Abstract
Kindlins are a novel family of intracellular adaptor proteins in integrin-containing focal adhesions. Kindlin-1 and -2 are expressed in the skin, but whether and how they cooperate in adult epithelial cells have remained elusive. We uncovered the overlapping roles of kindlin-1 and -2 in maintaining epithelial integrity and show that the phenotype of kindlin-1-deficient cells can be modulated by regulating kindlin-2 gene expression and vice versa. The experimental evidence is provided by use of human keratinocyte cell lines that express both kindlins, just kindlin-1 or kindlin-2, or none of them. Double deficiency of kindlin-1 and -2 had significant negative effects on focal adhesion formation and actin cytoskeleton organization, cell adhesion, survival, directional migration, and activation of β1 integrin, whereas deficiency of one kindlin only showed variable perturbation of these functions. Cell motility and formation of cell-cell contacts were particularly affected by lack of kindlin-2. These results predict that kindlin-1 and -2 can functionally compensate for each other, at least in part. The high physiologic and pathologic significance of the compensation was emphasized by the discovery of environmental regulation of kindlin-2 expression. UV-B irradiation induced loss of kindlin-2 in keratinocytes. This first example of environmental regulation of kindlin expression has implications for phenotype modulation in Kindler syndrome, a skin disorder caused by kindlin-1 deficiency.
Kindlin family proteins (also designated as fermitin family homologs) share a high structural and functional similarity and are considered to be essential for integrin activation.1 The three known family members localize to integrin adhesion sites in cells but have different tissue expression patterns.2–4 Kindlin-2 is expressed in embryonic stem cells and almost ubiquitously in tissues, whereas kindlin-1 is restricted to epithelial and kindlin-3 to hematopoietic and endothelial cells.3,5 The biological relevance of kindlins has been examined through in vitro biochemical, cell-based, and functional assays, as well as in mouse models, and their importance is highlighted through their association with several human genetic disorders.1 Kindlin-1 defects cause the Kindler syndrome (KS), a form of inherited epidermolysis bullosa that manifests with skin blistering, photosensitivity, and progressive generalized poikiloderma.6,7 Mutations in kindlin-3 cause leukocyte adhesion deficiency type 3, a rare inherited disease characterized by severe bleeding and impaired adhesion of leukocytes to inflamed epithelia.1 Thus far, inherited human disorders have not been associated with kindlin-2.1
Functional similarities between kindlin-1 and kindlin-2 have been postulated, including localization to cell-matrix adhesions and binding to and activation of β1 and β3 integrins.8–11 In epidermal keratinocytes, kindlin-1 colocalizes with kindlin-2,12 but whether and how the two kindlins interact with each other have remained unexplored. Kindlin-1 guides keratinocyte adhesion, proliferation, and migration13 and is required for GTPase-mediated lamellipodia formation.14 Kindlin-2 (encoded by the FERMT2 gene) has an essential role in development, but its functions in adult tissues are not well understood. In the dermis it regulates maturation of focal adhesions (FAs) and cytoskeletal organization in fibroblasts, in particular during regenerative processes such as wound healing.15 In zebra fish, knock down of kindlin-2 resulted in skeletal muscle dysfunction, ventricular hypoplasia, and reduced ventricular contractility due to disorganized intercalated disks.16
We investigated the impact of kindlins-1 and -2 on keratinocyte functions in vitro by comparing normal keratinocytes with kindlin-1-deficient, kindlin-2-deficient, or double-deficient cells. The data show that kindlin-1 and kindlin-2 have a number of overlapping functions and can compensate for each other in part but also have distinct functions, in particular in forming cell-cell contacts and in cell migration.
Materials and Methods
Cells and Short Hairpin RNA-Mediated Silencing
Four different cell lines with distinct kindlin expression patterns were generated for this study. As starting material, keratinocyte cell lines derived from normal human skin, designated NHK-E6E7, and from kindlin-1-deficient KS skin, designated KS-NM-E6E7,14,17 were used. The KS patient was compound heterozygous for two FERMT1 null mutations: the 3.9-kb deletion, including exons 10 and 11 and leading to frame shift and premature termination codon, and the nonsense mutation c.910G>T, p.E304X.18 Control cells containing both kindlin-1 and kindlin-2 (Co) were obtained by transducing NHK-E6E7 with control short hairpin RNA (shRNA) lentiviral particles (Santa Cruz Biotechnology, Santa Cruz, CA). Cells in which kindlin-2 was knocked down (K2−) were generated by transducing NHK-E6E7 with FERMT2 shRNA. Kindlin-1-deficient cells KS-NM-E6E7 containing only kindlin-2 were transduced either with control shRNA (K1−) or with FERMT2 shRNA to obtain double-deficient cells (K1−K2−). FERMT2 shRNA lentiviral particles are a pool of concentrated, transduction-ready viral particles containing three target-specific constructs that encode 19 to 25 nucleotides (plus hairpin) shRNA designed to knock down gene expression (Santa Cruz Biotechnology). The cells were cultured in keratinocyte growth medium (Invitrogen, Karlsruhe, Germany) as previously described.14 The transduction with kindlin-2-specific shRNA lentiviral particles or with irrelevant control shRNA was performed in the presence of polybrene. For stable transduction, cells were treated with puromycin, and resistant clones were selected. The expression of kindlins was assessed on RNA and protein level by RT-PCR and immunoblotting, respectively. Images of living cells were captured with the Nikon Biostation and were analyzed using Image J 1.43u.
