Abstract
Background and Purpose
The purpose of this study was to compare the biomechanics of recumbent cycling between adolescents with cerebral palsy (CP) classified at Gross Motor Function Classification System (GMFCS) levels III and IV and adolescents with typical development (TD).
Subjects
Twenty subjects, ages (X̄±SD) 15.2±1.6 years (10 with TD, 10 with CP), participated.
Methods
Lower-extremity kinematics and muscle activity were measured at 30 and 60 rpm while subjects pedaled on a recumbent cycle. Energy expenditure and perceived exertion were measured during a 5-minute test, and efficiency was calculated. Noncircular data were analyzed with analyses of variance. Circular data were analyzed using circular t tests.
Results
Differences were found between groups for joint kinematics for all motions. Subjects with CP displayed earlier onsets and later offsets of muscle activity, increased co-contraction of agonist and antagonist muscles, and decreased efficiency compared with subjects with TD. There were no differences in perceived exertion.
Discussion and Conclusion
Differences in cycling biomechanics between children with CP and children with TD may be due to decreased strength and motor control in the children with CP.
Children with cerebral palsy (CP) typically have progressive impairments that affect their function as children and later as adults, and they have decreased fitness levels versus children with typical development (TD). Cycling has been suggested as an intervention to address common impairments and improve fitness levels in this population. However, little is known about the biomechanics of cycling in children with CP.
Compared with children with TD, children with spastic CP have decreased muscle strength (force-generating capacity),1 muscle spasticity (hypertonicity),2 decreased joint range of motion (ROM),3 altered motor and postural control,2,4 and gait deviations.5,6 Several of these impairments are related to decreased function.7,8 In addition, muscle co-contraction in children with CP has received attention during isolated limb-segment movements as well as whole-body activities such as gait9,10 and is a contributor to decreased motor control.11
Fitness levels of people with disabilities recently have gained attention through Healthy People 2010.12,13 Inactivity in people with disabilities can lead to a cycle of deconditioning, adversely affecting the cardiovascular system, bone density, and circulation and leading to social isolation and decreased self-esteem.14-17 Children with CP often decline in ambulatory status as they become adults due to problems such as joint pain, joint deterioration, and overall fatigue.18 Adolescents and adults with CP experience secondary conditions, including fractures, osteoporosis, cardiovascular system impairments, degenerative joint disease, obesity, pain, contractures, depression, decreased mobility, dependency on other people for assistance, limited opportunities for recreation, and social isolation.19
However, most research on exercise interventions for individuals with CP has focused on the younger age groups,20 with less research directed toward adolescents,21 a group particularly at risk for deconditioning and potential negative health effects due to decreasing mobility that continues into adulthood.19,21,22 In addition, adolescents with higher Gross Motor Function Classification System23 (GMFCS) levels and therefore decreased mobility are particularly at risk as they transition from adolescence to adulthood.
Bicycling using a moving or stationary bicycle is a potential intervention for children with CP to address impairments while potentially minimizing joint stress,24 and studies have begun to examine outcomes of a therapeutic cycling program in children with CP.25,26 Only one study has examined the biomechanics of cycling in children with CP.27 A better understanding of how children with CP cycle may help to develop future interventions, such as volitional cycling programs or cycling assisted by functional electrical stimulation. Additionally, cycling may allow children with CP more opportunities for exercise to enhance overall fitness, which is currently a focus of the Pediatric Section of the American Physical Therapy Association, Healthy People 2010, and the President’s Council on Fitness.12
The purpose of this study was to determine the 3-dimensional kinematics, electromyographic (EMG) activity, gross mechanical efficiency (power output/metabolic input),28 and perception of effort of constant-load, low-intensity stationary recumbent cycling in adolescents with CP at GMFCS levels III and IV compared with those of adolescents with TD. The hypotheses were that subjects with CP would show increased joint movement in the frontal and transverse planes, altered muscle activation patterns, increased muscle co-contraction around the hip and knee, decreased gross mechanical efficiency, and greater perception of effort during cycling compared with subjects with TD.
Method
Subjects
Twenty adolescents participated in the study. Ten subjects (3 male, 7 female) had a diagnosis of spastic diplegic or quadriplegic CP (age [X̄±SD] = 15.6±1.8 years, body mass index=24.1±4.7), and 10 subjects (3 male, 7 female) were children with TD (age=14.9±1.4 years, body mass index=22.6±5.4). Sixty percent of the subjects with CP and 40% of the subjects with TD were from minority populations. Subjects with CP were recruited from Shriners Hospitals for Children, Philadelphia, Pa, and subjects with TD were recruited through advertisement. Subjects 18 years of age and older and a parent of subjects who were less than 18 years of age signed an informed consent form approved by the governing institutional review board (IRB). Subjects who were less than 18 years of age signed an IRB-approved assent form. All 20 screened subjects met the inclusion and exclusion criteria for the study (Tab. 1).
Table 1.
Inclusion and Exclusion Criteria
| Inclusion | Exclusion |
|---|---|
|
|
Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes: a statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation. 1996;94:850–856.
Subjects with cerebral palsy (CP) only.
Procedure
Each subject was tested while cycling on a stationary bicycle.* The bicycle was a compact free-standing stationary cycle consisting of a base, adjustable-length crank arms with adjustable pedals, handlebars, and a control pad (Fig. 1). The pedals differed from standard bicycle pedals by having a full footplate, and they differed in the location of the pedal spindle in relation to the foot. The pedal spindle is the point of pedal rotation in relation to the crank arm, and the pedal spindle on a standard bicycle pedal is located at the plantar surface of the foot. In contrast, the pedal spindle on the stationary bicycle used in this study was located closer to the lateral malleolus. The foot was placed on a footplate that was located 5.5 cm distal to the pedal spindle and was attached to the pedal spindle through a metal frame.
Figure 1.
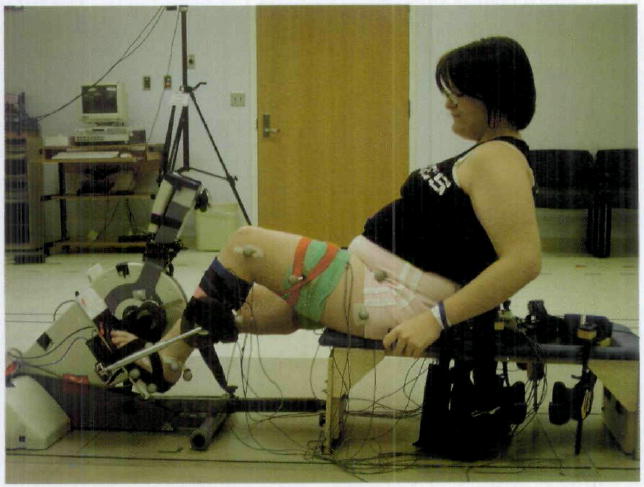
Subject with cerebral palsy using the cycle in the motion analysis laboratory. A therapy bench was attached to the cycle with an adjustable bar that was set based on subject anthropometrics. The seat back was reclined 25 degrees from vertical and was height-adjustable. The reflective markers on the subject and the pedal were used for the kinematic analysis.
Because the free-standing bicycle had no seat, subjects were seated on a therapy bench† attached to the base of the bicycle through an adjustable bar (Fig. 1). All subjects placed their hands on handgrips mounted on the sides of the bench. The specific ranges for adjustability of the back and the bar connecting the bicycle to the bench were determined by reviewing reported anthropometric data for children with TD, ages 6 to 18 years.29 Data on children younger than those in this study were included because children with CP tend to be shorter and lighter than their age-matched peers with TD.30
The bicycle was adjusted for each subject individually based upon anthropometric measurements (Fig. 2). The foot was positioned so that the second metatarsal head was aligned with the pedal spindle to maximize ankle power.31 This position was set by manipulating the footplate fastened with Velcro‡ to the pedal. The position of the footplate also was manipulated on the pedal to accommodate any lower-extremity rotational deformities for the subjects with CP. The footplate was rotated until the knee was aligned statically in the sagittal plane. The footplate was positioned flush to the medial side of the pedal for subjects with TD because no subject with TD displayed atypical torsional alignment. Subjects’ feet were secured to the footplate with soft straps.
