Abstract
The recent emergence of the swine-origin influenza A H1N1 virus (S-OIV) poses a serious global health threat. Rapid detection and differentiation of S-OIV from seasonal influenza is crucial for patient management and control of the epidemics. A one-step, single-tube accelerated and quantitative S-OIV-specific H1 reverse transcription loop-mediated isothermal amplification (RTLAMP) assay for clinical diagnosis of S-OIV by targeting the H1 gene is reported in this article. A comparative evaluation of the H1-specific RTLAMP assay vis-à-vis the World Health Organization-approved real-time polymerase chain reaction (RTPCR), involving 239 acute-phase throat swab samples, demonstrated exceptionally higher sensitivity by picking up all of the 116 H1N1-positive cases and 36 additional positive cases among the negatives that were sequence-confirmed as S-OIV H1N1. None of the real-time RTPCR-positive samples were missed by the RTLAMP system. The comparative analysis revealed that S-OIV RTLAMP was up to tenfold more sensitive than the World Health Organization real-time RTPCR; it had a detection limit of 0.1 tissue culture infectious dosage of 50/ml. One of the most attractive features of this isothermal gene amplification assay is that it seems to have an advantage in monitoring gene amplification by means of SYBR Green I dye-mediated naked-eye visualization within 30 minutes compared to 2 to 3 hours for a real-time reverse transcription polymerase chain reaction. This suggests that the RTLAMP assay is a valuable tool for rapid, real-time detection and quantification of S-OIV in acute-phase throat swab samples without requiring sophisticated equipment.
The recent emergence of the swine-origin influenza A H1N1 virus (S-OIV), which has a high efficiency of human-to-human transmission, raised concern about a global pandemic. The 2009 swine flu pandemic was caused by a novel S-OIV that had not been previously recognized in pigs or humans.1 This newly emerged virus is a quadruple reassortment of two swine strains, one human strain, and one avian strain of influenza viruses. The largest proportion of genes comes from swine influenza viruses (30.6% from North American swine influenza strains, 17.5% from Eurasian swine influenza strains), followed by North American avian influenza strains (34.4%) and human influenza strains (17.5%).2
Pigs play an important role in interspecies transmission of the influenza virus. Susceptible pig cells possess receptors for both avian (α 2-3-linked sialic acids) and human influenza strains (α 2-6-linked sialic acids), which allow for the reassortment of influenza virus genes from different species if a pig cell is infected with more than one strain.3 The S-OIV has caused a considerable number of deaths within a short time since its emergence.
The most common clinical findings of the 2009 H1N1 influenza A pandemic have been fever, cough, sore throat, malaise, and headache; vomiting and diarrhea have also been common, both of which are unusual features of seasonal influenza.1 The emergence of new strains will continue to pose challenges to public health and the scientific communities.4 Molecular tests for rapid and reliable diagnosis of novel S-OIV are therefore critical for patient management and control of epidemics.
A confirmed case of pandemic H1N1 influenza A can be made through real-time reverse transcription polymerase chain reaction (RTPCR) or culture. The recommended test to confirm the diagnosis of pandemic H1N1 influenza A virus is real-time RTPCR for influenza A, B, H1, and H3. Isolation of pandemic H1N1 influenza A virus using culture is diagnostic, but culture is usually too slow to help guide clinical management.5,6 A negative viral culture does not exclude infection by H1N1 influenza A. Clinicians may consider using rapid influenza antigen tests as part of their evaluation of patients suspected of having pandemic H1N1 influenza A, but results should be interpreted with caution.7,8
A large number of RTPCR tests have been reported, and they use various chemical and multiplexing formats.9–16 However, in the current scenario, the only real-time RTPCR validated by the Centers for Disease Control (CDC) is the one approved by the World Health Organization (WHO) for confirmation of pandemic novel swine-origin H1N1 infection. This CDC real-time RTPCR is based on a panel of oligonucleotide primers and dual-labeled hydrolysis probes that use the Superscript TM III Platinum one-step quantitative kit (Invitrogen, San Diego, CA) with four sets of primers and probes for universal influenza A, swine influenza A, swine H1(new H1N1), and housekeeping gene (RNase P) for testing the quality of the RNA template (http://www.who.int/csr/resources/publications/swine flu/CDC real-time RTPCR protocol). One of the limitations of the existing WHO-approved CDC real-time RTPCR is that the test system is very expensive, considering the large number of primer and probe sets that must be used. In addition, the requirement of expensive real-time polymerase chain reaction (PCR) instruments also restricts the system's application to a few referral laboratories that have good financial resources. Overall, the real-time RTPCR test system is expensive and time consuming—it requires 3 to 4 hours. Therefore, real-time RTPCR is not the method of choice in basic clinical settings in developing countries for clinical diagnosis of novel S-OIV. It is critical to develop simple and economical molecular tests that can be used in field conditions, especially at peripheral health care settings, as a routine test that does not require sophisticated instruments.
