Abstract
The proliferation of cholangiocytes occurs during the progression of cholestatic liver diseases and is critical for the maintenance and/or restoration of biliary mass during bile duct damage. The ability of cholangiocytes to proliferate is important in many different human pathologic conditions. Recent studies have brought to light the concept that proliferating cholangiocytes serve as a unique neuroendocrine compartment in the liver. During extrahepatic cholestasis and other pathologic conditions that trigger ductular reaction, proliferating cholangiocytes acquire a neuroendocrine phenotype. Cholangiocytes have the capacity to secrete and respond to a variety of hormones, neuropeptides, and neurotransmitters, regulating their surrounding cell functions and proliferative activity. In this review, we discuss the regulation of cholangiocyte growth by neuroendocrine factors in animal models of cholestasis and liver injury, which includes a discussion of the acquisition of neuroendocrine phenotypes by proliferating cholangiocytes and how this relates to cholangiopathies. We also review what is currently known about the neuroendocrine phenotypes of cholangiocytes in human cholestatic liver diseases (ie, cholangiopathies) that are characterized by ductular reaction.
The liver is formed by two types of epithelia: hepatocytes (which account for 70% of the nucleated liver population) and intrahepatic bile duct epithelial cells or cholangiocytes (which account for 3% to 5% of the endogenous liver cells).1,2 Cholangiocytes line the intrahepatic and extrahepatic bile ducts of the liver and participate in several cellular processes, including the modification of the bile of canalicular origin2 during transit through the biliary system before it reaches the duodenum and the detoxification of xenobiotics.1–4 The secretion of ductal bicarbonate is coordinately regulated by a variety of stimulatory or inhibitory factors, including gastrointestinal hormones (eg, secretin, somatostatin, and bombesin), neuropeptides, and neurotransmitters.5 Among these factors, secretin and its basolateral receptors (SR; expressed only by cholangiocytes in the liver)6 are the major players in the regulation of bicarbonate secretion.5 Secretin binds to SR, stimulating intracellular cAMP levels and inducing the phosphorylation of protein kinase A (PKA).7 Subsequently, PKA phosphorylation induces the activation of cystic fibrosis transmembrane conductance regulator (CFTR), leading to the secretion of Cl− at the apical membrane of cholangiocytes, resulting in membrane depolarization.8 The Cl− efflux from CFTR creates a Cl− gradient that induces activation of the apically located Cl−/HCO3− anion exchanger 2 (AE2),9,10 which results in secretin-stimulated bicarbonate-enriched bile.2 Signaling through SR plays a key role in the regulation of biliary growth/damage (see Secretin, Somatostatin, and cAMP-Dependent Signaling).
Cholangiocytes are the target cells in a variety of animal models of cholestasis (see Animal Models and in Vitro Systems) and human cholangiopathies.1,11 Proliferation of cholangiocytes is critical for maintenance of the homeostasis of the biliary tree and for secretory function during the pathogenesis of chronic cholestatic liver diseases, such as primary biliary cirrhosis (PBC), and primary sclerosing cholangitis.1 Interest in understanding the neuroendocrine nature of biliary proliferation has increased in the past several years with the identification of several neuroendocrine factors regulating the homeostasis of the biliary tree by autocrine and paracrine mechanisms in animal models of cholestasis11 and in human cholangiopathies.1 In addition, cholangiocytes display neuroendocrine phenotypes (such as chromogranin A, glycolipid A2-B4, S-100 protein, neural cell adhesion molecule, and the addition of neuroendocrine granules) during the progression of human cholestatic liver diseases.11,12 The number of factors that participate in the regulation of cholangiocyte proliferation has quickly expanded in the past several years. These neuroendocrine factors affecting the function and proliferation of cholangiocytes are thoroughly discussed in this review, and key factors are highlighted in Tables 1 and 2. Several previous reviews have highlighted the role of neuroendocrine factors in the regulation of biliary proliferation.4,5,11,52 However, owing to the rapid expansion of the list of factors that affect biliary proliferation, these reviews are not current and comprehensive. Although a variety of studies have emphasized the importance of proliferating cholangiocytes in the development of fibrosis, this topic is not discussed in this review.
Table 1.
Neurotransmitters and Neuromodulators That Affect Cholangiocyte Proliferation and Function
| Neurotransmitter/neuromodulator | Structure | Receptors | Effect on proliferation/function | References |
|---|---|---|---|---|
| Met-enkephalin | Delta, mu, and kappa ORs | Increase in opioid synthesis during cholestasis limits excessive cholangiocyte proliferation via delta OR | 13 | |
| Serotonin 5-hydroxytryptamine | 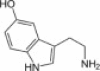 |
Serotonin 1A and 1B receptors | Autocrine loop based on serotonin secretion that limits the growth of the biliary tree during chronic cholestasis | 14 |
| Epinephrine/norepinephrine | 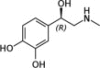 |
β1-ARβ2-AR | Required for cholangiocyte proliferation in response to BDL | 15–18 |
| α1-ARα2-AR | Regulation of secretin-stimulated ductal secretion | |||
| Acetylcholine | M3-ACh | Enhances the effect of secretin; required for cholangiocyte proliferation in response to BDL; maintains biliary mass | 19–22 | |
| GABA | GABAA, GABAB, GABAC receptors | After damage of large bile ducts by GABA, small ducts replenish the biliary tree by amplification of Ca2+-dependent signaling and de novo acquisition of large secretory phenotypes | 23 | |
| Anandamide | 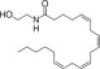 |
Cb1, VR1 | Anandamide inhibits cholangiocyte during BDL via activation of thioredoxin 1/redox factor 1 and AP-1 activation | 24 |
| Histamine | H1R, H3R | H3R agonist RAMH inhibits biliary growth of BDL rats; small mouse cholangiocytes proliferate in response to H1R stimulation | 25,26 |
AP-1, activator protein-1; AR, androgen receptor; OR, opiod receptor; RAMH, R-α-methylhistamine dihydrobromide.
Table 2.
Peptide Hormones and Other Neuroendocrine Factors That Affect Cholangiocyte Proliferation and Function
| Hormone | Receptors | Effect on proliferation | References |
|---|---|---|---|
| CGRP (α/β) | CLR, RAMP1, RCP | Stimulates an increase in biliary mass; lack of CGRP reduces biliary proliferation in response to BDL | 27 |
| Estrogen | ER-α/ER-β | Stimulates cholangiocyte proliferation | 28–31 |
| Gastrin | CCK-B/gastrin | Counteracts the effect of secretin; inhibits cholangiocyte proliferation during BDL | 32,33 |
| GH/IGF-1 | GH-R/IGF1R | Stimulates cholangiocyte proliferation; GH stimulates production and release of IGF-1 that modulates cell proliferation | 34 |
| GLP-1 (exendin-4) | GLP-1R | GLP-1 is expressed by cholestatic cholangiocytes; stimulates biliary proliferation; exendin-4 protects cholangiocytes from apoptosis | 35,36 |
| NGF | TrkA | Secreted by cholestatic cholangiocytes; supports the proliferative response to BDL | 37 |
| Progesterone | PR-A/B; mPR | Stimulates proliferation in normal and cholestatic cholangiocytes via autocrine/paracrine mechanisms | 38 |
| Secretin | SR | Stimulates proliferation of normal and cholestatic cholangiocytes; lack of SR reduces biliary hyperplasia in response to BDL | 39 |
| Somatostatin | SSTR2 | Counteracts the effect of secretin-stimulated biliary secretion; inhibits proliferation | 40–42 |
| VEGF | VEGR2 and 3 | Secreted by cholangiocytes during cholestasis and stimulates an increase in biliary proliferation; prevents cholangiocyte apoptosis induced by interruption of the blood flow of the hepatic artery; VEGF receptor inhibitor, SU-5416, blocked liver cyst growth in Pkd2WS25/– mice | 43–46 |
| ET-1 | ETA and ETB | ET-1 inhibits secretin-induced ductal secretion | 47 |
| Cytokines (IL-6, IL-8) | gp130 CXCR2 | CXCR2 agonists in ADPKD liver cyst fluids promote cholangiocyte proliferation; IL-6 and IL-8 are secreted by cholangiocytes | 48 |
| Prolactin | Prolactin receptor (short and long) | Stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms | 49,50 |
| FSH | FSH receptor | Induces biliary hyperplasia via an autocrine mechanism by the activation of cAMP-dependent ERK1/2 and Elk-1 | 51 |
CLR, calcitonin receptor-like receptor; Elk-1, ets-like gene-1; GH, growth hormone; IL-6, interleukin-6; RAMP1, receptor activity-modifying protein 1; RCP, receptor component protein; TrkA, neurotrophic tyrosine kinase receptor type 1.