Figure 2.
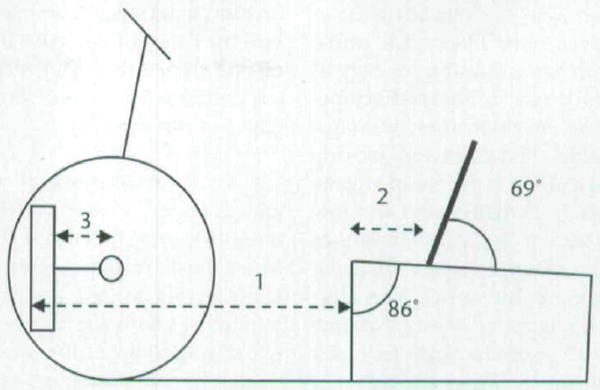
Bicycle setup. All components were adjusted based on subject anthropometrics. (1) Seat-to-pedal distance=85% of the distance from the greater trochanter to the base of the calcaneus. (2) Seat-to-greater trochanter distance=15% of the distance from the greater trochanter to the base of the calcaneus. (3) Crank arm length=30% of tibial length. The seat back was placed comfortably behind the subject while maintaining the seat to greater trochanter distance.
Each subject was instructed to pedal at a cadence of 30 revolutions per minute (rpm) and 60 rpm using the cycle’s tachometer for feedback. Subjects with TD required one short training session (less than 10 minutes), and subjects with CP required 1 or 2 training sessions with a maximum time of 20 minutes per session. All subjects were permitted to rest as needed.
Kinematic and EMG data were collected in a motion analysis laboratory. Three trials of 10 to 15 seconds’ duration were conducted for each subject for each cadence once the targeted cadence was reached. Six of the 10 subjects with CP were able to attain and maintain 60 rpm for short-duration trials (up to approximately 30 seconds). The 4 subjects with CP who could not cycle at 60 rpm in the motion laboratory could cycle at 30 rpm and were tested only at this lower cadence. The resistance load provided during cycling was based on each subject’s weight and was calculated using the following formula adapted from Dore et al32: Load (in newton-meters) = 0.49 N/kg × body weight (in kilograms) × crank arm length (in meters).
Kinematic Evaluation
Three-dimensional kinematics of the bilateral hip, knee, and ankle were collected using a 7-camera Vicon 370 motion analysis system§ and a standard marker set on the pelvis (bilateral anterior superior iliac spines and sacrum) and the bilateral lower extremities33 (Fig. 1). A rigid body marker setup was used, and joint centers were calculated. Data were collected at 60 Hz and digitally filtered using a low-pass filter of 6 Hz. Each subject underwent a series of anthropometric measures, which were required to process the kinematic data within the Vicon model.
A rotary encoder∥ mounted on the crank axis recorded the crank position during each revolution in increments of 0.3 degree. The crank arm was calibrated in a horizontal position prior to data collection, and 0 degrees was defined as the point at which the left crank arm was horizontal and farthest from the subject, as shown in Figure 2. All data were synchronized and processed with customized software using Vicon Plug-in-Gait (Version 1.9, Build 051).§ All kinematic data were analyzed in 1-degree increments of the crank position using customized software written in MATLAB version 7.5.#
Electromyography
Surface EMG data were collected from 8 lower-extremity muscles (gluteus maximus, rectus femoris, vastus lateralis, medial hamstrings, biceps femoris, anterior tibialis, lateral gastrocnemius, and soleus) bilaterally using standardized placement locations.31 These muscles were selected because they are major contributors to cycling in adults.34-36 The EMG data were used only to determine muscle timing and co-contraction during cycling.
The EMG data were collected at 1,200 Hz using a Motion Lab Systems MA-300 surface EMG recording system.** Each EMG sensor (MA-310**) consisted of 2 circular, stainless steel, dry button electrode contacts (12-mm diameter) that were pre-attached to double-differential preamplifiers located within the electrode housing. The EMG data were normalized across subjects by establishing a quiet baseline for each subject for 6 seconds. The EMG data were digitally filtered using a band-pass filter of 20 to 350 Hz. To determine the onset and offset of muscle activity, EMG data were rectified and then smoothed using a second-order low-pass Butterworth filter with phase correction and a cutoff frequency of 10 Hz to create a linear envelope. The linear envelope then was analyzed using 25-millisecond moving square windows. If the mean voltage within each 25-millisecond window was at least 3 standard deviations above the mean voltage during the quiet baseline, the muscle was identified as being active during that time period.37,38 From the data, the crank positions for the onset and offset of muscle activity were determined in 0.3-degree increments for each muscle and each subject. The duration of activity (in degrees) also was calculated.
Periods of co-contraction around each joint were identified based on the percentage of the cycling revolution in which each of 6 agonist and antagonist pairings around the bilateral hip and knee and the ankle were co-contracting. These pairings were the rectus femoris and biceps femoris muscles, the rectus femoris and medial hamstring muscles, the vastus lateralis and biceps femoris muscles, the vastus lateralis and medial hamstring muscles, the anterior tibialis and gastrocnemius muscles, and the anterior tibialis and soleus muscles.
Energy Expenditure and Gross Mechanical Efficiency
Energy expenditure data were collected via the breath-by-breath method utilizing a SensorMedics Vmax29 metabolic cart†† with subjects fasting for at least 2 hours prior to testing. Subjects wore a small airtight facemask‡‡ over the mouth and nose that held the flow sensor used to measured the volume of oxygen consumed (in milliliters per kilogram of body weight) (V̇o2/kg) for each breath. A gel liner‡‡ was placed inside the edges of the mask to ensure that air passed through the flow sensor and did not escape around the edges of the mask.
The V̇o2/kg measurements were obtained under 4 consecutive conditions: (1) sitting quietly for 5 minutes to establish resting values, (2) cycling at the desired cadence for 1 minute to allow the body to warm up, (3) cycling at the desired cadence for 5 minutes, and (4) sitting quietly to establish 3 minutes of recovery values. Power output data recorded once per second were downloaded from customized software on a pocket personal computer linked to the bicycle’s electronics. Following the test, gross mechanical efficiency was calculated by dividing the average power output (in watts) by the average metabolic input (in volume of oxygen consumed per kilogram of body weight) across the entire 5-minute cycling test.
Perception of Effort
Following each energy expenditure testing session, subjects were asked to rate their perception of effort using the Children’s OMNI Scale of Perceived Exertion.39 There are multiple versions of the OMNI scale depicting children doing a variety of activities. The version used in this study shows a child riding a bicycle uphill with a score of 0 (“not tired at all”) at the bottom of the hill and a score of 10 (“very, very tired”) at the top of the hill and has the child rate the exercise based on how tired he or she feels.39 The Children’s OMNI Scale of Perceived Exertion has been shown to yield reliable data in a study with adolescent girls40 and valid data for children ages 8 to 12 years of mixed sexes and races.41
Data Analysis
For kinematic and EMG analysis, 5 cycling revolutions closest to the targeted cadence with complete kinematic data were selected for analysis for each cadence for each subject. All data for the left and right sides were analyzed separately, and left- and right-side data were not compared. For brevity, only the left-side data are presented. For all data, rank transformations using normalized ranks were performed prior to analysis secondary to a nonnormal distribution.42 A P value of less than .05 was accepted for significance. For variables with multiple measures (ie, 16 muscles), the accepted P value was determined by dividing .05 by that number.