Loop-mediated isothermal gene amplification (LAMP) is a novel nucleic acid amplification method developed by the Eiken Chemical Company in Tokyo, Japan. It has the potential to replace the PCR because of its simplicity, rapidity, specificity, and cost-effectiveness.17 The reverse transcription LAMP (RTLAMP) assay is a simple diagnostic tool in which the reaction is carried out in a single tube by mixing the buffer, primers, reverse transcriptase, and DNA polymerase and incubating the mixture at 63°C for 30 minutes. Compared to RTPCR and real-time PCR, RTLAMP has the advantages of reaction simplicity and detection sensitivity.18,19 The LAMP assay has emerged as a powerful gene amplification tool for the rapid identification of microbial infections and is being increasingly used by various investigators for the rapid detection and typing of emerging viruses, such as West Nile, severe acute respiratory syndrome, dengue, Japanese encephalitis, chikungunya, and so forth.20–25
In the present study, we report the development of a one-step, real-time RTLAMP assay for rapid and real-time detection of novel S-OIV RNA in clinical specimens by targeting the HA gene. The particular importance is the substantial reduction in the time required for the confirmation of results: 30 minutes as opposed 2 to 3 hours in real time RT-PCR. The S-OIV H1 gene-specific RTLAMP assay reported in this study was found to be a simple, rapid, reliable, and inexpensive method for the detection as well as the identification of novel S-OIV without requiring any sophisticated instruments or fluorescent probes. The data concerning the sensitivity and specificity of the method is discussed, and the applicability of RTLAMP technology to clinical diagnosis of patients with swine flu is validated by evaluation by acute-phase throat swab samples collected from the ongoing epidemic in India.
Materials and Methods
Primer Design
We designed a set of six primers comprising two outer, two inner, and two loop primers that recognize eight distinct regions spanning more than 188 base pair (bp)-conserved regions of the structural HA gene of the novel H1N1 virus, corresponding to the genome position 529–716. The two outer primers were described as the forward outer primer (F3) and the backward outer primer (B3). The inner primers were described as the forward inner primer (FIP) and the backward inner primer (BIP). Further, two loop primers, forward loop primer (FLP) and backward loop primer (BLP), were designed to accelerate the amplification reaction. The usefulness of the selected primer set for correctly identifying all strains and isolates of S-OIV was established through sequence alignment of all available HA gene sequences in the GenBank, including the circulating strains responsible for the recent pandemic in India, using DNASIS software (MiraiBio, Yokohama, Japan). In addition, the homology study was used to ensure the maximum number of mismatched nucleotides between pandemic S-OIV sequences and seasonal flu (InfA), including other respiratory viruses, such as respiratory syncytial virus, para influenza, adeno virus, and so forth, so as to avoid cross-amplification. The details of the primer sequences with regard to genome position are depicted in Table 1.
Table 1.
Details of RTLAMP Primer Sets Designed for Rapid and Real-Time Detection of Novel Swine Flu H1N1 Virus⁎
| Name of primer | Genome position⁎ | Length of oligonucleotide (bp) | Sequence details |
|---|---|---|---|
| Forward outer (F3) | 529–547 | 19 | 5′-AAGCTCAGCAAATCCTACA-3′ |
| Backward outer (B3) | 699–716 | 18 | 5′-TCCCTCACTTTGGGTCTT-3′ |
| Forward inner primer (FIP) | F1c | 25 | 5′-GACTTTGTTGGTCAGCACTAGTAGA-3′ |
| [F1c+TTTT+F2] | 598–622 | ||
| F2 | 19 | 5′-AAAGGGAAAGAAGTCCTCG-3′ | |
| 556–574 | |||
| Backward inner primer (BIP) | B1 | 25 | 5′-TCTATCAGAATGCAGATGCATATGT-3′ |
| [B1+TTTT+B2c] | 623–647 | ||
| B2c | 18 | 5′-TGCTATTTCCGGCTTGAA-3′ | |
| 679–696 | |||
| Forward loop primer (FLP) | 576–595 | 20 | 5′-GATGGTGAATGCCCCATAGC-3′ |
| Backward loop primer (BLP) | 650–672 | 23 | 5′-TTGTGGGGTCATCAAGATACAGC-3′ |
GenBank Accession No. FJ966082; WHO-recommended referral strain.
Clinical Samples
A total of 239 acute-phase throat-swab samples were collected from patients when they were between 3 and 7 days of having a fever. The patients were referred to the National Centre for Disease Control in Delhi, India; the Post Graduate Institute of Medical Education and Research in Chandigarh, India; and the National Institute of Mental Health and Neuro Sciences in Bangalore, India, for S-OIV H1N1 testing. The RNA extracted from these samples was stored at −80°C at the respective centers. The testing of S-OIV H1 RTLAMP was performed within one month of storage at the centers. To check the cross-reactivity, 34 real-time RTPCR-confirmed InfA-positive samples were also included in the panel in addition to 20 throat-swab samples collected from apparently healthy individuals who served as negative controls.