Cell Proliferation and Adhesion Assays
Cell proliferation assays were performed in triplicates, as previously described.19 Equal numbers of cells were seeded in six-well plates, grown for 1 or 4 days, and counted. For adhesion and spreading assays, 96-well tissue culture plates were coated with 2 ng/μL of laminin 332 or 10 ng/μL of fibronectin overnight at 4°C. After saturation of the wells with 1% bovine serum albumin, equal numbers of cells were seeded. Cells were allowed to adhere for 1 hour at 37°C and thereafter rinsed with PBS, fixed with 70% ethanol for 30 minutes at room temperature, and stained with 0.5% crystal violet. Adherent cells were quantified by measuring the OD at 540 nm with an Infinite 200 spectrophotometer (Tecan Austria GmbH, Grödig, Austria). For morphologic assessment, the cells were documented photographically.
Cell Migration Assays
For monolayer wound healing assays, the cells were grown to confluence on Ibidi μ-dishes with culture inserts (Ibidi, Martinsried, Germany). After removal of the inserts, the cells were rinsed with PBS and incubated further in culture medium under 5% CO2 at 37°C in a Nikon Biostation immunofluorescence microscope (Nikon Instruments Inc., Melville, NY). Photographs were captured every 30 minutes for 24 hours, and the wound areas were measured using the Image J software. Motility of individual cells was monitored by the time-lapse imaging system Nikon's Biostation immunofluorescence microscope. The cells were seeded sparsely on Ibidi μ-dishes, and phase-contrast photographs were captured every 5 minutes for 4 hours with the Nikon Biostation IM system. The migration parameters and tracks of cells were analyzed with the Imaris 6.2.0 software. The processive index was defined as the ratio between the direct distance from start point to end point and the total track distance.
UV-B Irradiation of the Cells
Irradiation of keratinocytes with UV-B was performed as previously described.20 Briefly, 90% confluent untreated NHK-E6E7 and KS-NM-E6E7 cell monolayers were changed into PBS, put on ice, and exposed to 60 mJ/cm2 of UV-B (UV 801 BL; Herbert Waldmann GmbH & Co. KG, Villingen-Schwenningen, Germany). Control cells were treated identically but not irradiated. At different time points after irradiation, total RNA and proteins were extracted.
RT-PCR and Real-Time Quantitative PCR
First strand cDNA was synthesized from 1 μg of RNA using the Advantage RT-for-PCR Kit (Clontech, Mountain View, CA). The PCR reactions were performed in a 50-μL volume containing 250 ng of cDNA, 1× buffer with 2.5 mmol/L Mg2+, 0.2 mmol/L dNTP, 0.5 μmol/L each primer, and 1 U of Hot Master Polymerase (Eppendorf, Hamburg, Germany). Each experiment was performed in triplicate, and values were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Clontech). Primers used to amplify the FERMT2 cDNA were F: 5'-GAAGTTGATGAAGTTGATGCTGCCCTTTC-3' and R: 5'-AGACTGATTCGGATGGATGC-3'. Scanning densitometry of band intensity was performed with the Gel-Pro Express 4.0 software (Media Cybernetics Inc., Bethesda, MD).
Real-time quantitative PCR (qPCR) was performed using the iQ-SYBR-Green Supermix and an iCycler (Bio-Rad, München, Germany), and expression values were normalized to the housekeeping genes hypoxanthine phosphoribosyltransferase 1 (HPRT1) and 18S ribosomal RNA (18s). The data were processed using the BioRad CFX Manager Software (version 1.5). The primers used for amplification were as follows: for FERMT2, F: 5'-GAACAAGCAGATAACAGCGAGA-3' and R: 5'-TGGAACCTTGCAATGAAGTG-3'; for 18s, F: 5'-TCAAGAACGAAAGTCGGAGG-3' and R: 5'-GTGAGGTTTCCCGTGTTGAG-3'; and for HPRT1, F: 5'-AAGATGGTCAAGGTCGCAAG-3' and R: 5'-AAGCAGATGGCCACAGAACT-3'.
Flow Cytometry
For each analysis, 2 × 105 cells were trypsinized, washed twice with PBS, and then incubated with the antibody 4B7R to detect total β1 integrin and with the antibody 12G10 to recognize the active conformation of β1 integrin21 at room temperature for 15 minutes. After washing with PBS containing 1% bovine serum albumin and 0.05% NaN3, the cells were incubated with fluorescein isothiocyanate conjugated F(ab')2 goat anti-mouse IgG(H+C) antibody (Immunotech, Quebec, Canada) for a further 15 minutes. In a parallel experiment, isotype controls were used. Flow cytometry acquisition was performed using the BD FACSCantoII (BD Biosciences, Sparks, MD).