Kinematic data were analyzed using 3-way mixed-model analyses of variance (ANOVAs) with crank position used as a random factor to determine differences in the position of the joint (in degrees) based on group, cadence, and crank position in 1-degree increments. The inclusion of crank position in the analysis allows the comparison of the kinematic curves as a whole, accounting for differences in joint excursions and timing simultaneously. For EMG data, circular t tests using Oriana 2.0§§ were performed to determine differences in the crank position (in 0.3-degree increments) at which onset and offset of muscle activity occurred based on group and cadence. A Mardia-Watson-Wheeler test using rank transformations was used due to the nonnormal distribution of the data.43 A 2-way ANOVA for onset and offset data was not performed because a model for this statistic has not yet been developed for circular data. To analyze the duration of muscle activity, a 2-way ANOVA based on group and cadence was performed. Finally, an analysis of covariance (ANCOVA) based on group and targeted cadence with actual cadence as a covariate was used for the analysis of efficiency and perception of effort due to differences in cadences achieved between groups during the attempted 60-rpm energy expenditure test. There were no differences in achieved cadences between groups for the shorter-duration (10–15 seconds) trials for assessing joint kinematics and EMG activity in the motion laboratory, so an ANCOVA was not needed for analyzing those data.
Results
Differences were seen between groups and cadences for joint kinematics, muscle activity, and energy expenditure during cycling. As compared with subjects with TD, subjects with CP had increased joint movement in the frontal and transverse planes and altered sagittal-plane kinematics. Electromyographic activity was prolonged, co-contraction was increased, and efficiency was lower for the subjects with CP.
Kinematics
Joint kinematics of the left lower extremity differed based on cadence and crank position and interactions involving group, crank position, and cadence (Tab. 2). No differences were seen between groups unless crank position was taken into account (group × crank position interaction). In that case, there were differences in all joint motions, indicating that kinematic curves were different. There also were differences in 3 of 6 joint motions when looking at the interaction of group, crank position, and speed. Post hoc analyses were not performed for the interactions involving the crank position due to the large numbers of comparisons that would be required, greatly reducing the P value needed for significance.
Table 2.
Statistical Results (P Values) for the Kinematic Dataa
| Variable | Group | Cadence | Crank Position | Group × Cadence | Group × Crank Position | Group × Crank Position × Cadence |
|---|---|---|---|---|---|---|
| Left hip flexion and extension | .8767 | <.0001 | <.0001 | .0009 | <.0001 | .0300 |
| Left hip adduction and abduction | .7649 | <.0001 | .8664 | .0035 | <.0001 | .6809 |
| Left hip medial (internal) and lateral (external) rotation | .1040 | <.0001 | <.0001 | <.3202 | <.0001 | <.0001 |
| Left knee flexion and extension | .5121 | <.0001 | <.0001 | .1722 | <.0001 | .2217 |
| Left knee varus and valgus | .1570 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Left ankle dorsiflexion and plantar flexion | .1697 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
The values in bold type were significant (P<.004 for significance due to 12 joint motions being studied overall). The results that include the crank position (in degrees) reflect the comparison of the kinematic curves as a whole.
Figures 3 and 4 display the joint angles of the left lower extremity throughout the cycling revolution for each group and each cadence. In addition, there were differences between groups in the position of the foot in the transverse plane, with an average of 18.8±10.5 degrees of lateral (external) rotation for subjects with TD and 31.0±18.0 degrees of lateral rotation for subjects with CP (P=.013). Because the foot was fixed to the footplate, minimal motion was permitted in this plane.
Figure 3.
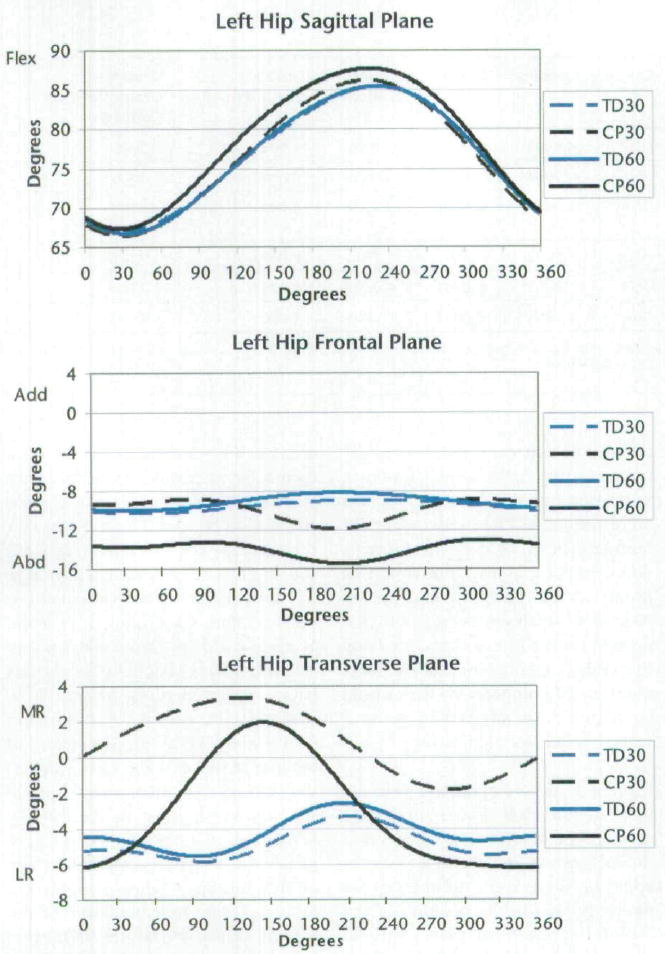
Joint kinematics of the left hip for all subjects. Zero degrees is the point at which the left crank arm was horizontal and farthest from the subject, as shown in Figure 2. Qualitative differences are evident, demonstrating the differences in magnitude and timing between groups and cadences. Subjects with cerebral palsy (CP) had greater excursions of motion in the frontal and transverse planes, greater hip flexion, greater knee extension, and greater dorsiflexion compared with the subjects with typical development (TD). The positive direction indicates flexion, adduction, and medial (internal) rotation for sagittal, frontal, and transverse planes, respectively. Flex=flexion, Add=adduction, Abd=abduction, MR=medial rotation, LR=lateral (external) rotation. TD30=subjects with TD at cadence of 30 rpm, CP30=subjects with CP at cadence of 30 rpm, TD60=subjects with TD at cadence of 60 rpm, CP60=subjects with CP at cadence of 60 rpm.
Figure 4.