RNA Extraction
The genomic viral RNA was extracted from 140 μL of throat and nasal swab suspensions using QIAamp viral RNA mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's protocols.
One-Step RT-LAMP
The amplification of the HA gene of H1N1 virus was carried out in a total 25 μL reaction volume using 12.5 μL of in-house buffer [20 mmol/L Tris-HCl pH8.8; 10 mmol/L KCl; 10 mmol/L (NH4)2SO4; 8 mmol/L MgSO4; 1.4 mmol/L dNTPs; 0.8M betaine; 0.1% Tween-20]; 3.75 μL c DNA mix (5x buffer, 10 mmol/L dNTP mix, and 0.25 μL of RNasin inhibitor); 1 μL of primer mix containing (50 pmol each of the primers FIP and BIP; 5 pmol each of the outer primers F3 and B3; 25 pmol each of loop primers FLP and BLP); 1 μL (8 units) of the BstDNA polymerase (New England Biolabs, Ipswich, MA); 0.25 μL of MMLV-RT; 1.5 μL of nuclease-free water; and 5 μL of RNA at 37°C for 30 minutes. The gene amplification was accomplished by incubating the reaction mixture at 63°C for 60 minutes in a routine laboratory water bath/dry heating bath. To rule out contamination issues, proper negative controls, including no template, no primer; and no enzyme, were always kept alongside each run.
Real-Time Monitoring of RTLAMP
The real-time monitoring of RTLAMP was observed through spectrophotometric analysis by recording optical density at 400 nm every 6 seconds with the help of a loop amp real-time turbidimeter (LA-200, Teramecs, Kyoto, Japan). The cut-off value for positivity by the real time RTLAMP assay was determined by taking into account the time of positivity, in minutes, at which the turbidity increases above the threshold value, fixed at 0.1, which is two times more than the average turbidity value of the negative controls of several replicates.
Agarose Gel Analysis
Following incubation at 63°C for 60 minutes, 10 μL aliquot of RTLAMP products were electrophoresed on 3% NuSieve 3:1 agarose gel (BMA, Rockland, ME) in Tris-borate buffer followed by staining with ethidium bromide and visualization on an UV transilluminator at 302 nm.
Naked-Eye Visualization
Following amplification, the tubes were inspected by naked eye for white turbidity after pulse spins to deposit the precipitate in the bottom of the tube. The inspection for amplification was also performed by observation of color change following the addition of 1 μL of SYBR Green 1 dye (Cambrex, East Rutherford, NJ) to the tube. In cases of positive amplification, the original orange color of the dye changes to green and can be seen in natural light and under UV light (302 nm). If there is no amplification, the original orange color of the dye is retained. However, to rule out bias in borderline cases, the result was recorded by three observers in the laboratory, and the sample was considered positive if the score was judged to be positive by more than two observers.
Sensitivity of S-OIV RTLAMP
The limit of detection of both S-OIV H1 RTLAMP as well as WHO-approved, CDC-recommended real-time RTPCR was determined by using the titrated infected culture fluid of the H1N1 virus isolate obtained from our clinical collaborators at the National Institute of Mental Health and Neuro Sciences in Bangalore, India. The serial tenfold dilutions of extracted RNA from the infected culture fluid were analyzed in triplicate. To generate the S-OIV H1 gene-specific RTLAMP standard curve, 188 bp target of the H1 gene was amplified by outer (F3) and backward outer (B3) primers. The amplicon was then purified and cloned using the pC R4-TOPO vector system (Invitrogen, San Diego, CA) according to the manufacturer's specifications. The sequence confirmed that plasmid was linearized using a vector-specific restriction enzyme (NdeI) and then quantified by Quantity One software (Bio-Rad, Hercules, CA). The concentration of the plasmid preparation was determined by measuring the OD at 260 nm. Conversion to genome equivalents was calculated as 1 OD260 equals 50g/ml and one bp equals 660 g/mol, resulting in a molecular weight for the plasmid (4156 bp) of 2.74 × 106 pg/pmol. The copy numbers per μL were determined by using the following formula:
Further serial tenfold dilution of the linearized plasmid was used in triplicate for detection of sensitivity and for construction of a standard curve using the cycle threshold values obtained from the known concentration of serially diluted plasmid constructs.
Evaluation of Throat Swab Samples
The applicability of S-OIV H1 RTLAMP for clinical diagnosis was validated by the study of 239 human throat swab samples, including those of 34 patients who had seasonal flu and 20 who were apparently healthy, negative controls. The results were compared with the results of the WHO-approved, CDC-recommended real-time RTPCR.