Anatomic and Morphologic Features of the Biliary Epithelium
The intrahepatic biliary epithelium is divided into extrahepatic and intrahepatic bile ducts.52,53 The intrahepatic biliary epithelium consists of the portion of the biliary tree proximal to the confluence of the hepatic ducts, extending from the canals of Hering to the large extrahepatic bile ducts.52,53 According to Ludwig,53 the human intrahepatic biliary epithelium is divided into small bile ductules (<15 μm in diameter), interlobular ducts (15 to 100 μm in diameter), septal ducts (>100–300 μm in diameter), segmental ducts (>300 to 800 μm in diameter), and hepatic ducts (>800 μm in diameter). In rodents, the intrahepatic biliary epithelium is morphologically heterogeneous and is formed by small (<15 μm in external diameter lined by small cholangiocytes, 8 μm in size) and large (>15 μm in external diameter lined by large cholangiocytes, 15 μm in size) bile ducts.54,55 We also isolated subpopulations of small (8-μm) and large (15-μm) cholangiocytes from small and large ducts, respectively, and demonstrated that these cell types are morphologically and functionally heterogeneous.3,8,40,55 This finding is particularly important because it allows for mapping of the in vitro studies in isolated small and large cholangiocytes to the different in situ portions (ie, small and large ducts) of the intrahepatic biliary epithelium.3,8,40,54–56 In support of the morphologic heterogeneity of the biliary epithelium, other groups have reconstructed the intrahepatic biliary system to resemble a tree, with the common and hepatic ducts corresponding to the trunk, the intrahepatic bile ducts corresponding to the large branches, and the small ductules corresponding to the smallest limbs of the tree.57,58
Innervation of the Biliary Epithelium
The liver is innervated by sympathetic and parasympathetic nerves and by spinal afferent nerves (originating from the dorsal root ganglia) with variations in localization of the innervation by species.59 In rat liver, sympathetic and parasympathetic nerve fibers are located around the hepatic artery, portal vein, and intrahepatic and extrahepatic bile ducts.59 Sensory nerves also possess an efferent function that is mediated by the release of sensory neuropeptides [ie, calcitonin gene-related peptide (CGRP) and substance P] from their peripheral terminals in tissues they innervate, regulating cellular functions independent of sensation. In rodent liver, CGRP-positive innervation is present as dense networks in the fibromuscular layer of the biliary tree, surrounding the portal vein, and in the stromal compartment of portal areas.60
Vascularization of the Biliary Epithelium
The intrahepatic and extrahepatic biliary epithelium is nourished by a vascular network of minute vessels [the peribiliary vascular plexus (PBVP)] that originate from branches of the hepatic artery and flow principally into the hepatic sinusoids, either directly (lobular branch) or via portal vein branches (prelobular branches).61 A well-defined monolayered PBVP is observed around large bile ducts, whereas the PBVP is progressively reduced up to a single capillary around small bile ducts because the plexus gets smaller proportionally to bile duct size.61 After bile duct ligation (BDL), the PBVP undergoes marked proliferation around large bile ducts,61 and this may explain why only large cholangiocytes in large ducts undergo mitosis in this cholestatic model.40 Because the blood flows in an opposite direction with respect to bile flow (from large toward small ducts), the PBVP presents a counter current stream of biliary reabsorbed substances to hepatocytes.61
Animal Models and in Vitro Systems for Studying Biliary Growth/Damage
Cholangiocytes have low mitotic activity in the normal state.40 A variety of animal models that mimic cholestatic liver diseases (ie, primary sclerosing cholangitis and PBC) and liver injury have been used to expand our current knowledge of the intracellular signaling mechanisms regulating cholangiocyte proliferation and bile duct damage. These models include BDL, partial hepatectomy, feeding of the bile salts taurocholate and taurolithocholate, and acute carbon tetrachloride (CCl4) administration.3,9,19,32,40,62 Among these models of biliary hyperplasia, the cholestatic BDL model, which is characterized by biliary hyperplasia confined to portal tracts, has been the most commonly used in the literature.1–4,6,8,19,27,28,32,37–40 Cholangiocytes also display a heterogeneous profile regarding the proliferative/apoptotic responses to liver injury/toxins.3,19,40,52,56 After BDL, large (but not small) cholangiocytes proliferate through the activation of cAMP-dependent signaling, leading to increased intrahepatic bile duct mass.3,32,40,56 Regarding small cholangiocytes, studies have suggested that the activation of D-myo-inositol 1,4,5-triphosphate (IP3)/Ca2+–dependent signaling plays an important role in regulating the function of these cells.25 For example, small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/Ca2+–dependent calmodulin-dependent protein kinase I/cAMP-response element binding protein pathway.25 In pathologic conditions of damage of large cholangiocytes (eg, after treatment with CCl4 or GABA), small ducts replenish the damaged biliary tree by amplification of Ca2+-dependent signaling and de novo acquisition of large cholangiocyte phenotypes.3,23
An important advancement in the field came from the isolation and characterization of pure preparations of pooled and small (mean diameter, ∼8 μm) and large (mean diameter, ∼15 μm) cholangiocytes (originating from small and large bile ducts, respectively) and intrahepatic bile duct units from normal, hyperplastic, and regenerating rodent livers.3,8,9,19,32,55,56,62,63 Of particular importance, the isolation of small and large cholangiocytes and intrahepatic bile duct units (obtained from different portions of the biliary epithelium) has allowed us to begin to define the morphologic and functional heterogeneity of intrahepatic bile ducts. A variety of polarized cholangiocyte (from pooled, small and large) culture systems or immortalized cell lines from normal and diseased livers have further expanded our knowledge of the pathobiologic features of human and rodent biliary epithelia.25,64,65 This topic is beyond the purpose of this review and is not discussed in detail herein.
Neural Modulators of Biliary Growth
Parasympathetic System
The first evidence of the role of the sympathetic system in the regulation of biliary functions came from an elegant study20 that showed that acetylcholine, by acting on M3 receptor subtypes, induces a Ca2+-calcineurin–mediated potentiation of secretin-stimulated Cl−/HCO3− AE 2 activity, a functional index of biliary growth.2,9 In support of the notion that cholinergic innervation stimulates biliary growth, interruption of the parasympathetic system (by total vagotomy) induces biliary apoptosis and decreases cholangiocyte proliferation and impairs secretin-stimulated cAMP levels and ductal secretion.19 Vagotomy-induced effects on biliary apoptosis and proliferation were reversed by the maintenance of cholangiocyte cAMP levels by means of long-term administration of forskolin.19 Vagotomy-induced damage of bile ducts is also prevented by the feeding of the bile salts taurocholate and ursodeoxycholate.21,22 At the clinical level, antibodies against acetylcholine receptors have been identified in PBC sera.66 Autonomic parasympathetic and sensory nerve dysfunction also has been observed in patients with chronic liver diseases, such as alcoholic cirrhosis and PBC.67 Taken together, the data support the concept that up-regulation of the parasympathetic system can be beneficial in ductopenic diseases.
Adrenergic Innervation
Adrenergic innervation regulates the proliferation of hepatocytes and cholangiocytes during liver regeneration induced by partial hepatectomy.68 In BDL rats, the α1-adrenergic receptor agonist phenylephrine potentiates SR expression and secretin-stimulated cAMP levels and bile flow (functional indices of biliary growth)2,3,9,32,40 by activation of Ca2+-dependent protein kinase C (PKC) signaling.15 In addition, the α2-adrenergic receptor agonist UK-14304 inhibits secretin-stimulated ductal secretion in BDL rats by down-regulation of cAMP signaling.16 Consistent with the notion that adrenergic innervation is key in maintaining the homeostasis of the biliary tree, sympathetic denervation (by intraportal administration of 6-hydroxydopamine to BDL rats) induces the damage of bile ducts (by apoptosis) and functional loss of secretin-stimulated secretion that was prevented by activation of the cAMP-dependent pathway by the long-term administration of clenbuterol (a β2-adrenergic agonist) and dobutamine (a β1-adrenergic agonist).17 6-Hydroxydopamine–induced effects on secretin-stimulated choleresis and biliary hyperplasia were also prevented by taurocholic acid feeding,18 a finding that suggests that bile acids may interact with nerve receptors.
Dopaminergic Innervation
Limited information exists regarding the role of dopaminergic fibers in the modulation of biliary growth. We have shown that cholangiocytes express D2 dopaminergic receptors and that quinelorane (a D2 dopamine receptor agonist) inhibits secretin-stimulated choleresis by increased PKC-γ expression and decreased PKA activity.7
Serotoninergic Innervation
There is growing evidence regarding the role of the neuroendocrine hormone serotonin in the regulation of cholangiocyte proliferation/loss. A variety of studies have suggested the role of serotonin in the origin of pruritus and fatigue in patients with PBC.69 Recently, we showed that i) cholangiocytes express the serotonin receptor types 1A and 1B and ii) activation of these receptor subtypes decreases the cholangiocyte hyperplasia typical of BDL by IP3/Ca2+-dependent down-regulation of cAMP-dependent signaling.14 This study demonstrates that proliferating cholangiocytes secrete serotonin and that immunoneutralization of serotonin induces an increase in cholangiocyte proliferation in vitro and in vivo.14 The data suggest that autocrine modulation of cholangiocyte serotonin secretion may be important in the regulation of the balance between cholangiocyte growth/apoptosis during the progression of cholangiopathies.14
Sensory Innervation
Sensory neuropathy has been associated with PBC.67,70 We recently provided the first evidence that sensory innervation regulates the hyperplasia of the biliary epithelium in cholestatic states.27 Using a knockout mouse model, which lacks the sensory neuropeptide α-CGRP, BDL induced lower cholangiocyte hyperplasia compared with that of BDL WT mice.27 The BDL-induced biliary hyperplasia in WT mice was associated with increases in circulating α-CGRP levels.27 In vitro, α- and β-CGRP increased biliary proliferation by the activation of cAMP-dependent PKA and cAMP-response element binding protein DNA binding.27 Preliminary data from Dr. Gianfranco Alpini's laboratory (Temple, TX) have shown that i) substance P stimulates the growth of mouse cholangiocytes and ii) knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in BDL mice (S.S. Glaser and G. Alpini, unpublished observations).
