found that MHC class I expression and SIN presentation on Lenti-LucOS tumor cell lines was regulated by IFNγ and that antigen-specific T cell recognition in vitro was sufficient to upregulate MHC class I and allow for the specific killing of Lenti-LucOS tumor cells (Figure S4). Therefore, the rapid decline in T cell infiltration into tumors may result from the combined effect of reduced anti-tumor T cell activity that lowers MHC class I expression on tumor cells, ultimately decreasing the potential for T cells to recognize tumors. This model was supported in vivo by the antigen-specific retention in Lenti-LucOS tumors of fully-functional in vitro-activated 2C T cells transferred into tumor-bearing mice 16 weeks after tumor initiation, a time when few tumors retain endogenous lymphocytic infiltrates (Figure 4F). Furthermore, by staining tumor sections with the epithelial marker cytokeratin 8, we could observe tumor-infiltrating 2C T cells making direct contacts with the epithelial cancer cells themselves rather than cytokeratin 8-negative stromal cells (Figure 4G, 4H). Thus, the dissipating T cell response to Lenti-LucOS tumors during the later stages of tumor progression is not due to the escape of tumors that lose the capacity to present the tumor antigens.
Figure 4. Antigen expression and presentation is maintained in tumors.
(A) Luciferase activity (RLU/μg protein) in tumor lysates from K-rasLSL-G12D/+;p53fl/fl or K-rasLSL-G12D/+;p53fl/fl;Rag-2−/− mice at 16 or 20 weeks after tumor initiation. n= 8–14 tumors per mouse per group.
(B) FACS analysis comparing the accumulation of SIY-reactive, 2C T cells in the DLN three days after adoptive transfer of naïve 2C cells into Lenti-x (n= 1) or Lenti-LucOS (n= 3) tumor-bearing mice 24 weeks after tumor initiation. CFSE dilution of CD8+1B2+ cells in mice with Lenti-LucOS (open histogram) or Lenti-x (filled histogram) tumors.
(C) Freshly isolated Lenti-LucOS tumors generated in Rag-2null (dashed line) and Rag-2+ mice (solid line) 20 weeks after tumor initiation were pooled and compared by FACS analysis for H-2Kb surface expression after gating-out cells positive for CD45, CD31, Ter119, and IA/IE (MHCII). Plots representative of three pooled tumor samples from Rag-2null or Rag-2+ mice. Filled histogram is a negative control stain for CD8.
(D) Freshly isolated Lenti-LucOS (solid line) and Lenti-x tumors (dashed line) at 16 and 24 weeks after tumor initiation compared by FACS analysis for H-2Kb surface expression as in C. Plots representative of pooled and individual tumor samples from two independent experiments. Filled histograms are negative control stains for H-2Kd.
(E) H-2Kb mean fluorescent intensity on Lenti-LucOS and Lenti-x tumors at 16 and 24 weeks after tumor initiation. n= 2–3 mice, 6–11 tumor samples per group per time-point.
(F) Activated 2C T cells, or α-CD3/CD28 activated polyclonal CD8+ T cells, labeled with CFSE were transferred into Lenti-LucOS or Lenti-x tumor-bearing mice 16 weeks post tumor initiation and tumors were analyzed for the presence of CFSE+ cells (green) 24 hours later. DAPI counter stained. Tumors are outlined and quantification of the number of CFSE+ cells/tumor is indicated. Scale = 50μm. n= 2–4 mice, 21–38 tumors, per group.
(G) Cytokeratin 8 (red) stained Lenti-LucOS tumors from F that received activated CFSE+ 2C T cells (green) 16 weeks after tumor initiation. Scale = 50μm.
(H) High magnification of box outlined in G. Scale = 10μm.
Data are mean ± SEM. See also Figure S4.
