Abstract
Diet and obesity are important risk factors for cancer development. Many studies have suggested an important role for several dietary nutrients in the progression and development of breast cancer. However, few studies have specifically addressed the role of components of a Western diet as important factors involved in breast cancer initiation and progression. The present study examined the role of cholesterol in the regulation of tumor progression in a mouse model of mammary tumor formation. The results suggest that cholesterol accelerates and enhances tumor formation. In addition, tumors were more aggressive, and tumor angiogenesis was enhanced. Metabolism of cholesterol was also examined in this mouse model. It was observed that plasma cholesterol levels were reduced during tumor development but not prior to its initiation. These data provide new evidence for an increased utilization of cholesterol by tumors and for its role in tumor formation. Taken together, these results imply that an increase in plasma cholesterol levels accelerates the development of tumors and exacerbates their aggressiveness.
Breast cancer is the most commonly occurring cancer in women in Western societies.1 It is estimated that 207,090 new cases will be diagnosed and that in the United States, 39,840 women will die of the disease in 2010 (American Cancer Society, Cancer Facts & Figures 2010, http://www.cancer.org/Research/cancer-facts-and-figures-2010, last accessed November 15, 2010). Interestingly, the incidence is about 5 times higher in Western countries than in developing countries.2 Moreover, relocation and migrational studies have demonstrated that migration from a region with low incidence to a region with high incidence increases breast cancer incidence in the immigrant population.3 These observations suggest a strong environmental influence on breast cancer development.
Diet and obesity are now considered important risk factors for cancer development.4,5 However, despite major modifications of its metabolism during obesity development and its function in tissue pathogenesis, little is known about the metabolism and role of plasma cholesterol in cancer development. Epidemiological studies have demonstrated that patients with cancer have abnormal levels of high-density lipoprotein (HDL)–cholesterol and low-density lipoprotein (LDL)–cholesterol,6,7 which are the major lipoprotein carriers of cholesterol in human plasma. In addition, numerous studies have established that transformed cells and tumors exhibit abnormal regulation of several genes that are under the control of cholesterol. The products of these genes include the LDL receptor (LDL-R), hydroxy-methyl-glutaryl coenzyme A reductase (HMG-CoA Reductase), and their regulators, the sterol regulatory element binding proteins.8–13 As a consequence, these genes are dysregulated at the transcriptional level during tumorigenesis. These data suggest that transformed cells may require or utilize more cholesterol than normal cells, and this may be associated with their increased rate of proliferation. More recent studies have implicated HDL during tumor formation in breast cancer.14–16 However, their precise function remains controversial. The present study was performed to test the hypothesis that dietary cholesterol and plasma cholesterol levels have an important role in the regulation of breast cancer onset and progression.
The role of cholesterol in tumor development was examined in a mouse model used to study the development of mammary tumors. MMTV-PyMT transgenic mice were used to directly explore this hypothesis. MMTV-PyMT transgenic mice express high levels of the transforming oncogene polyoma middle T (PyMT) antigen under the control of the mouse mammary tumor virus long terminal repeat promoter, which specifically directs expression to the mammary epithelium.17 All virgin female PyMT transgenic PyMTTg mice spontaneously develop widespread multifocal adenocarcinomas in the mammary gland, with dysplastic foci occurring as early as age 3 weeks. Importantly, this mouse tumor model recapitulates human breast cancer progression from early hyperplasia to malignant breast carcinoma.18,19
Materials and Methods
Materials
Antibodies used included rabbit polyclonal anti–estrogen receptor-α (ERα; MC-20 and H-184), mouse monoclonal anti-cyclin D1 (DCS-6), rabbit polyclonal anti-caveolin-1 (N-20), and rabbit polyclonal anti-Rho GDIα (A-20) (all from Santa Cruz Biotechnology, Inc, Santa Cruz, CA); rabbit polyclonal anti-cyclin D1 (NeoMarkers, Inc, Fremont, CA); rabbit polyclonal anti-scavenger receptor class BI (SR-BI; Novus Biologicals, Inc, Littleton, CO); rabbit anti-LDL receptor (Research Diagnostics, Inc, division of Fitzgerald Intl, Concord, MA); and rabbit anti-CD31 and mouse anti–pan-cytokeratin (Abcam, Inc, Cambridge, MA).
Animal Studies
All animals were housed and maintained in a barrier facility at the Kimmel Cancer Center of Thomas Jefferson University (Philadelphia, PA). Transgenic FVB/N mice expressing the polyoma middle T antigen under the control of the mouse mammary tumor virus long terminal repeat promoter were obtained as described previously.17 Breeding was performed with male mice hemizygote for the PyMT transgene and female wild types. Genotyping was performed as described by The Jackson Laboratory (Bar Harbor, ME). Mice were kept on a 12-hour light-dark cycle with free access to food. For the tumor studies, mice were fed a standard chow diet until age 4 weeks. Female mice hemizygous for the PyMT transgene were given either a Western diet (57BD; LabDiet, Richmond, IN) containing 20.2% fat, 16.8% protein, and 48.0% carbohydrate, or a chow diet (5010; LabDiet) containing 4.5% fat, 23.0% protein, and 50.1% carbohydrate, at age 4 weeks and thereafter ad libitum. Although fat content of the diet was increased, carbohydrate content was not altered. Moreover, energy values of the 2 diets were similar (4.43 kcal/g and 4.14 kcal/g for Western and chow diets, respectively). Whole mounts of mammary glands were obtained from 3- and 4-week-old female mice whose mothers received either a chow or Western diet during the pregnancy and the lactation period. Animal protocols used in this study were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Tumor Palpation and Excision
Eight-week-old female mice were palpated in each of the 10 mammary glands to determine the presence of detectable tumors. These animals were sacrificed at age 12 weeks, and all mammary tumors were carefully excised and weighed. Portions of the tumors were frozen in liquid nitrogen or fixed in 10% neutral buffered formalin.
Lung Metastasis Analysis
After removal of the tumors, lungs from 12-week-old female PyMTTg mice were injected with 1 ml of 10% neutral buffered formalin via tracheal cannulation to fix the inner spaces and inflate the lung lobes. Lungs were then excised and placed in formalin for 24 hours. The left lung of each animal was paraffin-embedded, sectioned at 50-μm intervals, and stained with H&E. Lung metastases were scored as the total number of metastatic foci (defined as a cluster of at least 10 cells) per lung, as previously described.20
Whole-Mount Analysis of Mammary Glands
Right inguinal mammary glands of 3- and 4-week-old female mice were excised, spread on glass slides, fixed, and stained with carmine alum essentially as previously described.21 Whole mounts were digitally photographed with a ruler, and total area measurements for the dysplastic lesions were quantified using Image J software.22
Histologic Analysis of Mammary Tumors
Right inguinal mammary tumors of 8-week-old female mice were excised, fixed with 10% neutral buffered formalin for 24 hours, and embedded in paraffin after dehydration. Subsequently, 5-μm sections were cut, stained with H&E, and evaluated by an experienced histopathologist (IM) without knowledge of the experimental group. Each tissue section was graded as normal (N), hyperplastic (H), well-differentiated (WD) carcinoma, moderately differentiated (MD) carcinoma, or poorly differentiated (PD) carcinoma. Analyses and descriptions were performed in accordance with previously established guidelines.23
Immunohistochemistry
Paraffin-embedded mammary tumor sections were first deparaffinized via treatment with xylene, and rehydrated via passage through a graded series of ethanol. Antigen retrieval was performed by microwaving the slides in sodium citrate buffer, and endogenous peroxide activity was quenched by incubation in 3% hydrogen peroxide. Slides were then washed with PBS, blocked with 10% normal goat serum (Vector Laboratories, Inc, Burlingame, CA) in PBS for 1 hour, and incubated with the primary antibody diluted in blocking solution overnight at 4°C. Sections were then incubated with a biotin-streptavidin detection system (LSAB2 System-HRP; Dako North America, Inc, Carpinteria, CA), and bound antibodies were visualized using 3,3′-diaminobenzidine as a substrate. Finally, the slides were washed in PBS, counterstained with hematoxylin, dehydrated, and mounted with coverslips.