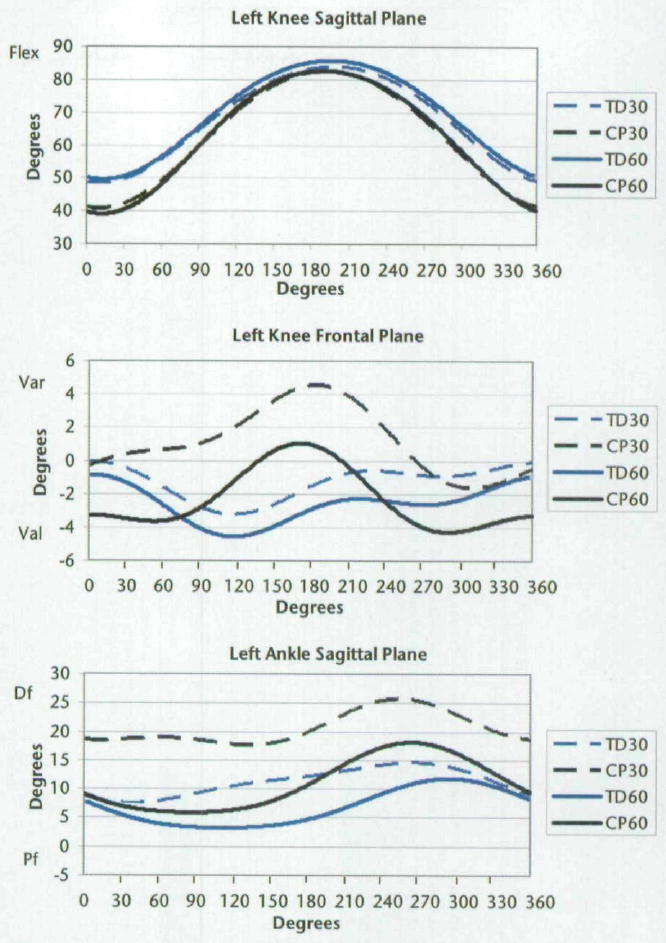
Joint kinematics of the left knee and ankle for all subjects. Zero degrees is the point at which the left crank arm was horizontal and farthest from the subject, as shown in Figure 2. Qualitative differences are evident, demonstrating the differences in magnitude and timing between groups and cadences. The positive direction indicates flexion (dorsiflexion) and varus for sagittal and frontal planes, respectively. Flex=flexion, Var=varus, Val=valgus, Df=dorsiflexion, Pf=plantar flexion. TD30=subjects with TD at cadence of 30 rpm, CP30=subjects with CP at cadence of 30 rpm, TD60=subjects with TD at cadence of 60 rpm, CP60=subjects with CP at cadence of 60 rpm.
Electromyography
Subjects with CP had earlier onsets, later offsets, and longer durations of muscle activity for some muscles of the left lower extremity compared with subjects with TD (Tab. 3). Onset, offset, and duration of muscle activity for some muscles were affected by increasing cadence, with more activity seen at 60 rpm as compared with 30 rpm. Figure 5 displays the patterns for the onsets and offsets of EMG activity for each muscle for each group at each cadence. For subjects with CP cycling at 60 rpm, average crank positions for the onsets and offsets of activity for the left lateral gastrocnemius muscle indicate that the muscle was working only briefly (Tab. 3). However, in looking at the data for each subject, the lateral gastrocnemius muscle was active for most of the cycling revolution. Therefore, the average values do not represent the activity of this muscle. Figure 5 displays a more accurate representation of the activity of the lateral gastrocnemius muscle for the subjects with CP at 60 rpm.
Table 3.
Crank Position (in Degrees) for the Onset, Offset, and Duration of Muscle Activity (Mean±Standard Deviation)a
| Muscle | Onset | Offset | Duration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TD Group at Cadence of 30 rpm | CP Group at Cadence of 30 rpm | TD Group at Cadence of 60 rpm | CP Group at Cadence of 60 rpm | TD Group at Cadence of 30 rpm | CP Group at Cadence of 30 rpm | TD Group at Cadence of 60 rpm | CP Group at Cadence of 60 rpm | TD Group at Cadence of 30 rpm | CP Group at Cadence of 30 rpm | TD Group at Cadence of 60 rpm | CP Group at Cadence of 60 rpm | |
| Left gluteus maximus | 292.0±27.0 | 6.6±27.2 | 257.1±29.5 | 206.1±86.3 | 307.3±28.1 | 9.4±33.2 | 302.9±28.7 | 324.3±100.1 | 15.4±3.6 | 210.2±183.0 | 33.0±19.6 | 179.9±131.6 |
| Left biceps femoris | 326.6±66.7 | 240.9±45.3 | 227.1±68.5 | 54.4±67.4 | 47.4±62.6 | 352.9±73.6 | 33.8±78.3 | 12.9±48.3 | 123.1±103.6 | 138.6±82.0 | 194.8±90.7 | 298.9±59.2 |
| Left rectus femoris | 153.4±25.2 | 21.0±40.0 | 131.4±40.4 | 344.6±44.8 | 239.5±47.7 | 292.7±62.1 | 231.4±43.6 | 297.2±63.6 | 84.4±54.8 | 220.8±114.9 | 105.5±70.7 | 270.5±120.5 |
| Left vastus lateralis | 157.6±37.9 | 27.6±84.2 | 147.0±31.7 | 26.4±115.7 | 244.7±28.1 | 341.7±75.1 | 253.7±43.4 | 345.3±48.9 | 87.4±47.8 | 231.0±122.5 | 108.2±62.7 | 308.5±68.3 |
| Left medial hamstrings | 290.2±29.9 | 255.5±36.2 | 268.5±39.0 | 180.5±52.9 | 106.7±52.4 | 40.8±50.8 | 96.6±50.3 | 33.9±55.8 | 170.6±63.8 | 148.3±60.6 | 187.3±70.1 | 275.5±103.0 |
| Left anterior tibialis | 104.2±81.9 | 22.1±87.1 | 99.0±89.5 | 5.7±69.8 | 199.5±53.7 | 357.9±74.0 | 198.8±82.7 | 2.5±52.1 | 94.5±78.8 | 274.5±110.2 | 109.7±78.3 | 342.2±33.2 |
| Left lateral gastrocnemius | 312.3±82.3 | 242.7±92.5 | 213.6±59.0 | 3.4±75.9 | 13.9±51.7 | 70.6±83.7 | 90.1±67.4 | 6.6±64.0 | 136.2±110.6 | 225.5±106.3 | 254.6±76.4 | 342.3±33.4 |
| Left soleus | 230.2±84.4 | 184.7±30.4 | 191.1±49.7 | 80.7±58.9 | 0.4±46.4 | 46.9±73.9 | 48.5±51.7 | 40.3±42.5 | 185.6±109.7 | 206.9±84.3 | 230.5±54.7 | 319.4±41.7 |
Zero degrees is the point at which the left crank arm was horizontal and farthest from the subject, as shown in Figure 1. Interaction effects of group and cadence were seen for the duration for the left biceps femoris muscle, the left medial hamstring muscle, and the left soleus muscle. Significance defined as P<.0031 due to 16 muscles being studied overall. Light gray=difference between groups, black=difference between cadences, dark gray=difference between groups and between cadences. CP=cerebral palsy, TD=typical development.
Figure 5.
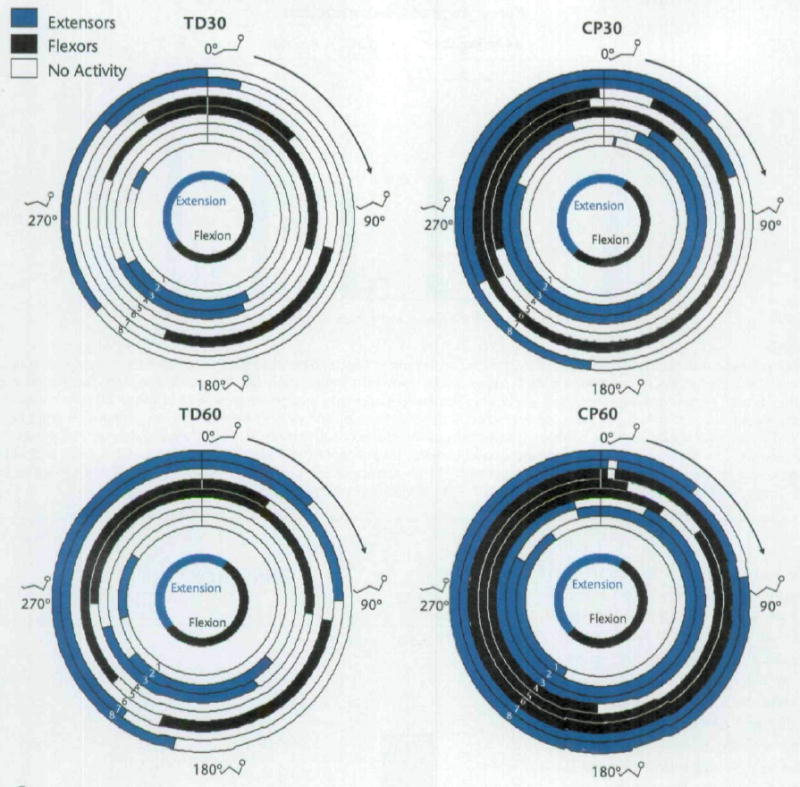
Polar plots of mean onsets and offsets of muscle activity of the left lower extremity for all subjects. Zero degrees occurred when the left crank arm was horizontal and farthest from the subject (Fig. 2). Muscles other than the gluteus maximus were labeled as primary extensors and flexors based on their actions at the knee and at the ankle. The duration of activity may differ from those shown in Table 3 because the durations here represent the difference between mean onset and mean offset. From these plots, the relationship of activity of muscles, including co-contraction, can be seen. The innermost circle represents when hip flexion and extension occurred in order to identify the phase. The stick figures show the approximate position of the lower extremities at that point in the revolution, and the arrow indicates forward movement of the crank when viewed from the right side of the cycle. 1=gluteus maximus muscle, 2=rectus femoris muscle, 3=vastus lateralis muscle, 4=medial hamstring muscle, 5=biceps femoris muscle, 6=anterior tibialis muscle, 7=lateral gastrocnemius muscle, 8=soleus muscle. TD30=subjects with typical development (TD) at cadence of 30 rpm, CP30=subjects with cerebral palsy (CP) at cadence of 30 rpm, TD60=subjects with TD at cadence of 60 rpm, CP60=subjects with CP at cadence of 60 rpm.
