Abstract
Maternal immune tolerance of the semiallogeneic fetus is a complex phenomenon. Macrophages are an abundant cell population in the human decidua, and changes in distribution or phenotype may be involved in the development of preeclampsia. The aim of this study was to assess the distribution and phenotype of macrophages in preterm preeclamptic, preterm control, and term control placentas. Placentas of preterm preeclamptic (n = 6), preterm control (n = 5), and term control pregnancies (n = 6) were sequentially immunohistochemically stained for CD14, CD163, DC SIGN, and IL-10. The distributions of CD14+, CD163+, DC SIGN+, IL-10+, CD163+/CD14+, DC SIGN+/CD14+, and Flt-1/CD14+ cells were determined by double staining and by digital image analysis of sequential photomicrographs. CD14 and CD163 expression increased significantly in preterm preeclamptic decidua basalis compared with preterm control pregnancies (P = 0.0006 and P = 0.034, respectively). IL-10 expression was significantly lower in the decidua parietalis of preterm preeclamptic pregnancies compared with preterm control pregnancies (P = 0.03). The CD163/CD14 ratio was significantly lower in the decidua basalis (P = 0.0293) and the DC SIGN/CD14 ratio was significantly higher in the decidua basalis (P < 0.0001) and parietalis (P < 0.0001) of preterm preeclamptic pregnancies compared with preterm control pregnancies. CD14+ macrophages did express Flt-1. Alterations in distribution and phenotype of macrophages in the decidua of preterm preeclamptic pregnancies compared with control pregnancies may contribute to the pathogenesis of preeclampsia.
Maternal immune tolerance of the semiallogeneic fetus and placenta is important in uncomplicated human pregnancy. Maternal immune cells at the feto-maternal interface are exposed directly to fetal antigens at three locations.1 First, the maternal tissue lining the fetal membranes, the decidua parietalis, interacts with the trophoblast cells of the chorion. Second, the maternal part of the placenta, the decidua basalis, is infiltrated by invading extravillous trophoblast. Third, after the establishment of the utero-placental circulation, maternal peripheral blood contacts with the syncytiotrophoblast. Several mechanisms, some of them implying a special role for macrophages at the three interfaces, have been postulated to promote an immunomodulatory state.2,3
Macrophages are antigen-presenting cells that account for the second most numerous type of leukocytes in the human decidua.4 They are mononuclear phagocytotic cells involved in the innate and adaptive immune system. Macrophages promote inflammation by production of inflammatory molecules during an innate immune response and are able to present antigens to T cells as part of the adaptive immune system. Macrophages may have a role in immunosuppression in the human decidua, as suggested by their ability to suppress a one-way mixed lymphocyte reaction.5 Furthermore, macrophages express costimulation molecules CD80 and CD86 in low levels and indoleamine 2,3-dioxygenase, both preventing T lymphocyte activation.6 An alteration in the quantity or distribution of these cells may be involved in the development of preeclampsia. Preeclampsia is a relatively common but potentially dangerous disorder in human pregnancy, leading to maternal and neonatal morbidity and mortality. It affects 1% to 7% of nulliparous women, who have a three times higher risk than multiparous women.7,8 The disease is characterized by inadequate transformation of the spiral arteries9 and generalized maternal soluble fms-like tyrosine kinase-1 (sFlt-1)–mediated endothelial cell dysfunction.10 Furthermore, immunological factors are involved in the pathogenesis of preeclampsia, because earlier exposure with paternal antigens decreases the risk of preeclampsia.11,12
The exact role of macrophages in the human decidua and their function in preeclampsia remain unknown. The numbers of macrophages have been studied by several groups with varying results. Decreased number of CD14+ macrophages,13 no alteration in number,14 and increased number of macrophages15 have all been found in decidua from preeclamptic compared with control women. Because of these discrepancies in the literature, we decided to study the role and distribution of macrophages in the decidua of preeclamptic and control women. For phenotypic characterization of the macrophage subsets, three different markers were tested. CD14, a glycosylphosphatidylinositol-anchored membrane protein, is present on monocytes and macrophages. CD163, the macrophage scavenging receptor, is a mononuclear phagocyte–restricted cell surface glycoprotein antigen present on type 2 macrophages (M2 cells), which have been reported to exert an anti-inflammatory function.16 Gene expression profiling shows that the human decidua contains mainly M2 cells, which contribute to the immunosuppressive state favorable to the maintenance of the semiallogeneic fetus.17 In contrast to M2 cells, macrophages stimulated with Th1 cytokines polarize toward pro-inflammatory type 1 macrophages (M1 cells). These cells are able to defend against utero-placental infections, but they do not contribute to the tolerance of the fetus.18 Finally, we used dendritic cell–specific intercellular adhesion molecule-3–grabbing nonintegrin (DC SIGN) for phenotypic characterization. DC SIGN is highly expressed on immature dendritic cells (DCs) but also present on macrophages in the human decidua.19,20
We also stained the interleukin-10 (IL-10) and Flt-1 expression by immunohistochemistry in the decidua basalis and parietalis. IL-10 is an immunosuppressive molecule, produced by T cells, monocytes or macrophages, and B cells. This cytokine is produced spontaneously in high levels by decidual macrophages.6 It is a Th2-type cytokine and appears to be pregnancy protective.21 Decreased villous trophoblast staining of IL-10 has been demonstrated in women with preeclampsia compared to normal pregnancy with correlated gestational age.22
Coexpression of CD14 and CD68 as a general macrophage marker, with CD163, DC SIGN, or sFlt, was studied to define the phenotype of cells. We determined the number and type of macrophages in the decidua of preterm preeclamptic, preterm control, and term control pregnancies and defined the natural polarization of decidual macrophages and alterations of the phenotype of these cells.
