Abstract
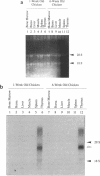
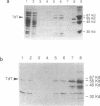
A cDNA clone coding for avian terminal deoxynucleotidyl transferase (TdT) has been isolated and sequenced. The size of this cDNA was 2545 bp with an open reading frame of 1521 bp and a predicted translation product of 58 kDa. Comparison of this TdT sequence with other known TdT sequences has revealed a very high degree of homology at both the DNA and predicted amino acid levels. The chicken TdT cDNA was expressed in a bacterial system and the protein was purified by affinity chromatography. The purified recombinant enzyme, with a specific activity of approximately 1700 U/mg protein, was significantly less active than TdTs from mammalian species. This finding correlates with the observation that TdT isolated from avian thymus has lower activity than that isolated from any mammalian thymus source. Northern blot hybridization analyses and reverse transcription PCR of RNA preparations were carried out with the chicken cDNA. The data generated from these experiments revealed that the TdT RNA was only expressed in the thymus and not in the bone marrow or the bursa of Fabricius during pre- and post hatching chicken development. These data suggest that while TdT is probably involved in N region addition in chicken T-cell receptor genes, it is unlikely to play a role in diversification of immunoglobulin genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollum F. J. Antibody to terminal deoxynucleotidyl transferase. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4119–4122. doi: 10.1073/pnas.72.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J., Chang L. M. Immunological detection of a conserved structure for terminal deoxynucleotidyltransferase. J Biol Chem. 1981 Aug 25;256(16):8767–8770. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Multiple roles of divalent cation in the terminal deoxynucleotidyltransferase reaction. J Biol Chem. 1990 Oct 15;265(29):17436–17440. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coleman M. S. Terminal deoxynucleotidyl transferase: characterization of extraction and assay conditions from human and calf tissue. Arch Biochem Biophys. 1977 Aug;182(2):525–532. doi: 10.1016/0003-9861(77)90533-1. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Chen C. L., Bucy R. P., Thompson C. B. Avian T cell ontogeny. Adv Immunol. 1991;50:87–117. doi: 10.1016/s0065-2776(08)60823-8. [DOI] [PubMed] [Google Scholar]
- Davis M. M. T cell receptor gene diversity and selection. Annu Rev Biochem. 1990;59:475–496. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Biochemical properties of purified human terminal deoxynucleotidyltransferase. J Biol Chem. 1980 May 10;255(9):4206–4212. [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Purification of a high molecular weight human terminal deoxynucleotidyl transferase. J Biol Chem. 1979 Sep 10;254(17):8634–8640. [PubMed] [Google Scholar]
- Demolder J., Fiers W., Contreras R. Efficient synthesis of secreted murine interleukin-2 by Saccharomyces cerevisiae: influence of 3'-untranslated regions and codon usage. Gene. 1992 Feb 15;111(2):207–213. doi: 10.1016/0378-1119(92)90688-l. [DOI] [PubMed] [Google Scholar]
- Evans R. K., Beach C. M., Coleman M. S. Photoaffinity labeling of terminal deoxynucleotidyl transferase. 2. Identification of peptides in the nucleotide binding domain. Biochemistry. 1989 Jan 24;28(2):713–720. doi: 10.1021/bi00428a045. [DOI] [PubMed] [Google Scholar]
- Farrar Y. J., Evans R. K., Beach C. M., Coleman M. S. Interactions of photoactive DNAs with terminal deoxynucleotidyl transferase: identification of peptides in the DNA binding domain. Biochemistry. 1991 Mar 26;30(12):3075–3082. doi: 10.1021/bi00226a014. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S., Dierich A., Lemeur M., Benoist C., Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993 Aug 27;261(5125):1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gregoire K. E., Barton R. W., Bollum F. J. Demonstration of terminal deoxynucleotidyl transferase in thymocytes by immunofluorescence. Proc Natl Acad Sci U S A. 1977 Feb;74(2):734–738. doi: 10.1073/pnas.74.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel T. W., Chen C. L., Lahti J., Kubota T., Kuo C. L., Aebersold R., Hood L., Cooper M. D. Identification of T-cell receptor alpha-chain genes in the chicken. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1094–1098. doi: 10.1073/pnas.91.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E., Schwager J., Alt F. W. Evolution of immunoglobulin genes: VH families in the amphibian Xenopus. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8010–8014. doi: 10.1073/pnas.86.20.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach S., Doyen N., Fanton d'Andon M., Rougeon F. Three lymphoid-specific factors account for all junctional diversity characteristic of somatic assembly of T-cell receptor and immunoglobulin genes. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2799–2803. doi: 10.1073/pnas.89.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- Koiwai O., Yokota T., Kageyama T., Hirose T., Yoshida S., Arai K. Isolation and characterization of bovine and mouse terminal deoxynucleotidyltransferase cDNAs expressible in mammalian cells. Nucleic Acids Res. 1986 Jul 25;14(14):5777–5792. doi: 10.1093/nar/14.14.