Immunoblot Analysis
Left inguinal mammary glands of 8-week-old female mice were homogenized and prepared as previously described.24 Protein concentrations were quantified using the bicinchoninic acid protein kit (Thermo Fisher Scientific, Inc, Rockford, IL). Equal amounts of protein were loaded, separated using sodium dodecylsulfate–polyacrylamide gel electrophoresis (6% to 12% acrylamide), and transferred to nitrocellulose. Immunoblot analyses for ERα, cyclin D1, caveolin-1, SR-BI, LDL-R, Rho-GDI, and cytokeratin were performed using specific antibodies.
Determination of Serum Lipid Levels
Serum from female PyMT mice was obtained at age 4 weeks (for determination of basal levels) and at age 8 weeks via tail bleeding, and via cardiac puncture after the mice had been sacrificed at age 12 weeks. Serum cholesterol levels were determined using a colorimetric assay system (Wako Chemicals USA, Inc, Richmond, VA).
Lipoprotein Profiles
Lipoprotein profiles were determined using fast protein liquid chromatography. Fasting plasma samples obtained from 10 mice in each group were pooled to obtain a total volume of 150 μL, and loaded onto a Superose 6 column (GE Healthcare Bio-Sciences Corp, Piscataway, NJ) to achieve a total bed volume of 25 ml and a void volume of 7.5 ml. Plasma was passed over the column at a flow rate of 0.25 ml/min, and 0.5-ml fractions were collected. Total cholesterol content of each fraction was determined (Wako Chemicals USA, Inc) and plotted against elution volume.
Cholesterol Content of Mammary Gland and Tumors
Portions (∼0.1 g) of 12-week-old female mammary tumors were homogenized in 3 ml of 10 mmol/L Tris, 1 mmol/L of EDTA, and 1 mmol/L of β-mercaptoethanol. An aliquot of the lysate was used to determine protein concentrations using the bicinchroninic acid protein kit (Thermo Fisher Scientific, Inc). Lipids were extracted using the method of Bligh and Dyer.25 Total cholesterol content of each tumor was determined (Wako Chemicals USA, Inc). Results were expressed as micrograms of cholesterol per milligram of tissue protein.
Statistical Analysis
Values are given as mean (± SE). Comparisons between control and treated mouse samples were performed using the Student t test or analysis of variance when appropriate. The number of mice used for each experiment is indicated in the corresponding figure legend.
Results
Dietary Cholesterol Accelerates Mammary Tumor Onset and Burden in PyMT Transgenic Mice
To assess the role of dietary cholesterol on mammary tumor development and progression, 4-week-old PyMTTg female mice were fed a regular chow diet (<0.03% cholesterol) or a Western-type diet (0.2% cholesterol). After receiving the diet for 4 weeks, mice were examined by palpation for the presence of tumors in all 10 mammary glands. Eight-week-old female mice fed a cholesterol-rich diet developed more palpable tumors, on average, 1.91 tumors per mouse, compared with only 1.00 tumor per mouse in the group fed a chow diet (1.9-fold induction; P = 0.02) (Figure 1A). These female mice were given the same diet for 4 additional weeks, and were sacrificed at age 12 weeks. All of the tumors were carefully excised and weighed. By recording the total tumor weight per mouse, it was observed that PyMTTg mice fed a Western-type diet developed significantly larger tumors (1.5-fold larger; P = 0.01) compared with mice fed a chow diet (Figure 1B). Representative images of 12-week-old PyMTTg female mice given each of the diets are shown in Figure 1C. Note the increased number of large and bulky tumors in the PyMT mouse fed a high-cholesterol diet compared with the mouse fed a regular chow diet, indicating increased tumor multiplicity and tumor size. Body weight was determined at sacrifice after the mice had been fed either a chow or a Western-type diet for 4 (8-week-old female mice) and 8 weeks (12-week-old female mice). In PyMTTg female mice fed a chow or a Western diet, no significant difference in mean (± SE) total body weight could be detected in 8-week-old mice (20.74 ± 1.49 g vs. 20.43 ± 2.09 g, respectively) nor in 12-week-old mice (25.3 ± 1.7 g vs. 26.0 ± 3.5 g, respectively).
Figure 1.

Increased tumor incidence and burden in PyMTTg mice fed a Western diet. A: Female mice were palpated at 8 weeks of age for development of tumors in mammary glands. Mice were examined in a genotype-blinded fashion and palpated in each of the 10 mammary glands. The presence of tumors was examined in mice from each group (PyMTTg fed a chow or Western diet for 4 weeks). The number of tumors per mouse was determined. Results are given as mean ± SE (n = 15). Significant difference at *P < 0.05. B: Tumors were isolated from mice in each group (PyMTTg fed a chow or Western diet for 8 weeks) and weighed. Results are given as mean ± SE (n = 15). Significant difference at *P < 0.05. C: Representative images of tumors obtained from 12-week-old female PyMTTg mice fed a chow or a Western-type diet.
In summary, administration of a Western-type diet resulted in accelerated tumor onset, and increased tumor incidence, multiplicity, and burden. These data suggest an important role for dietary cholesterol in tumor formation.
PyMTTg Mice Fed a Western-Type Diet Show Increased Lung Metastasis
To further evaluate the aggressiveness of the tumors formed in PyMTTg mice fed a Western-type diet, lung metastasis development was evaluated. Serial sections of the left lung were obtained and stained with H&E, and the number of metastases was determined. The results suggest a trend toward an increased number of metastases in the lungs of PyMTTg mice fed a Western diet compared with mice fed a chow diet (Figure 2, A and B). The percentage of mice that developed at least 1 metastatic focus is shown in Figure 2A. The number of metastatic foci was scored for each animal. The mean (± SE) number of metastatic foci in PyMTTg mice fed a chow diet compared with those fed a Western-type diet was 7.89 ± 3.39 and 16.22 ± 6.68, respectively. Despite the important increase in the number of metastatic foci in the lung, no statistical significance was reached owing to the high variability in the number of lung metastases (P = 0.28). In this context, the data suggest a trend toward an increased number of metastasis in the lungs of PyMTTg mice fed a Western diet compared to mice fed a chow diet. To better illustrate this tendency, data are presented as the percentage of mice that developed 0, 1 to 10, or more than 10 metastatic foci per left lung (Figure 2B).