Cannabinoid System
A recent study demonstrated i) the presence of cannabinoid receptors (Cb1, Cb2, and VR1) on normal and BDL mouse cholangiocytes and ii) that the endogenous cannabinoid anandamide inhibits cholangiocyte hyperplasia in BDL mice via a Cb2-dependent mechanism by activation of the thioredoxin 1/redox factor 1 and activator protein-1 pathways.24 Modulation of the endocannabinoid system may be important in the treatment of cholangiopathies. The hepatic expression of endocannabinoid receptors and their novel polymorphisms was recently evaluated in patients with PBC.71 The study showed that Cb1 was heavily expressed in hepatocytes and cholangiocytes in PBC samples but was absent in control liver samples.71 The Cb2 was expressed in hepatocytes and in cholangiocytes but not in mesenchymal cells.71 The mRNA for Cb1 and Cb2 was expressed in PBC liver samples.71 Logistic regression analysis demonstrated that advanced histologic stage and the lack of response to ursodeoxycholic acid treatment were significantly correlated with the polymorphism of Cb1.71
Regulation of Biliary Growth by Gastrointestinal Hormones
Secretin, Somatostatin, and cAMP-Dependent Signaling
The cAMP-dependent PKA/Rous sarcoma virus/MEK/extracellular signal-regulated kinase (ERK1/2) pathway has been shown to play a key role in the regulation of large cholangiocyte functions. For example, long-term administration of forskolin to normal rats increased intrahepatic bile duct mass, cAMP levels, and secretin-induced bicarbonate-rich bicarbonate secretion of large cholangiocytes.63 Recently, we showed that the Ca2+/calmodulin-dependent adenylyl cyclase AC8 is expressed mostly in large cholangiocytes, where it regulates secretin-stimulated large biliary function because Ca2+/calmodulin inhibitors and AC8 gene silencing inhibited choleresis and cAMP production stimulated by secretin.72 Also, the cAMP-dependent mitogen-activated protein kinase isoform ERK1/2 is up-regulated in proliferating BDL cholangiocytes, where it regulates the growth of these cells.29
Secretin and its receptors (constitutively expressed only by cholangiocytes in humans and rodents)6,8,54–56,73 have been shown to regulate biliary secretion by activation of cAMP-dependent signaling.8,10,54–56 Specifically, in normal rodents, secretin stimulates ductal secretion in isolated large cholangiocytes and in large bile duct units (the only cholangiocyte subpopulation that expresses SR in normal rodent models)8,54,55 by activation of cAMP⇒PKA⇒CFTR/Cl−/HCO3− AE2.54–56 At the functional level, the secretin-stimulated cAMP⇒PKA⇒CFTR/Cl−/HCO3− AE2 pathway has been associated with changes in the degree of cholangiocyte hyperplasia/damage.2,3,6,9,23,32 For example, in pathologic conditions of cholangiocyte hyperplasia (eg, after BDL, taurocholate feeding, or partial hepatectomy) there is enhanced SR expression6,9,32,40 and secretin-stimulated cAMP⇒PKA⇒CFTR/Cl−/HCO3− AE2,3,8,9,23,32,40,62 leading to enhanced bicarbonate secretion in ductal bile.2,9 Conversely, in pathologic conditions of damage of large cAMP-dependent bile ducts (eg, after acute administration of the toxin CCl4 or long-term administration of the neurotransmitter GABA), there is decreased proliferation and down-regulation of SR and secretin-stimulated cAMP⇒PKA⇒CFTR/Cl−/HCO3− AE2 in large cholangiocytes with decreased bicarbonate secretion in bile.3,23 Concomitant with damage of large cholangiocytes,3,23 small cholangiocytes (whose function is not regulated by cAMP-dependent signaling)3,8,25,40,54,55 acquire phenotypes of large cholangiocytes, and the de novo respond to secretin with changes in ductal secretory activity.3,23 In addition to acquiring phenotypes of large cholangiocytes, small cholangiocytes can proliferate by activation of IP3/Ca2+-dependent signaling, a phenomenon that may be key in replenishing the biliary epithelium during abnormalities of large cAMP-responsive bile ducts, such as in cystic fibrosis.1,52,74 As strong support for the important role of the cAMP⇒PKA⇒CFTR/Cl−/HCO3− AE2 pathway in the development of cholangiopathies, a recent study demonstrated that AE2a,b–deficient mice develop antimitochondrial antibodies and other features resembling PBC.75
Although these findings suggested that the secretin-SR axis modulates cholangiocyte growth, we have shown direct evidence of secretin-dependent cholangiocyte proliferation.39 In this study,39 we demonstrated that knockout of SR significantly decreased large cholangiocyte hyperplasia induced by cholestasis, which was associated with enhanced apoptosis. Reduced cAMP-dependent ERK1/2 phosphorylation was also observed in large cholangiocytes from SR−/− BDL compared with WT BDL mice. In vivo, long-term administration of secretin to normal WT mice increased cholangiocyte proliferation and biliary mass. In vitro, stable knockdown of SR expression reduced basal proliferation of large cholangiocytes. The decreased basal proliferative rates that we observed in large cholangiocytes with stable knockdown of SR suggest that the regulation of cholangiocyte growth by secretin occurs perhaps in an autocrine mechanism. In support of this concept, preliminary data from our group (S.S. Glaser and G. Alpini, unpublished observations) demonstrated that i) cholangiocytes (in addition to S cells in the duodenum in the crypts of Lieberkühn) express the message for secretin and secrete this gastrointestinal hormone and ii) knockout of the gene for secretin inhibits large cholangiocyte hyperplasia in cholestatic mice by means of an autocrine mechanism. Modulation of secretin expression/secretion by cholangiocytes may be important as a therapy for ductopenic liver diseases and associated changes in ductal secretory activity. In support of the importance of secretin in clinical therapy, secretin-stimulated magnetic resonance cholangiopancreatography is used to detect bile duct injuries in diseases of the biliary and pancreatic ducts.76 Furthermore, Prieto et al77 demonstrated the importance of positron emission tomography for assessing biliary function by measuring secretin-stimulated biliary bicarbonate secretion in humans. In this study, absence of response to secretin was observed in patients with extrahepatic biliary obstruction and untreated PBC. The choleretic response to secretin was observed in patients with PBC undergoing treatment with ursodeoxycholic acid.77
Somatostatin (growth hormone–inhibiting hormone or somatotropin release–inhibiting factor) is a peptide hormone that modulates the endocrine system and affects neurotransmission and cell mitosis by interaction with G-protein–coupled somatostatin receptor subtypes (SSTR1 through SSTR5). In cholestatic BDL rats, a study has shown that somatostatin (by selectively interacting with SSTR2) inhibits secretin-stimulated bicarbonate-rich choleresis in bile fistula rats and secretin-stimulated cAMP levels and exocytosis in purified cholangiocytes.41 In a study in BDL rats, somatostatin inhibited in vivo and in vitro large cholangiocyte proliferation and bile duct mass by down-regulation of cAMP-dependent signaling.40 Similarly, a decrease in biliary hyperplasia and fibrosis was observed when BDL rats were treated with an octreotide, an analog of somatostatin known to inhibit cAMP synthesis.42 The importance of somatostatin in clinical practice has been evidenced by a recent elegant study showing that octreotide inhibits hepatic cyst growth in in vitro and in vivo rat models of autosomal recessive polycystic kidney disease by decreasing cAMP levels.78 For more information on the role of somatostatin on the management of cholangiociliopathies, we refer to a recent review article.79
Gastrin
In the biliary epithelium of cholestatic BDL rats, gastrin inhibits secretin-stimulated cAMP levels and bile secretion (functional variables of biliary growth)2,9 by down-regulation of cAMP signaling.33 Also, gastrin decreased in vivo and in vitro cholangiocyte growth in cholestatic rats by interaction with cholecystokinin B by Ca2+-dependent membrane translocation of PKC isoform.32 Recently, glucagonlike peptide-1 (GLP-1) has been shown to regulate the adaptive proliferative responses of cholangiocytes to cholestasis induced by BDL.35 These findings are particularly relevant because i) cholangiocytes undergo a neuroendocrine transdifferentiation during cholestasis11 and ii) GLP-1, secreted by neuroendocrine cells, sustains β-cell survival in experimental diabetes and induces the neuroendocrine transdifferentiation of pancreatic ductal cells.80 Cholangiocytes express the GLP-1 receptor, whose expression increases after BDL.80 Both GLP-1 and exendin-4 (a selective GLP-1 agonist) increase in vivo and in vitro cholangiocyte growth.80 The GLP-1 receptor–dependent activation is mediated by activation of the phosphatidyl-inositol-3-kinase, cAMP/PKA, and Ca2+–calmodulin-dependent protein kinase IIα pathways.80 The GLP-1 receptor agonist exendin-4 also has been shown to protect in vivo (after CCl4 acute administration) and in vitro (after treatment with glycochenodeoxycholic acid) cholangiocytes from apoptosis.36 A recent study demonstrated that ectopically expressed pancreatic and duodenal homeobox factor-1 in liver cells (hepatocytes and cholangiocytes) initiates endocrine and exocrine pancreas differentiation.81