Immunogenic tumors exhibit delayed tumor progression
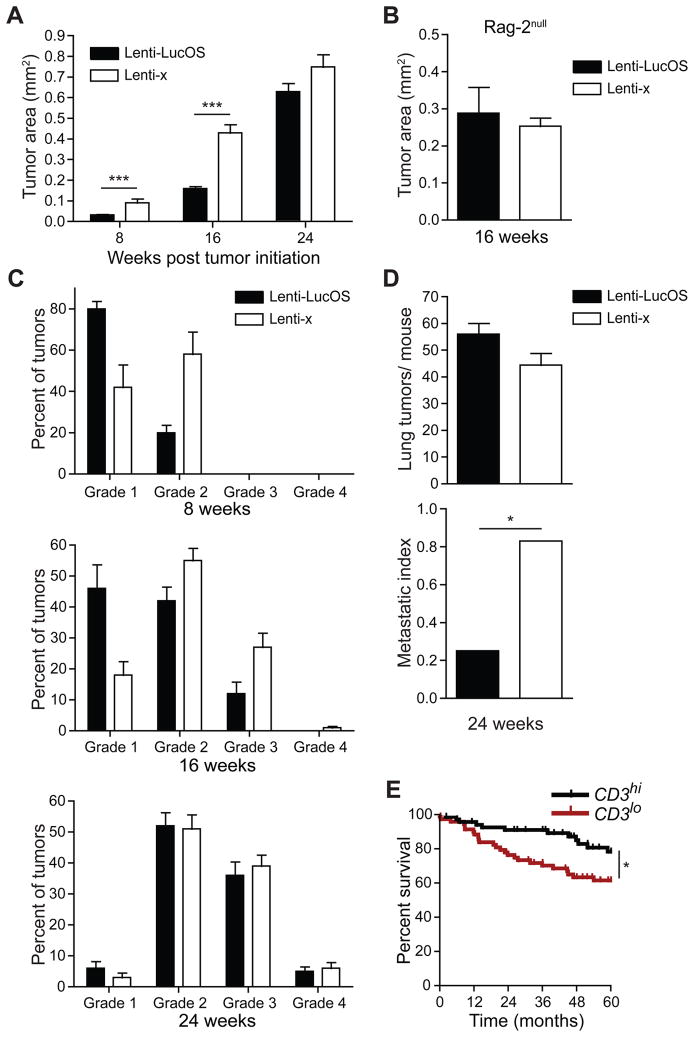
Despite the suboptimal anti-tumor T cell response, it remained possible that the immune response might still affect the course of the disease. To understand the impact of the T cell response on tumor progression, we first compared the size of “immunogenic” Lenti-LucOS tumors to “non-immunogenic” Lenti-x tumors over the lifespan of tumor-bearing mice. As shown in Figure 5, at each time point examined, immunogenic tumors were smaller than non-immunogenic tumors, with the differences in size being most significant at 8 and 16 weeks after tumor initiation, coincident with T cell infiltration into tumors (Figure 5A). Analysis of tumors 8 weeks after initiation revealed an increase in apoptosis in Lenti-LucOS compared to Lenti-x tumors but no difference in proliferation (Figure S5A–B). Importantly, the difference in size between Lenti-LucOS and Lenti-x tumors required the presence of the adaptive immune system, because the expression of the LucOS antigens had no effect on tumor size when induced in Rag-2−/− mice (Figure 5B). We also followed tumor progression by examining the histological tumor grades at each time-point. At 8 and 16 weeks after tumor initiation, Lenti-LucOS tumors were of lower grade compared to Lenti-x tumors (Figure 5C). By 24 weeks post tumor initiation, the primary lung tumor grades were similar; however, far fewer mice with immunogenic tumors had metastatic disease (Figure 5D). Thus, early anti-tumor T cell responses can have long-lasting effects on tumor progression that delay the most deadly phase of the disease. Analysis of human lung adenocarcinoma gene expression datasets indicated that high expression of CD3 genes in tumor samples, as well as other genes expressed specifically in T and B cells, was predictive of better patient outcomes (Figure 5E and Figure S5C–F).
Figure 5. Immunogenic tumors have delayed tumor progression.
(A) Tumor area of Lenti-LucOS and Lenti-x lung tumors at 8, 16, and 24 weeks after tumor initiation. p-values are ~10−10, ~10−14, and ~0.108, respectively, from n= 9–15 mice, 44–892 tumors, per group.
(B) Tumor area of Lenti-LucOS and Lenti-x lung tumors in K-rasLSL-G12D/+;p53fl/fl;Rag-2−/− mice 16 weeks after tumor initiation. p ~0.561 from n= 6–7 mice, 45–223 tumors, per group.
(C) Tumor grades for Lenti-LucOS and Lenti-x lung tumors at 8, 16 and 24 weeks after tumor initiation. n= 9–22 mice, 49–1594 tumors, per group.
(D) The number of lung tumors/mouse and metastatic index (number of mice with detectable metastases/total examined, p < 0.02) in Lenti-LucOS and Lenti-x tumor-bearing mice 24 weeks after tumor initiation. n= 8–12 mice per group.
(E) Kaplan-Meyer plot comparing the survival of Stage I/II (lymph node metastases free, N0) lung adenocarcinoma patients with high or low CD3 expression in tumors. Patients were ranked from highest to lowest based on CD3 expression in tumors (average expression of CD3δ, CD3γ, CD3ε, and CD247 (CD3ζ)) and the top versus bottom quartiles were compared, p < 0.02.
Data are mean ± SEM. See also Figure S5.