Materials and Methods
Patient Selection
After a pilot study of five preterm preeclamptic and five term control placentas, six preterm preeclamptic, five preterm control, and six term control placentas were collected. Criteria for inclusion in the preeclamptic group were presence of hypertension (diastolic blood pressure ≥95 mm Hg), proteinuria (>0.3 g/L/24 hours), and a gestational age less than 34 0/7 weeks. Term placentas were collected from healthy women after normal, uncomplicated pregnancies with deliveries at 37 to 42 weeks' gestational age. Preterm placentas were collected if delivered before 34 weeks' gestational age after an uncomplicated pregnancy with no signs of infection. This group contained a quadruplet pregnancy, and the placentas were analyzed separately. The values obtained in the singleton preterm control placenta were in the same range as those observed in the placentas of the quadruplet pregnancy. No significant differences were present between the singleton preterm control placenta and the quadruplet preterm control placentas for the staining of CD14, CD163, and DC SIGN, as well as in the decidua basalis or parietalis (data not shown). Tissue samples were collected within 5 hours of delivery of the placenta after primary caesarean section or vaginal delivery. The study was approved by the ethics committee of the Leiden University Medical Centre (LUMC), and informed consent of every patient was obtained.
Immunohistochemistry
Tissue blocks of the placenta and rolls of fetal membranes were taken at three locations, fixed in 4% formalin, and routinely embedded in paraffin. Sequential serial sections (4 μm) were cut on adhesive-coated glasses and dried overnight at 37°C. Tissue sections were deparaffinized and hydrated by xylene in decreasing alcohol concentration to demi-H2O. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 20 minutes. After washing with demi-H2O, antigen retrieval was performed by boiling the sections for 10 minutes in citrate buffer (pH 6.0). The slides were cooled down for 20 minutes followed by another washing. The optimal dilution for each primary antibody was determined in positive decidual tissue selected on the basis of maximal specific reactivity and minimal background staining (Table 1). As a control, the primary antibody was replaced by normal serum. The primary antibody was incubated for 1 hour at room temperature at the appropriate dilutions in PBS with 1% bovine serum albumin (except for IL-10, which was pretreated with normal goat serum for 30 minutes and incubated overnight). After washing three times in PBS, the slides were incubated for 30 minutes with EnVision (Dako North America, Inc., Carpenteria, CA). Another washing was followed by 5 minutes of incubation with diaminobenzidine (Dako). Demi-H2O was used to stop the reaction. The tissue sections were subsequently counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO). The slides were mounted in mounting medium (Surgipath Medical Industries, Inc., Richmond, IL) and covered.
Table 1.
Antibody Characteristics
| Antibody | Isotype | Dilution | Source |
|---|---|---|---|
| CD14 | IgG2a | 1:200 | Novocastra Laboratories Ltd., Newcastle, UK |
| CD68 | IgG1 | 1:250 | Dako North America, Inc., Carpenteria, CA |
| CD163 | IgG1 | 1:20 | Abcam, Cambridge, UK |
| DC SIGN (CD209) | IgG2b | 1:4000 | Miltenyi Biotec GmbH, Bergisch Gladbach, Germany |
| IL-10 | Polyclonal IgG | 1:50 | Hycult Biotechnology, Uden, The Netherlands |
| Flt-1 | Polyclonal IgG | 1:250 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA |
Double-Label Immunohistochemistry of CD68 and CD163 or DC SIGN
To determine whether cells were double positive for CD68 and CD163 or DC SIGN, in addition to using sequential slides, double labeling was performed. Extensive investigation showed that the combination of CD14 and CD163 or DC SIGN did not give reliable results. Therefore, double labeling with CD163 or DC SIGN and CD68, a general and a pan-macrophage marker, was performed. The sections were deparaffinized in xylene followed by 100% alcohol. Blocking was performed with 0.3% H2O2 in methanol. The sections were rehydrated and rinsed with PBS. The Tris-HCL buffer (pH8.2, 100 mmol/L) was preheated in a water bath at 97°C. The sections were incubated with the buffer for 30 minutes at 97°C and cooled down for 45 minutes on ice. Thereafter, sections were incubated with the first antibody (CD163 or DC SIGN) for 1 hour at room temperature and afterward rinsed with PBS. The sections were incubated with EnVision-HRP (horseradish peroxidase) anti-mouse (Dako) for 30 minutes and rinsed with PBS. The sections were incubated with Vector NovaRED (Vector Laboratories, Inc., Burlingame, CA) for 7 minutes at room temperature and rinsed with PBS. Then the sections were incubated with the second antibody (CD68) for 1 hour, followed by incubation with rabbit anti-mouse (Dako) for 30 minutes, APAAP mouse (Dako) for 30 minutes, and VECTOR blue substrate (Vector) for 25 minutes. The slides were rinsed with PBS between each step. Finally, the sections were dried and covered with mounting medium (Pertex, Histolab Products, Gothenburg, Sweden).