Protein Extraction and Immunoblotting
Confluent cell monolayers or peripheral blood mononuclear cells were lysed and homogenized in 25 mmol/L Tris-HCl, pH 7.5, 0.1 M NaCl, 1% NP-40, 10 mmol/L EDTA, and 1 mmol/L PEFA-Bloc, with 1% protease inhibitor cocktail (Calbiochem, Darmstadt, Germany). The lysates were centrifuged at 14,000 × g for 30 minutes at 4°C, and the protein concentration in the supernatants was determined with the DC Protein Assay (Bio-Rad). For immunoblotting, the proteins were separated on 8% or 10% SDS-polyacrylamide gel electrophoresis under reducing conditions and transferred to nitrocellulose membranes. The blots were incubated with antibodies to kindlin-1,14 kindlin-2,15 kindlin-3 (ProSci, San Diego, CA), or GAPDH (clone 6C5; Millipore) overnight at 4°C, followed by incubation with horseradish peroxidase-labeled anti-mouse or anti-rabbit IgG (Bio-Rad) for 1 hour at room temperature. The visualization was performed with the ECL Plus System (Amersham, Billerica, MA).
Immunofluorescence Microscopy
Cells grown on coverslips were fixed with 2% paraformaldehyde in PBS on ice for 15 minutes, washed three times with PBS, and treated for 5 minutes with 0.1% Triton-X in PBS at room temperature. The incubation with primary antibodies was at 4°C overnight or for 1 hour at room temperature. The following antibodies were used: anti-talin (clone 8d4), anti-β1 integrin (clone 4B7R and clone 12G10), rabbit polyclonal anti-β-catenin (Abcam, Cambridge, MA), and anti-desmoplakin (clone 2Q400). As secondary antibodies, Alexa anti-mouse or anti-rabbit IgG (Invitrogen) were used. Fibrillar actin was stained with phalloidin-TRITC (Millipore) and nuclei with DAPI (Chemicon, Rosemont, IL). Stained cells were observed with a confocal laser scanning microscope (LSM510; Carl Zeiss, Jena, Germany) or with an epifluorescence microscope (Zeiss Axio Imager; Carl Zeiss). Images were captured using Zeiss internal software and processed using ImageJ version 1.43u. To assess apoptosis, TUNEL staining of cultured cells was performed according to the manufacturer's recommendations (Roche, Grenzach-Wyhlen, Germany).
Statistical Analysis
Quantitative data were compared using a two-tailed t-test. P values to determine statistical significance are indicated in the text. Except for the flow cytometry and adhesion assays for which two independent experiments were performed, for all assays three independent experiments were performed.
Results
Both Kindlin-1 and -2 Modulate Cell Shape and Size
The cell lines used in this study, NHK-E6E7 and KS-NM-E6E7, are both keratinocytes immortalized with the same method.14 They express the β1 integrin subunit but not β3. In a separate study using a proteomics approach, we demonstrated that these cell lines are similar to primary keratinocytes.17 To distinguish between the functions of kindlin-1 and kindlin-2 in epithelial cells, we generated four different cell lines with distinct kindlin expression patterns: i) Co, keratinocytes expressing both kindlins; ii) K1−, kindlin-1-negative cells containing only kindlin-2; iii) K2− cells containing kindlin-1 and significantly reduced amounts of kindlin-2; and iv) K1−K2−, cells that are kindlin-1 negative and have significantly reduced expression of kindlin-2. Immunoblotting demonstrated the specificity of the cell lines, that both K1− and K1−K2− cells were completely devoid of kindlin-1, and that a knockdown of kindlin-2 by approximately 90% and 80% was achieved in K2− and K1−K2− cells, respectively (Figure 1, A and B). These cell lines did not express any kindlin-3 (Figure 1B).
Figure 1.