Figure 2.

PyMTTg mice fed a Western-type diet exhibit increased lung metastasis. Lungs obtained from 12-week-old female PyMTTg mice were obtained as described in the Materials and Methods section. They were sectioned at 50-μm intervals and stained with H&E. Lung metastasis was scored as the total number of metastatic foci (defined as a cluster of at least 10 cells) per lung. Nine animals were analyzed for each diet. A: Incidence of metastasis in PyMTTg mice fed a chow or Western-type diet. B: Distribution of lung metastases in PyMTTg mice fed a chow or Western-type diet.
Dietary Cholesterol Accelerates the Development of Multifocal Dysplastic Lesions in the Mammary Gland
One of the earliest premalignant lesions is hyperplasia, or mammary intraepithelial neoplasia or hyperplastic atypias. These lesions are thought to develop into tumors.18 These dysplastic lesions are present in most mammary glands by age 3 weeks in female PyMTTg mice, and spread throughout the entire mammary fat pad by age 7 weeks. To determine whether dietary cholesterol affects development of multifocal dysplastic lesions, whole-mount mammary gland analyses were performed at exactly age 3 and 4 weeks. To expose 3- and 4-week-old mice to a high-cholesterol diet, and considering that these animals obtain most of their nutrients from milk, their mothers were fed a Western-type diet during pregnancy and the lactation period.26–28 Moreover, embryos were exposed to increased plasma cholesterol levels in utero, and pups had access to the dams' diet until weaning at age 21 days.
Representative images of mammary whole-mount preparations obtained from mice at age 3 and 4 weeks are shown in Figure 3, A and B, respectively. Thus, compared with mice fed a chow diet, 3-week-old PyMTTg mice fed a high-cholesterol diet showed a striking increase (2.5-fold; P = 0.008) in the number of these multifocal dysplastic lesions (Figure 3C). Quantification of the total area occupied by dysplastic lesions was performed for each mammary gland, and revealed that a Western-type diet was associated with a 2.5-fold increase (P = 0.006) in the lesion area in 3-week-old female mice (Figure 3D). Similar results were obtained in 4-week-old female mice, with a 1.7-fold increase (P = 0.004) in the area occupied by dysplastic lesions (Figure 3E). These data demonstrate that tumor onset is accelerated in mice fed a Western-type diet.
Figure 3.

PyMTTg mice fed a Western-type diet demonstrate accelerated development of multifocal dysplastic lesions in the mammary gland. Whole mounts of mammary glands were obtained from 3-week-old (A) and 4-week-old (B) virgin female mice whose mothers had received either a chow or a Western diet during pregnancy and the lactation period. Right inguinal mammary glands were fixed in ethanol and acetic acid for 2 to 4 hours and stained overnight with carmine dye. Representative images are shown for each age and diet group. A mammary whole-mount preparation obtained from a wild-type mouse is included for comparison. The primary duct (PD), which originates from the nipple area, is visible in the top left corner of all images. The subiliac lymph node (LN) is visible to the right of the images. Arrows indicate a few examples of dysplastic foci. C: Quantification of the number of hyperplastic lesions per gland in 3-week-old female mice. Results are given as the mean ± SE (n = 8 and 6 for chow and Western-diet groups, respectively; **P < 0.01). D: Quantification of the total area occupied by multifocal dysplastic lesions in 3-week-old female PyMTTg mice. Data are given as mean ± SE (n = 8 and 6 for chow and Western diet groups, respectively; **P < 0.01). E: Quantification of total lesion area in 4-week-old female PyMTTg mice. Results are given as mean ± SE (n = 6 and 9 for chow and Western diet groups, respectively; ***P < 0.001).
Dietary Cholesterol Increases the Extent and Histologic Grade of Mammary Lesions
In addition to following the onset and growth of dysplastic foci, the present study also examined whether dietary cholesterol could affect the histologic grade of these lesions. Experiments were performed using 8-week-old PyMTTg female mice given a chow or a Western-type diet for 4 weeks to observe tumor formation and also to prevent development of exceedingly large tumors. Longitudinal sections of the right inguinal mammary gland were prepared for each animal. With the lymph node as a guide, two different areas were analyzed: the distal area where the primary tumor originates and the proximal area surrounding the lymph node where a secondary nucleus of lesions appears. In general, these secondary growths are one or two stages behind the primary tumor in the same mammary gland. The percentage of tissues in a given pathological stage and the corresponding representative histological images are shown for both proximal and distal regions (Figure 4, A and B, respectively) and for each experimental condition. Proximal areas corresponding to mice fed a chow diet were all classified as well-differentiated (epithelial proliferation still confined by a basement membrane, with minimal cytological atypia and no evidence of invasion). In 75% of mice fed a Western diet, tissues obtained from the proximal area of the mammary gland had progressed to a moderately differentiated stage (Figure 4A). Sections from the distal area in mice fed a chow diet were predominantly classified as moderately differentiated. However, in the group fed a Western diet, 50% of the sections were classified as poorly differentiated, the most malignant stage (Figure 4B). Noted were the presence of solid sheets of malignant cells with marked variation in cell shape, size, and nuclear structure, increased mitotic index, leukocyte infiltration, increased vessel density, and lack of organized basement membrane. These properties are characteristic of the most advanced stage of carcinoma. Therefore, consumption of a Western-type diet resulted in worsening of the histological grade of mammary tumor lesions.
Figure 4.
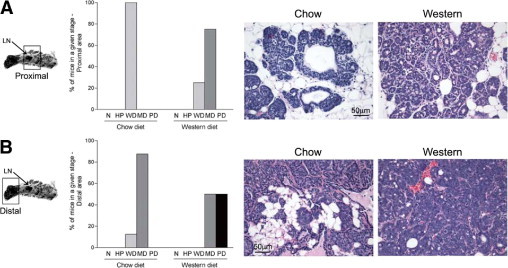
PyMT mice fed a Western diet exhibit more advanced mammary carcinogenic lesions. Right inguinal mammary glands were dissected from 8-week-old PyMT transgenic mice fed a regular chow diet or a Western-type diet, fixed, embedded in paraffin, sectioned, and stained with H&E. Using the lymph node as a reference, the proximal (secondary lesions) and distal (primary tumors) areas were analyzed for each mouse. Each tissue section was classified as being normal (N), hyperplastic (HP), well-differentiated carcinoma (WD), moderately differentiated carcinoma (MD), or poorly differentiated carcinoma (PD). The percentage of mice with mammary glands in a given histopathological stage in the proximal (A) and distal (B) areas is represented. Representative histological images are shown for each diet and each tissue section examined. Eight animals were analyzed for each diet. Proximal and distal indications refer to lymph node location. Original magnification, ×40.