Real-Time RTPCR
Before the RTLAMP assay, all the samples were analyzed by the CDC-recommended real-time RTPCR. The assay is based on TaqMan assays (Applied Biosystems) that involve a panel of oligonucleotide primers and a dual-labeled hydrolysis probe using the Superscript TM III Platinum One-Step quantitative kit (Invitrogen). The amplification was carried out in a 25 μL reaction volume according to the manufacturer's instructions and the recommended thermal profile.
SYBR Green I-Based Real-Time RTPCR
Analysis of the discrepant additional positive samples was carried out by sequencing more than one target gene—that is, not only RTLAMP, but also 141bp of HA (1010–1150) and 173 bp of NA (1137–1309). We used SYBR Green I–based, one-step, real-time RTPCR in an MX 3000P quantitative PCR system (Stratagene, La Jolla, CA) for further confirmation. Following optimization with standard RNA using Brilliant SYBR Green Single-Step QRTPCR Master Mix (Stratagene), test samples were assayed in a 25-μL reaction mixture containing 12.5 μL of 2x reaction mix, 0.4 μL of reference dye (ROX), 1 μL (10 pmol) of each forward (F3) and reverse (B3) primers, 1 μL of RNA, 0.1 μL of reverse transcriptase, and 9.0 μL of nuclease-free water. The thermal profile consists of 1 cycle of 30 minutes of reverse transcription at 50°C and 10 minutes of TaqDNA polymerase activation at 95°C, followed by 40 cycles of PCR at 95°C for 30 seconds (denaturation), 55°C for 60 seconds (annealing), and 72°C for 30 seconds (extension).
Sequence Analysis
To establish the authenticity of the S-OIV H1 RTLAMP, the HA and NA genes (resulting amplicons of the real-time RTPCR) were sequenced with two outer LAMP primers (F3 and B3) using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The sequencing was carried out using a 3130 DNA analyzer (Applied Biosystems), and all of the sequences were assembled using Lasergene software (DNA Star, Madison, WI). Along with the RTLAMP target, we also sequenced 141 bp of the HA gene (1010–1150) and 173 bp of the NA (1137–1309) for confirmation of discrepant additional positive samples.
Results
A one-step, real-time, accelerated quantitative RTLAMP assay was standardized for rapid detection of novel S-OIV. A set of six primers, comprising two outer primers (F3 and B3), two internal primers (FIP and BIP), and two loop primers (FLP and BLP), were designed using the H1 gene strain recommended by the WHO (Human H1N1 virus isolates, California, 2009, Gene Bank Accession No FJ966082). Each primer's position in the genomic sequences is shown in Table 1.
The monitoring of gene amplification was accomplished by real-time monitoring of turbidity, which is recorded at 400 nm every 6 seconds with the help of a loopamp real-time turbidity meter (Teramecs, Los Angeles, CA). The result indicated that the minimum time required for the initiation of amplification was 15 minutes. It was also observed that there was continuous amplification of the target sequence, as revealed by the increased turbidity; in the negative control, the turbidity remained fixed at about 0.01, well below the threshold value (Figure 1). None of the positive samples, which were tested multiple times, showed increased turbidity after 30 minutes. Therefore, a sample having time of positivity (Tp) values of 30 minutes or less and turbidity above the threshold value of 0.1 or more was considered to be positive.
Figure 1.
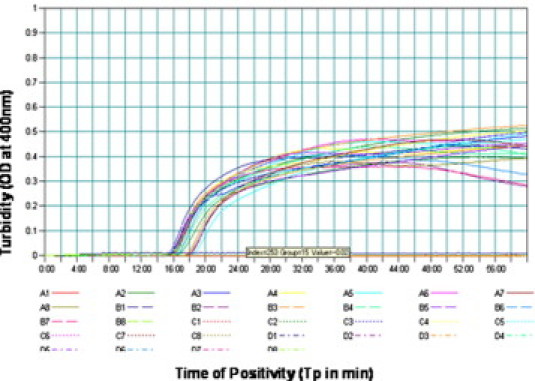
Real-time kinetics of S-OIV H1 gene-specific RTLAMP showing the amplification curve. The x axis depicts the time of positivity (Tp); the y axis shows the turbidity value in terms of optical density at 400 nm.
Sensitivity and Specificity of S-OIV H1 RTLAMP
The comparative limit of detection of S-OIV H1 RTLAMP as well as WHO-approved, CDC-recommended real-time RTPCR were determined by using serial tenfold dilutions of extracted RNA from the infected culture fluid in triplicate. The limit of detection for the S-OIV H1 RTLAMP assay was found to be 0.1 tissue culture infection dosage 50/ml as compared to 1 tissue culture infection dosage 50/ml for WHO-CDC real-time RTPCR. Therefore, S-OIV H1 RTLAMP is as much as tenfold more sensitive than the WHO-CDC real-time RTPCR.