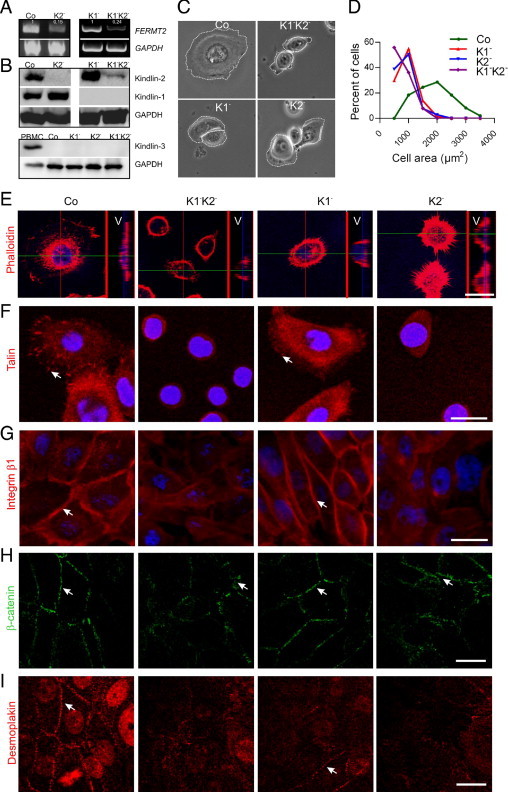
Double deficiency of kindlin-1 and -2 exerts a dramatic effect on the morphology of keratinocytes. RT-PCR (A) and immunoblot (B) analysis demonstrate efficient knockdown of kindlin-2 in K2− and K1−K2− cells and absence of kindlin-1 in K1− and K1−K2− cells. GAPDH was used to control loading. The numbers in panel A represent the mean relative intensities of the bands for FERMT2 from three experiments after normalization to GAPDH. B: Lower panel shows a kindlin-3 immunoblot of peripheral blood mononuclear cells as a positive control and of Co, K1−, K2−, and K1−K2− cells. Antibodies to GAPDH were used as loading control. C: Phase contrast microscopy reveals shape abnormalities and small surface area of K1−K2−, K1−, and K2− cell lines. Note that K1−K2− cells are rounded and have completely lost the polygonal shape and lamellae still evident in K1− and K2− cells. White lines demark cell borders. D: The cell surface area was measured using the software Image J. The graph shows the significantly different distribution of cell areas for K1− (red), K2− (blue), and K1−K2− (purple) compared with control cells (green). E: F-actin was visualized with phalloidin staining and confocal microscopy. For each cell type, the right narrow panel shows the vertical section of the marked cell (V). Note the abnormal distribution of actin and the impaired spreading of K1−K2−, K1−, and K2− cells. Instead of the flat elongated appearance of control cells, all kindlin-deficient cells remained round, just attached but not spread. F: FAs were visualized by staining with anti-talin antibodies (arrows). Note the diffuse distribution of talin in the cytoplasm of K1−K2− and K2− cells. Twenty-four hours after seeding, these cells remained rounded and talin was not targeted to FAs. G: After calcium switch, staining with antibodies to β1 integrin demonstrates localization at cell-cell contacts (arrows) in control and K1− cells. In contrast, β1 integrin has a rather diffuse distribution in K1−K2− and K2− cells. After calcium switch, immunofluorescence staining with anti-β-catenin (green) antibodies (H) was performed to demonstrate adherence junctions and with anti-desmoplakin (red) antibodies (I) to visualize desmosomes. In control and K1− cells, both markers localize to cell-cell junctions (arrows). In K2− and K1−K2− cells, irregular β-catenin–positive adhesion junctions are visible (arrows), whereas desmoplakin remains in the cytoplasm. Nuclei are stained with DAPI (blue). Scale bars in all panels = 20 μm.
Phase contrast microscopy demonstrated a significant effect of kindlin double deficiency on cell size and shape (Figure 1C). K1−K2− cells were rounded and had completely lost the polygonal shape and lamellae, which were still seen to some extent in K1− and K2− cells. The cell surface areas were significantly smaller (mean ± SD cell areas: Co, 1921 ± 90 μm2, n = 49; K1−, 930 ± 25 μm2, n = 151; K2−, 897 ± 32 μm2, n = 103; and K1−K2−, 758 ± 26 μm2, n = 127), as also indicated by histograms with clearly different distribution patterns of cell areas in Co, K1−, K2−, and K1−K2− cells (Figure 1D). Conspicuously altered shape and spreading in K1−K2−, K1−, and K2− cells were demonstrated by confocal microscopy. Vertical sections (V panels in Figure 1E) verified attachment but not spreading of kindlin-deficient cells, in contrast to controls, which appear flat and elongated. The defects were particularly prominent in K1−K2− cells, which also exhibited an abnormal actin cytoskeleton (Figure 1E).
Kindlin-2 Is Important for Formation of FAs and Cell-Cell Contacts
Although both kindlin-1 and kindlin-2 seem to modulate the morphology and size of the cells, immunofluorescence staining revealed distinct differences. Confocal microscopy with talin staining showed that K2− and K1−K2− cells were unable to target talin to FAs 24 hours after seeding (Figure 1F). In contrast, in K1− cells talin staining was only slightly reduced compared with controls (Figure 1F). Formation of new cell-cell adhesions, adherence junctions, and desmosomes was induced by adding 1.2 mmol/L Ca2+ to the cultures. This resulted in targeting of β1 integrin, β-catenin, and desmoplakin to cell-cell adhesions in Co and K1− cells but not in cells with significantly reduced expression of kindlin-2. In K2− and K1−K2− cells, β-catenin staining had an irregular pattern demonstrating abnormal adherence junctions, whereas β1 integrin and desmoplakin remained diffusely distributed in the cytoplasm (Figure 1, G–I).
Kindlin-1 and -2 Can Compensate for Each Other in Integrin Activation
Because integrin activation is regarded as one of the main functions of kindlins, we analyzed this by flow cytometry using the antibody 12G10, which recognizes the active conformation of β1 integrin. Deficiency of both kindlins had a considerable impact on β1 integrin activation; only approximately 20% active integrin was present on the cell surface (Figure 2A). The single-deficient cells exhibited more subtle effects. β1 integrin activation was reduced to approximately 60% in K1− cells and 90% in K2− cells (Figure 2A). These observations strongly suggest that kindlin-1 and kindlin-2 compensate for each other as integrin activators in keratinocytes.