Loss of antigen expression in transplantable models of lung cancer
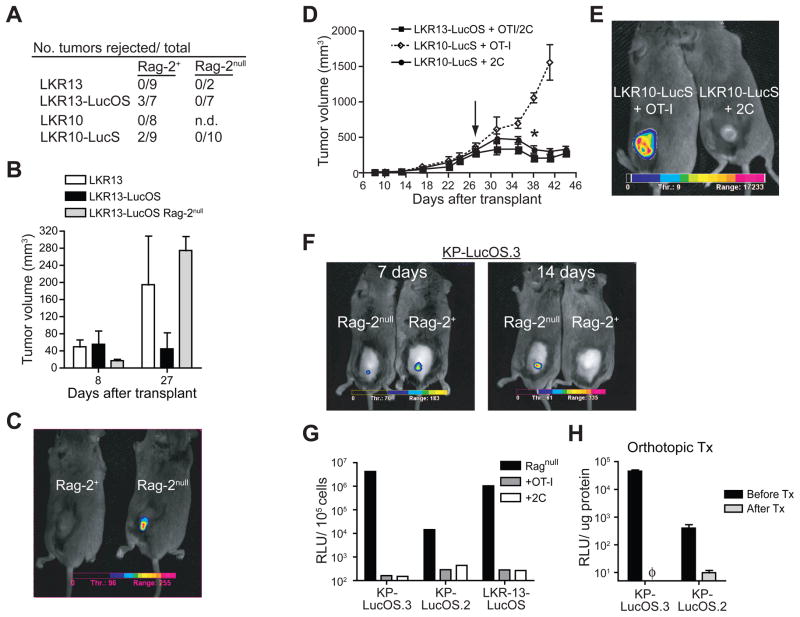
Tumor antigen loss has been reported as a mechanism of tumor immune evasion in transplantable models of cancer (Spiotto et al., 2004; Uyttenhove et al., 1983; Ward et al., 1990; Zhou et al., 2004). Since the endogenously arising lung tumors did not lose antigen expression or presentation in the face of an anti-tumor immune response, we hypothesized that this may be the consequence of a weaker immune response against endogenous tumors compared to transplanted tumors. To compare immune responses in the context of transplantable or endogenously arising models of cancer, we transduced cell lines from the K-rasG12D-driven lung tumor model with lentiviral vectors to introduce either SIY fused to luciferase (LKR10-LucS) or ovalbumin (containing SIN and OVA323–339) and SIY fused to luciferase (LKR13-LucOS), and transplanted the cell lines subcutaneously into syngeneic immune-competent mice (Figure S4A). While the parental LKR10 and LKR13 cell lines produced tumors upon transplantation, the antigen-expressing LKR10-LucS and LKR13-LucOS were rejected or exhibited delayed growth and generated CD8+ T cells specific to SIN and/or SIY (Figure 6A, 6B, Figure S6A–D). In stark contrast to the situation with autochthonous tumors, all the tumors that grew in immune-competent mice lost expression of the antigens (Figure 6C). Transferring naïve 2C or OT-I T cells into immune-compromised mice bearing established LKR10-LucS or LKR13-LucOS tumors led to reductions in tumor volume and antigen expression in an antigen-specific manner (Figure 6D and 6E). Thus, T cell responses to a single antigen expressed in transplanted lung tumor cell lines are sufficient to elicit tumor rejection or induce the selective outgrowth of tumors that lose antigen expression.
Figure 6. Transplanted lung tumors induce strong T cell responses that force tumor elimination or tumor antigen loss.
(A) Number of tumors rejected of the total transplanted subcutaneously into immune-competent or immune-compromised (Rag-2null) 129S4/SvJae mice (n.d.= not done). Results are cumulative from three independent experiments and transplantation of 105–106 cells.
(B) Size of LKR13 and LKR13-LucOS tumors 8 and 27 days after subcutaneous transplantation into immune-competent 129S4/SvJae mice or LKR13-LucOS into Rag-2null 129S4/SvJae mice. LKR13-LucOS tumors that were rejected are not included. Results are cumulative from three independent experiments. n= 2–5 mice per group.
(C) Representative in vivo luciferase activity of LKR10-LucS or LKR13-LucOS tumors transplanted subcutaneously into Rag-2+ or Rag-2null 129S4/SvJae mice. (n= 23 mice, 6–7 LKR10-LucS, 4–6 LKR13-LucOS per group).
(D) Tumor growth after subcutaneous transplantation of LKR10-LucS or LKR13-LucOS into Rag-2null mice followed by transfer of naïve OT-I or 2C T cells (arrow) and measurement of luciferase activity (star) (OTI/2C = OT-I or 2C transfer). n= 3–4 mice per group.
(E) Representative in vivo luciferase activity of LKR10-LucS tumors in Rag-2null mice that received OT-I or 2C T cells from D. All LKR13-LucOS tumors lost detectable luciferase activity with transfer of OT-I or 2C T cells.
(F) Representative in vivo luciferase activity 7 and 14 days after subcutaneous transplantation of a Lenti-LucOS induced lung tumor cell line (KP-LucOS.3) into Rag-2null or Rag-2+ 129S4/SvJae mice.
(G) Similar to D, Lenti-LucOS induced lung tumor cell lines (KP-LucOS.3 and KP-LucOS.2) or LKR13-LucOS were transplanted subcutaneously into Rag-2null mice, then OT-I, 2C, or no T cells were transferred and luciferase activity was measured in tumor cell lines generated from the tumors >28 days after transplantation.
(H) Lenti-LucOS induced lung tumor cell lines were orthotopically transplanted into 129S4/SvJae mice and luciferase activity in the grafted lung tumors was assayed and compared to the activity before transplantation (φ = nzot detectable).
Data are mean ± SEM. See also Figure S6.
It has been reported that spontaneous tumors induce a state of systemic tolerance such that T cells can no longer respond to transplanted tumors (Willimsky and Blankenstein, 2005). In this model, however, mice bearing autochthonous lung tumors expressing model tumor antigens, still produced effective T cell responses against transplanted tumors expressing the same antigens (Figure S6E). We suspect that leaky expression of tumor antigens in normal tissues, rather than tumor-specific expression, may be responsible for the systemic tolerance to tumor antigens in these transgenic models (Bai et al., 2008; Cheung et al., 2008; Drake et al., 2005; Willimsky and Blankenstein, 2005).