A standard curve was generated by plotting a graph between different concentrations of gene copy numbers (106 to 1) and time of positivity (Tp) in minutes through real-time monitoring (Figure 2A–B). The quantification of the virus load in the positive samples was extrapolated on the basis of their Tp by using the standard curve.
Figure 2.
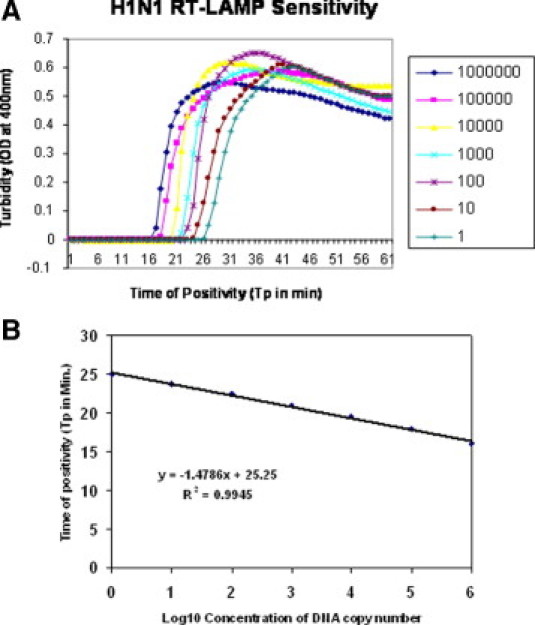
A: Sensitivity of S-OIV H1 gene-specific RTLAMP assay as monitored by real-time measurement of turbidity. Shown (left to right) are the amplification curves of decreasing concentration of virus, ranging from 106 to 1 copy number of the 188 bp template (HA) gene in serial tenfold dilution. B: S-OIV H1 RTLAMP-specific standard curve as generated from the amplification plot between different copy number of the template (serial tenfold dilution from 106 to 1 copy number) and Tp. The Tp value shown here is the average of triplicates run for each concentration.
The specificity of the RTLAMP primers for the H1 gene of S-OIV was established by ruling out the cross-reactivity with seasonal flu viruses having similar clinical symptoms. The S-OIV H1 gene-specific RTLAMP primers showed a high degree of specificity for the novel H1N1 virus by amplifying the H1 gene of the novel H1N1 virus only while yielding negative results for seasonal flu as well as for healthy throat-swab samples. Further confirmation of the structures of the amplified products was also carried out by sequencing; the sequences we obtained perfectly matched the expected nucleotide sequences (data not shown).
Evaluation of Efficacy in Clinical Samples
The feasibility of using S-OIV H1 RTLAMP for clinical diagnosis was validated by studying a panel of 239 human throat-wash samples suspected to be part of the ongoing epidemic. The comparative evaluation of the S-OIV H1-specific RTLAMP assay using WHO-recommended, CDC-approved, real-time RTPCR demonstrated exceptionally higher sensitivity by picking up 36 additional positive borderline cases (as indicated by higher Tp values).
Further analysis of the discrepant additional positive samples was carried out by sequencing both HA and NA genes in addition to the RTLAMP target. All these additional positive samples were amplified by SYBR Green I real-time RTPCR, and the amplicons were then purified and sequenced as indicated earlier. Sequence analysis of partial HA genes in all of these samples revealed >99% nucleotide identity with the A/California/04/2009 (H1N1) prototype strain. None of the CDC real-time RTPCR positive samples were missed by the RTLAMP system (Table 2).
Table 2.
Comparative Evaluation of Swine Flu H1-Specific RTLAMP Assay and WHO-approved, CDC Real-Time RT-PCR Assay for the Detection of the HA Gene of H1N1 Virus in Suspected Human-Patient Throat-Swab Samples⁎
| Real-time RTPCR | RT-LAMP | No. of samples |
|---|---|---|
| + | + | 116 |
| + | − | 0 |
| − | + | 36† |
| − | − | 87 |
+, positive; −, negative; PPV, positive predictive value; NPV, negative predictive value.
Total no of samples = 239; concordance, 85%; sensitivity, 100%; specificity, 100%; ppv, 100%; npv, 100%.
Additional positive samples turned out to be true H1N1 positives as confirmed by real-time RTPCR followed by sequencing.
Furthermore, the S-OIV H1 RTLAMP assay was found to be specific for the H1 gene of the novel H1N1 virus because it did not cross-react with any of the 34 seasonal flu and 20 apparently healthy human throat-swab samples included in the panel as negative controls. The virus load in the various clinical samples, as determined by S-OIV H1-specific RTLAMP, a standard curve based on Tp, was found to be in the range of 106−1 copy numbers (Figure 3). The intra-assay reproducibility and variability of the S-OIV H1 gene-specific RTLAMP assay was determined by repeated experimental setups in several replicates. The details of intra-assay variability (mean, SD, and CV) are tabulated in Table 3. The positive predictive value and negative predictive value of the reported assay was found to be 100%.
Figure 3.