Figure 2.
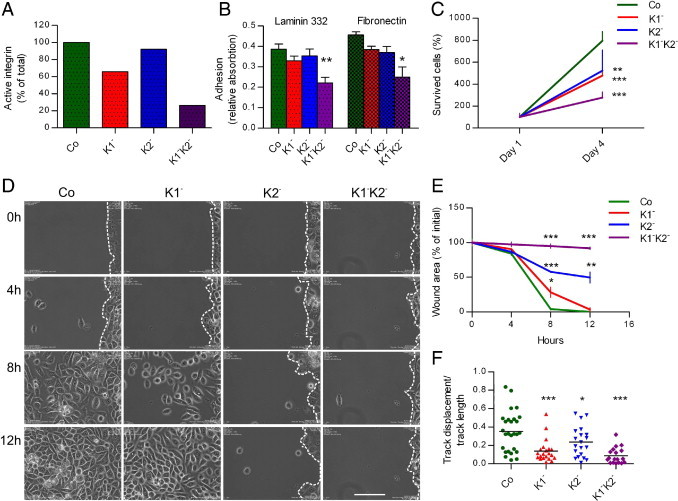
Kindlin-1 and -2 have overlapping functions in keratinocytes. A: To assess the levels of the active integrin on cell surface, flow cytometry was performed. The binding of the antibody 12G10, which recognizes the active conformation of β1 integrin, was quantified by subtracting background values from mean fluorescence intensity values normalized to total integrin expression. The active β1 integrin levels shown are normalized to the total β1 integrin amount present in every cell type. The graph shows the average values from two independent experiments (n = 2). B: Adhesion assays of control and kindlin-depleted keratinocytes on laminin 332 and fibronectin were performed as described. Data are presented as means of eight measurements from two independent experiments; error bars show SD of the mean (t-test: *P < 0.05; **P < 0.01). C: To assess the effect of kindlin knockdown on proliferation and survival, the number of viable Co, K1−K2−, K1−, and K2− cells relative to day 1 after seeding was measured. Shown are mean values from three experiments (n = 3) performed in parallel; error bars show SD (t-test: **P < 0.01; ***P < 0.001). D: Two-dimensional wound closure assays were performed using Ibidi μ-dishes with inserts. Wounds at 0, 4, 8, and 12 hours are shown. Note the delayed wound closure by kindlin-deficient cells, more prominent in the case of K2− and in particular K1−K2− cells, which do not migrate toward the wound area at all. The dashed lines indicate the wound margins. Scale bar = 50 μm. E: The graph shows the quantification of the wound area after 4, 8, and 12 hours as percentage of the initial area. Three wounds were analyzed for each cell type (n = 3). The statistical difference between the deficient cell types and control cell is significant (t-test: **P < 0.01, ***P < 0.0001). F: Cell migration was recorded for 4 hours by time lapse video microscopy. The migration routes of single cells were analyzed and used to determine processive indexes for control (green, n = 27), K1− (red, n = 21), K2− (blue, n = 21), and K1−K2− (purple, n = 19) cells. The mean values are marked with bars; the statistical difference between the deficient cell types and control cells is significant (t-test: ***P < 0.0001, *P < 0.05, and ***P < 0.0001, respectively).
Kindlin-1 and -2 Have Overlapping Functions in Adhesion, Survival, and Migration of Keratinocytes
Because cell adhesion, survival, and migration are generally governed by kindlins, these cellular functions were assessed in the different kindlin-deficient keratinocytes. First, adhesion assays showed that double-deficient K1−K2− cells adhered and spread poorly on both laminin 332 (P < 0.01) and fibronectin (P < 0.05), whereas single-deficient K1− and K2− cells adhered with an efficiency of 80% to 90% of the Co cells (difference not statistically significant), suggesting that in these cell lines the two kindlins mutually compensate for this function (Figure 2B).
Cell survival and migration were also significantly impaired by the deficiency of both kindlins. The survival of K1−K2− cells was approximately 25% of controls (P < 0.001). If only one kindlin was abolished, the cell survival was diminished to about 50% of that of controls (K1−, P < 0.001; K2−, P < 0.01; Figure 2C). In a two-dimensional wound healing model, kindlin-deficient cells exhibited clearly delayed wound closure compared with controls. Co cells migrated into the wounded area and closed it within 8 hours, whereas K1−K2− cells did not migrate at all (Figure 2, D and E). K1− cells migrated slower than Co cells but still covered the wound to approximately 75% within 8 hours. A more prominent migration defect was evident in K2− cells; scattered cells were found within the open wound area after 8 hours but no significant progress had occurred after 12 hours. Quantification of the wound area revealed a highly significant difference between Co and K2− and K1−K2− cells at all time points (P < 0.001; Figure 2E). Single-cell tracking corroborated the migration anomalies of kindlin-deficient cells. A high processive index reflects continued directional migration of single cells, as was seen with Co cells. In contrast, K1− and K1−K2− cells had significantly reduced processive indexes, indicating undirected, random motility. Similar abnormal movement, but to a lesser extent, was observed in K2− cells (Figure 2F).