Finally, Lenti-LucOS cell lines, derived from autochthonous lung tumors that had escaped the immune response in situ without losing antigen expression, lost antigen expression in a T cell-dependent manner when transplanted subcutaneously into immune-competent mice (Figure 6F and 6G). These cell lines also lost antigen expression after orthotopic transplantation into the lung, indicating that transplantation, even into a tumor’s native site, can induce T cell responses that drive tumor antigen loss (Figure 6H).
Enhancing the anti-tumor T cell response with vaccination causes tumor elimination and tumor antigen loss
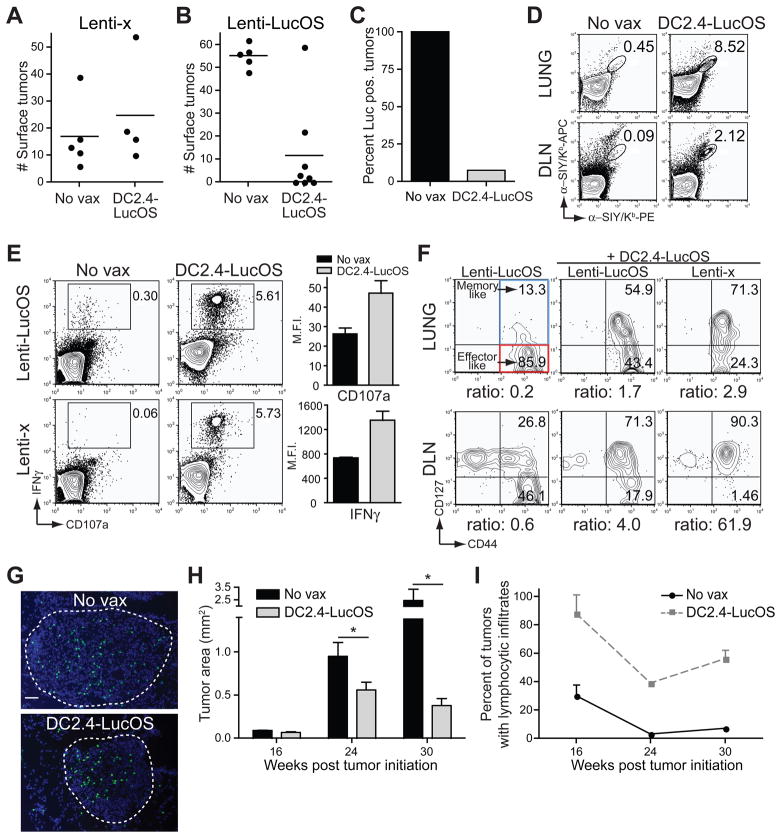
Enhanced T cell priming in the context of transplanted tumors is a likely explanation for tumor elimination and tumor antigen loss in this setting. Therefore, priming by vaccination might elicit similar anti-tumor T cell responses against autochthonous tumors. To examine this possibility, we vaccinated mice prophylactically with a dendritic cell line engineered to express the LucOS antigens (DC2.4-LucOS) and subsequently induced Lenti-LucOS or Lenti-x tumors. Vaccination did not affect the number of Lenti-x tumors, but dramatically reduced the number of Lenti-LucOS tumors and antigen expression in tumors that formed (Figure 7A–C). This was likely due to an enlarged T cell response early during tumor development, as well as a functionally enhanced response characterized by increased secretion of effector proteins and the maintenance of CD127+ memory-like cells (Figure 7D–F).
Figure 7. Vaccination in autochthonous lung tumor model reduces tumor burden and promotes loss of antigen expression.
(A and B) Tumor number on the lungs of mice with Lenti-x or Lenti-LucOS tumors 16 weeks after tumor initiation with or without prior DC2.4-LucOS vaccination. p-values are ~0.50 and ~0.0008, respectively.
(C) Freshly explanted Lenti-LucOS tumors were assayed for luciferase activity and the percent of tumors that were luciferase positive (> 50 RLU/μg protein) +/- DC2.4-LucOS vaccination is plotted. n= 5–6 mice, 27–34 tumors, per group.
(D) Representative FACS plots of SIY/Kb-specific T cells (gated on CD8) from the lung and DLN +/- DC2.4-LucOS vaccination, 9 days after tumor initiation with Lenti-LucOS. n≥ 2 mice per group.
(E) FACS for surface-exposed CD107a and captured IFNγ (gated on CD8) after stimulation of DLN cells with SIY and SIN from Lenti-LucOS or Lenti-x tumor-bearing mice +/− DC2.4-LucOS vaccination (16 weeks post tumor initiation). Mean fluorescent intensities of CD107a and IFNγ on reactive T cells is plotted. n= 2–4 mice per group.