In the analysis of agonist and antagonist muscle co-contraction of the left lower extremity, subjects with CP had increased co-contraction for 4 out of the 6 agonist and antagonist pairings compared with subjects with TD, and co-contraction was greater when cycling at 60 rpm compared with 30 rpm for all subjects for 4 out of the 6 pairings (Fig. 6). Subjects with CP also had a greater increase in co-contraction with increasing cadence (group × cadence interaction) compared with subjects with TD for all 6 pairings. Bonferroni post hoc testing showed differences (P<.0042) in co-contraction percentage between the subjects with TD and the subjects with CP when cycling at 60 rpm for all 6 significant pairings.
Figure 6.
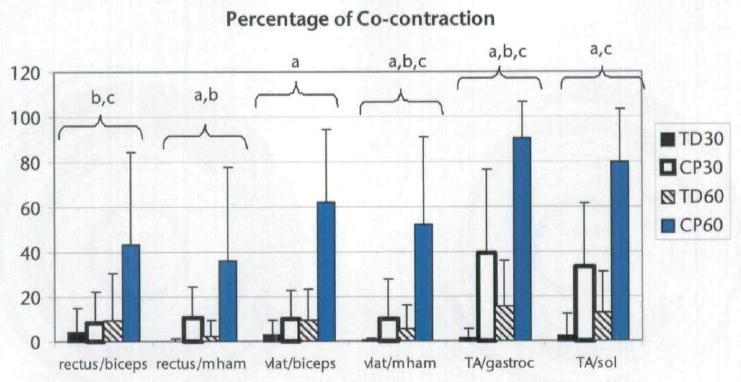
Percentage (mean and standard deviation) of the cycling revolution in which co-contraction occurred for each agonist and antagonist pairing around the left knee and ankle for each subject. Subjects with cerebral palsy had increased co-contraction compared with the subjects with typical development, and all subjects displayed increased co-contraction when cycling at 60 rpm compared with 30 rpm. Rectus=rectus femoris muscle, biceps=biceps femoris muscle, vlat=vastus lateralis muscle, mham=medial hamstring muscle, TA=anterior tibialis muscle, gastroc=gastrocnemius muscle, sol=soleus muscle. aSignificant main effect of group. bSignificant main effect of cadence. cInteraction of group and cadence. Significance defined as P<.004 due to 12 agonist and antagonist pairings being studied (6 per side). TD30=subjects with TD at cadence of 30 rpm, CP30=subjects with CP at cadence of 30 rpm, TD60=subjects with TD at cadence of 60 rpm, CP60=subjects with CP at cadence of 60 rpm.
Energy Expenditure and Gross Mechanical Efficiency
Using cadence as a covariate, efficiency was greater for the subjects with TD than for the subjects with CP (F=7.66, P=.0127) and greater for all subjects when cycling at 30 rpm compared with the attempts at 60 rpm (F=6.51, P=.0068) (Fig. 7). There was no interaction effect of group and cadence (F=0.41, P=.5288). All subjects could all cycle at 30 rpm (subjects with TD at 30.6±1.1 rpm and subjects with CP at 29.4±3.0 rpm) throughout the energy expenditure test. However, during the attempted 60-rpm test, subjects with CP were unable to maintain this cadence throughout the test. The 5 subjects with CP who attempted the energy expenditure test at 60 rpm cycled at 46.5±5.8 rpm, whereas the subjects with TD cycled at 57.9±1.2 rpm.
Figure 7.
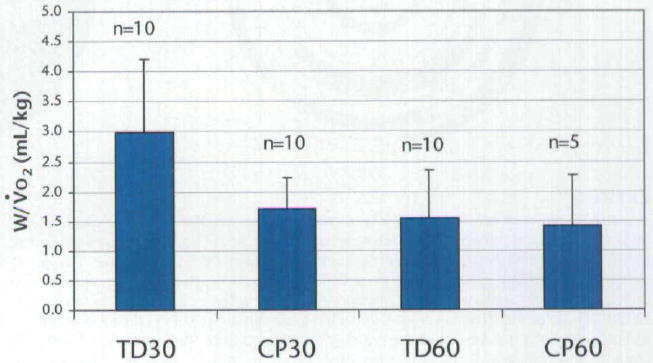
Cycling efficiency (mean and standard deviation) for all subjects. When actual cadence (rather than targeted cadence) was used as a covariate, there were significant main effects of group (P=.0127) and cadence (P=.0068); however, the interaction was not significant. V̇o2=volume of oxygen consumed, TD30=subjects with typical development (TD) at cadence of 30 rpm, CP30=subjects with cerebral palsy (CP) at cadence of 30 rpm, TD60=subjects with TD at cadence of 60 rpm, CP60=subjects with CP at cadence of 60 rpm.
There were no differences between groups (F=2.88, P=.4605) or between cadences (F=5.06, P=.1186) for perceived effort as measured with the Children’s OMNI Scale of Perceived Exertion, and there was no interaction effect (F=36.4, P=.3694). Subjects with TD reported median OMNI scores of 0 (range=0–1) and 1 (range=0–6) at 30 rpm and 60 rpm, respectively, and subjects with CP reported median OMNI scores of 1 (range=0–6) and 5 (range=2–10) at 30 rpm and the attempted 60 rpm, respectively. With the sample size and standard deviations in this study and with alpha=.05 (2-tailed) and 80% power, a mean score of 4.48 in the subjects with CP (effect size=2.38) would have been necessary to attain statistical significance in OMNI scores between groups.
Discussion
Differences were seen in cycling biomechanics between adolescents with CP and adolescents with TD. Subjects were successful with short bouts of cycling at 30 or 60 rpm in this study, and no subjects were excluded from the study due to failure to learn to cycle. It should be noted that the cycle design used in this study is different from that typically used in cycling studies, and the results may reflect this specific design. Although only the left-side data are presented, results for the right lower extremity were overall similar to those of the left lower extremity.
Kinematics
Subjects with CP displayed differences in joint kinematics around the hip, knee, and ankle in all 3 planes of motion compared with the subjects with TD. Although the findings of differences in the frontal and transverse planes were anticipated due to differences in gait kinematics in these planes,6,21,44 differences in the sagittal plane of increased hip flexion and increased knee extension for the subjects with CP were unanticipated because the positioning on the bicycle was based on anthropometrics. However, ankle dorsiflexion was increased, thus effectively shortening the limb, and therefore might have affected the position of the hip and knee in the sagittal plane. The subjects with CP may have had this increased ankle dorsiflexion due to decreased strength and motor control of the plantar flexors.