Histamine
A variety of studies have recently emphasized the importance of histamine and its receptors (H1HR, H2HR, H3HR, and H4HR) in the regulation of hyperplastic and neoplastic growth of the biliary epithelium.25,26 We demonstrated that cholangiocyte proliferation is regulated by a balance between the stimulatory (by activation of H1HR and H2HR) and inhibitory (by activation of H3HR and H4HR) effects of endogenous histamine.26 Indeed, activation of H3HR induces inhibition of large biliary growth of BDL rats by down-regulation of the cAMP-dependent PKA/ERK1/2/ets-like gene-1 pathway.26 Furthermore, activation of H1HR stimulates small cholangiocyte proliferation by activation of the IP3/calmodulin-dependent protein kinase I/cAMP-response element binding protein pathway.25
Sex Hormones
Several studies have shown that sex hormones, including estrogen, progesterone, prolactin, and follicle-stimulating hormone (FSH), sustain the growth of a variety of cell types, including cholangiocytes. A large body of information has recently flourished regarding the role of estrogens in the regulation of hyperplastic and neoplastic biliary growth. Alvaro et al29 showed that i) normal and proliferating cholangiocytes express both subtypes (α and β) of estrogen receptor (ER) ii) and in vitro 17β-estradiol increased large cholangiocyte proliferation by activation of Rous sarcoma virus–Shc–ERK1/2 signaling through an interaction likely with ER-β, whose expression increases in cholangiocytes after BDL. Conclusive support for the role of estrogens in sustaining biliary growth came from an elegant in vivo study in which administration of the ER antagonists tamoxifen or ICI182,760 or ovariectomy to BDL rats reduced cholangiocyte hyperplasia via enhanced biliary apoptosis.28 In support of the concept that estrogens prevent the progression of cholangiopathies toward ductopenia, the administration of this sex hormone maintains bile duct mass and reduces apoptosis after biliodigestive anastomosis in cholestatic BDL rats.30 Moreover, ezrin-radixin-moesin-binding phosphoprotein, a scaffold protein regulated by estrogens, has been shown to sustain cholangiocyte proliferation in BDL rat cholangiocytes and human cell lines.82 In humans, a recent study has also shown that i) the expression of ER is decreased in the biliary epithelium of patients at late stages of PBC31 and ii) ER-α positivity in cholangiocytes of patients with PBC was markedly lower than that of cholangiocytes in primary sclerosing cholangitis and alcoholic cirrhosis.31 The low expression of ER-α in PBC and their disappearance in the advanced histologic stages suggest that an estrogenic deficiency may trigger the development of PBC toward ductopenia.31 In support of the concept that estrogens may be beneficial in ductopenic diseases, ER modulators have been shown to improve serum variables of cholestasis in patients with PBC.83 The clinical importance of estrogens in sustaining biliary growth is also supported by the fact that in middle-aged women (mainly affected by PBC), estrogen and progesterone levels are decreased.84,85
There is limited information regarding the role of progesterone in the regulation of biliary functions. For example, positivity for progesterone receptor (PR) of gallbladder specimens was demonstrated more in patients with secondary (46.0%) compared with primary (23.5%) hepatolithiasis.86 Recently, we demonstrated that i) cholangiocytes express the PR-B nuclear receptor and the membrane PR (PRGMC1, PRGMC2, and mPRα) and ii) progesterone stimulates in vivo and in vitro the proliferation of male and female cholangiocytes via an autocrine mechanism.38 Of particular importance, we have shown that normal and proliferating cholangiocytes expressed the biosynthetic pathway (ie, STAR, 3β-HSD, p450scc) related to the synthesis of progesterone and secreted progesterone, findings supporting the concept that progesterone stimulates biliary proliferation via an autocrine loop.38 In in vitro studies, supernatants containing progesterone increased cholangiocyte proliferation, which was partially inhibited by preincubation with an antiprogesterone antibody.38 There are two different isoforms (short and long forms) of the prolactin receptor, isoforms that are encoded by a single gene by alternative splicing.49 Although hepatocytes and cholangiocytes of normal and cholestatic livers express prolactin receptors, limited information exists on the role of prolactin on the regulation of biliary hyperplasia.87 Other studies have shown that the long isoform of prolactin receptor is highly expressed by cholangiocytes and further increases under obstructive cholestasis, whereas the short isoform of prolactin receptor predominates in hepatocytes.50 We have recently shown that female cholangiocytes express the long and short forms of prolactin receptors.49 The administration of prolactin to normal rats increased cholangiocyte proliferation. In vitro, prolactin increased cholangiocyte proliferation by increased phosphorylation of the Ca2+-dependent PKC-βI and decreased phosphorylation of the Ca2+-dependent PKC-α phosphorylation.49 Reduction of the circulating levels of prolactin (by the administration of an immunoneutralizing antiprolactin antibody to BDL female rats) decreased cholangiocyte proliferation in these animals.49 Furthermore, we have shown that cholangiocytes express and secrete prolactin, a finding supporting the novel concept that prolactin sustains cholangiocyte proliferation via an autocrine mechanism.49 Prolactin may be an important therapeutic approach for the management of ductopenic diseases that affect female patients. The evaluation of prolactin levels can be an important diagnostic tool for liver diseases because elevated levels of this growth hormone are observed in patients with nonalcoholic chronic liver disease.88
The central hormone of mammalian reproduction, FSH, is synthesized in the anterior pituitary gland of the brain. Although some studies suggested a link between liver damage and FSH, there is limited information regarding FSH regulation of biliary growth. The derangement of hypothalamic-pituitary function has been suggested to play a role in sexual dysfunction in male cirrhotic patients.89 In the biliary epithelium, we provided the first evidence that i) cholangiocytes express FSH mRNA and secrete FSH and ii) FSH induces biliary hyperplasia via an autocrine mechanism by the activation of cAMP-dependent ERK1/2 and ets-like gene-1.51 Furthermore, the administration of antide and anti-FSH antibody to BDL rats decreased ductal mass and secretin-stimulated cAMP levels.51 A recent study has also shown that estrogens directly or coordinately with synergizing growth factors, such as FSH, nerve growth factor (NGF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF), regulate polycystic liver diseases.90
Opioid System
A recent study supports the important role of endogenous opioids in the regulation of biliary growth because the blockage of endogenous opioid peptides by naloxone sustains cholangiocyte growth in in vivo and in vitro models. The increase in opioid peptide synthesis during cholestasis seems to limit the aberrant growth of the biliary epithelium during cholestasis by interaction with the delta opioid receptors on cholangiocytes.13 Furthermore, the fact that delta opioid receptor-1 is expressed by proliferating bile ductules in rats with cholestasis suggests the possible role of this receptor during biliary regeneration.91 In patients with PBC, the appearance of liver failure symptoms is associated with Met-enkephalin concentration increases in plasma while the Met-enkephalin levels decrease in the liver tissue.92 In addition, hepatic Met-enkephalin immunoreactivity is enhanced in patients with PBC.93
Angiogenic Factors