(F) FACS analysis of CD8+ anti-SIY/Kb+- and anti-SIN/Kb+-specific T cells for CD127 and CD44 surface expression from the lungs or DLN of Lenti-LucOS or Lenti-x tumor-bearing mice +/− DC2.4-LucOS vaccination (8 weeks post tumor initiation, n= 2–3 mice per group). The ratio of CD44+CD127+ (memory-like) to CD44+CD127− (effector-like) is shown below each FACS plot.
(G) Lenti-LucOS tumors from mice 8 weeks after tumor initiation and 24 hours after transfer of SIY-reactive, CFSE-labeled activated T cells (green) +/− DC2.4-LucOS vaccination. DAPI counter stained. Tumors are outlined. Scale = 50μm.
(H) Mean tumor area of Lenti-LucOS lung tumors at 16, 24 and 30 weeks after tumor initiation +/− DC2.4-LucOS vaccination. p-values are ~0.050, ~0.038, and ~0.027, respectively, from n= 3–10 mice, 70–506 tumors, per group at 16 and 24 weeks, and n= 1–2 mice, 15–46 tumors, per group at 30 weeks.
(I) Percent of Lenti-LucOS tumors/mouse containing infiltrating lymphocytes at 16, 24 and 30 weeks after tumor initiation +/− DC2.4-LucOS vaccination (analysis of mice from H).
Data are mean ± SEM. See also Figure S7.
Despite the dramatic reduction of antigen expression in Lenti-LucOS tumors from vaccinated mice, there were still significant numbers of tumor-infiltrating lymphocytes 8 weeks after tumor initiation. Interestingly, in vitro-activated T cells specific to the model antigens could still recognize Lenti-LucOS tumors in DC2.4-LucOS vaccinated mice after adoptive transfer (Figure 7G). The maintenance of a T cell response to the tumor antigens in vaccinated mice led us to ask whether the progression of Lenti-LucOS tumors was altered in vaccinated compared to unvaccinated mice. Although Lenti-LucOS tumors in vaccinated mice appeared only modestly smaller 16 weeks after tumor initiation, the differences in tumor size between vaccinated and unvaccinated mice increased at 24 and 30 weeks after tumor initiation (Figure 7H, Figure S7A). The delayed tumor growth in vaccinated mice correlated with a prolonged retention of lymphocytic infiltrates into tumors (Figure 7I and Figure S7B), again highlighting the potential for tumor-infiltrating lymphocytes to delay tumor progression.
DISCUSSION
Several important conclusions regarding immune-tumor interactions and their role in regulating tumor progression are evident from our study that could not be directly addressed using previous cancer models. Thus far, using genetically engineered mouse models, it has not been possible to compare tumors expressing or lacking tumor-specific antigens (Clark et al., 2007; Huijbers et al., 2006; Willimsky and Blankenstein, 2005). With the recent sequencing of several cancer genomes, including lung adenocarcinomas, it is clear that human tumors contain hundreds of mutated proteins and many of these have the potential to be presented on MHC molecules, thus providing a means for T cells to recognize tumors as distinct from their surrounding normal tissues (Ding et al., 2008; Segal et al., 2008; Sjoblom et al., 2006). Our ability to specifically control the induction of tumor formation and tumor antigen expression has allowed us to closely recapitulate the effects of T cell responses to tumor-specific antigens from inception to malignant transformation.
In contrast to transgenic models of tumor-associated antigen expression, here we followed endogenous T cell responses against antigens expressed in autochthonous lung tumors in an attempt to model T cell responses to tumor neoantigens. By tracking the response of endogenous T cells as well as transferred T cells, we find that suboptimal T cell responses against tumors are not necessarily due to immune ignorance (Nguyen et al., 2002; Ochsenbein et al., 2001; Speiser et al., 1997; Spiotto et al., 2002). However, the expansion of T cells specific to the tumor antigens was markedly delayed, potentially allowing tumor escape (Hanson et al., 2000; Ochsenbein et al., 2001), and the T cell response eventually declined in both magnitude and quality as tumors progressed. Despite their ability to proliferate and migrate to tumors, T cells had an altered phenotypic state that was exemplified by a poor capacity to produce and secrete effector cytokines and a rapid loss of TNFα production in the lung. The reduced capacity of T cells to produce effector cytokines such as IFNγ and TNFα as tumors progressed may account for their inefficient migration to tumors over time (Calzascia et al., 2007; Muller-Hermelink et al., 2008). The ability to precisely time tumor initiation revealed a dynamic relationship between anti-tumor T cells and tumor cells. Loss of T cell activity can contribute to the rapid decline in tumor-infiltrating lymphocytes by allowing for reduced MHC class I expression on tumor cells. Tumor-reactive T cells were also found to express high levels of PD-1 and low levels of CD127, features that resemble T cell responses during chronic infections (Bucks et al., 2009; Wherry et al., 2007). Indeed, the T cell response to lung tumors was quite distinct from the response to the same antigens expressed by recombinant influenza virus after infection of the lung. Signaling through the inhibitory PD-1/PD-L1 pathway alone was not responsible for the loss of T cell reactivity against tumors in this model because blocking the function of this pathway with the use of antibodies or PD-1-deficient T cells did not rescue the activity of tumor-reactive T cells (not shown). Instead, T cell exhaustion might be exacerbated by an ineffective anti-tumor immune response that allows prolonged T cell exposure to antigens expressed from tumors. Chronic T cell stimulation with cognate antigen has been shown to be sufficient to drive exhaustion in several settings (Bucks et al., 2009; Drake et al., 2005; Mueller and Ahmed, 2009; Redmond and Sherman, 2005). We also found that T regulatory cells were recruited specifically to tumors expressing the LucOS antigens, and T regulatory cells have been shown to suppress anti-tumor responses in several cancer models (Clark et al., 2007; Ercolini et al., 2005; Pellegrini et al., 2009). Despite the evidence of an incomplete anti-tumor T cell response, the immune response still delayed tumor progression, indicating that suboptimal T cell responses that do not eliminate tumors are capable of slowing cancer progression (Koebel et al., 2007). This mirrors observations made here and in several retrospective studies of human cancer patients in which immune infiltration into tumors significantly correlated with improved patient outcomes (Buckanovich et al., 2008; Budhu et al., 2006; Dunn et al., 2002; Finak et al., 2008; Galon et al., 2006; Molldrem et al., 2000; Pages et al., 2005; Piersma et al., 2007).