Double-Label Immunohistochemistry of CD14 and Flt-1
Double immunohistochemical staining of CD14 and Flt-1 was performed using the Dako EnVision G/2 Doublestain system (code K5361) following the manufacturer's protocol. Briefly, slides were deparaffinized and hydrated via graded alcohols to demi-H2O. Heat-induced antigen-retrieval was performed with a citrate buffer (pH 6.0) for 20 minutes in a microwave, followed by washing in PBS. Endogenous alkaline phosphatase and peroxidase activity was blocked for 5 minutes by dual endogenous enzyme block. The sections were incubated with primary antibody anti–Flt-1 (dilution 1:250; Santa Cruz Biotechnology, sc-316) followed by incubation with Polymer/HRP reagent, using diaminobenzidine as chromogen. Next a blocking step with double stain block reagent was performed. The sections were incubated with the second primary antibody anti-CD14 (dilution 1:200 in 1% bovine serum albumin/PBS; Novocastra, clone 1F6). Afterward a rabbit/mouse link was added (EnVision, Dako Inc., Carpinteria, CA), followed by incubation with Polymer/AP reagent, using permanent red as chromogen. As a control, primary antibodies were replaced with isotype control antibodies to obtain single immunohistochemical staining. Double-stained sections were counterstained with hematoxylin.
Quantification of Staining
Equivalent fields containing decidua of sequential sections were digitized blinded by study group (Zeiss Axioskop 40, magnification ×200, Zeiss Axiocam MRc 5 camera, 150 × 150 dpi). For every staining of one placenta, a total of 15 pictures of the decidua parietalis and 15 of the basalis were taken (3 locations and 5 pictures per location). Only the decidual stroma was selected for evaluation; irrelevant structures like blood vessels and shadows were removed digitally. The number of positive pixels per area was measured, indicating the level of expression using Image-J software (National Institutes of Health, Bethesda, MD). The program is able to identify and measure positive cells by setting a threshold. For every staining, a macro was made, predefining the threshold of a positive cell. This threshold was independently defined by two observers. Of the 15 pictures taken, the mean and SD of the number of pixels per area were calculated.
The CD163/CD14 ratio and the DC SIGN/CD14 ratio were calculated for every side-matched picture. All analyses were performed blinded for the pregnancy group. Placentas included in the preterm preeclamptic group all showed histological characteristics of preeclampsia (increased syncytial knots, chronic villitis, decidual vasculopathy, thickening of trophoblastic basement membrane, and infarction),23 blindly observed in H&E staining.
Statistical Analysis
The total amount of pixels per area for every antibody staining was compared between preterm preeclamptic versus preterm control placentas and preterm control versus term control placentas. The CD163/CD14 and DC SIGN/CD14 ratios were calculated to define the amount of CD163+ and DC SIGN+ cells within the macrophage population. Descriptive statistical analysis was performed using Graph Pad Prism (Graph Pad Software, Inc., La Jolla, CA) and SPSS Statistics 17 (SPSS, IBM Corporation, Somers, NY). A P value <0.05 was considered statistically significant. The one-way analysis of variance and the nonparametric Mann-Whitney test were used to identify differences between the data.
Results
Pilot Findings and Patient Characteristics
In a pilot study of five other preterm preeclamptic and term control placentas, a difference was found in the level of expression of CD14, CD163, and DC SIGN in the preterm preeclamptic and term control groups. A higher expression rate of CD14 and CD163 and a lower expression rate of DC SIGN were found in the decidua basalis of preterm preeclamptic placentas compared with term control placentas (data not shown). Because a difference in gestational age in preterm preeclamptic and term control placentas (40 weeks versus 30 weeks, respectively; P < 0.05) could have an effect on these outcomes, a preterm control group was collected for the current study. Patient characteristics are shown in Table 2. Patients in the preterm preeclampsia group had a significantly lower gestational age and higher systolic and higher diastolic blood pressure (P < 0.05) compared with term control and preterm control placentas (Table 2). The gestational age of the preterm preeclampsia and preterm control groups was 33 and 34 weeks, respectively (P = 0.033).
Table 2.