The differences in joint kinematics may have been due to decreased strength and motor control for the subjects with CP. Interestingly, the subjects with CP could physically attain the degree of hip lateral rotation achieved by the subjects with TD, demonstrating that ROM was not limiting the ability to reach this joint position. Therefore, issues such as motor control and strength may be more relevant factors. Muscles can increase or decrease the amount of rotation observed during functional movements in people with bony rotational deformities.44 Thus, muscle activity rather than rotational deformities may have led to some of the differences seen. In addition, it is possible that foot position altered the kinematics at the more proximal joints. The goal was to position the foot to accommodate deformities to allow the hip and knee to be better aligned in the sagittal plane. Because cycling occurs primarily in the sagittal plane, motion out of this plane may place additional stresses on the hip and knee joints. Further study is needed to determine the forces to which these joint are exposed.
Electromyography
The EMG patterns differed for some muscles between groups. In general, subjects with CP displayed earlier onset and later offset of muscle activity within the cycling revolution than did subjects with TD (Tab. 3, Fig. 5). These results are similar to those of Kaplan,27 who performed a more limited biomechanical study comparing cycling between children with and without CP. Again, subjects with CP may have experienced differences due to decreased strength and motor control and therefore activated as many muscles as possible to both stabilize the joints and allow movement. This pattern of increased activity may contribute to decreased efficiency and greater effort during cycling and may help to explain why some children with CP may have difficulty with the task.
There appeared to be differences in how subjects with TD and subjects with CP used their muscles while cycling. For the subjects with TD, the rectus femoris and vastus lateralis muscles appeared to act mainly as knee extensors, whereas the subjects with CP appeared to use the rectus femoris muscle for hip flexion in addition to using both muscles for knee extension. Both groups appeared to use the medial hamstring and biceps femoris muscles for a combination of hip extension and hip deceleration; however, for knee flexion, subjects with TD appeared to use the medial hamstring muscles, whereas the subjects with CP used the biceps femoris muscle.
Around the ankle, subjects with TD used the anterior tibialis muscle only during flexion, but the subjects with CP used this muscle throughout the revolution except for a brief period near the end of the extension phase. Subjects with TD used the gastrocnemius and soleus muscles primarily during the extension phase and into the flexion phase at the higher cadence, potentially as a decelerator or a knee flexor. Subjects with CP used these muscles primarily during the extension phase at 30 rpm but increased the activity to nearly continuous at 60 rpm. These differences may have been due to poor motor planning and the inability to dissociate the activity of some muscles, resulting in co-contraction of muscles around a joint. For the subjects with CP, it was difficult to determine which muscle was contributing primarily to the motion observed. In order to determine this, future work would need to examine the magnitude of EMG activity.34,36,45
Greater co-contraction of muscles around the hip, knee, and ankle was seen in subjects with CP compared with subjects with TD. Co-contraction has been reported to occur normally during cycling in both children27 and adults34 who are healthy. The increase in co-contraction for the subjects with CP may reflect an attempt to stabilize the joints while allowing movement to occur. In addition, co-contraction increased for subjects with CP when cycling at 60 rpm compared with cycling at 30 rpm to a greater extent than was seen for the subjects with TD, indicating a greater task demand and the potential need for greater stabilization of joints at the higher cadence.
In this study, onset of muscle activity was determined by EMG amplitude being at least 3 standard deviations above a quiet baseline. For most subjects, obtaining a quiet baseline was not problematic; however, a quiet baseline was unobtainable for every muscle for every subject, which may have underestimated muscle activity. In addition, subjects with CP often showed constant or almost constant activity that was 3 standard deviations above the baseline throughout the cycling revolution. In some of these subjects, phasic increases in muscle activity could be visually identified above this level of activity. Therefore, the muscle activity likely represented a combination of postural demands as well as the demands required for cycling. Future work should determine the differences between postural demands on muscles versus demands for activity.
In contrast, some subjects with CP displayed continuous, nonphasic activity throughout the cycling revolution. This finding is similar to what was described by Kaplan,27 who suggested that the coordination of the cycling pattern may be less affected by continuous activity of some muscles due to the strength and timing of the muscles that are acting phasically. However, this pattern may be relatively inefficient for cycling.
In addition, subjects with CP in the present study displayed co-contraction around joints almost continuously for some muscle combinations, especially around the ankle, with a greater effect at the higher cadence. The subjects with CP may have been using co-contraction to stabilize the foot and ankle. Minimal dorsiflexion and plantar-flexion movement was noted for both groups. It is possible that the pedal design used in this study affected ankle motion, as it was a full-length pedal in which the entire foot maintained contact with the surface. In addition, the pedal weight favored movement toward dorsiflexion, and the location of the pedal spindle in relation to the foot differed. Perhaps greater stabilization of the foot and ankle was required by all subjects due to this pedal design, because subjects with TD displayed greater co-contraction around the ankle than that reported in the literature for cycling in adults who are healthy.34 However, Kaplan27 reported similar percentages of co-contraction around the ankle in children with TD, as was seen with the subjects with TD in this study. Subjects in this study were adolescents, so it is unknown whether co-contraction is related to an immature system or to pedal design. As gait matures before adolescence,46 it would be anticipated that adolescents would behave more like adults while cycling, which requires a similar repetitive task but with more constraints to movement than with gait.
Efficiency
Subjects with TD cycled more efficiently than did the subjects with CP, and all subjects were more efficient when cycling at 30 rpm compared with cycling when attempting 60 rpm. Increased viscous resistance to motion of contracting muscle filaments47 as well as failure of a muscle to relax between contractions could potentially contribute to an increased metabolic cost of cycling.48 These issues may have contributed to a decreased efficiency for the subjects with CP due to increased metabolic demand. In addition, spasticity can increase the energy demand due to involuntary movements, the need to fight the spasticity in order to move, and the need to stabilize the body on a cycle.15 Subjects with CP in the present study displayed greater muscle co-contraction than subjects with TD, which may have contributed to the decrease in efficiency seen in the subjects with CP.
When attempting the 60 rpm cadence, subjects with CP and with TD cycled with fairly comparable efficiency (1.4±0.9 and 1.6±0.8 W/V̇o2, respectively) when directly comparing the values. However, although the targeted cadence for each group was the same (ie, 60 rpm), subjects with CP had difficulty attaining and maintaining this cadence during the 5-minute cycling test, despite being able to achieve this cadence during the short trials in the motion analysis laboratory. In addition, efficiency declines with increasing cycling cadence due to the linearly or exponentially increased demand for oxygen.49-51 Therefore, subjects with CP in the present study may have cycled less efficiently if they had been able to cycle at 60 rpm during the 5-minute cycling test.
No differences were seen between groups or cadences in the perception of effort as measured by the Children’s OMNI Scale of Perceived Exertion. Unexpectedly, several subjects with CP reported a low perception of effort using the OMNI scale during the 5-minute cycling test. Seven of the 10 subjects with CP reported an OMNI score of 2 or less (“a little tired” or less) for the 30 rpm test, and 2 out of 6 subjects reported this score for the attempted 60 rpm test. There was variability among the other subjects, with scores of 3, 5, and 6 for the remaining 3 subjects at the 30 rpm test and scores of 5, 6, and 10 for the remaining 4 subjects during the attempted 60 rpm test. This variability was unanticipated due to the similar task demand among subjects. As the resistance provided was based on body weight, basing the resistance on lean body mass or muscle volume may have been more appropriate due to potential differences in the proportion of fat and lean tissue among subjects. All subjects with TD reported OMNI scores of 0 or 1 at 30 rpm. There was greater variability at 60 rpm, with 6 of the 10 subjects reporting scores of 0 or 1, and the remaining subjects reporting scores of 3, 4, 5, and 6.