Prophylactic vaccination provided substantial protection from autochthonous tumors, but it also led to prolonged immune infiltration into the Lenti-LucOS tumors, further delaying tumor progression even though antigen expression was dramatically reduced. Delayed tumor progression despite antigen loss may occur through direct targeting of tumor cells by T cells or via indirect elimination of tumor cells during T cell-mediated destruction of stromal cells that cross-present tumor antigens in the tumor microenvironment (Spiotto et al., 2004). This result has important implications for immunotherapy because it indicates that generating large numbers of highly functional, tumor-reactive T cells that persist long-term can effectively inhibit tumor progression even after the selective outgrowth of tumors that express very low levels of the targeted antigen.
The majority of studies investigating immunity to cancer have focused on transplantable models of cancer (Dunn et al., 2002; Hanson et al., 2000; Khong and Restifo, 2002; Koebel et al., 2007; Ochsenbein et al., 2001; Schreiber, 2003; Shankaran et al., 2001; Spiotto et al., 2002; Street et al., 2002; Thomas and Massague, 2005). These studies have led to the discovery of several important mechanisms in both T cells and tumor cells that can modulate the effectiveness of anti-tumor T cell responses or allow for tumor escape. However, these models may not accurately reflect the human disease (Khong and Restifo, 2002). Here we have used comparable models of transplantable and autochthonous lung cancer to show that transplantation induces anti-tumor T cell responses capable of driving tumor immunoediting. Even orthotopic transplantation of cell lines derived from Lenti-LucOS-induced tumors that had escaped immune surveillance in their autochthonous setting led to an adaptive immune response that forced the outgrowth of tumors with reduced antigen expression, a phenomenon that never occurred with autochthonous tumors expressing these antigens. This was due in part to suboptimal priming of T cells in the context of autochthonous tumors. T cells activated in vitro had enhanced activity compared to T cells stimulated in vivo and priming of T cells by vaccination led to responses against autochthonous tumors that were comparable to responses against transplanted tumors. From our comparison of autochthonous and transplanted tumors, we conclude that the immune system recognizes tumors that originate from transformation of somatic cells in an inherently different way than transplanted tumor cell lines.
Future experiments should address the diversity of anti-tumor immune responses that likely stem from subtle differences in the models used for the investigations. The development of models that link the tumor antigen to the driving oncogenic event will be useful for investigating whether T cell responses to oncogenic antigens, whose expression in tumors cannot easily be downregulated to evade an immune response, instigate alternative mechanisms of tumor escape (Willimsky and Blankenstein, 2005). Models in which tumor initiation and expression of tumor-specific antigens are temporally separable may discern whether T cell responses are different if the induction of antigen expression occurs in established tumors, where mutated proteins most likely arise in human cancers. Indeed, using these lentiviral vectors to initiate tumor development and introduce the antigens has some important limitations. Specifically, these lentiviruses require viral infection concomitant with tumor initiation, and they cannot completely restrict the expression of the antigens to tumor cells. However, preliminary experiments with lentiviral systems that can restrict the expression of the antigens to lung epithelial cells, as well as experiments in which we delayed antigen expression to several days after the lentiviral infection, indicate that inducing the expression of these antigens in lung epithelial cells is sufficient to generate antigen-specific T cell responses. Further investigation is necessary to determine whether priming during tumor initiation in the context of the lentiviral infection alters the outcome of the anti-tumor immune response. Finally, discoveries made in a particular model of cancer have often been accepted as broadly applicable to all immune-tumor interactions. However, the immune response and tumor response are likely to vary greatly depending on the originating tissue and the genetic pathology of the disease. Therefore, it will be important to extend these analyses to other genetically engineered mouse models of cancer to discern whether T cell responses and tumor development change depending on the context of the disease.