A recent study provided the first evidence that VEGF regulates biliary growth via an autocrine mechanism.43 Specifically, the study demonstrated that cholangiocytes i) express the message/protein for VEGF-A/C and the VEGF receptors VEGFR2 and VEGFR3 and ii) VEGF-A/C stimulates cholangiocyte proliferation in vivo and in vitro via autocrine and paracrine mechanisms.43 In support of this autocrine loop, immunoneutralization of VEGF-A and VEGF-C decreases biliary hyperplasia in the cholestatic BDL rat model.43 The interaction between cholangiocytes (that may mediate the adaptive changes of biliary cells and the microvascular system in cholestatic liver diseases) and vascular cells is also supported by an elegant study by Gaudio et al,61 who demonstrated that proliferation of cholangiocytes precedes the expansion of the PBVP in the biliary epithelium. In the normal state, where cholangiocytes are mitotically dormant, interruption of the hepatic artery blood flow on its own does not induce biliary damage, suggesting that accessory arteries, collateral vessels, or anastomosis between the PBVP and the portal system overcomes the interruption of arterial flow in the main hepatic artery.94 In support of these observations, short-term ligation of the hepatic artery in normal guinea pigs does not alter biliary secretory activity.95 Conversely, in cholestatic BDL rats, we demonstrated that i) interruption of the flow of the hepatic artery by ligation induced loss of the PBVP and a decrease in cholangiocyte VEGF secretion, increased cholangiocyte apoptosis, and impaired biliary growth and secretin-stimulated ductal choleresis and ii) hepatic artery by ligation–induced effects on biliary functions were prevented by administration of recombinant VEGF-A by maintaining the integrity of the PBVP after ischemic injury.44 These findings suggest that manipulation of VEGF expression/secretion may be an important factor in the balance between cholangiocyte proliferation/apoptosis in the development of cholangiopathies. Several studies in normal and diseased cholangiocytes have emphasized the importance of angiogenic factors in the regulation of cholangiopathies. For example, a recent study evaluated the expression and effect of angiogenic factors in cholangiocytes from patients with autosomal dominant polycystic kidney disease (ADPKD) and ADPKD mice (Pkd2WS25/–) and the effect of angiogenic factors on cholangiocyte growth.45 The study demonstrated that i) VEGF, VEGFRs, angiopoietin-1, and its receptor Tie-2 are all up-regulated in cholangiocytes from polycystic liver diseases and ii) VEGF and angiopoietin-1 have autocrine trophic effects on cholangiocyte growth and paracrine effects on portal vasculature, stimulating growth of the cysts and their vascular supply.45
Furthermore, consistent with the key role of angiogenic factors in the management of liver cysts in ADPKD, the VEGFR inhibitor SU-5416 blocked liver cyst growth in Pkd2WS25/– mice.46 Moreover, a recent study demonstrated that i) PKA-dependent up-regulation of mammalian target of rapamycin regulates the proliferative, antiapoptotic, and proangiogenic effects of IGF-1 and VEGF in polycystin-2–defective mice and ii) a link between PKA, ERK, mammalian target of rapamycin, and HIF1α-mediated VEGF secretion.96 The study suggests that mammalian target of rapamycin inhibitors may be used in ADPKD and other pathologic conditions with cholangiocyte proliferation.96
Another recent study has also shown that cholangiocytes are a major source of hepatic endothelin (ET)-1 production during the development of hepatopulmonary syndrome in cholestasis induced by BDL.97 Also, hepatopulmonary syndrome develops in biliary cirrhosis and is associated with increased plasma ET-1 and tumor necrosis factor-α levels.98 The ET-1 inhibits secretin-stimulated cAMP levels and bicarbonate-rich choleresis by interaction with ETA but not ETB receptors.47
Growth Factors
A recent study showed that the receptors for NGF, neurotrophic tyrosine kinase receptor type 1, were expressed by normal rat cholangiocytes and were up-regulated during cholestasis.37 In vitro, NGF stimulates (synergistically with estrogens) cholangiocyte proliferation by activation of the ERK phosphatidylinositol 3-kinase pathway.37 In vivo, immunoneutralization of NGF decreases cholangiocyte proliferation and intrahepatic ductal mass, findings suggesting a possible autocrine loop for NGF in the regulation of biliary mass.37 The expression and secretion of putative growth factors, including NGF, may be important in the autocrine regulation of liver homeostasis in clinical conditions after transplantation. Recently, a study has shown that growth hormone stimulates the synthesis and release of IGF-1 from cholangiocytes with a subsequent increase in biliary growth by IGF-1.34 Furthermore, the same group has demonstrated the expression of IGF-1 isoforms in rodent hepatocytes and cholangiocytes and their role in the protection against liver injury.99 The interaction of CD44 (a multifunctional cell adhesion molecule expressed by cholangiocytes) and hyaluronic acid (the main component of the periportal extracellular matrix and the primary ligand of CD44) has been shown to sustain cholangiocyte growth in cholestatic livers.100 A recent study demonstrated that i) CXCR2 agonists are secreted at the basolateral and apical membrane of ADPKD liver cyst fluids and ii) promote cholangiocyte proliferation. In this study, the authors identified interleukin-8 as a potential candidate for the trophic effect of CXCR2 agonists.48
Overview of Signaling Mechanisms Regulating Growth and Remodeling of the Biliary System
The factors that regulate biliary proliferation are numerous and possess some degree of redundancy in the signaling mechanisms on which they act. Previous studies by our group and others have highlighted the importance of understanding the heterogeneous responses of small and large cholangiocytes (lining small and large bile ducts, respectively)54–56 to cholestasis and during biliary injury.3,8,23,25,55 As described previously herein, large (but not small) cholangiocytes proliferate during the BDL model of extrahepatic cholestasis.8 However, small cholangiocytes proliferate and de novo express SR in response to the damage of large functionally active cholangiocytes induced by CCl4 and GABA administration.3,23 Numerous studies have shown that large cholangiocyte proliferation is predominantly regulated via the activation of cAMP-dependent signaling.27,63 Factors that activate cAMP signaling pathways, such as secretin, forskolin, α-CGRP, and H3HR agonists, stimulate large cholangiocyte proliferation.27,63 As expected, factors that down-regulate cAMP signaling, such as gastrin, also down-regulate large cholangiocyte proliferation, which most often involves the negative cross talk between Ca2+/PKC-dependent signaling mechanisms.32,33 Differential activation of PKC isoforms seems to play a key role in determining whether the factor is inhibitory or stimulatory for large cholangiocyte proliferation. For example, gastrin inhibits cholangiocyte proliferation during BDL through activation of the Ca2+-dependent PKC-α.32,33 In contrast, prolactin stimulates cholangiocyte proliferation via increased phosphorylation of Ca2+-dependent PKC-βI and decreased phosphorylation of Ca2+-dependent PKC-α.49 The importance of cAMP signaling in the regulation of large cholangiocyte proliferation is clearly highlighted in instances when augmentation of intracellular cAMP levels prevents the functional damage of large cholangiocytes. For example, adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt.17
Although the basic understanding of the intracellular signaling mechanisms has been developed, the mechanisms that regulate the phenotypic switch between small and large cholangiocytes remains to be completely addressed. In addition, the most interesting aspect of the neuroendocrine regulation of biliary proliferation will be how these factors activate, control, and regulate biliary proliferation during cholestatic liver diseases. Information is currently lacking in this area. Although, evidence from several studies indicates that VEGF and its regulation of cholangiocyte and vascular endothelial cell proliferation play in important role in regulating biliary mass during cholestasis.43,44 Preventing expansion of the PBVP during cholestasis limits biliary proliferation.43,44 However, future studies on the interplay between cholangiocytes and other cell types during biliary remodeling should reveal interesting signaling mechanisms that may be potential therapeutic targets for the treatment of cholestatic liver diseases.
Summary and Future Perspectives
Our understanding of the neuroendocrine phenotype of proliferating cholangiocytes has greatly expanded, and there is an ever-growing list of factors that are secreted by cholangiocytes and positively and negatively regulate their proliferative activity. A summary of the regulators of small and large cholangiocyte proliferation is illustrated in Figure 1. As our knowledge of the neuroendocrine nature of cholangiocytes develops, in the future our focus will need to be to determine how cholangiocytes interact with other liver cell types that reside in the biliary microenvironment. Several of the currently known and postulated interactions are summarized in Figure 2. In particular, an understanding of how proliferating neuroendocrine cholangiocytes contribute to the progression of cholestatic liver diseases, especially biliary fibrosis, should be developed. We hope that our thorough review of the gastrointestinal hormones, neuropeptides, neurotransmitters, and neuromodulators that regulate cholangiocyte proliferation and function stimulates interest in understanding how cholangiocytes contribute to liver disease processes through interacting with hepatocytes, vascular endothelial cells, hepatic stellate cells, and immune cells in paracrine mechanisms and by regulating their own proliferation in autocrine/paracrine mechanisms.
Figure 1.
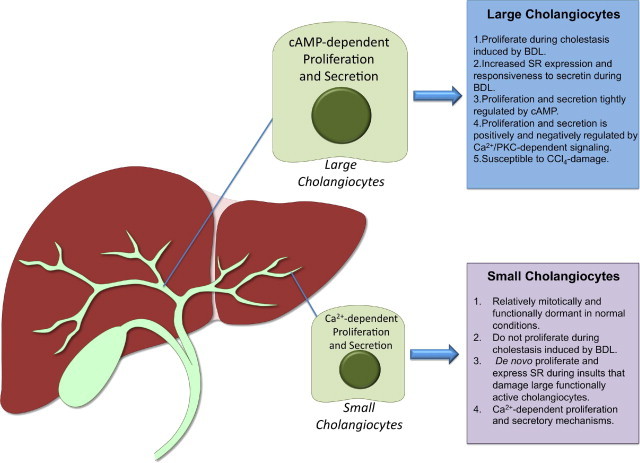
Schematic drawing of the growth factors, gastrointestinal hormones, and neuropeptides/neuromodulators that affect cholangiocyte proliferation with decreases or increases with biliary mass.
Figure 2.