Dietary Cholesterol Is Associated with a Protein Expression Pattern Characteristic of Advanced Tumorigenic Mammary Lesions
To confirm the aggressive nature of the cancer in animals fed a cholesterol-rich diet, the expression level of several biomarkers of tumor progression was examined using Western blot analysis (Figure 5A) and immunohistochemistry (Figure 5, B–D). Cyclin D1 protein expression was significantly elevated in PyMTTg mice fed a high-cholesterol diet, compared with matched samples derived from PyMTTg mice fed a chow diet (Figure 5, A and C). Immunohistochemistry confirmed this increase, but also revealed differences in the staining pattern consistent with a more advanced stage of carcinoma. Thus, in PyMTTg mice fed a chow diet, immunostaining of cyclin D1 was primarily located at the outer layer of the tumor acini, with little or no staining observed at the center of the lesion. In contrast, in PyMTTg mice fed a Western-type diet, cyclin D1 immunostaining was present in virtually all of the epithelial cells, and extended to the center of the lesion (Figure 5C). On the other hand, the expression levels of both ERα and caveolin-1 were reduced, as expected in more advanced tumor lesions (Figure 5A).21,29 Cytokeratin protein levels were not affected by the treatment (Figure 5D). Moreover, normalization with cytokeratin indicated that caveolin-1 and ERα protein levels were reduced 1.5- and 1.3-fold, respectively, in tumors obtained from animals fed a Western diet. Confirming these results, ERα immunostaining demonstrated areas containing a high density of ERα-positive cells in PyMTTg mice fed a regular diet that were no longer visible in mice fed a high-cholesterol diet (Figure 5B).
Figure 5.
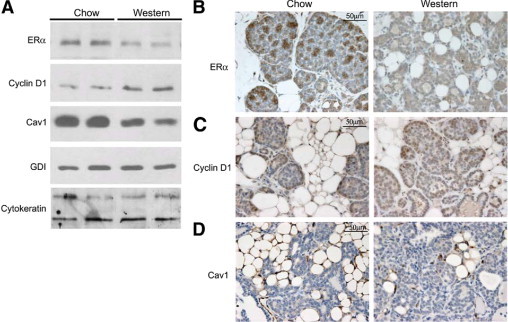
PyMT mice fed a Western diet exhibit expression patterns of progression markers consistent with more advanced mammary gland carcinoma. A: Left inguinal mammary glands isolated from 8-week-old female PyMTTg mice were lysed and subjected to immunoblot analysis for ERα, cyclin D1, and caveolin-1. GDI and cytokeratin were used as controls for equal protein loading and epithelial cell content, respectively. Two representative images of the 6 animals examined in each dietary group are shown. B–D, Right inguinal mammary glands from 8-week-old female PyMT mice were excised, formalin-fixed, paraffin-embedded, sectioned at 5-μm intervals, and subjected to immunohistochemical analysis. Representative images are shown for ERα (B), cyclin D1 (C), and caveolin-1 (D) immunohistochemistry in each of the dietary conditions. Six animals were analyzed for each diet. Original magnification, ×60.
Considering that plasma cholesterol levels have been correlated with angiogenic activity,30 microvessel density in tumors obtained from these mice was also examined (Figure 6, A and B). For these experiments, tissue sections were stained with CD31 antibody, a specific marker of endothelial cells. Microvascular density was remarkably increased in tumors obtained from animals fed a cholesterol-rich diet. These data suggest that high plasma cholesterol levels are associated with increased angiogenesis in mammary tumors.
Figure 6.
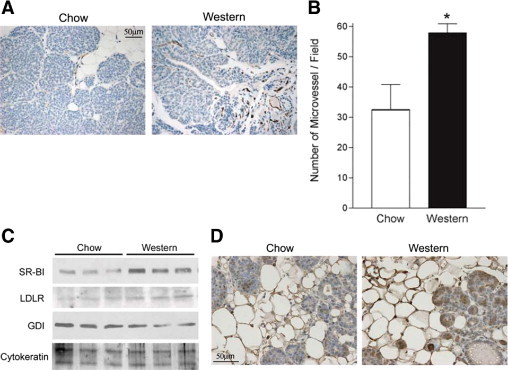
Increased plasma cholesterol is associated with increased tumor angiogenesis and lipoprotein receptor in PyMTTg mice. Right inguinal mammary glands from 8-week-old female PyMTTg mice were excised, formalin-fixed, paraffin-embedded, cut to obtain 5-μm sections, and subjected to immunohistochemical analysis. A: Representative images are shown for CD31 immunohistochemistry in each of the dietary conditions. Three animals were analyzed for each diet. Original magnification, ×40. B: Microvessel quantification was performed by counting the number of CD-31–positive vessels in 6 representative fields for each section. Results are given as mean ± SE. Significant difference at *P < 0.05. C: Left inguinal mammary glands isolated from 8-week-old female PyMTTg mice were lysed and subjected to immunoblot analysis for SR-BI and LDL-R. GDI and cytokeratin were used as controls for equal protein loading and epithelial cell content, respectively. Three representative images of the 6 animals examined in each dietary group are shown. D: Right inguinal mammary glands from 8-week-old female PyMTTg mice were excised, formalin-fixed, paraffin-embedded, cut to obtain 5-μm sections, and subjected to immunohistochemical analysis for SR-BI. Representative images are shown for each dietary condition. Six animals were analyzed for both chow and Western-type diet groups. Original magnification, ×60.
Mammary Tumorigenesis Is Associated with Reduced Plasma Cholesterol and HDL Levels in PyMTTg Mice
To study the metabolism of plasma cholesterol during tumor formation, fasting plasma samples were collected in wild-type and PyMTTg female mice at age 4 weeks (before the mice had been given the Western-type diet), 8 weeks (after receiving the diet for 4 weeks), and 12 weeks (after receiving the diet for 8 weeks). Total plasma cholesterol and lipoprotein levels before and after tumorigenesis were subsequently determined (Table 1 and Figure 7). Our results indicate that plasma cholesterol levels in 12-week-old PyMTTg mice fed a cholesterol-rich diet were significantly reduced compared with those of age-matched wild-type mice fed the same diet. This finding suggests that tumor formation may be responsible for this reduction. A similar trend (but not significant) was observed in mice fed a chow diet. A more detailed analysis of the type of lipoprotein affected revealed that the amount and size of the HDL particles were reduced in mice that developed tumors when they were fed a Western diet, compared with age-matched wild-type mice fed the same diet (Figure 7B). The reduced size of the HDL particles may reflect their reduced cholesteryl ester content, which forms most of the core of mouse HDL. It is possible that plasma cholesterol levels may be a rate-limiting factor for tumor progression. In agreement with this hypothesis, the mean (± SE) ratio plasma cholesterol to tumor burden remained constant in both groups of animals (48 ± 18 mg of cholesterol/dL/g of tumor in animals fed a chow diet versus 52 ± 20 mg cholesterol/dL/g of tumor in animals fed a Western diet). No differences in plasma triglyceride levels were detected after consumption of a Western diet in both 12-week-old nontransgenic female mice (125.6 ± 12.3 mg/dL and 120.3 ± 21.2 mg/dL for mice fed a chow or Western diet, respectively) and 12-week-old PyMTTg female mice (124.0 ± 4.4 mg/dL and 119.8 ± 17.2 mg/dL for mice fed a chow or a Western diet, respectively).
Table 1.