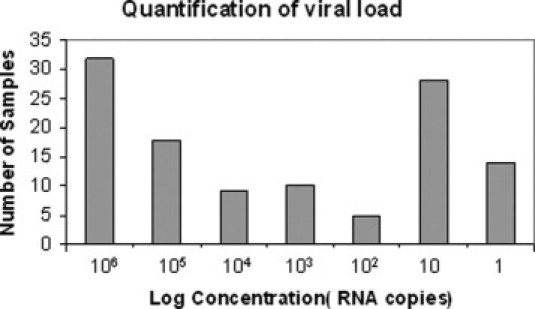
Quantitative determination of the virus load in terms of genome copy number in acute-phase throat-swab samples as determined from the S-OIV H RTLAMP-specific standard curve based on their Tp values.
Table 3.
Intra-Assay Reproducibility and Variability of the S-OIV H1 Gene-Specific RTLAMP Assay
| Virus concentration (copy numbers) | Mean (Tp) | SD | CV (%) |
|---|---|---|---|
| 106 | 16 | 0.21 | 1.31 |
| 105 | 18 | 0.29 | 1.61 |
| 104 | 19.5 | 0.29 | 1.48 |
| 103 | 21 | 0.35 | 1.66 |
| 102 | 22.5 | 0.29 | 1.38 |
| 10 | 23.7 | 0.21 | 0.88 |
| 1 | 25 | 0.43 | 1.72 |
Tp, time of positivity; SD, standard deviation; CV, coefficient of variation.
The field applicability of RTLAMP assay was also validated by using the SYBR Green I-mediated naked-eye visualization test. Following incubation at 63°C for 30 minutes in the water bath, the monitoring of RTLAMP amplification was accomplished by naked-eye observation enabled by the addition of 1 μL of SYBR Green I dye (1:1000) to the amplified products.
Discussion
The emergence of novel S-OIV with transmissibility from human to human poses pandemic concern. During outbreaks of emerging infectious diseases, accurate and rapid diagnosis is critical for minimizing further spread through timely implementation of appropriate vaccines, antiviral treatments, and prophylactic measures.4 A number of different diagnostic laboratory tests can be used for detecting the presence of influenza viruses in respiratory specimens, including direct antigen-detection tests, virus isolation in cell culture, and detection of influenza-specific RNA by real-time RTPCR. These tests differ in their sensitivity and specificity in detecting influenza viruses. The confirmatory and reliable early laboratory diagnosis of S-OIV is currently based on a WHO-approved, CDC-recommended, real-time RT-PCR–based detection method followed by either isolation of the novel H1N1 influenza virus or detection of a fourfold rise of neutralization antibodies to this virus.
Of the diagnostic tests discussed, only the real-time RTPCR-based detection method allows rapid detection of the swine-like H1N1 influenza virus within a few hours. Real-time is more rapid and sensitive than traditional techniques, including virus isolation by cell culture. Various investigators have reported several formats for real-time RTPCR, but none of those assays were validated by a defined sample panel. Therefore, the WHO approved only the CDC-recommended real-time RTPCR assay for confirmation of infection by pandemic S-OIV H1N1 influenza. However, the existing WHO-approved, CDC real-time RTPCR system has several limitations, including the high cost because of the use of large number of primer and probe sets. One of the major limitations of the WHO-approved, CDC real-time RTPCR is the requirement of an expensive real-time PCR instrument that can be afforded only by referral laboratories with good financial resources. Still another limitation of the real-time RTPCR test system is that it is time consuming, requiring 3 to 4 hours. In addition, the limitation of all PCR methods is that false-negative results may occur because of sequence variations in primer and probe targets, and that is particularly relevant for the detection of emerging viruses. However, the use of multiple targets can reduce such limitations and may serve as a means of confirming positive results.
The present study describes the development of a one-step, single-tube isothermal RTLAMP gene-amplification assay for rapid detection of novel S-OIV H1N1 viruses. This is an alternative real-time test system for rapid detection and for identification of novel S-OIV H1N1 viruses. The assay is based on a simple isothermal gene-amplification tool using a specially designed primer set that specifically amplifies the H1 gene of novel S-OIV only. Unlike PCRs, in which the target gene is flanked by two primers, in the case of LAMP, a set of six primers flanks a small target of approximately 200 bp through recognition of eight different sequences of the target gene. LAMP is therefore selective and target specific. Unless all of the eight different sequences are identified, the amplification will not proceed.17 Therefore, a sequence homology of 95% or higher is required for LAMP. The in-silico specificity testing of S-OIV H1 RTLAMP primer set revealed more than 98% to 99% homology among the circulating S-OINV strains, including the California/Paris/China/Singapore/Indian strains. However, the homology between S-OIV and InfA strains in swine H1N1 strains was below 90%. Further analysis of other respiratory viruses, such as respiratory synctial, adeno, para influenza, and so forth revealed less than 50% homology. The in-silco analysis of the S-OIV H1 RTLAMP primer set clearly indicates that the designed primer set is highly specific for the S-OIV H1N1 virus strain only.