Patient Characteristics
| Preeclampsia | Preterm | Term | P value⁎ | |
|---|---|---|---|---|
| Maternal age (years) | 31 ± 6 | 28 ± 2.5 | 30 ± 2.5 | NS |
| Gestational age (weeks) | 33 ± 2 | 34 ± 0.5 | 39 ± 1.5 | <0.05† |
| Highest systole (mm Hg) | 185 ± 10 | 123 ± 2 | 126 ± 14 | <0.05‡ |
| Highest diastole (mm Hg) | 106 ± 11.5 | 76 ± 7.5 | 77 ± 12.5 | <0.05‡ |
| Gravidity | 1 | 2 | 1 | NS |
| Parity | 0 | 0 | 0 | NS |
| Medication | 4 × antihypertensive | No | No | <0.05§ |
Plus-minus values are ranges; NS, not significant.
One-way analysis of variance (ANOVA).
One-way ANOVA followed by t-test showed significant differences between the comparisons of all groups (preeclampsia versus term, P < 0.0001; preeclampsia versus preterm, P = 0.033; preterm versus term P = 0.0023).
One-way ANOVA followed by t-test showed significant differences between preeclampsia versus term and preeclampsia versus preterm.
Kruskal-Wallis test.
The decidua of preterm preeclamptic, preterm control, and term control placentas all showed positive cells for the used antibodies. Negative control slides were all negative. In general, the average amount of expression for every antigen is higher in the decidua basalis compared to the decidua parietalis, regardless of the pregnancy group (Figure 1, A–D). The staining location of CD14+, CD163+, and DC SIGN+ cells was, in general, similar at both locations (decidua basalis and parietalis, Figure 1E).
Figure 1.
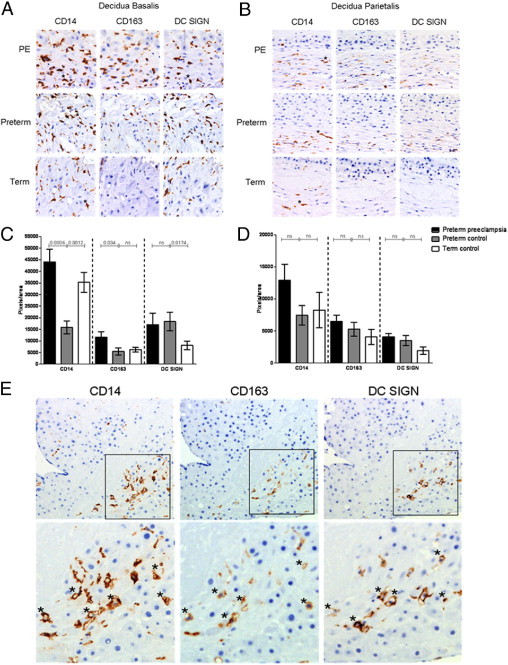
A and B: Photomicrographs of sequential sections stained immunohistochemically for CD14, CD163, and DC SIGN of the decidua basalis (A) and decidua parietalis (B). Positive cells are brown; nuclei are stained blue. Original magnification, ×400. The upper row shows the staining in preterm preeclamptic (PE) pregnancies. In the decidua basalis, more CD14+ and CD163+ staining is present in the PE group compared with the preterm control pregnancies. The amount of DC SIGN staining does not differ between the PE group and the preterm control group. In the decidua parietalis, no significant differences are present. Asterisks indicate examples of positive cells. C and D: Graphs illustrating the amount of positive pixels per area in the decidua basalis (C) and decidua parietalis (D) for each antibody in preterm preeclamptic, preterm control, and term control placentas. Statistical differences were determined using the nonparametric Mann-Whitney test. Values are presented as means; the error bars indicate the SEM. E: Photomicrographs of sequential sections of the decidua basalis stained immunohistochemically for CD14, CD163, and DC SIGN. Positive cells are brown; nuclei are stained blue. Original magnification, ×200. The upper panel shows the same pattern of staining for the three antigens. The lower panel shows a magnification ×2.2 in which asterisks indicate positive cells for CD14, CD163, and DC SIGN.
Comparison in Level of Expression of CD14, CD163, and DC SIGN in Decidua Basalis and Parietalis between Preterm Preeclampsia and Preterm Control
To compare the phenotype of decidual macrophages of the preterm preeclamptic, preterm control, and term control placentas, first the expression of the markers CD14 and CD163 were analyzed. The level of expression of CD14 and CD163 was significantly higher in the preterm preeclamptic decidua basalis compared with the decidua basalis of preterm control pregnancies (P = 0.0006 and P = 0.034, respectively; Figure 1C). No significant differences were present in the level of expression of DC SIGN+ cells in the decidua basalis. In the decidua parietalis, no significant differences were present between preterm preeclamptic and preterm control pregnancies for CD14, CD163, or DC SIGN (Figure 1, A–D).
Comparison in Level of Expression of CD14, CD163, and DC SIGN in Decidua Basalis and Parietalis between Preterm Control and Term Control
Because gestational age could have an effect on study outcomes in comparing outcomes of the level of expression in macrophage markers, we also analyzed the differences between the preterm and term control groups. Significant differences are present for CD14 and DC SIGN. CD14 expression is significantly lower in the preterm control group compared with the term control group (P = 0.0012; Figure 1C). CD163 is significantly higher in the preterm control group compared with the term control group (P = 0.0174; Figure 1C). In the decidua parietalis, no significant differences were present between preterm and term control pregnancies (Figure 1D).