EXPERIMENTAL PROCEDURES
Mice and tumor induction
129S4/SvJae strains backcrossed 8 generations were used for all tumor transplant experiments and most autochthonous tumor studies. C57/BL6 mice backcrossed 5 generations were used when transgenic T cells from C57/BL6 mice were adoptively transferred (Figures 3 and 4B). Trp53fl mice were provided by A. Berns, K-rasLSL-G12D were generated in our laboratory, and Rag-2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Lung tumors were induced in K-rasLSL-G12D/+;p53fl/fl mice by intratracheal intubation and inhalation of viruses expressing Cre recombinase as reported (DuPage et al., 2009). J. Chen provided the 2C, OT-I, and OT-II TCR transgenic mice that were C57/BL6 and crossed into Rag-2−/−. All animal studies and procedures were approved by the Massachusetts Institute of Technology’s Committee for Animal Care.
Lentiviral production
Lentiviruses were produced by transfection of 293T cells with Δ8.2 (gag/pol) and CMV-VSV-G as described (DuPage et al., 2009).
Histology, tumor size, tumor grading
Mice were killed at designated time points and lung tissues were preserved by fixation with 3.6% formaldehyde solution or frozen in O.C.T. Bioquant Image Analysis software was used to measure the area of individual tumors in histological sections. Grading was performed by R.B. as described (DuPage et al., 2009). Metastasis was monitored by gross examination of mice at necropsy and histological examination of lung DLNs.
Immunohistochemistry
We performed immunohistochemical analysis on formalin-fixed paraffin sections after heat-mediated antigen retrieval in sodium citrate (see Abcam protocol). We used α-CD3 (1:100, Dako), α-B220 (1:50, RA3-6B2), α-Mac-3 (1:10, M3/84) (BD Pharmingen), α-Foxp3 (1:25, FJK-16s) (eBioscience), α-cytokeratin 8 (1:25) primary antibodies and Vectastain ABC secondary reagents followed by DAB substrate detection (Vector Laboratories).
Flow cytometry
Cell suspensions from lymphoid organs were prepared by mechanical disruption between frosted slides or from lungs by mincing and digesting the tissues for ~1 hour at 37°C in 125 U/ml Collagenase Type I (Gibco) and 60 U/ml Hyaluronidase (Sigma). Cells were stained with antibodies for 20–30 min after treatment with FcBlock (BD Pharmingen). α-CD8α (53-6.7), α-CD45.1 (A20), α-mouse IgG1 (X56), α-PD-1 (J43), α-IFNγ (XMG1.2), α-TNFα (MP6-XT22), α-CD107a (ID4B), α-CD44 (IM7), α-CD62L (MEL-14), α-CD69 (H1.2F3), and DimerX I (Dimeric Mouse H-2Kb:Ig) were from BD Pharmingen. α-CD127 (A7R34) was from eBioscience. J. Chen provided the 1B2 antibody recognizing the 2C TCR. H. Eisen provided the 25-D1.16 antibody that recognizes SIN-loaded Kb/MHCI. All antibodies were used at 1:200. Peptide-loaded DimerX reagents were prepared as directed and used at 1:75. To improve the sensitivity of the DimerX reagent, we utilized both PE and APC labeled dimers to co-stain CD8+ T cells. Propidium iodide was used to exclude dead cells. Cells were read on a FACSCalibur or LSR II (BD Biosciences) and analyzed using Flowjo software (Tree Star).
Cytokine production
Cells were resuspended in the presence or absence of SIYRYYGL and/or SIINFEKL peptides in OPTI-MEM I (Gibco) supplemented with GolgiPlug (BD Pharmingen) for ~4 hours at 37°C, 5% CO2. Cells were then fixed and stained for intracellular cytokines using the Cytofix/Cytoperm kit (BD Biosciences). For IFNγ-capture, cells were stained with the capture and detection antibodies as directed (Miltenyi Biotec).
Luciferase detection
Freshly explanted tumors or cell lines were lysed in Cell Culture Lysis Reagent, mixed with Luciferase Assay Reagent (Promega), and relative light units (RLU) were detected using the Optocomp I luminometer (MGM Instruments). RLUs were standardized based on the total number of cells or total protein (Bio-Rad Protein Assay). In vivo bioluminescence images were obtained using the NightOWLII LB983 (Berthold Technologies) after intraperitoneal injection of 1.5 mg Beetle Luciferin (Promega).
CFSE-labeling and adoptive transfer
Naïve and activated T cells were labeled with 5μM CFSE (Molecular Probes) for 10 min at 37°C. Naïve TCR transgenic T cells were harvested from lymphoid organs, stained, and 5×105–106 CD8+ cells were transferred intravenously. Activated TCR transgenic cells were stimulated in vitro with cognate peptide for 16–20 hrs and then cultured for 6–7 days in complete-RPMI supplemented with 5 ng/ml IL-2 (R&D Systems). 107 in vitro-activated T cells were transferred intravenously.
Tumor cell lines and transplantation
LKR10 and LKR13 cell lines were derived from K-rasG12D-driven mouse lung tumors from 129S4/SvJae mice. LKR10-LucS and LKR13-LucOS were generated by lentiviral infection with LucS or LucOS constructs (no Cre). KP-LucOS and KP-x cell lines were derived from Lenti-LucOS- or Lenti-x-induced lung tumors, respectively, from 129S4/SvJae mice. 105–106 cells were transplanted subcutaneously (sc) or intravenously (for orthotopic). 129S4/SvJae immune-competent and Rag-2−/− transplant recipients came from the same mouse strains used to generate the autochthonous tumors. Sc tumor volumes were calculated by multiplying length × width × height of each tumor.