The comparative evaluation of CDC real-time RTPCR that includes a reasonably good number of samples revealed 100% concordance for picking up positive cases. Overall, S-OIV RTLAMP picked up some additional positive cases that were being missed by WHO-CDC real-time RTPCR and that were found to be true-positives following sequencing. The presence of the discrepant-additional positive samples is attributed to the higher sensitivity of S-OIV RTLAMP, as already indicated through comparative analytical sensitivity tests for limit of detection. The lower sensitivity of WHO-CDC real-time RTPCR has been reported by Ellis et al.16 in 2009 in their comparative study that evaluated various H1N1 primer and probe sets. These discrepant additional positive samples were further confirmed as true positives by using SYBR Green I-based real-time RTPCR as the third test, and nucleotide sequencing is considered a polished gold standard.26 The higher sensitivity and specificity of the reaction is attributed to the continuous amplification under isothermal conditions and the use of six primers that recognize eight distinct regions of the target.19 The reproducibility and versatility of the reported S-OIV H1 gene-specific RTLAMP is indicated by the fact that the evaluation is being carried out at three centers and by at least 10 different investigators over a period of three months, using four to five lots of reagents. The quantification of the virus load by the RTLAMP assay can also be a useful marker to assess the therapeutic efficacy of antiviral drug as well as for the quarantine of patients so as to restrict the spread of infection. As indicated by the results, the S-OIV H1 RTLAMP could pick up the borderline cases in which the virus load was in the range of 10 to 1 copy numbers. The fact that most of the patients may have either a high or a low virus load, depicting bimodal distribution, may be attributed to late reporting in most cases because all three of the testing centers are being designated referral laboratories for H1N1 testing.
One of the most attractive features of this isothermal gene amplification assay seems to be its great advantage in terms of monitoring the amplification that can be accomplished by SYBR Green I dye–mediated naked-eye visualization. The assay is rapid and cost-effective because gene amplification can be accomplished in a heating block/water bath and followed by the naked eye as it monitors the visual fluorescence in the form of color change. These simple attributes of the assay make it favorable for adoption as a field gene-amplification technology without requiring any expensive instruments. The method is simple and obviates the need for expensive equipment such as real-time PCR instruments, which cost $40,000 to $50,000. The better appreciation of apple-green fluorescence can be achieved by using a simple UV hand-held torch. The test is rapid; results can be obtained in 60 minutes as compared to the 3 to 4 hours required by conventional gene amplification assays. The method is highly sensitive and specific and can detect low copy number of viruses, especially in the borderline cases that would be missed by conventional RT-PCR techniques. These findings suggest that the S-OIV H1 gene-specific RTLAMP assay is a valuable tool for rapid, real-time detection as well as for quantification of the H1N1 virus in acute-phase throat-swab samples. It requires no sophisticated equipment, so it is useful for clinical diagnosis in developing countries.
Acknowledgments
The authors are thankful to Dr. Krishnamurthy Sekhar, CC R & D (Missiles), Chief Controller, Research and Development, for his keen interest in and constant inspiration for this study. The authors are also thankful to the directors of the National Institute of Mental Health and Neuro Sciences (Bangalore, India), the National Centre for Disease Control (Delhi, India), and the Postgraduate Centre for Medical Education and Research (Chandigarh, India) for their kind evaluation of the clinical samples.
Footnotes
Supported by the Defence Research and Development Establishment, Ministry of Defence, Government of India.
None of the authors disclosed any relevant financial relationships.