Serum Cholesterol Levels during Tumorigenesis
| Chow diet |
Western diet |
|||
|---|---|---|---|---|
| WT | PyMT | WT | PyMT | |
| 4w | 112.7 ± 7.9 | 112.3 ± 6.1 | 110.1 ± 5.8 | 109.4 ± 6.8 |
| 8w | 135.9 ± 3.9 | 131.2 ± 3.2 | 221.9 ± 5.3a | 210.9 ± 5.5a |
| 12w | 132.3 ± 4.4 | 127.6 ± 5.0 | 205.7 ± 5.0a | 190.3 ± 6.3a,b |
Total serum cholesterol content was determined from fasting plasma samples isolated from wild-type (WT) and PyMTTg mice using a colorimetric assay. Serum from 4-week-old, 8-week-old, and 12-week-old female mice on a chow or a Western-type diet for 0, 4, or 8 weeks were analyzed. Note that, as expected, animals fed a cholesterol-rich diet showed increased levels of total serum cholesterol compared to mice fed a chow diet (a; P < 0.001). Importantly, in animals fed a Western-type diet, 12-week-old PyMTTg mice displayed reduced serum cholesterol levels compared to the corresponding WT animals (b; P < 0.05). Total serum cholesterol levels are expressed in mg of cholesterol/dL ± SE (n=13 and 17 for WT and PyMT mice fed a chow diet, respectively, and n=15 for WT or PyMT mice group fed a Western-type diet).
Figure 7.
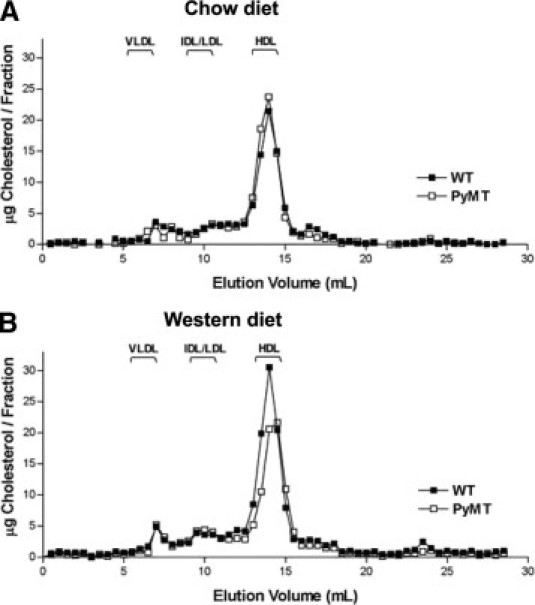
PyMT transgenic mice show decreased plasma cholesterol levels after tumor formation. Fasting plasma samples isolated from 10 mice from each group were pooled and loaded on two Superose 6 columns. Fractions were collected and analyzed for cholesterol content using a colorimetric assay. Lipoprotein profiles obtained from 12-week-old wild-type and PyMTTg female mice fed a chow diet (A) or a Western-type diet (B) for 8 weeks. Note the reduction in HDL-cholesterol levels and HDL size (peak shifted to the right) observed in PyMTTg mice compared with wild-type mice that did not develop any tumors.
Increased Expression in Tumors of the Protein SR-BI Responsible for the Selective Uptake of Cholesteryl Ester from HDL
Expression of the protein SR-BI, responsible for the selective uptake of cholesteryl ester from HDL, was examined. PyMTTg female mice fed a Western-type diet demonstrated a striking increase (1.8-fold; P = 0.002, as calculated by densitometric analysis normalized for epithelial cell content) in expression of SR-BI in the mammary gland, compared with age-matched samples from PyMTTg mice fed a chow diet (Figure 6C). Moreover, immunohistochemistry for SR-BI confirmed this remarkable increase in both the mammary fat pad and the epithelial proliferating cells (Figure 6D). In addition, despite the increased expression of SR-BI in the mammary gland of PyMTTg mice fed a cholesterol-rich diet, no differences in the total cholesterol content of these mammary tumors were detected (Figure 8). This suggests that even if tumor cholesterol uptake is increased, cholesterol does not accumulate in the tissue; rather, it is probably used to sustain a high level of cellular proliferation.
Figure 8.
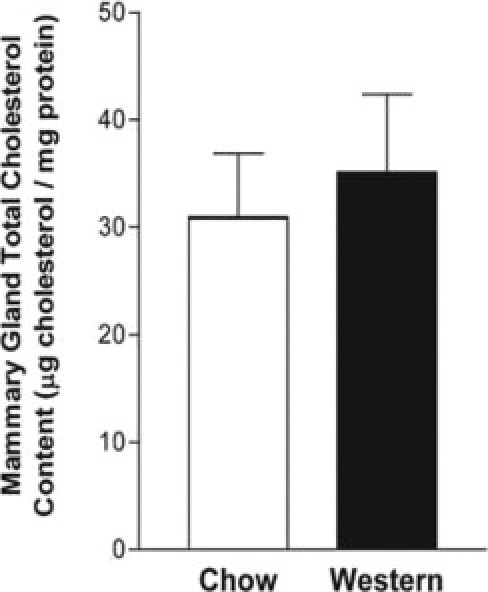
Mammary tumor cholesterol content is not affected by a Western-type diet in PyMTTg mice. Total cholesterol content of mammary tumors was determined with tissues obtained from 12-week-old PyMTTg mice fed a chow or a Western-type diet for 8 weeks. Portions of the tumors were homogenized, lipids were extracted, and total cholesterol content was determined using a colorimetric assay. Results are given as mean ± SE (n = 10 tumors analyzed for each dietary group). No statistical differences were observed between groups.
Discussion
Diet, Cholesterol, and Cancer
Predisposing genetic factors play an important role in the development of cancer.31 However, environmental factors have also been shown to play an important role in the regulation of cancer initiation, development, and aggressiveness.32 One such factor that is now well-recognized to play an important role in this process is the diet.33 Dietary cholesterol has an important role in the regulation of plasma cholesterol metabolism. Its role in the development of coronary artery disease, in particular, has been well documented, and numerous studies have demonstrated its worsening effect on the development of atherosclerosis.34 However, its role in cancer development has not been fully investigated, and preliminary studies have yielded contradictory results. While it is generally well accepted that particularly low plasma cholesterol levels can be a marker of the disease, the role of cholesterol in the initiation and progression of tumor formation is generally more controversial. The so-called preclinical cancer effect has been suggested for patients presenting low plasma cholesterol levels before diagnosis.35 It has been proposed that cancer was responsible for the observed low plasma cholesterol values. In addition, some types of cancer are more likely to decrease plasma cholesterol levels. For example, patients with liver cancer often exhibit reduced plasma cholesterol levels, which seems to be a symptom rather than a cause of cancer.36
For more than two decades, statins have been used to treat hypercholesterolemia and reduce coronary artery disease.37 Statins reduce plasma cholesterol by inhibiting the enzyme HMG-CoA reductase, which promotes a rate-limiting reaction regulating the synthesis of cholesterol. Several studies have demonstrated an important role for statins in the regulation of cellular proliferation and apoptosis of transformed cells. However, the effect of statin therapy on cancer is still controversial as some studies have suggested a negative effect, and others have proposed a positive or no effect.38–41 Nevertheless, statins may regulate important cellular signaling pathways associated with proliferation and apoptosis. In these cases, statins affect the isoprenylation (from geranylgeranyl or farnesyl) of several target proteins such as members of the Ras protein superfamily,42 which have an important role in oncogenesis. Therefore, it may be difficult to determine whether the reduced plasma cholesterol levels observed in patients treated with statins are responsible for the drugs' role in tumor formation.