Training and Resistance
In this study, all subjects received short training sessions, with a maximum of 2 sessions required to accomplish the task demands. Because the task and cycle design were novel, increased practice may have led to differences in cycling patterns, including joint kinematics, muscle activity, co-contraction, and efficiency. With practice, co-contraction may have decreased in all subjects, potentially leading to increased efficiency. Future work should examine the changes seen with increased practice.
Resistance in this study was based on each subject’s body weight in order to allow comparison between groups. However, this resistance may have been too great for the subjects with CP. Future work should examine cycling biomechanics at differing levels of resistance to determine its effects. Literature on cycling in adults who are healthy has shown differences in cycling efficiency50 and EMG patterns52 with differing workloads, and similar effects may be seen in subjects with CP.
Clinical Relevance
As differences were seen in kinematics, EMG, and efficiency, cycling interventions may provide different benefits for adolescents with CP compared with adolescents with TD. Further research is needed to study specific outcomes, including strengthening and cardiovascular improvements. Based on the results of this study, adolescents with CP may not be able to cycle for longer periods of time due to inefficiency of the task. Although this may lead to higher heart rates, which are desirable for cardiovascular benefits, it may lead to early fatigue as well as dissatisfaction with cycling as a mode of exercise.
In addition, some adolescents in this study were not able to achieve cadences higher than 30 rpm. Cycling at this low cadence may prevent the attainment of a heart rate high enough to achieve the cardiovascular benefits of exercise. Altered kinematics, prolonged muscle activity, and co-contraction likely interfered with the ability to cycle efficiently, and methods to encourage appropriate muscle activity may be beneficial. Extended training and cues such as “push out” at the appropriate time may lead to improvements in cycling. Another possibility is the use of electrical stimulation both as a way of providing information on appropriate muscle timing as well as to activate muscles to achieve a higher cadence. Further research is needed to determine whether techniques such as these can be successful.
Conclusions
During cycling, adolescents with CP displayed differences in joint kinematics in all 3 planes, altered muscle activation patterns, and increased co-contraction compared with their peers with TD, all of which may have contributed to the decreased cycling efficiency seen in the subjects with CP. Many of the differences seen may be due to issues such as decreased strength and motor control for the subjects with CP. The information from this study can assist in the development of future intervention studies, which should examine whether a cycling intervention can lead to improvements in strength and cardiovascular conditioning in adolescents with CP.
Acknowledgments
This work was completed in partial fulfillment of the requirements for Dr Johnston’s doctoral degree at Temple University.
All authors provided concept/idea/research design and project management. Dr Johnston and Dr Barr provided writing and data analysis. Dr Johnston and Dr Lee provided data collection and fund procurement. Dr Johnston provided subjects. Dr Lee provided facilities/equipment. Dr Barr and Dr Lee provided institutional liaisons and consultation (including review of manuscript before submission). Dr Johnston acknowledges her doctoral dissertation committee members Kim Nixon-Cave, PT, PhD, and Brian Clark, PhD, as well as the following people from Shriners Hospitals for Children who assisted in many different ways: Carrie Stackhouse, MS, Emily Slater, MS, Richard Lauer, PhD, Brian Smith, MS, James McCarthy, MD, Kyle Watson, PT, DPT, and Patricia Shewokis, PhD (also at Drexel University). The authors also acknowledge John Gaughan, PhD, of Temple University, who assisted with the statistical analysis.
This project received funding from Shriners Hospitals for Children (grant 8530) and from a Clinical Research Grant from the Pediatric Section of the American Physical Therapy Association. Dr Lee also was supported by National Institutes of Health grant HD043859.
Footnotes
Restorative-Therapies Inc, 907 S Lakewood St, Baltimore, MD 21224.
Kaye Products, 535 Dimmocks Mill Rd, Hillsborough, NC 27278.
Velcro USA Inc, 406 Brown Ave, Manchester, NH 03103.
Vicon Motion Systems, 9 Spectrum Pointe Dr, Lake Forest, CA 92630.
US Digital Corp, 1400 NE 136th Ave, Vancouver, WA 98684.
The MathWorks Inc, 3 Apple Hill Dr, Natick, MA 01760-2098.
Motion Lab Systems, 15045 Old Hammond Hwy, Baton Rouge, LA 70816-1244.
SensorMedics Corp, 22745 Savi Ranch Pky, Yorba Linda, CA 92887.
Hans Rudolph Inc, 7200 Wyandotte, Kansas, MO 64114.
Computing Services, 85 Nant-y-Felin Pentraeth, Isle of Anglesey, LL75 8UY, Wales, United Kingdom.
Contributor Information
Therese E Johnston, Shriners Hospitals for Children, 3551 N Broad St, Philadelphia, PA 19140 (USA).
Ann E Barr, College of Health Professions, Temple University, Philadelphia, Pa.
Samuel CK Lee, Department of Physical Therapy, University of Delaware, Newark, Del, and Research Associate, Shriner’s Hospitals for Children.
References
- 1.Damiano DL, Martellotta TL, Quinlivan JM, Abel MF. Deficits in eccentric versus concentric torque in children with spastic cerebral palsy. Med Sci Sports Exerc. 2001;33:117–122. doi: 10.1097/00005768-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Burtner PA, Qualls C, Woollacott MH. Muscle activation characteristics of stance balance control in children with spastic cerebral palsy. Gait Posture. 1998;8:163–174. doi: 10.1016/s0966-6362(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 3.Bleck EE. Orthopedic Management in Cerebral Palsy. London, United Kingdom: MacKeith Press; 1987. [Google Scholar]
- 4.Woollacott MH, Burtner P. Neural and musculoskeletal contributions to the development of stance balance control in typical children and in children with cerebral palsy. Acta Paediatr Suppl. 1996:416, 58–62. doi: 10.1111/j.1651-2227.1996.tb14279.x. [DOI] [PubMed] [Google Scholar]
- 5.Sussman MD. Crouched gait consensus. In: Sussman MD, editor. The Diplegic Child: Evaluation and Management. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 1992. pp. 337–339. [Google Scholar]
- 6.Wren TA, Rethlefsen S, Kay RM. Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery. J Pediatr Orthop. 2005;25:79–83. doi: 10.1097/00004694-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Damiano DL, Quinlivan J, Owen BF, et al. Spasticity versus strength in cerebral palsy: relationships among involuntary resistance, voluntary torque, and motor function. Eur J Neurol. 2001;8(suppl 5):40–49. doi: 10.1046/j.1468-1331.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 8.Abel MF, Damiano DL, Blanco JS, et al. Relationships among musculoskeletal impairments and functional health status in ambulatory cerebral palsy. J Pediatr Orthop. 2003;23:535–541. [PubMed] [Google Scholar]
- 9.Damiano DL, Martellotta TL, Sullivan DJ, et al. Muscle force production and functional performance in spastic cerebral palsy: relationship of co-contraction. Arch Phys Med Rehabil. 2000;81:895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- 10.Crenna P, Inverno M, Frigo C, et al. Pathophysiological profile of gait in children with cerebral palsy. Med Sport Sci. 1992;36:186–198. [Google Scholar]