References
- 1.Neumann G., Noda T., Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinde V., Bridges C.B., Uyeki T.M. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–2625. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 3.Dawood F.S., Jain S., Finelli L., Shaw M.W., Lindstrom S., Garten R.J. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2616–2625. doi: 10.1056/NEJMoa0903810. 360:2605-2615. [DOI] [PubMed] [Google Scholar]
- 4.Michaelis M., Doerr H.W., Cinatl J. Novel swine-origin influenza A virus in humans: another pandemic knocking at the door. Med Microbiol Immunol. 2009;198:175–183. doi: 10.1007/s00430-009-0118-5. [DOI] [PubMed] [Google Scholar]
- 5.He J., Bose M.E., Beck E.T., Fan J., Tiwari S., Metallo J. Rapid multiplex reverse transcription-PCR typing of influenza A and B virus, and subtyping of influenza A virus into H1, 2, 3, 5, 7, 9, N1 (human), N1 (animal), N2, and N7, including typing of novel swine origin influenza A (H1N1) virus, during the 2009 outbreak in Milwaukee, Wisconsin. J Clin Microbiol. 2009;47:2772–2778. doi: 10.1128/JCM.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBlanc J.J., Li Y., Bastien N., Forward K.R., Davidson R.J., Hatchette T.F. Switching gears for an influenza pandemic: validation of a duplex reverse transcriptase PCR assay for simultaneous detection and confirmatory identification of pandemic (H1N1) 2009 influenza virus. J Clin Microbiol. 2009;47:3805–3813. doi: 10.1128/JCM.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faix D.J., Sherman S.S., Waterman S.H. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;361:728–729. doi: 10.1056/NEJMc0904264. [DOI] [PubMed] [Google Scholar]
- 8.Ginocchio C.C., Zhang F., Manji R., Arora S., Bornfreund M., Falk L. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45:191–195. doi: 10.1016/j.jcv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong H., Zhang Y., Xiong H. Detection of human novel influenza A (H1N1) viruses using multi-fluorescent real-time RT-PCR. Virus Res. 2009;147:85–90. doi: 10.1016/j.virusres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel J.J., Walch H., Bollwein M., Niller H.H., Ankenbauer W., Mauritz R., Höltke H.J. Library of prefabricated locked nucleic acid hydrolysis probes facilitates rapid development of reverse-transcription quantitative real-time PCR assays for detection of novel influenza A/H1N1/09 virus. Clin Chem. 2009;55:2218–2222. doi: 10.1373/clinchem.2009.136192. [DOI] [PubMed] [Google Scholar]
- 11.Panning M., Eickmann M., Landt O. Detection of influenza A(H1N1)v virus by real-time RT-PCR. Euro Surveill. 2009;14(36):1–6. [PubMed] [Google Scholar]
- 12.Yang J.R., Lo J., Liu J.L. Rapid SYBR Green I and modified probe real-time reverse transcription PCR assays identify influenza H1N1 viruses and distinguish between pandemic and seasonal strains. J Clin Microbiol. 2009;47:3714–3716. doi: 10.1128/JCM.01646-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pabbaraju K., Wong S., Wong A.A. Design and validation of real-time reverse transcription PCR assays for detection of pandemic (H1N1) 2009 virus. J Clin Microbiol. 2009;47:3454–3460. doi: 10.1128/JCM.01103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr M.J., Gunson R., Maclean A. Development of a real-time RT-PCR for the detection of swine-lineage influenza A (H1N1) virus infections. J Clin Virol. 2009:45196–45199. doi: 10.1016/j.jcv.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon L.L., Chan K.H., Smith G.J. Molecular detection of a novel human influenza (H1N1) of pandemic potential by conventional and real-time quantitative RT-PCR assays. Clin Chem. 2009 doi: 10.1373/clinchem.2009.130229. 551555–55158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis J., Iturriza M., Allen R. Evaluation of four real-time PCR assays for detection of influenza A(H1N1) viruses. Euro Surveill. 2009;4:14. doi: 10.2807/ese.14.22.19230-en. [DOI] [PubMed] [Google Scholar]
- 17.Notomi T., Okayama H., Masubuchi H. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori Y., Nagamine K., Tomita N. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophy Res Com. 2001;289:150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 19.Nagamine K., Hase T., Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Prob. 2002;16:223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- 20.Parida M.M., Santhosh S.R., Dash P.K., Rao P.V.L., Morita K. Loop-mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol. 2008;18:407–421. doi: 10.1002/rmv.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parida M.M., Guillermo P., Inoue S., Hasebe F., Morita K. Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol. 2004;42:257–263. doi: 10.1128/JCM.42.1.257-263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong T.C., Mai Q.L., Cuong D.V., Parida M., Minekawa H., Notomi T., Hasebe F., Morita K. Development and evaluation of a novel loop-mediated isothermal amplification (LAMP) method for rapid detection of SARS corona virus. J Clin Microbiol. 2004;42:1956–1961. doi: 10.1128/JCM.42.5.1956-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parida M.M., Horioke K., Ishida H. Rapid detection and differentiation of dengue virus serotypes by real-time reverse transcription loop-mediated isothermal amplification assay. J Clin Microbiol. 2005;43:2895–2903. doi: 10.1128/JCM.43.6.2895-2903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parida M.M., Santhosh S.R., Dash P.K., Tripathi N.K., Saxena P., Srivastav A., Sahni A.K., Lakshmana Rao P.V., Morita K. Development and evaluation of reverse transcription loop-mediated isothermal amplification assay for rapid and real-time detection of Japanese encephalitis virus. J Clin Microbiol. 2006;44:4172–4178. doi: 10.1128/JCM.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parida M.M., Santhosh S.R., Dash P.K. Rapid and real-time detection of chikungunya virus by reverse transcription loop-mediated isothermal amplification assay. J Clin Microbiol. 2007;45:351–357. doi: 10.1128/JCM.01734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mcadam A.J. Discrepant analysis: how can we test a test? J Clin Microbiol. 2000;38:2027–2029. doi: 10.1128/jcm.38.6.2027-2029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