To better examine the specific role of cholesterol in the regulation of tumor growth, Solomon et al30 used the drug ezetimibe, which reduces intestinal cholesterol absorption and, therefore, reduces plasma cholesterol levels in animals fed a cholesterol-rich diet. In that study, ezetimibe therapy reduced tumor growth in mice bearing human prostate tumor xenograft and fed a cholesterol-rich diet.30 The authors also showed that hypercholesterolemia accelerates tumor growth and that ezetimibe can retard this process by inhibiting tumor angiogenesis.
Previous works have demonstrated an important role for obesity in the development of breast cancer. In mouse models, it was shown that obesity could lead to an abnormal development of the mammary gland.43 In particular, reduced branching frequency and impaired alveolar development, but no effects on growth or proliferation, were observed. These observations were made in animals with abnormally large mammary fat pads. This was not the case in the present study. These studies may suggest that the mammary gland microenvironment can affect cellular growth. In agreement with this hypothesis, studies by Cleary et al44 have demonstrated that a high-fat diet and increased adiposity may accelerate tumor formation in a mouse model of breast cancer.
Cholesterol and Breast Cancer
To date, the relationship between breast cancer and plasma cholesterol has been a matter of controversy. A role for cholesterol in the development of prostate cancer has previously been suggested. However, in comparison with the present study, the situation is quite different since prostate tumors accumulate cholesterol, whereas Swyer45 did not observe any increase in the cholesterol content of mammary tumors. An important aspect to consider when examining the correlation between plasma cholesterol and breast cancer is that estrogen concentrations are also correlated with plasma HDL-cholesterol. This is even more important because increased exposure to estrogen is associated with increased risk of breast cancer development.
In the present study, the role of plasma cholesterol in the development of mammary tumors was examined in a mouse model. The data showed that increased plasma cholesterol, in association with oncogenic stimuli, lead to accelerated tumor formation and increased tumor burden. Mechanistically, it was demonstrated that in mammary tumors increased plasma cholesterol levels are associated with increased expression of cyclin D1, a marker associated with tumor formation, and decreased expression of markers associated with protection. Moreover, increased expression of SR-BI and the LDL receptor in these tumors was also demonstrated. These observations must be considered in light of the fact that plasma cholesterol levels were reduced in animals bearing tumors compared with control animals fed the same diet. The latter findings may suggest that reduced plasma cholesterol is associated with enhanced cholesterol uptake by tumors. Alternatively, other organs may also alter cholesterol metabolism to respond to increased physiological requirements. In addition, it is not unreasonable to assume that liver function may be affected in this disease. It may follow that plasma lipoprotein levels could be influenced by reduced hepatic lipoprotein secretion.
Cholesterol and Cellular Proliferation
Previous studies have suggested that alteration in the cellular plasma membrane cholesterol content may be a specific hallmark of transformed or tumorigenic cells. In agreement with this hypothesis, Zhuang et al46 have demonstrated that a cholesterol-rich diet can accelerate tumor formation in mice engrafted with prostate cancer cell lines. Their findings suggest that increased cholesterol content of lipid rafts is associated with greater cell survival of these prostate cancer cell lines. Additionally, regulation of the cholesterol-synthesis pathway may also affect cellular proliferation. In proliferating cells, mevalonate derivatives may be diverted away from cholesterol synthesis toward isoprenoid formation.47,48 This diversion may further increase production of proteins involved in control of cholesterol synthesis and its cellular uptake. Therefore, increased cholesterol uptake by proliferating cells may be allowed by the diversion of mevalonate derivatives toward the posttranslational modification of protein targets with an important role in the regulation of cellular proliferation. As a consequence, transformed cells may require additional cholesterol to maintain normal cellular cholesterol homeostasis. Statin therapy of tumorigenic cells would therefore reduce the neosynthesis of cholesterol and, importantly, the cellular supply of mevalonate.
The effect of phytoestrogen has been evaluated in other studies using animal models and in epidemiological studies. Phytoestrogens are plant polyphenols with a chemical structure similar to that of estrogens.49 A complicated role for these compounds has been proposed. For example, at low doses, phytoestrogens may prevent tamoxifen-induced reduction in mammary tumor formation. However, at higher doses, they may inhibit tumor growth and cellular proliferation of cancer cells.50 The latter effect may be related to the selective estrogen receptor modulator activity that has been associated with phytoestrogens. Since cholesterol is a sterol like estrogen, it may also have some selective estrogen receptor modulator activity. In that case, its activity (agonist or antagonist) may depend on its concentration or the type of lipoprotein it is associated with. At present, this is only speculative; however, results of the present study suggest that in the model used, cholesterol may promote tumor progression.
Role of Lipoproteins in Tumor Formation
Lipoproteins may have a direct role in the formation of tumors. HDL is the major plasma cholesterol carrier in the mouse model. Importantly, HDL regulates the mitogen-activated protein kinase pathway51 via SR-BI.52 This observation is especially important since, in our studies, both HDL and SR-BI proteins were elevated in animals fed a cholesterol-rich diet. In addition to its role in the regulation of cellular proliferation, plasma cholesterol may also have an important role in the regulation of vascular leakage and in the regulation of angiogenesis. Both pathways are regulated by HDL via the SR-BI receptor. In endothelial cells, endothelial nitric oxide synthase (eNOS) has a critical role in the regulation of angiogenesis and vascular leakage.53 Increased vascular leakage is a well-known characteristic of tumors.54 It is believed to have an important role in the regulation of plasma protein deposition in tumor. This process may enable proliferation of tumorigenic cells in the particular tumor microenvironment. Interestingly, HDL binding to SR-BI increases eNOS activity.55 To further support the proangiogenic role of HDL, previous studies have shown that HDL can promote endothelial cell proliferation.56 In agreement with this interpretation, inhibition of eNOS via N(G)-nitro-l-arginine methyl ester administration inhibits tumor angiogenesis.57 In addition, Orucevic et al58 have demonstrated that murine mammary adenocarcinoma can also produce nitric oxide since they express both inducible nitric oxide synthase and eNOS. In agreement with a role for cholesterol and HDL in the regulation of angiogenesis, we were able to demonstrate an increased angiogenic activity in tumors obtained from animals fed a cholesterol-rich diet (Figure 6A).
Estradiol has an important role in tumor development since it may regulate cellular proliferation and survival.59 Gong et al.60 demonstrated that HDL-associated estradiol can also stimulate eNOS activity The increased availability of HDL may increase estradiol access to the tumor. While lipoproteins may regulate endothelial function in the tumor, they may also affect the transport of estrogen. In addition, SR-BI may promote estradiol uptake.61 The increased SR-BI expression observed in mammary tumors may further enhance the signaling pathways activated by estradiol in transformed cells.