The CD163/CD14 Ratio Is Lower and the DC SIGN/CD14 Ratio Is Higher in Preterm Preeclamptic Decidua Basalis Compared with Preterm Control Pregnancies
In general, the sequential stained slides showed a similar staining pattern for CD14, CD163, and DC SIGN, although not all CD14+ cells are positive for CD163 or DC SIGN (Figure 1E). Double labeling was performed to prove that cells were double positive for CD68 and CD163 or DC SIGN, next to the use of sequential slides. The double staining of CD68 and CD163 or DC SIGN confirms that some cells that were positive for a general macrophage marker are also positive for the M2 marker (Figure 2, A and B). To examine the natural polarization of decidual macrophages and alterations of the phenotype, the CD163/CD14 and DC SIGN/CD14 ratios of subsequent areas were calculated. Although the individual level of expression of CD163 is higher in preterm preeclamptic decidua basalis compared with the preterm control group (Figure 1C), the number of CD163+ cells in the fraction of CD14+ cells (CD163/CD14) was significantly lower in preterm preeclamptic decidua basalis compared with preterm control decidua basalis (P = 0.0293; Figure 3A). By contrast, the level of DC SIGN in the fraction of CD14+ cells (DC SIGN/CD14) was significantly higher in preterm preeclamptic placentas than in preterm control placentas (P < 0.0001; Figure 3A). As in the decidua basalis, the DC SIGN/CD14 ratio in the decidua parietalis was significantly higher in preterm preeclamptic and preterm control pregnancies (P < 0.0001; Figure 3B).
Figure 2.

A: Example of cells in the decidua parietalis that are double positive for CD68 (blue) and CD163. No nuclear counterstaining was used. Lower panel shows a magnification from the pictures in the upper panel. Original magnification, ×400. B: Example of cells in the decidua parietalis that are double positive for CD68 (blue) and DC SIGN (red). No nuclear counterstaining was used. Lower panel shows a magnification from the pictures in the upper panel. Original magnification, ×400. C: Example of cells in the decidua basalis that are double positive for CD14 (red) and Flt-1 (brown). The nuclei are stained blue. Lower panel shows a magnification from the pictures in the upper panel. Double-positive cells are indicated with asterisks.
Figure 3.

CD163/CD14 and DC SIGN/CD14 ratios calculated from subsequent pictures. A: The CD163/CD14 ratio is significantly lower (P = 0.0293) and the DC SIGN/CD14 ratio is significantly higher (P < 0.0001) in preterm preeclamptic decidua basalis compared with preterm control pregnancies. B: The CD163/CD14 and DC SIGN/CD14 ratios in the decidua parietalis. The ratio CD163/CD14 is not significantly different and the DC SIGN/CD14 ratio is significantly higher (P < 0.0001) in preterm preeclamptic decidua parietalis compared with preterm control pregnancies.
The CD163/CD14 ratio and DC SIGN/CD14 ratio are significantly higher in decidua basalis of the preterm control group compared with the term control group (P = 0.0190 and <0.0001 respectively; Figure 3A). In the decidua parietalis the DC SIGN/CD14 ratio is significantly lower in the preterm control group compared with the term control group (P < 0.0001; Figure 3B).
CD14+ Macrophages Are Flt-1+
As suggested, decidual macrophages are a possible additional source of sFlt-1 production and thereby could contribute to the pathogenesis of preeclampsia. Therefore, we investigated whether macrophages are positive for Flt-1. Double labeling of CD14 and Flt-1 shows that macrophages in the decidua basalis did express Flt-1 (Figure 2C).
Lower Expression of IL-10 in Decidua Parietalis of Preterm Preeclamptic Pregnancies Compared with Preterm Control Pregnancies
To functionally characterize cells in the decidua, immunohistochemical staining of IL-10 was performed on placental tissue. The level of expression of IL-10 in preterm preeclamptic decidua parietalis is significantly lower compared with preterm control pregnancies (P = 0.03). No significant differences were found among the expression of IL-10 in the decidua basalis of preterm preeclamptic, preterm control, and term control placentas (Figure 4).
Figure 4.

A: Photomicrographs of sections stained immunohistochemically for IL-10 in preterm preeclamptic and preterm control decidua parietalis. Asterisks indicate examples of positive cells. Original magnification, ×400. B: In the decidua basalis (left), no significant differences are present in the amount of IL-10+ cells among preterm preeclamptic, preterm control, and term control pregnancies. In the decidua parietalis (right), less IL-10 staining is present in the preterm preeclampsia group compared with preterm controls (P = 0.03).