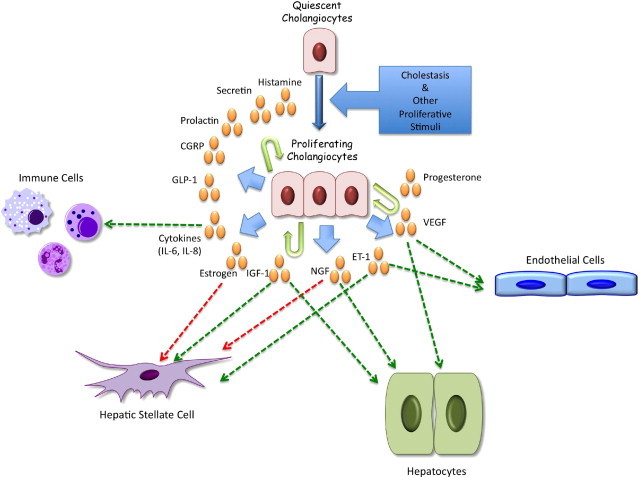
Illustration of the neuroendocrine factors secreted by cholangiocytes that regulate their proliferation and secretory function and their known and postulated interactions with various cell types in the portal track microenvironment. IL-6, interleukin-6.
Footnotes
Supported in part by a grant award from Scott & White and National Institutes of Health grant RO1 DK081442 (S.S.G.); by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, the VA Research Career Scientist Award, a VA Merit Award, and National Institutes of Health grants DK76898, DK58411, and DK62975 (G.A.); and by University and Federate Athenaeum funds from University of Rome “La Sapienza” and Ministero dell'Istruzione, dell'Università e della Ricerca grants (PRIN#2007, prot. 2007HPT7BA_001) (E.G.).
References
- 1.Alpini G., Prall R.T., LaRusso N.F. The pathobiology of biliary epithelia. The Liver: Biology & Pathobiology, ed 4, ch 29. In: Arias I.M., Boyer J.L., Chisari F.V., Fausto N., Jakoby W., Schachter D., Shafritz D.A., editors. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. pp 421–435. [Google Scholar]
- 2.Alpini G., Lenzi R., Sarkozi L., Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia: evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeSage G., Glaser S., Marucci L., Benedetti A., Phinizy J.L., Rodgers R., Caligiuri A., Papa E., Tretjak Z., Jezequel A.M., Holcomb L.A., Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 4.Glaser S., Gaudio E., Miller T., Alvaro D., Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanno N., LeSage G., Glaser S., Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 6.Alpini G., Ulrich C.D., II, Phillips J.O., Pham L.D., Miller L.J., LaRusso N.F. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 7.Glaser S., Alvaro D., Roskams T., Phinizy J.L., Stoica G., Francis H., Ueno Y., Barbaro B., Marzioni M., Mauldin J., Rashid S., Mancino M.G., LeSage G., Alpini G. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-γ expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284:G683–G694. doi: 10.1152/ajpgi.00302.2002. [DOI] [PubMed] [Google Scholar]
- 8.Alpini G., Ulrich C., Roberts S., Phillips J.O., Ueno Y., Podila P.V., Colegio O., LeSage G., Miller L.J., LaRusso N.F. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–G297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 9.LeSage G., Glaser S., Gubba S., Robertson W.E., Phinizy J.L., Lasater J., Rodgers R.E., Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 10.Banales J.M., Arenas F., Rodriguez-Ortigosa C.M., Saez E., Uriarte I., Doctor R.B., Prieto J., Medina J.F. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- 11.Alvaro D., Mancino M.G., Glaser S., Gaudio E., Marzioni M., Francis H., Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Roskams T., van den Oord J.J., De Vos R., Desmet V.J. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990;137:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 13.Marzioni M., Alpini G., Saccomanno S., de Minicis S., Glaser S., Francis H., Trozzi L., Venter J., Orlando F., Fava G., Candelaresi C., Macarri G., Benedetti A. Endogenous opioids modulate the growth of the biliary tree in the course of cholestasis. Gastroenterology. 2006;130:1831–1847. doi: 10.1053/j.gastro.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Marzioni M., Glaser S., Francis H., Marucci L., Benedetti A., Alvaro D., Taffetani S., Ueno Y., Roskams T., Phinizy J.L., Venter J., Fava G., LeSage G., Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–137. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.LeSage G., Alvaro D., Glaser S., Francis H., Marucci L., Roskams T., Phinizy J.L., Marzioni M., Benedetti A., Taffetani S., Barbaro B., Fava G., Ueno Y., Alpini G. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116–1127. doi: 10.1002/hep.20424. [DOI] [PubMed] [Google Scholar]
- 16.Francis H., LeSage G., DeMorrow S., Alvaro D., Ueno Y., Venter J., Glaser S., Mancino M.G., Marucci L., Benedetti A., Alpini G. The α2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol. 2007;293:C1252–C1262. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- 17.Glaser S., Alvaro D., Francis H., Ueno Y., Marucci L., Benedetti A., De Morrow S., Marzioni M., Mancino M.G., Phinizy J.L., Reichenbach R., Fava G., Summers R., Venter J., Alpini G. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol. 2006;290:G813–G826. doi: 10.1152/ajpgi.00306.2005. [DOI] [PubMed] [Google Scholar]
- 18.Marzioni M., Ueno Y., Glaser S., Francis H., Benedetti A., Alvaro D., Venter J., Fava G., Alpini G. Cytoprotective effects of taurocholic acid feeding on the biliary tree after adrenergic denervation of the liver. Liver Int. 2007;27:558–568. doi: 10.1111/j.1478-3231.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 19.LeSage G., Alvaro D., Benedetti A., Glaser S., Marucci L., Baiocchi L., Eisel W., Caligiuri A., Phinizy J.L., Rodgers R., Francis H., Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–199. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 20.Alvaro D., Alpini G., Jezequel A.M., Bassotti C., Francia C., Fraioli F., Romeo R., Marucci L., Le Sage G., Glaser S., Benedetti A. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100:1349–1362. doi: 10.1172/JCI119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzioni M., Francis H., Benedetti A., Ueno Y., Fava G., Venter J., Reichenbach R., Mancino M.G., Summers R., Alpini G., Glaser S. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168:398–409. doi: 10.2353/ajpath.2006.050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzioni M., LeSage G., Glaser S., Patel T., Marienfeld C., Ueno Y., Francis H., Alvaro D., Tadlock L., Benedetti A., Marucci L., Baiocchi L., Phinizy J.L., Alpini G. Taurocholate prevents the loss of intrahepatic bile ducts due to vagotomy in bile duct-ligated rats. Am J Physiol Gastrointest Liver Physiol. 2003;284:G837–G852. doi: 10.1152/ajpgi.00398.2002. [DOI] [PubMed] [Google Scholar]
- 23.Mancinelli R., Franchitto A., Gaudio E., Onori P., Glaser S., Francis H., Venter J., Demorrow S., Carpino G., Kopriva S., White M., Fava G., Alvaro D., Alpini G. After damage of large bile ducts by γ-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMorrow S., Francis H., Gaudio E., Ueno Y., Venter J., Onori P., Franchitto A., Vaculin B., Vaculin S., Alpini G. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506–G519. doi: 10.1152/ajpgi.00304.2007. [DOI] [PubMed] [Google Scholar]
- 25.Francis H., Glaser S., Demorrow S., Gaudio E., Ueno Y., Venter J., Dostal D., Onori P., Franchitto A., Marzioni M., Vaculin S., Vaculin B., Katki K., Stutes M., Savage J., Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis H., Franchitto A., Ueno Y., Glaser S., DeMorrow S., Venter J., Gaudio E., Alvaro D., Fava G., Marzioni M., Vaculin B., Alpini G. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–487. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser S., Ueno Y., DeMorrow S., Chiasson V.L., Katki K.A., Venter J., Francis H.L., Dickerson I.M., DiPette D.J., Supowit S.C., Alpini G. Knockout of α-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest. 2007;87:914–926. doi: 10.1038/labinvest.3700602. [DOI] [PubMed] [Google Scholar]
- 28.Alvaro D., Alpini G., Onori P., Franchitto A., Glaser S., Le Sage G., Gigliozzi A., Vetuschi A., Morini S., Attili A.F., Gaudio E. Effect of ovariectomy on the proliferative capacity of intrahepatic rat cholangiocytes. Gastroenterology. 2002;123:336–344. doi: 10.1053/gast.2002.34169. [DOI] [PubMed] [Google Scholar]
- 29.Alvaro D., Onori P., Metalli V.D., Svegliati-Baroni G., Folli F., Franchitto A., Alpini G., Mancino M.G., Attili A.F., Gaudio E. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology. 2002;36:297–304. doi: 10.1053/jhep.2002.34741. [DOI] [PubMed] [Google Scholar]