Conclusions
Results of the present study suggest that plasma cholesterol is an important determinant involved in the control of breast cancer. However, further research is needed to evaluate the various pathways affected in this disease. A cholesterol-based approach to the treatment of breast cancer could be implemented in the future with additional evidence. Drugs that target cholesterol metabolism could be used in addition to drugs that may facilitate the elimination of breast cancer cells. A synergistic effect would definitely help treat tumors, and may reduce the toxicity of existing therapies.
Footnotes
Supported by the Jane Barsumian / Mary Lyons Trust, and the Susan G. Komen Foundation (P.G.F.); and by a postdoctoral fellowship from the Spanish Ministry of Education and Science and a FIS Postdoctoral Fellowship from the Spanish Health Ministry (G.L.).
A guest editor acted as editor-in-chief for the manuscript. No person at Thomas Jefferson University or Albert Einstein College of Medicine was involved in the peer review process or final disposition of this article.
References
- 1.Key T.J., Verkasalo P.K., Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey J.L. Breast cancer epidemiology: summary and future directions. Epidemiol Rev. 1993;15:256–263. doi: 10.1093/oxfordjournals.epirev.a036112. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu H., Ross R.K., Bernstein L., Yatani R., Henderson B.E., Mack T.M. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowey S., Hardy R.W. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillan D.C., Sattar N., McArdle C.S. ABC of obesity: obesity and cancer. BMJ. 2006;333:1109–1111. doi: 10.1136/bmj.39042.565035.BE1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantafora A., Blotta I. Neutral lipids production, transport, utilization. Anticancer Res. 1996;16:1441–1449. [PubMed] [Google Scholar]
- 7.Tomiki Y., Suda S., Tanaka M., Okuzawa A., Matsuda M., Ishibiki Y., Sakamoto K., Kamano T., Tsurumaru M., Watanabe Y. Reduced low-density-lipoprotein cholesterol causing low serum cholesterol levels in gastrointestinal cancer: a case control study. J Exp Clin Cancer Res. 2004;23:233–240. [PubMed] [Google Scholar]
- 8.Vitols S., Norgren S., Juliusson G., Tatidis L., Luthman H. Multilevel regulation of low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in normal and leukemic cells. Blood. 1994;84:2689–2698. [PubMed] [Google Scholar]
- 9.Tatidis L., Gruber A., Vitols S. Decreased feedback regulation of low density lipoprotein receptor activity by sterols in leukemic cells from patients with acute myelogenous leukemia. J Lipid Res. 1997;38:2436–2445. [PubMed] [Google Scholar]
- 10.Tatidis L., Vitols S., Gruber A., Paul C., Axelson M. Cholesterol catabolism in patients with acute myelogenous leukemia and hypocholesterolemia: suppressed levels of a circulating marker for bile acid synthesis. Cancer Lett. 2001;170:169–175. doi: 10.1016/s0304-3835(01)00592-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91:41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Caruso M.G., Notarnicola M., Cavallini A., Di Leo A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity and low-density lipoprotein receptor expression in diffuse-type and intestinal-type human gastric cancer. J Gastroenterol. 2002;37:504–508. doi: 10.1007/s005350200078. [DOI] [PubMed] [Google Scholar]
- 13.Tatidis L., Masquelier M., Vitols S. Elevated uptake of low density lipoprotein by drug resistant human leukemic cell lines. Biochem Pharmacol. 2002;63:2169–2180. doi: 10.1016/s0006-2952(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 14.Chang S.J., Hou M.F., Tsai S.M., Wu S.H., Hou L.A., Ma H., Shann T.Y., Wu S.H., Tsai L.Y. The association between lipid profiles and breast cancer among Taiwanese women. Clin Chem Lab Med. 2007;45:1219–1223. doi: 10.1515/CCLM.2007.263. [DOI] [PubMed] [Google Scholar]
- 15.Kucharska-Newton A.M., Rosamond W.D., Mink P.J., Alberg A.J., Shahar E., Folsom A.R. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol. 2008;18:671–677. doi: 10.1016/j.annepidem.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y., Park S.K., Han W., Kim D.H., Hong Y.C., Ha E.H., Ahn S.H., Noh D.Y., Kang D., Yoo K.Y. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol Biomarkers Prev. 2009;18:508–515. doi: 10.1158/1055-9965.EPI-08-0133. [DOI] [PubMed] [Google Scholar]
- 17.Guy C.T., Cardiff R.D., Muller W.J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maglione J.E., Moghanaki D., Young L.J., Manner C.K., Ellies L.G., Joseph S.O., Nicholson B., Cardiff R.D., MacLeod C.L. Transgenic polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 19.Lin E.Y., Jones J.G., Li P., Zhu L., Whitney K.D., Muller W.J., Pollard J.W. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams T.M., Medina F., Badano I., Hazan R.B., Hutchinson J., Muller W.J., Chopra N.G., Scherer P.E., Pestell R.G., Lisanti M.P. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo: role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 21.Williams T.M., Cheung M.W., Park D.S., Razani B., Cohen A.W., Muller W.J., Di Vizio D., Chopra N.G., Pestell R.G., Lisanti M.P. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell. 2003;14:1027–1042. doi: 10.1091/mbc.E02-08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramoff M.D., Magelhaes P.J., Ram S.J. Image processing with Image J. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 23.Cardiff R.D., Anver M.R., Gusterson B.A., Hennighausen L., Jensen R.A., Merino M.J., Rehm S., Russo J., Tavassoli F.A., Wakefield L.M., Ward J.M., Green J.E. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 24.Williams T.M., Sotgia F., Lee H., Hassan G., Di Vizio D., Bonuccelli G., Capozza F., Mercier I., Rui H., Pestell R.G., Lisanti M.P. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. Am J Pathol. 2006;169:1784–1801. doi: 10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can.J. Biochem. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Connor W.E., Lin D.S. Placental transfer of cholesterol-4-14C into rabbit and guinea pig fetus. J Lipid Res. 1967;8:558–564. [PubMed] [Google Scholar]
- 27.Green M.H., Dohner E.L., Green J.B. Influence of dietary fat and cholesterol on milk lipids and on cholesterol metabolism in the rat. J Nutr. 1981;111:276–286. doi: 10.1093/jn/111.2.276. [DOI] [PubMed] [Google Scholar]
- 28.Whatley B.J., Green J.B., Green M.H. Effect of dietary fat and cholesterol on milk composition, milk intake and cholesterol metabolism in the rabbit. J Nutr. 1981;111:432–441. doi: 10.1093/jn/111.3.432. [DOI] [PubMed] [Google Scholar]
- 29.Lapidus R.G., Nass S.J., Davidson N.E. The loss of estrogen and progesterone receptor gene expression in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:85–94. doi: 10.1023/a:1018778403001. [DOI] [PubMed] [Google Scholar]