Regulation of Kindlin-2 Expression as a Phenotype Modifier in KS
These findings clearly indicate an overlap of kindlin-1 and kindlin-2 functions in keratinocytes. Therefore, we postulate that in naturally occurring kindlin-1 deficiency in KS, kindlin-2 is able to compensate at least in part for the functions normally exerted by kindlin-1 in epidermal keratinocytes. A direct consequence is that regulation of kindlin-2 expression can modulate the biological and clinical phenotype in KS. To test this, we investigated kindlin-2 expression after UV-B irradiation because photosensitivity is an unexplained symptom that aggravates the skin condition in KS. Keratinocytes isolated from control and kindlin-1-deficient KS skin were irradiated with 60 mJ/cm2 of UV-B, and kindlin-2 mRNA expression was assessed by semiquantitative RT-PCR and qPCR. Both methods demonstrated a substantial down-regulation of kindlin-2 expression as a consequence of UV-B irradiation. In both normal and KS keratinocytes, kindlin-2 mRNA levels were reduced by approximately 70% to 80%, 6 to 12 hours after UV treatment, and slowly recovered after 12 hours in NHK and after 24 hours in KS-NM cells (Figure 3, A and B). As shown in Figure 3C, these correlate with reduced kindlin-2 protein levels after UV irradiation especially in KS-NM cells. To exclude the possibility that down-regulation reflected cell death, we analyzed as a control the mRNA levels of two other relevant genes, kindlin-1 and α3 integrin, and found no evidence for significant changes (not shown). These experiments clearly indicate that environmental factors can modify kindlin-2 gene expression and modulate the phenotype in KS by abolishing compensatory cellular functions.
Figure 3.

In keratinocytes derived from normal and KS skin, kindlin-2 expression was down-regulated after treatment with UV-B. A: Representative agarose gel electrophoresis showing RT-PCR products on total RNA extracted from NHK-E6E7 (NHK) and KS-NM-E6E7 (KS-NM) cells treated with UV (+) or not treated (−). It demonstrates the reduction of kindlin-2 expression in UV-irradiated keratinocytes. For quantification, band intensities were measured in three independent experiments and normalized to total GAPDH levels. In each experiment, the kindlin-2 levels for not irradiated cells (gray bars, UV–) were set as 100, and those for irradiated cells (black bars, UV+) were calculated as a percentage. Mean values and the SDs are shown. The statistical difference between treated and not treated cells is significant (t-test: **P < 0.001 and ***P < 0.0001, respectively). B: Total RNA was extracted from NHK-E6E7 (NHK) and KS-NM-E6E7 keratinocytes 0, 6, 9, 12, or 24 hours after treatment with 60 mJ/cm2 of UVB (+, gray bars). At the same time points, untreated cells were considered as controls (−, white bars). The graph shows the normalized fold expression of the FERMT2 gene obtained by qPCR. 18s and HPRT1 were used for normalization. C: In the upper panel, lysates obtained from the same experiment as in panel B, collected 24 hours after treatment with (+) or without (−) UV-B were immunoblotted with antibodies to kindlin-2 and GAPDH.
Discussion
In this study, we uncovered the overlapping roles of kindlin-1 and kindlin-2 in maintaining epithelial integrity. Cell adhesion, spreading, proliferation, and migration were significantly impaired by loss of both kindlins, suggesting that they have at least in part redundant functions. Despite complete lack of FAs and cell-cell junctions, the kindlin double-deficient cells survived but showed only minimal proliferation and were not able to spread or migrate. In addition, they exhibited slightly increased apoptosis in culture 1 day after seeding (not shown). Deficiency of only one kindlin yielded considerably fewer severe cellular changes, corroborating the pivotal need for at least one kindlin in the above epithelial cell processes. A similar observation has been reported in Drosophila, where the kindlin homologues fermitin 1 and fermitin 2 were shown to act in a partially redundant manner to maintain muscle integrity.22
An interesting finding in our model was that kindlin-1 and -2 were able to mutually compensate for the activation of β1 integrin, at least in part, as shown by the fact that adhesion was only minimally reduced in single-deficient K1− and K2− cells. This is in agreement with data derived from the kindlin-1 knockout mouse, which has a very minor skin phenotype, and in which kindlin-1-negative keratinocytes showed only slightly reduced activation of β1 integrins.8 At present, the amounts of kindlin-1 and -2 proteins in keratinocytes cannot be compared on the basis of immunoblots because the sensitivity of kindlin-1 antibodies is much higher than that of kindlin-2 antibodies. Therefore, to address the mechanisms for how and to what extent the kindlins compensate for each other requires development of new molecular tools and approaches, such as quantitative proteomics to elucidate the kinetics of kindlin-containing protein complexes in keratinocytes.23
However, it became obvious that kindlin-1 and -2 also have distinct dominant functions. Although both kindlins had similar effects on cell morphology and size, kindlin-2 deficiency seemed to affect formation of cell-cell junctions and FAs in a more drastic manner, as demonstrated by diffuse cytoplasmic distribution of β1 integrin, β-catenin, desmoplakin, and talin in K2− and K1−K2− cells, instead of a membrane-associated localization. Despite the higher levels of integrin activation, the impact of kindlin-2 deficiency on cell motility was stronger than that of kindlin-1. This suggests that the abnormalities of K2− cells may not result only from defective integrin activation. Because kindlin-1 and -2 have partially overlapping functional roles, major questions moving forward include understanding which interactions are responsible for the similar and distinct functions, what binding partners mediate the unique interactions, and which ones mediate kindlin-1- or kindlin-2-specific interactions. Studies addressing these questions are ongoing in the laboratory.