- 30.Svegliati-Baroni G., Ghiselli R., Marzioni M., Alvaro D., Mocchegiani F., Saccomanno S., Sisti V., Ugili L., Orlando F., Alpini G., Saba V., Benedetti A. Estrogens maintain bile duct mass and reduce apoptosis after biliodigestive anastomosis in bile duct ligated rats. J Hepatol. 2006;44:1158–1166. doi: 10.1016/j.jhep.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Alvaro D., Invernizzi P., Onori P., Franchitto A., De Santis A., Crosignani A., Sferra R., Ginanni-Corradini S., Mancino M.G., Maggioni M., Attili A.F., Podda M., Gaudio E. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J Hepatol. 2004;41:905–912. doi: 10.1016/j.jhep.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Glaser S., Benedetti A., Marucci L., Alvaro D., Baiocchi L., Kanno N., Caligiuri A., Phinizy J.L., Chowdhury U., Papa E., LeSage G., Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate- Ca(2+)-, and protein kinase C α-dependent mechanisms, Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 33.Glaser S., Rodgers R.E., Phinizy J.L., Robertson W.E., Lasater J., Caligiuri A., Tretjak Z., LeSage G., Alpini G. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G1061–G1070. doi: 10.1152/ajpgi.1997.273.5.G1061. [DOI] [PubMed] [Google Scholar]
- 34.Alvaro D., Metalli V.D., Alpini G., Onori P., Franchitto A., Barbaro B., Glaser S., Francis H., Cantafora A., Blotta I., Attili A.F., Gaudio E. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875–883. doi: 10.1016/j.jhep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Marzioni M., Alpini G., Saccomanno S., Candelaresi C., Venter J., Rychlicki C., Fava G., Francis H., Trozzi L., Glaser S., Benedetti A. Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology. 2007;133:244–255. doi: 10.1053/j.gastro.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Marzioni M., Alpini G., Saccomanno S., Candelaresi C., Venter J., Rychlicki C., Fava G., Francis H., Trozzi L., Benedetti A. Exendin-4, a glucagon-like peptide 1 receptor agonist, protects cholangiocytes from apoptosis. Gut. 2009;58:990–997. doi: 10.1136/gut.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gigliozzi A., Alpini G., Baroni G.S., Marucci L., Metalli V.D., Glaser S., Francis H., Mancino M.G., Ueno Y., Barbaro B., Benedetti A., Attili A.F., Alvaro D. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198–1209. doi: 10.1053/j.gastro.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Glaser S., DeMorrow S., Francis H., Ueno Y., Gaudio E., Vaculin S., Venter J., Franchitto A., Onori P., Vaculin B., Marzioni M., Wise C., Pilanthananond M., Savage J., Pierce L., Mancinelli R., Alpini G. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol. 2008;295:G124–G136. doi: 10.1152/ajpgi.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Glaser S., Lam I.P., Franchitto A., Gaudio E., Onori P., Chow B.K., Wise C., Kopriva S., Venter J., White M., Ueno Y., Dostal D., Carpino G., Mancinelli R., Butler W., Chiasson V., DeMorrow S., Francis H., Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alpini G., Glaser S., Ueno Y., Pham L., Podila P.V., Caligiuri A., LeSage G., LaRusso N.F. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 41.Tietz P.S., Alpini G., Pham L.D., LaRusso N.F. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol. 1995;269:G110–G118. doi: 10.1152/ajpgi.1995.269.1.G110. [DOI] [PubMed] [Google Scholar]
- 42.Tracy T.F., Jr, Tector A.J., Goerke M.E., Kitchen S., Lagunoff D. Somatostatin analogue (octreotide) inhibits bile duct epithelial cell proliferation and fibrosis after extrahepatic biliary obstruction. Am J Pathol. 1993;143:1574–1578. [PMC free article] [PubMed] [Google Scholar]
- 43.Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Ueno Y., Meininger C.J., Franchitto A., Onori P., Marzioni M., Taffetani S., Fava G., Stoica G., Venter J., Reichenbach R., De Morrow S., Summers R., Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 44.Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Franchitto A., Onori P., Ueno Y., Marzioni M., Fava G., Venter J., Reichenbach R., Summers R., Alpini G. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–G317. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 45.Fabris L., Cadamuro M., Fiorotto R., Roskams T., Spirli C., Melero S., Sonzogni A., Joplin R.E., Okolicsanyi L., Strazzabosco M. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 46.Amura C.R., Brodsky K.S., Groff R., Gattone V.H., Voelkel N.F., Doctor R.B. VEGF receptor inhibition blocks liver cyst growth in pkd2(WS25/-) mice. Am J Physiol Cell Physiol. 2007;293:C419–C428. doi: 10.1152/ajpcell.00038.2007. [DOI] [PubMed] [Google Scholar]
- 47.Caligiuri A., Glaser S., Rodgers R.E., Phinizy J.L., Robertson W., Papa E., Pinzani M., Alpini G. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1998;275:G835–G846. doi: 10.1152/ajpgi.1998.275.4.G835. [DOI] [PubMed] [Google Scholar]
- 48.Amura C.R., Brodsky K.S., Gitomer B., McFann K., Lazennec G., Nichols M.T., Jani A., Schrier R.W., Doctor R.B. CXCR2 agonists in ADPKD liver cyst fluids promote cell proliferation. Am J Physiol Cell Physiol. 2008;294:C786–C796. doi: 10.1152/ajpcell.00457.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taffetani S., Glaser S., Francis H., DeMorrow S., Ueno Y., Alvaro D., Marucci L., Marzioni M., Fava G., Venter J., Vaculin S., Vaculin B., Lam I.P., Lee V.H., Gaudio E., Carpino G., Benedetti A., Alpini G. Prolactin stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms. BMC Physiol. 2007;7:6. doi: 10.1186/1472-6793-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bogorad R.L., Ostroukhova T.Y., Orlova A.N., Rubtsov P.M., Smirnova O.V. Long isoform of prolactin receptor predominates in rat intrahepatic bile ducts and further increases under obstructive cholestasis. J Endocrinol. 2006;188:345–354. doi: 10.1677/joe.1.06468. [DOI] [PubMed] [Google Scholar]
- 51.Mancinelli R., Onori P., Gaudio E., DeMorrow S., Franchitto A., Francis H., Glaser S., Carpino G., Venter J., Alvaro D., Kopriva S., White M., Kossie A., Savage J., Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–G26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Kanno N., LeSage G., Glaser S., Alvaro D., Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–561. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- 53.Ludwig J. New concepts in biliary cirrhosis. Semin Liver Dis. 1987;7:293–301. doi: 10.1055/s-2008-1040584. [DOI] [PubMed] [Google Scholar]
- 54.Alpini G., Glaser S., Robertson W., Rodgers R.E., Phinizy J.L., Lasater J., LeSage G. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 55.Alpini G., Roberts S., Kuntz S.M., Ueno Y., Gubba S., Podila P.V., LeSage G., LaRusso N.F. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 56.Glaser S., Gaudio E., Rao A., Pierce L.M., Onori P., Franchitto A., Francis H.L., Dostal D.E., Venter J.K., DeMorrow S., Mancinelli R., Carpino G., Alvaro D., Kopriva S.E., Savage J.M., Alpini G. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sparks E.E., Huppert K.A., Brown M.A., Washington M.K., Huppert S.S. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 2010;51:1391–1400. doi: 10.1002/hep.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masyuk T.V., Ritman E.L., LaRusso N.F. Quantitative assessment of the rat intrahepatic biliary system by three-dimensional reconstruction. Am J Pathol. 2001;158:2079–2088. doi: 10.1016/S0002-9440(10)64679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reilly F.D., McCuskey P.A., McCuskey R.S. Intrahepatic distribution of nerves in the rat. Anat Rec. 1978;191:55–67. doi: 10.1002/ar.1091910106. [DOI] [PubMed] [Google Scholar]
- 60.Akiyoshi H., Gonda T., Terada T. A comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig, dog and human livers. Liver. 1998;18:352–359. doi: 10.1111/j.1600-0676.1998.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 61.Gaudio E., Onori P., Pannarale L., Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–1124. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 62.Alpini G., Glaser S., Ueno Y., Rodgers R., Phinizy J.L., Francis H., Baiocchi L., Holcomb L.A., Caligiuri A., LeSage G. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 63.Francis H., Glaser S., Ueno Y., LeSage G., Marucci L., Benedetti A., Taffetani S., Marzioni M., Alvaro D., Venter J., Reichenbach R., Fava G., Phinizy J.L., Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Alpini G., Invernizzi P., Gaudio E., Venter J., Kopriva S., Bernuzzi F., Onori P., Franchitto A., Coufal M., Frampton G., Alvaro D., Lee S.P., Marzioni M., Benedetti A., DeMorrow S. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184–9193. doi: 10.1158/0008-5472.CAN-08-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alpini G., Phinizy J.L., Glaser S., Francis H., Benedetti A., Marucci L., LeSage G. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1066–G1073. doi: 10.1152/ajpgi.00260.2002. [DOI] [PubMed] [Google Scholar]