Discussion
This study investigated the phenotype and natural polarization of decidual macrophages by comparing the myeloid cell markers CD14, CD163, and DC SIGN in decidua basalis and parietalis of preterm preeclamptic, preterm control, and term control pregnancies using immunohistochemical analysis and an objective quantification method. We found significantly more CD14+ cells in the decidua basalis of preterm preeclamptic pregnancies compared with preterm control pregnancies. In addition, the specific M2 marker CD163 was significantly up-regulated in the decidua basalis, in preterm preeclamptic pregnancies compared with preterm control pregnancies. Insight into the functional importance of the phenotypic differences in decidual macrophages is limited by lack of M1 markers; therefore, the M2 ratio of CD163/CD14 was used. In the decidua basalis the number of M2 cells (CD163/CD14 ratio) was significantly lower in placentas from preterm preeclamptic pregnancies compared with preterm control pregnancies. The DC SIGN/CD14 ratio was significantly higher in decidua basalis and parietalis of preterm preeclamptic pregnancies compared with preterm control pregnancies. In addition to the preterm control group, we compared the term control group with the preterm control group. A significantly lower level of expression of CD14 was present in the decidua basalis of the preterm control group compared with the term control group (P = 0.0012). This indicates that it is important to have a gestational age–matched control group when investigating macrophages in preterm preeclamptic pregnancies.
The most abundant differences are found in the decidua basalis (and not in the decidua parietalis), which might be explained by the invasion of trophoblast that occurs in the decidua basalis and not in the decidua parietalis.
Maternal tolerance toward the semiallogeneic fetus is important for an uncomplicated pregnancy. The decidual cell population consists of several immunological cells, and a disturbance in the distribution of phenotype of these cells may lead to pregnancy complications. Macrophages and DCs are present in the human decidua,6,19,24,25 and an alteration of the phenotype and distribution may be involved in the pathogenesis of preeclampsia.26
The sequential stained immunohistochemical slides showed that, in general, CD14+ cells also can be DC SIGN+ and CD163+. Our study confirms earlier reports of predominant polarization to M2 macrophages in the term placenta (reviewed by Nagamatsu and Schust).27 The amount of CD14+ or CD163+ cells in the decidua basalis was significantly higher in placentas from preterm preeclamptic pregnancies compared with preterm control pregnancies. Severity of preeclampsia could contribute to this higher number and the different functionality of macrophages present in the decidua. Therefore, two placentas from most severe cases of preeclampsia (based on the level of diastolic pressure, amount of proteinuria, and gestational age) demonstrated the highest number of cells in the decidua basalis.
The number of M2 macrophages in relation to all macrophages (CD163/CD14 ratio) was lower in placentas from preterm preeclamptic pregnancies compared with preterm control pregnancies. To our knowledge, this is the first study that describes a decrease in M2 macrophages in the decidua basalis of preterm preeclamptic pregnancies compared with preterm control pregnancies. We speculate that this lower amount of M2 macrophages may contribute to the cause of preeclampsia. Furthermore, we have shown an increase of the ratio of DC SIGN+ cells in placentas from preterm preeclamptic pregnancies. The phenotypic plasticity of myeloid cells such as DCs and macrophages is substantial, and a subset distinction is difficult to make. Only a few markers are known that really make the distinction between macrophages and DCs. Gardner and Moffett19 already postulated that DC SIGN is present on decidual macrophages but not on decidual DCs. It remains unclear whether this cell subset, also called intermediate antigen-presenting cells, is a subset of macrophages or of DCs. Cells positive for CD14 and DC SIGN are reported in other human tissues,28,29 and these cells produce large amounts of proinflammatory cytokines.30 In line with the study of Gardner and Moffett,19 our study also shows, in general, a similar staining pattern between CD14+ and DC SIGN+ cells. The presence of this subset of DC SIGN+ macrophages in the decidua is pregnancy associated, and these cells may play a crucial role in local immune response. Therefore, alterations in the function and distribution of this cell type may result in pathological pregnancies, like preeclampsia, which has been shown by Huang et al.31 Preeclamptic decidua contained an infiltrate of DC SIGN+ cells in contrast to their sparse presence in the decidua of uncomplicated pregnancies. This study also confirms an increased level of DC SIGN expression in preterm preeclamptic decidua compared with preterm control decidua. However, the current study relates DC SIGN+ cells with macrophages instead of DCs because of their colocalization and as shown by double staining. In contrast to our study, Scholz et al32 found no significant differences between preeclamptic and control placentas in the amount of DC SIGN+ cells using immunohistochemical analysis. They did find, however, a higher amount of DC SIGN+ cells in placentas from patients who developed HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome. It is possible that our preterm preeclamptic group is more comparable with the HELLP group of the study of Scholz et al,32 because our study included only very severely preterm preeclamptic patients with deliveries at gestational age less than 34 0/7 weeks.