Despite overlapping functions, the two kindlins cannot fully rescue the loss of the other in keratinocytes. For example, knockdown of kindlin-2 aggravates the cellular phenotype of K1− cells. In the light of this, it is conceivable that the progressive phenotype in KS results from modulated expression of kindlin-2. Because all KS patients have kindlin-1 null mutations, the phenotypic variability is believed to depend on genetic and environmental modifiers,24 one of them being UV light. To further dissect these hypotheses, we examined the regulation of kindlin-2 expression after UV-B irradiation in normal and KS keratinocytes. Intriguingly, kindlin-2 was strongly down-regulated after irradiation with UV-B; the mRNA levels decreased to 15% to 20% of controls. The depletion of kindlin-2 in kindlin-1-negative keratinocytes could explain the photosensitivity observed in KS patients and the aggravation of the symptoms in sun-exposed skin areas. Gene regulation by UV irradiation is well studied in human skin and keratinocytes. A ninefold increase of α3 integrin and a 20-fold decrease of actin protein levels by UV-B25 have been reported. Interestingly, in sun-exposed skin, expression of β1 integrin protein by epidermal basal cells was reduced, paralleling a down-regulation of β1 integrin mRNA.26
Taken together, the present study demonstrates that the highly homologous kindlin-1 and -2 display partly distinctive and partly overlapping functions in epidermal keratinocytes and that they can compensate for each other to a certain extent. Loss of the compensation leads to a more severe cellular phenotype, as seen in KS after UV irradiation. This represents the first example of environmental phenotype modulation of KS.
Acknowledgments
We thank Melanie Boerries for providing primers and Nadja Chmel, Florian Scheithauer, and Margit Schubert for expert technical assistance. We are grateful to Yannik Gache for generating NHK-E6E7 and KS-NM-E6E7 cells.
Footnotes
Supported by grant HA 5663/1-1 from the German Research Foundation (C.H. and L.B.T.); by the International Kindler Syndrome Network Kindlernet grant from ERANET, project 01GM0812, by the Network Epidermolysis Bullosa grant from the Federal Ministry for Education and Research (L.B.T.); and by the Freiburg Institute for Advanced Studies (L.B.T.).
References
- 1.Meves A., Stremmel C., Gottschalk K., Fassler R. The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 2009;19:504–513. doi: 10.1016/j.tcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Montanez E., Ussar S., Schifferer M., Bosl M., Zent R., Moser M., Fassler R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ussar S., Wang H.V., Linder S., Fassler R., Moser M. The Kindlins: subcellular localization and expression during murine development. Exp Cell Res. 2006;312:3142–3151. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Larjava H., Plow E.F., Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9:1203–1208. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bialkowska K., Ma Y.Q., Bledzka K., Sossey-Alaoui K., Izem L., Zhang X., Malinin N., Qin J., Byzova T., Plow E.F. The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. J Biol Chem. 2010;285:18640–18649. doi: 10.1074/jbc.M109.085746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel D.H., Ashton G.H., Penagos H.G., Lee J.V., Feiler H.S., Wilhelmsen K.C., South A.P., Smith F.J., Prescott A.R., Wessagowit V., Oyama N., Akiyama M., Al Aboud D., Al Aboud K., Al Githami A., Al Hawsawi K., Al Ismaily A., Al-Suwaid R., Atherton D.J., Caputo R., Fine J.D., Frieden I.J., Fuchs E., Haber R.M., Harada T., Kitajima Y., Mallory S.B., Ogawa H., Sahin S., Shimizu H., Suga Y., Tadini G., Tsuchiya K., Wiebe C.B., Wojnarowska F., Zaghloul A.B., Hamada T., Mallipeddi R., Eady R.A., McLean W.H., McGrath J.A., Epstein E.H. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jobard F., Bouadjar B., Caux F., Hadj-Rabia S., Has C., Matsuda F., Weissenbach J., Lathrop M., Prud'homme J.F., Fischer J. Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet. 2003;12:925–935. doi: 10.1093/hmg/ddg097. [DOI] [PubMed] [Google Scholar]