DC2.4-LucOS vaccination
DC2.4-LucOS cells were generated from the dendritic cell line (DC2.4) after infection with lentivirus that expresses LucOS (no Cre). Mice were vaccinated by intraperitoneal injection of 5×105 cells >1 month prior to tumor initiation.
Influenza
WSN-SIY and WSN-SIN were provided by J. Chen. Mice were infected with 20 pfu of virus by intratracheal inhalation.
Statistical analyses
P-values from unpaired two-tailed student’s T-tests were used for statistical comparisons with the following exceptions: Figure 7F utilized Welch’s correction, Figure 5D utilized the Fischer exact probability test, and Figure 5E utilized the logrank test.
SIGNIFICANCE.
Our immune system may naturally protect us from cancer by recognizing and eliminating nascent tumors. However, the study of immune-tumor interactions has relied upon T cell responses to transplanted tumors or tumor-associated antigens expressed from transgenic mouse cancer models. Here we report a system to assess how T cells respond and adapt to endogenously arising lung tumors expressing exogenous antigens that model tumor neoantigens. We uncover critical differences in the way that the immune system responds to tumors that originate from transformation of somatic cells versus transplanted tumor cell lines. Rather than eliminate tumors, T cells suppress tumor progression, reflecting data in cancer patients. This model provides a valuable system for the optimization of immune therapies to eradicate human cancers.
Supplementary Material
Acknowledgments
We would like to thank A. Charest and P. Sandy for providing lentiviral vectors, G. Paradis for assistance with flow cytometry, E. Vasile for assistance with microscopy, H. Eisen for reagents and experimental discussions, and A.G. DuPage, D. Feldser, T. Staton, and C. Kim for critical reading of this manuscript. This work was supported by Grant 1 U54 CA126515-01 from the NIH and partially by Cancer Center Support (core) grant P30-CA14051 from the National Cancer Institute and the Margaret A. Cunningham Immune Mechanisms in Cancer Research Fellowship Award (M.D.) from the John D. Proctor Foundation. M.M.W. was a Merck fellow of the Damon Runyon Cancer Research Foundation and is a Genentech postdoctoral fellow. T.J. is a Howard Hughes Investigator and a Daniel K. Ludwig Scholar.
References
- Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol. 2009;182:6697–6708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Calzascia T, Pellegrini M, Hall H, Sabbagh L, Ono N, Elford AR, Mak TW, Ohashi PS. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117:3833–3845. doi: 10.1172/JCI32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AF, Dupage MJ, Dong HK, Chen J, Jacks T. Regulated expression of a tumor-associated antigen reveals multiple levels of T-cell tolerance in a mouse model of lung cancer. Cancer Res. 2008;68:9459–9468. doi: 10.1158/0008-5472.CAN-08-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Finn OJ. Immunological weapons acquired early in life win battles with cancer late in life. J Immunol. 2008;181:1589–1592. doi: 10.4049/jimmunol.181.3.1589. [DOI] [PubMed] [Google Scholar]
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Getnet D, Maris CH, Hipkiss EL, Grosso JF, Harris TJ, Yen HR, Bruno TC, Wada S, Adler A, Georgantas RW, et al. Tumor recognition and self-recognition induce distinct transcriptional profiles in antigen-specific CD4 T cells. J Immunol. 2009;182:4675–4685. doi: 10.4049/jimmunol.0803400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- Huijbers IJ, Krimpenfort P, Chomez P, van der Valk MA, Song JY, Inderberg-Suso EM, Schmitt-Verhulst AM, Berns A, Van den Eynde BJ. An inducible mouse model of melanoma expressing a defined tumor antigen. Cancer Res. 2006;66:3278–3286. doi: 10.1158/0008-5472.CAN-05-3216. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J Immunol. 2004;172:6558–6567. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Elford AR, Murakami K, Garza KM, Schoenberger SP, Odermatt B, Speiser DE, Ohashi PS. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J Exp Med. 2002;195:423–435. doi: 10.1084/jem.20010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, Jungbluth AA, Allison JP. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319:215–220. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- Schreiber H. Tumor Immunology. In: Paul WE, editor. Fundamental Immunology. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 1557–1592. [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol. 1997;9:1355–1365. doi: 10.1093/intimm/9.9.1355. [DOI] [PubMed] [Google Scholar]
- Speiser DE, Miranda R, Zakarian A, Bachmann MF, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel RM, Ohashi PS. Self antigens expressed by solid tumors Do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med. 2002;196:129–134. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Maryanski J, Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med. 1983;157:1040–1052. doi: 10.1084/jem.157.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PL, Koeppen HK, Hurteau T, Rowley DA, Schreiber H. Major histocompatibility complex class I and unique antigen expression by murine tumors that escaped from CD8+ T-cell-dependent surveillance. Cancer Res. 1990;50:3851–3858. [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal changes in tumor antigenicity and antigen-specific T cell function during tumor progression. J Exp Med. 2004;200:1581–1592. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.