In addition to the presence of macrophage antigens in the decidua, this study investigated the production of IL-10 in placental tissue. During pregnancy, IL-10 is an important cytokine. It plays a role in the prevention of placental rejection. Human pregnancy is a type 2 immune state shown by a shift in cytokine production from type 1 to type 2. This balance is different in preeclampsia, in which a decrease in IL-10 compared to the pro-inflammatory cytokines is present. IL-10 is secreted by cytotrophoblast, and it can suppress an allogeneic immune response in vitro.33 It is possible that IL-10 may be involved in protecting the semiallogeneic fetus in normal pregnancy.21 To our knowledge, only one earlier published study performed IL-10 immunohistochemical staining on placental tissue.22 Hennesy et al showed a change in IL-10 immunolocalization in term placentas from women with preeclampsia compared with those with a normal pregnancy outcome. They showed a general decrease in cytoplasmic trophoblast villi IL-10 content in preeclamptic pregnancies. Additionally, a decrease in IL-10+ trophoblast cells located in the decidual tissue was present. A lower level of IL-10 in the decidua basalis suggests an impaired protective mechanism of the mother toward the allogeneic fetus in cases of preeclampsia. Our digital analysis shows that the number of IL-10+ cells is lower in the decidua parietalis of preeclamptic pregnancies compared with preterm pregnancies. This indicates that there is a difference in the defense mechanism between the decidua parietalis and decidua basalis. The decidua parietalis contacts the noninvading trophoblast of the chorion; the decidua basalis interacts with invading villous trophoblast. It seems that the contact between the chorion in the decidua parietalis in preterm pregnancies synthesizes the trophoblast cells to produce IL-10, which does not appear in preeclamptic decidua parietalis. Because this study showed a lower amount of IL-10+ cells in the decidua parietalis of preeclamptic pregnancies compared with preterm pregnancies, we speculate that a high level of IL-10 is necessary to maintain pregnancy without complications and that a down-regulation of IL-10 produced by the decidua parietalis is a permissive condition for the development of preeclampsia.
Recently, it has been shown in chronic kidney disease that monocytes may be a possible source of sFlt-1.34 Increase of sFlt-1 leads to endothelial dysfunction, and increased levels have been found in patients with preeclampsia.10,35 Double-labeling immunohistochemical staining of CD14+ and Flt-1 shows that macrophages in the decidua basalis are positive for Flt-1. Because we found an increase in the amount of CD14+ cells in preeclamptic decidua basalis compared with preterm decidua basalis (P = 0.0006), it is possible that decidual macrophages are responsible for the increased sFlt-1 production that may contribute to the cause of preeclampsia.
Tolerance of the genetically foreign fetus by the maternal immune system is a complex phenomenon and remains to be fully understood. Multiple mechanisms are involved in maintaining the pregnancy. Localized secretion of immunoregulatory cytokines may prevent immune rejection of the placenta. In addition, the presence of immunomodulatory cells may be important in dampening an inflammatory immune response. Preeclampsia is a state in which the immune system has to work harder to maintain pregnancy. Alterations in immunomodulatory cells in the decidua basalis and parietalis of preterm preeclamptic pregnancies compared to control pregnancies may contribute to the etiology of preeclampsia. The question is whether alterations in the immune system lead to the pathogenesis of preeclampsia or its prevention in subsequent pregnancies. In conclusion, the present study shows that macrophages can be DC SIGN+ as well as CD163+, based on double staining and based on the similar staining pattern of these antigens. An increase of CD163+ cells in preterm preeclamptic placentas was found compared with preterm control placentas. However, the total amount of CD14+ cells is also increased in preterm preeclamptic placentas compared with preterm control placentas. The amount of CD163+ cells in the fraction of CD14+ cells is lower in preterm preeclamptic placentas compared with preterm control placentas. Furthermore, this study found an increase in DC SIGN/CD14 myeloid cells in the decidua parietalis and basalis of preterm preeclamptic pregnancies compared with preterm control pregnancies. This study suggests that further investigation of the distribution and phenotype of macrophages is possibly relevant to further understand the immunology at the fetal-maternal interface.
Acknowledgments
The authors thank Pieter Hiemstra and Simone van Wijngaarden for their help with the immunohistochemical staining and Angela van Lochem and Tamara Tilburgs for their helpful discussion and critical insights on this topic.
Footnotes
D.S. and M.-L.v.d.H. contributed equally to this manuscript.