- 66.Kyriatsoulis A., Manns M., Gerken G., Lohse A.W., Maelicke A., Wessler I., Reske K., Meyer zum Buschenfelde K.H. Immunochemical characterization of anti-acetylcholine receptor antibodies in primary biliary cirrhosis. J Hepatol. 1988;6:283–290. doi: 10.1016/s0168-8278(88)80044-8. [DOI] [PubMed] [Google Scholar]
- 67.Keresztes K., Istenes I., Folhoffer A., Lakatos P.L., Horvath A., Csak T., Varga P., Kempler P., Szalay F. Autonomic and sensory nerve dysfunction in primary biliary cirrhosis. World J Gastroenterol. 2004;10:3039–3043. doi: 10.3748/wjg.v10.i20.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwai M., Shimazu T. Alteration in sympathetic nerve activity during liver regeneration in rats after partial hepatectomy. J Auton Nerv Syst. 1992;41:209–214. doi: 10.1016/0165-1838(92)90060-t. [DOI] [PubMed] [Google Scholar]
- 69.Jones E.A., Bergasa N.V. The pathogenesis and treatment of pruritus and fatigue in patients with PBC. Eur J Gastroenterol Hepatol. 1999;11:623–631. doi: 10.1097/00042737-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Charron L., Peyronnard J.M., Marchand L. Sensory neuropathy associated with primary biliary cirrhosis: histologic and morphometric studies. Arch Neurol. 1980;37:84–87. doi: 10.1001/archneur.1980.00500510042006. [DOI] [PubMed] [Google Scholar]
- 71.Floreani A., Lazzari R., Macchi V., Porzionato A., Variola A., Colavito D., Leon A., Guido M., Baldo V., De Caro R., Bergasa N.V. Hepatic expression of endocannabinoid receptors and their novel polymorphisms in primary biliary cirrhosis. J Gastroenterol. 2010;45:68–76. doi: 10.1007/s00535-009-0122-y. [DOI] [PubMed] [Google Scholar]
- 72.Strazzabosco M., Fiorotto R., Melero S., Glaser S., Francis H., Spirli C., Alpini G. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez-Anso E., Castillo J.E., Diez J., Medina J.F., Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- 74.Marzioni M., Glaser S., Francis H., Phinizy J.L., LeSage G., Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227–240. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- 75.Salas J.T., Banales J.M., Sarvide S., Recalde S., Ferrer A., Uriarte I., Oude Elferink R.P., Prieto J., Medina J.F. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 76.Hellund J.C., Skattum J., Buanes T., Geitung J.T. Secretin-stimulated magnetic resonance cholangiopancreatography of patients with unclear disease in the pancreaticobiliary tract. Acta Radiol. 2007;48:135–141. doi: 10.1080/02841850601128983. [DOI] [PubMed] [Google Scholar]
- 77.Prieto J., Garcia N., Marti-Climent J.M., Penuelas I., Richter J.A., Medina J.F. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology. 1999;117:167–172. doi: 10.1016/s0016-5085(99)70564-0. [DOI] [PubMed] [Google Scholar]
- 78.Masyuk T.V., Masyuk A.I., Torres V.E., Harris P.C., LaRusso N.F. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 79.Masyuk T., Masyuk A., LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol. 2009;25:265–271. doi: 10.1097/MOG.0b013e328328f4ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drucker D.J. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- 81.Miyatsuka T., Kaneto H., Kajimoto Y., Hirota S., Arakawa Y., Fujitani Y., Umayahara Y., Watada H., Yamasaki Y., Magnuson M.A., Miyazaki J., Hori M. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun. 2003;310:1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- 82.Fouassier L., Rosenberg P., Mergey M., Saubamea B., Claperon A., Kinnman N., Chignard N., Jacobsson-Ekman G., Strandvik B., Rey C., Barbu V., Hultcrantz R., Housset C. Ezrin-radixin-moesin-binding phosphoprotein (EBP50), an estrogen-inducible scaffold protein, contributes to biliary epithelial cell proliferation. Am J Pathol. 2009;174:869–880. doi: 10.2353/ajpath.2009.080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Invernizzi P., Alvaro D., Crosignani A., Gaudio E., Podda M. Tamoxifen in treatment of primary biliary cirrhosis. Hepatology. 2004;39:1175–1176. doi: 10.1002/hep.20164. [DOI] [PubMed] [Google Scholar]
- 84.Beckmann M.W., Jap D., Djahansouzi S., Nestle-Kramling C., Kuschel B., Dall P., Brumm C., Bender H.G. Hormone replacement therapy after treatment of breast cancer: effects on postmenopausal symptoms, bone mineral density and recurrence rates. Oncology. 2001;60:199–206. doi: 10.1159/000055319. [DOI] [PubMed] [Google Scholar]
- 85.Reddy A., Prince M., James O.F., Jain S., Bassendine M.F. Tamoxifen: a novel treatment for primary biliary cirrhosis. Liver Int. 2004;24:194–197. doi: 10.1111/j.1478-3231.2004.00920.x. [DOI] [PubMed] [Google Scholar]
- 86.Sheen-Chen S.M., Ho H.T., Chen W.J., Sheen C.W., Eng H.L., Chou F.F. Progesterone receptor in patients with hepatolithiasis. Dig Dis Sci. 2001;46:2374–2377. doi: 10.1023/a:1012347130235. [DOI] [PubMed] [Google Scholar]
- 87.Zenkova T.Y., Kulikov A.V., Bogorad R.L., Rozenkrants A.A., Platonova L.V., Shono N.I., Gal'perin E.I., Smirnova O.V. Expression of prolactin receptors in human liver during cholestasis of different etiology and secondary liver cancer. Bull Exp Biol Med. 2003;135:566–569. doi: 10.1023/a:1025429318932. [DOI] [PubMed] [Google Scholar]
- 88.Ilan Y., Oren R., Tur-Kaspa R. Elevated growth hormone levels in patients with non-alcoholic chronic liver disease. J Gastroenterol Hepatol. 1993;8:448–450. doi: 10.1111/j.1440-1746.1993.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y.J., Wu J.C., Lee S.D., Tsai Y.T., Lo K.J. Gonadal dysfunction and changes in sex hormones in postnecrotic cirrhotic men: a matched study with alcoholic cirrhotic men. Hepatogastroenterology. 1991;38:531–534. [PubMed] [Google Scholar]
- 90.Onori P., Franchitto A., Mancinelli R., Carpino G., Alvaro D., Francis H., Alpini G., Gaudio E. Polycystic liver diseases. Dig Liver Dis. 2010;42:261–271. doi: 10.1016/j.dld.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nicoll J., Axiotis C.A., Bergasa N.V. The delta opioid receptor 1 is expressed by proliferating bile ductules in rats with cholestasis: implications for the study of liver regeneration and malignant transformation of biliary epithelium. Med Hypotheses. 2005;65:1099–1105. doi: 10.1016/j.mehy.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 92.Owczarek D., Garlicka M., Pierzchala-Koziec K., Skulina D., Szulewski P. Met-enkephalin plasma concentration and content in liver tissue in patients with primary biliary cirrhosis (in Polish) Przegl Lek. 2003;60:461–466. [PubMed] [Google Scholar]
- 93.Bergasa N.V., Liau S., Homel P., Ghali V. Hepatic Met-enkephalin immunoreactivity is enhanced in primary biliary cirrhosis. Liver. 2002;22:107–113. doi: 10.1034/j.1600-0676.2002.01458.x. [DOI] [PubMed] [Google Scholar]
- 94.Castaing D., Houssin D., Bismuth H. Anatomy of the liver and portal system: Hepatic and Portal Surgery in the Rat, ch 6. In: Castaing D., Houssin D., Bismuth H., editors. Masson; Paris: 1980. pp. 27–45. [Google Scholar]
- 95.Tavoloni N., Schaffner F. The intrahepatic biliary epithelium in the guinea pig: is hepatic artery blood flow essential in maintaining its function and structure. Hepatology. 1985;55:666–672. doi: 10.1002/hep.1840050424. [DOI] [PubMed] [Google Scholar]
- 96.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S., Tian X., Somlo S., Strazzabosco M. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology. 2010;51:1778–1788. doi: 10.1002/hep.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo B., Tang L., Wang Z., Zhang J., Ling Y., Feng W., Sun J.Z., Stockard C.R., Frost A.R., Chen Y.F., Grizzle W.E., Fallon M.B. Cholangiocyte endothelin 1 and transforming growth factor β1 production in rat experimental hepatopulmonary syndrome. Gastroenterology. 2005;129:682–695. doi: 10.1016/j.gastro.2005.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo B., Liu L., Tang L., Zhang J., Ling Y., Fallon M.B. ET-1 and TNF-α in HPS: analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G294–G303. doi: 10.1152/ajpgi.00298.2003. [DOI] [PubMed] [Google Scholar]
- 99.Gatto M., Drudi-Metalli V., Torrice A., Alpini G., Cantafora A., Blotta I., Alvaro D. Insulin-like growth factor-1 isoforms in rat hepatocytes and cholangiocytes and their involvement in protection against cholestatic injury. Lab Invest. 2008;88:986–994. doi: 10.1038/labinvest.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Y., Wu G.D., Sadahiro T., Noh S.I., Wang H., Talavera D., Vierling J.M., Klein A.S. Interaction of CD44 and hyaluronic acid enhances biliary epithelial proliferation in cholestatic livers. Am J Physiol Gastrointest Liver Physiol. 2008;295:G305–G312. doi: 10.1152/ajpgi.90229.2008. [DOI] [PubMed] [Google Scholar]