- 30.Solomon K.R., Pelton K., Boucher K., Joo J., Tully C., Zurakowski D., Schaffner C.P., Kim J., Freeman M.R. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174:1017–1026. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowin P.A., Anglesio M., Etemadmoghadam D., Bowtell D.D. Profiling the cancer genome. Annu Rev Genomics Hum Genet. 2010;11:133–159. doi: 10.1146/annurev-genom-082509-141536. [DOI] [PubMed] [Google Scholar]
- 32.Schottenfeld D., Beebe-Dimmer J.L. Advances in cancer epidemiology: understanding causal mechanisms and the evidence for implementing interventions. Annu Rev Public Health. 2005;26:37–60. doi: 10.1146/annurev.publhealth.26.021304.144402. [DOI] [PubMed] [Google Scholar]
- 33.Forman M.R., Hursting S.D., Umar A., Barrett J.C. Nutrition and cancer prevention: a multidisciplinary perspective on human trials. Annu Rev Nutr. 2004;24:223–254. doi: 10.1146/annurev.nutr.24.012003.132315. [DOI] [PubMed] [Google Scholar]
- 34.Gordon T., Kannel W.B. Predisposition to atherosclerosis in the head, heart, and legs: the Framingham study. JAMA. 1972;221:661–666. [PubMed] [Google Scholar]
- 35.Kritchevsky S.B., Kritchevsky D. Serum cholesterol and cancer risk: an epidemiologic perspective. Annu Rev Nutr. 1992;12:391–416. doi: 10.1146/annurev.nu.12.070192.002135. [DOI] [PubMed] [Google Scholar]
- 36.Iso H., Ikeda A., Inoue M., Sato S., Tsugane S. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer. 2009;125:2679–2686. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 37.Tobert J.A. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat Rev Drug Discov. 2003;2:517–526. doi: 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- 38.Blais L., Desgagne A., LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 39.Cauley J.A., Zmuda J.M., Lui L.Y., Hillier T.A., Ness R.B., Stone K.L., Cummings S.R., Bauer D.C. Lipid-lowering drug use and breast cancer in older women: a prospective study. J Womens Health (Larchmt) 2003;12:749–756. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 40.Graaf M.R., Beiderbeck A.B., Egberts A.C., Richel D.J., Guchelaar H.J. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Poynter J.N., Gruber S.B., Higgins P.D., Almog R., Bonner J.D., Rennert H.S., Low M., Greenson J.K., Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 42.Liao J.K. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flint D.J., Travers M.T., Barber M.C., Binart N., Kelly P.A. Diet-induced obesity impairs mammary development and lactogenesis in murine mammary gland. Am J Physiol Endocrinol Metab. 2005;288:E1179–E1187. doi: 10.1152/ajpendo.00433.2004. [DOI] [PubMed] [Google Scholar]
- 44.Cleary M.P., Grande J.P., Maihle N.J. Effect of high fat diet on body weight and mammary tumor latency in MMTV-TGF-alpha mice. Int J Obes Relat Metab Disord. 2004;28:956–962. doi: 10.1038/sj.ijo.0802664. [DOI] [PubMed] [Google Scholar]
- 45.Swyer G. The cholesterol content of normal and enlarged prostates. Cancer Res. 1942;2:372–375. [Google Scholar]
- 46.Zhuang L., Kim J., Adam R.M., Solomon K.R., Freeman M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairbanks K.P., Witte L.D., Goodman D.S. Relationship between mevalonate and mitogenesis in human fibroblasts stimulated with platelet-derived growth factor. J Biol Chem. 1984;259:1546–1551. [PubMed] [Google Scholar]
- 48.Witte L.D., Fairbanks K.P., Barbu V., Goodman D.S. Studies on cell proliferation and mevalonic acid metabolism in cultured human fibroblasts. Ann NY Acad Sci. 1985;454:261–269. doi: 10.1111/j.1749-6632.1985.tb11866.x. [DOI] [PubMed] [Google Scholar]
- 49.Oseni T., Patel R., Pyle J., Jordan V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656–1665. doi: 10.1055/s-0028-1088304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu B., Edgerton S., Yang X., Kim A., Ordonez-Ercan D., Mason T., Alvarez K., McKimmey C., Liu N., Thor A. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res. 2005;65:879–886. [PubMed] [Google Scholar]
- 51.Deeg M.A., Bowen R.F., Oram J.F., Bierman E.L. High density lipoproteins stimulate mitogen-activated protein kinases in human skin fibroblasts. Arterioscler Thromb Vasc Biol. 1997;17:1667–1674. doi: 10.1161/01.atv.17.9.1667. [DOI] [PubMed] [Google Scholar]
- 52.Grewal T., de Diego I., Kirchhoff M.F., Tebar F., Heeren J., Rinninger F., Enrich C. High density lipoprotein–induced signaling of the MAPK pathway involves scavenger receptor type BI-mediated activation of Ras. J Biol Chem. 2003;278:16478–16481. doi: 10.1074/jbc.C300085200. [DOI] [PubMed] [Google Scholar]
- 53.Gratton J.P., Lin M.I., Yu J., Weiss E.D., Jiang Z.L., Fairchild T.A., Iwakiri Y., Groszmann R., Claffey K.P., Cheng Y.C., Sessa W.C. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 54.Dvorak H.F. Leaky tumor vessels: consequences for tumor stroma generation and for solid tumor therapy. Prog Clin Biol Res. 1990;354A:317–330. [PubMed] [Google Scholar]
- 55.Yuhanna I.S., Zhu Y., Cox B.E., Hahner L.D., Osborne-Lawrence S., Lu P., Marcel Y.L., Anderson R.G., Mendelsohn M.E., Hobbs H.H., Shaul P.W. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 56.Tauber J.P., Cheng J., Massoglia S., Gospodarowicz D. High density lipoproteins and the growth of vascular endothelial cells in serum-free medium. In Vitro. 1981;17:519–530. doi: 10.1007/BF02633513. [DOI] [PubMed] [Google Scholar]
- 57.Jadeski L.C., Lala P.K. Nitric oxide synthase inhibition by N(G)-nitro-l-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol. 1999;155:1381–1390. doi: 10.1016/S0002-9440(10)65240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orucevic A., Bechberger J., Green A.M., Shapiro R.A., Billiar T.R., Lala P.K. Nitric-oxide production by murine mammary adenocarcinoma cells promotes tumor-cell invasiveness. Int J Cancer. 1999;81:889–896. doi: 10.1002/(sici)1097-0215(19990611)81:6<889::aid-ijc9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 59.Butt A.J., Sutherland R.L., Musgrove E.A. Live or let die: oestrogen regulation of survival signalling in endocrine response. Breast Cancer Res. 2007;9:306. doi: 10.1186/bcr1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong M., Wilson M., Kelly T., Su W., Dressman J., Kincer J., Matveev S.V., Guo L., Guerin T., Li X.A., Zhu W., Uittenbogaard A., Smart E.J. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J Clin Invest. 2003;111:1579–1587. doi: 10.1172/JCI16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brodeur M.R., Brissette L., Falstrault L., Luangrath V., Moreau R. Scavenger receptor of class B expressed by osteoblastic cells are implicated in the uptake of cholesteryl ester and estradiol from LDL and HDL3. J Bone Miner Res. 2008;23:326–337. doi: 10.1359/jbmr.071022. [DOI] [PubMed] [Google Scholar]