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Okada A., Stewart V., Alt F. W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993 Aug 27;261(5125):1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Lahti J. M., Chen C. L., Tjoelker L. W., Pickel J. M., Schat K. A., Calnek B. W., Thompson C. B., Cooper M. D. Two distinct alpha beta T-cell lineages can be distinguished by the differential usage of T-cell receptor V beta gene segments. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10956–10960. doi: 10.1073/pnas.88.23.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Houssaint E., Jotereau F. V., Belo M. Origin of hemopoietic stem cells in embryonic bursa of Fabricius and bone marrow studied through interspecific chimeras. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2701–2705. doi: 10.1073/pnas.72.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Hsu E. Isolation and characterization of the Xenopus terminal deoxynucleotidyl transferase. J Immunol. 1994 May 1;152(9):4500–4507. [PubMed] [Google Scholar]
- Lydyard P. M., Grossi C. E., Cooper M. D. Ontogeny of B cells in the chicken. I. Sequential development of clonal diversity in the bursa. J Exp Med. 1976 Jul 1;144(1):79–97. doi: 10.1084/jem.144.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Carlson L. M., Petryniak B., Barth C. F., Humphries E. H., Thompson C. B. Chicken IgL gene rearrangement involves deletion of a circular episome and addition of single nonrandom nucleotides to both coding segments. Cell. 1989 Mar 10;56(5):785–791. doi: 10.1016/0092-8674(89)90683-1. [DOI] [PubMed] [Google Scholar]
- Meier J. T., Lewis S. M. P nucleotides in V(D)J recombination: a fine-structure analysis. Mol Cell Biol. 1993 Feb;13(2):1078–1092. doi: 10.1128/mcb.13.2.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A., Owen J. J. Experimental studies on the development of the bursa of Fabricius. Dev Biol. 1966 Aug;14(1):40–51. doi: 10.1016/0012-1606(66)90004-2. [DOI] [PubMed] [Google Scholar]
- Peterson R. C., Cheung L. C., Mattaliano R. J., White S. T., Chang L. M., Bollum F. J. Expression of human terminal deoxynucleotidyl transferase in Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10495–10502. [PubMed] [Google Scholar]
- Pink J. R., Ratcliffe M. J., Vainio O. Immunoglobulin-bearing stem cells for clones of B (bursa-derived) lymphocytes. Eur J Immunol. 1985 Jun;15(6):617–620. doi: 10.1002/eji.1830150616. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Vainio O., Rijnbeek A. M. Clones of B lymphocytes in individual follicles of the bursa of Fabricius. Eur J Immunol. 1985 Jan;15(1):83–87. doi: 10.1002/eji.1830150116. [DOI] [PubMed] [Google Scholar]
- Pénit C., Gelabert M. J., Transy C., Rouget P. Purification and properties of chick terminal deoxynucleotidyl transferase (TdT). Adv Exp Med Biol. 1982;145:61–73. doi: 10.1007/978-1-4684-8929-3_6. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Weill J. C. The chicken D locus and its contribution to the immunoglobulin heavy chain repertoire. Eur J Immunol. 1991 Nov;21(11):2661–2670. doi: 10.1002/eji.1830211104. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Anquez V., Weill J. C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989 Oct 6;59(1):171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- Riley L. K., Morrow J. K., Danton M. J., Coleman M. S. Human terminal deoxyribonucleotidyltransferase: molecular cloning and structural analysis of the gene and 5' flanking region. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2489–2493. doi: 10.1073/pnas.85.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Rouget P., Penit C. Terminal deoxynucleotidyl transferase during the development of chicken thymus. Cell Differ. 1980 Dec;9(6):329–337. doi: 10.1016/0045-6039(80)90032-9. [DOI] [PubMed] [Google Scholar]
- Saedler H., Nevers P. Transposition in plants: a molecular model. EMBO J. 1985 Mar;4(3):585–590. doi: 10.1002/j.1460-2075.1985.tb03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Bollum F. J. Terminal deoxynucleotidyl transferase (TdT) in chick embryo lymphoid tissues. J Immunol. 1979 Feb;122(2):392–397. [PubMed] [Google Scholar]
- Thompson C. B., Neiman P. E. Somatic diversification of the chicken immunoglobulin light chain gene is limited to the rearranged variable gene segment. Cell. 1987 Feb 13;48(3):369–378. doi: 10.1016/0092-8674(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Tjoelker L. W., Carlson L. M., Lee K., Lahti J., McCormack W. T., Leiden J. M., Chen C. L., Cooper M. D., Thompson C. B. Evolutionary conservation of antigen recognition: the chicken T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7856–7860. doi: 10.1073/pnas.87.20.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trangas T., Coleman M. S. Tissue-specific expression of human terminal deoxynucleotidyl transferase is regulated at the transcriptional level. Biochem Biophys Res Commun. 1989 Oct 31;164(2):750–757. doi: 10.1016/0006-291x(89)91523-4. [DOI] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A., Lassila O., Pink J. R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1986 May;83(10):3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Gathy K. N., Coleman M. S. Mutational analysis of residues in the nucleotide binding domain of human terminal deoxynucleotidyl transferase. J Biol Chem. 1994 Apr 22;269(16):11859–11868. [PubMed] [Google Scholar]