References
- 1.Huppertz B. The feto-maternal interface: setting the stage for potential immune interactions. Semin Immunopathol. 2007;29:83–94. doi: 10.1007/s00281-007-0070-7. [DOI] [PubMed] [Google Scholar]
- 2.Hunt J.S. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsi B.L., Hunt J.S., Atkinson J.P. Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. J Reprod Immunol. 1991;19:209–223. doi: 10.1016/0165-0378(91)90036-p. [DOI] [PubMed] [Google Scholar]
- 4.Vince G.S., Starkey P.M., Jackson M.C., Sargent I.L., Redman C.W. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990;132:181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno M., Aoki K., Kimbara T. Functions of macrophages in human decidual tissue in early pregnancy. Am J Reprod Immunol. 1994;31:180–188. doi: 10.1111/j.1600-0897.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 6.Heikkinen J., Mottonen M., Komi J., Alanen A., Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saftlas A.F., Olson D.R., Franks A.L., Atrash H.K., Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163:460–465. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 8.Sibai B.M., Gordon T., Thom E., Caritis S.N., Klebanoff M., McNellis D., Paul R.H. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study: The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995;172:642–648. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 9.Roberts J.M., Redman C.W. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 10.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F., Schisterman E.F., Thadhani R., Sachs B.P., Epstein F.H., Sibai B.M., Sukhatme V.P., Karumanchi S.A. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 11.Koelman C.A., Coumans A.B., Nijman H.W., Doxiadis I.I., Dekker G.A., Claas F.H. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol. 2000;46:155–166. doi: 10.1016/s0165-0378(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 12.Kho E.M., McCowan L.M., North R.A., Roberts C.T., Chan E., Black M.A., Taylor R.S., Dekker G.A. Duration of sexual relationship and its effect on preeclampsia and small for gestational age perinatal outcome. J Reprod Immunol. 2009;82:66–73. doi: 10.1016/j.jri.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Burk M.R., Troeger C., Brinkhaus R., Holzgreve W., Hahn S. Severely reduced presence of tissue macrophages in the basal plate of pre-eclamptic placentae. Placenta. 2001;22:309–316. doi: 10.1053/plac.2001.0624. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.S., Romero R., Cushenberry E., Kim Y.M., Erez O., Nien J.K., Yoon B.H., Espinoza J., Kim C.J. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta. 2007;28:571–576. doi: 10.1016/j.placenta.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Lockwood C.J., Matta P., Krikun G., Koopman L.A., Masch R., Toti P., Arcuri F., Huang S.T., Funai E.F., Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bockle B.C., Solder E., Kind S., Romani N., Sepp N.T. DC-sign+ CD163+ macrophages expressing hyaluronan receptor LYVE-1 are located within chorion villi of the placenta. Placenta. 2008;29:187–192. doi: 10.1016/j.placenta.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson C., Mjosberg J., Matussek A., Geffers R., Matthiesen L., Berg G., Sharma S., Buer J., Ernerudh J. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One. 2008;3:e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 19.Gardner L., Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003;69:1438–1446. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 20.Breburda E.E., Dambaeva S.V., Slukvin I.I., Golos T.G. Selective distribution and pregnancy-specific expression of DC-SIGN at the maternal-fetal interface in the rhesus macaque: DC-SIGN is a putative marker of the recognition of pregnancy. Placenta. 2006;27:11–21. doi: 10.1016/j.placenta.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Lin H., Mosmann T.R., Guilbert L., Tuntipopipat S., Wegmann T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- 22.Hennessy A., Pilmore H.L., Simmons L.A., Painter D.M. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 23.Roberts D.J., Post M.D. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61:1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 24.Kammerer U., Schoppet M., McLellan A.D., Kapp M., Huppertz H.I., Kampgen E., Dietl J. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol. 2000;157:159–169. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Repnik U., Tilburgs T., Roelen D.L., van der Mast B.J., Kanhai H.H., Scherjon S., Claas F.H. Comparison of macrophage phenotype between decidua basalis and decidua parietalis by flow cytometry. Placenta. 2008;29:405–412. doi: 10.1016/j.placenta.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Darmochwal-Kolarz D., Rolinski J., Tabarkiewicz J., Leszczynska-Gorzelak B., Buczkowski J., Wojas K., Oleszczuk J. Myeloid and lymphoid dendritic cells in normal pregnancy and pre-eclampsia. Clin Exp Immunol. 2003;132:339–344. doi: 10.1046/j.1365-2249.2003.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagamatsu T., Schust D.J. Review: the immunomodulatory roles of macrophages at the maternal–fetal interface. Reprod Sci. 2010;17:209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa M.T., Loncaric A., Krutzik S.R., Becker T.C., Modlin R.L. “Dermal dendritic cells” comprise two distinct populations: cD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamada N., Hisamatsu T., Honda H., Kobayashi T., Chinen H., Kitazume M.T., Takayama T., Okamoto S., Koganei K., Sugita A., Kanai T., Hibi T. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009;183:1724–1731. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 30.Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K., Akagawa K.S., Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S.J., Chen C.P., Schatz F., Rahman M., Abrahams V.M., Lockwood C.J. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 32.Scholz C., Toth B., Santoso L., Kuhn C., Franz M., Mayr D., Jeschke U., Friese K., Schiessl B. Distribution and maturity of dendritic cells in diseases of insufficient placentation. Am J Reprod Immunol. 2008;60:238–245. doi: 10.1111/j.1600-0897.2008.00619.x. [DOI] [PubMed] [Google Scholar]
- 33.Roth I., Corry D.B., Locksley R.M., Abrams J.S., Litton M.J., Fisher S.J. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Marco G.S., Reuter S., Hillebrand U., Amler S., Konig M., Larger E., Oberleithner H., Brand E., Pavenstadt H., Brand M. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol. 2009;20:2235–2245. doi: 10.1681/ASN.2009010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKeeman G.C., Ardill J.E., Caldwell C.M., Hunter A.J., McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]