- 8.Ussar S., Moser M., Widmaier M., Rognoni E., Harrer C., Genzel-Boroviczeny O., Fassler R. Loss of kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai-Cheong J.E., Parsons M., Tanaka A., Ussar S., South A.P., Gomathy S., Mee J.B., Barbaroux J.B., Techanukul T., Almaani N., Clements S.E., Hart I.R., McGrath J.A. Loss-of-function FERMT1 mutations in kindler syndrome implicate a role for fermitin family homolog-1 in integrin activation. Am J Pathol. 2009;175:1431–1441. doi: 10.2353/ajpath.2009.081154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y.Q., Qin J., Wu C., Plow E.F. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harburger D.S., Bouaouina M., Calderwood D.A. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284:11485–11497. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai-Cheong J.E., Ussar S., Arita K., Hart I.R., McGrath J.A. Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol. 2008;128:2156–2165. doi: 10.1038/jid.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herz C., Aumailley M., Schulte C., Schlotzer-Schrehardt U., Bruckner-Tuderman L., Has C. Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem. 2006;281:36082–36090. doi: 10.1074/jbc.M606259200. [DOI] [PubMed] [Google Scholar]
- 14.Has C., Herz C., Zimina E., Qu H.Y., He Y., Zhang Z.G., Wen T.T., Gache Y., Aumailley M., Bruckner-Tuderman L. Kindlin-1 is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am J Pathol. 2009;175:1442–1452. doi: 10.2353/ajpath.2009.090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y., Esser P., Schacht V., Bruckner-Tuderman L., Has C. Role of kindlin-2 in fibroblast functions: implications for wound healing. J Invest Dermatol. 2011;131:245–256. doi: 10.1038/jid.2010.273. [DOI] [PubMed] [Google Scholar]
- 16.Dowling J.J., Gibbs E., Russell M., Goldman D., Minarcik J., Golden J.A., Feldman E.L. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res. 2008;102:423–431. doi: 10.1161/CIRCRESAHA.107.161489. [DOI] [PubMed] [Google Scholar]
- 17.Sprenger A., Kuttner V., Biniossek M.L., Gretzmeier C., Boerries M., Mack C., Has C., Bruckner-Tuderman L., Dengjel J. Comparative quantitation of proteome alterations induced by aging or immortalization in primary human fibroblasts and keratinocytes for clinical applications. Mol Biosyst. 2010;6:1579–1582. doi: 10.1039/c003962d. [DOI] [PubMed] [Google Scholar]
- 18.Has C., Wessagowit V., Pascucci M., Baer C., Didona B., Wilhelm C., Pedicelli C., Locatelli A., Kohlhase J., Ashton G.H., Tadini G., Zambruno G., Bruckner-Tuderman L., McGrath J.A., Castiglia D. Molecular basis of Kindler syndrome in Italy: novel and recurrent Alu/Alu recombination, splice site, nonsense, and frameshift mutations in the KIND1 gene. J Invest Dermatol. 2006;126:1776–1783. doi: 10.1038/sj.jid.5700339. [DOI] [PubMed] [Google Scholar]
- 19.Raghavan S., Vaezi A., Fuchs E. A role for alphabeta1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev Cell. 2003;5:415–427. doi: 10.1016/s1534-5807(03)00261-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferrero L., Pissavini M., Doucet O. How a calculated model of sunscreen film geometry can explain in vitro and in vivo SPF variation. Photochem Photobiol Sci. 2010;9:540–551. doi: 10.1039/b9pp00183b. [DOI] [PubMed] [Google Scholar]
- 21.Humphries J.D., Schofield N.R., Mostafavi-Pour Z., Green L.J., Garratt A.N., Mould A.P., Humphries M.J. Dual functionality of the anti-beta1 integrin antibody, 12G10, exemplifies agonistic signalling from the ligand binding pocket of integrin adhesion receptors. J Biol Chem. 2005;280:10234–10243. doi: 10.1074/jbc.M411102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai J., Binari R., Ni J.Q., Vijayakanthan M., Li H.S., Perrimon N. RNA interference screening in Drosophila primary cells for genes involved in muscle assembly and maintenance. Development. 2008;135:1439–1449. doi: 10.1242/dev.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphries J.D., Byron A., Bass M.D., Craig S.E., Pinney J.W., Knight D., Humphries M.J. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal. 2009;2:ra51. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai-Cheong J.E., Tanaka A., Hawche G., Emanuel P., Maari C., Taskesen M., Akdeniz S., Liu L., McGrath J.A. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233–242. doi: 10.1111/j.1365-2133.2008.08976.x. [DOI] [PubMed] [Google Scholar]
- 25.Perluigi M., Di Domenico F., Blarzino C., Foppoli C., Cini C., Giorgi A., Grillo C., De Marco F., Butterfield D.A., Schinina M.E., Coccia R. Effects of UVB-induced oxidative stress on protein expression and specific protein oxidation in normal human epithelial keratinocytes: a proteomic approach. Proteome Sci. 2010;8:13. doi: 10.1186/1477-5956-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosset S., Bonnet-Duquennoy M., Barre P., Chalon A., Lazou K., Kurfurst R., Bonte F., Schnebert S., Disant F., Le Varlet B., Nicolas J.F. Decreased expression of keratinocyte beta1 integrins in chronically sun-exposed skin in vivo. Br J Dermatol. 2003;148:770–778. doi: 10.1046/j.1365-2133.2003.05159.x. [DOI] [PubMed] [Google Scholar]