- 11.Giuliani CA. Dorsal rhizotomy for children with cerebral palsy: support for concepts of motor control. Phys Ther. 1991;71:248–259. doi: 10.1093/ptj/71.3.248. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention and President’s Council on Physical Fitness and Sports. Healthy People 2010. Chapter 22. US Department of Health and Human Services; 2000. [August 24, 2006]. Physical Activity and Fitness. Available at: http://www.healthypeople.gov/Document/pdf/Volume2/22Physical.pdf. [Google Scholar]
- 13.Centers for Disease Control and Prevention and National Institute on Disability and Rehabilitation Research, US Department of Education. Healthy People 2010. Chapter 6. US Department of Health and Human Services; 2000. [August 24, 2006]. Disability and secondary conditions. Available at: www.healthypeople.gov/Document/pdf/Volume1/06Disability.pdf. [Google Scholar]
- 14.Durstine JL, Painter P, Franklin BA, et al. Physical activity for the chronically ill and disabled. Sports Med. 2000;30:207–219. doi: 10.2165/00007256-200030030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg A. Maximal aerobic capacity of young people with spastic cerebral palsy. Dev Med Child Neurol. 1978;20:205–210. doi: 10.1111/j.1469-8749.1978.tb15205.x. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg A. Longitudinal study of physical working capacity of young people with spastic cerebral palsy. Dev Med Child Neurol. 1984;26:328–334. doi: 10.1111/j.1469-8749.1984.tb04449.x. [DOI] [PubMed] [Google Scholar]
- 17.Rimmer JH. Physical fitness levels of persons with cerebral palsy. Dev Med Child Neurol. 2001;43:208–212. [PubMed] [Google Scholar]
- 18.Bottos M, Gericke C. Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol. 2003;45:786–790. doi: 10.1017/s0012162203001452. [DOI] [PubMed] [Google Scholar]
- 19.Gajdosik CG, Cicirello N. Secondary conditions of the musculoskeletal system in adolescents and adults with cerebral palsy. Physical & Occupational Therapy in Pediatrics. 2001;21:49–68. doi: 10.1300/j006v21n04_04. [DOI] [PubMed] [Google Scholar]
- 20.Damiano DL, Vaughan CL, Abel MF. Muscle response to heavy resistance exercise in children with spastic cerebral palsy. Dev Med Child Neurol. 1995;37:731–739. doi: 10.1111/j.1469-8749.1995.tb15019.x. [DOI] [PubMed] [Google Scholar]
- 21.Bell KJ, Ounpuu S, DeLuca PA, Romness MJ. Natural progression of gait in children with cerebral palsy. J Pediatr Orthop. 2002;22:677–682. [PubMed] [Google Scholar]
- 22.Bottos M, Feliciangeli A, Sciuto L, et al. Functional status of adults with cerebral palsy and implications for treatment of children. Dev Med Child Neurol. 2001;43:516–528. doi: 10.1017/s0012162201000950. [DOI] [PubMed] [Google Scholar]
- 23.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 24.Gregor RJ, Fowler E. Biomechanics of cycling. In: Zachazewski JE, Magee DJ, Quillen WS, editors. Athletic Injuries and Rehabilitation. Philadelphia, Pa: WB Saunders Co; 1996. pp. 367–388. [Google Scholar]
- 25.Bloswick DS, King EM, Brown D, et al. Evaluation of a device to exercise hip extensor muscles in children with cerebral palsy: a clinical and field study. Assist Technol. 1994;6:147–151. doi: 10.1080/10400435.1994.10132239. [DOI] [PubMed] [Google Scholar]
- 26.Physical Therapy Clinical Research Network. [August 24, 2006]; Available at: http://pt.usc.edu/clinresnet/index.html.
- 27.Kaplan SL. Cycling patterns in children with and without cerebral palsy. Dev Med Child Neurol. 1995;37:620–630. doi: 10.1111/j.1469-8749.1995.tb12050.x. [DOI] [PubMed] [Google Scholar]
- 28.Eston RG, Brodie DA. Responses to arm and leg ergometry. Br J Sports Med. 1986;20:4–6. doi: 10.1136/bjsm.20.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford SM. Anthropometry. In: Docherty D, editor. Measurement in Pediatric Exercise Science. Champaign, Ill: Human Kinetics Inc; 1996. pp. 17–86. [Google Scholar]
- 30.Chad KE, McKay HA, Zello GA, et al. Body composition in nutritionally adequate ambulatory and non-ambulatory children with cerebral palsy and a healthy reference group. Dev Med Child Neurol. 2000;42:334–339. doi: 10.1017/s001216220000058x. [DOI] [PubMed] [Google Scholar]
- 31.Ericson MO, Nisell R, Arborelius UP, Ekholm J. Muscular activity during ergometer cycling. Scand J Rehabil Med. 1985;17:53–61. [PubMed] [Google Scholar]
- 32.Dore E, Bedu M, Franca NM, et al. Testing peak cycling performance: effects of braking force during growth. Med Sci Sports Exerc. 2000;32:493–498. doi: 10.1097/00005768-200002000-00035. [DOI] [PubMed] [Google Scholar]
- 33.Davis RB, Ounpuu S, Tyisurski D, Gage JR. A gait analysis data collection and reduction technique. Hum Move Sci. 1991;20:575–587. [Google Scholar]
- 34.Jorge M, Hull ML. Analysis of EMG measurements during bicycle pedalling. J Biomech. 1986;19:683–694. doi: 10.1016/0021-9290(86)90192-2. [DOI] [PubMed] [Google Scholar]
- 35.Raasch CC, Zajac FE. Locomotor strategy for pedaling: muscle groups and biomechanical functions. J Neurophysiol. 1999;82:515–525. doi: 10.1152/jn.1999.82.2.515. [DOI] [PubMed] [Google Scholar]
- 36.Ericson MO. Muscular function during ergometer cycling. Scand J Rehabil Med. 1988;20:35–41. [PubMed] [Google Scholar]
- 37.Di Fabio RP. Reliability of computerized surface electromyography for determining the onset of muscle activity. Phys Ther. 1987;67:43–48. doi: 10.1093/ptj/67.1.43. [DOI] [PubMed] [Google Scholar]
- 38.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101:511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 39.Robertson RJ. Perceived Exertion for Practitioners: Rating Effort With the OMNI Picture System. Champaign, Ill: Human Kinetics Inc; 2004. [Google Scholar]
- 40.Pfeiffer KA, Pivarnik JM, Womack CJ, et al. Reliability and validity of the Borg and OMNI rating of perceived exertion scales in adolescent girls. Med Sci Sports Exerc. 2002;34:2057–2061. doi: 10.1097/00005768-200212000-00029. [DOI] [PubMed] [Google Scholar]
- 41.Robertson RJ, Goss FL, Boer NF, et al. Children’s OMNI scale of perceived exertion: mixed gender and race validation. Med Sci Sports Exerc. 2000;32:452–458. doi: 10.1097/00005768-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 42.Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. American Statistician. 1981;35:124–129. [Google Scholar]
- 43.Sparto PJ, Schor RH. Directional statistics. In: Stergiou N, editor. Innovative Analysis of Human Movement: Analytical Tools for Human Movement Research. Champaign, Ill: Human Kinetics Inc; 2004. pp. 121–161. [Google Scholar]
- 44.Aktas S, Aiona MD, Orendurff M. Evaluation of rotational gait abnormality in the patients cerebral palsy. J Pediatr Orthop. 2000;20:217–220. [PubMed] [Google Scholar]
- 45.Hakansson NA, Hull ML. Functional roles of the leg muscles when pedaling in the recumbent versus the upright position. J Biomech Eng. 2005;127:301–310. doi: 10.1115/1.1865192. [DOI] [PubMed] [Google Scholar]
- 46.Sutherland DH, Olshen R, Cooper L, Woo SL. The development of mature gait. J Bone Joint Surg Am. 1980;62:336–353. [PubMed] [Google Scholar]
- 47.Elliott GF, Worthington CR. Muscle contraction: viscous-like frictional forces and the impulsive model. Int J Biol Macromol. 2001;29:213–218. doi: 10.1016/s0141-8130(01)00163-5. [DOI] [PubMed] [Google Scholar]
- 48.McDaniel J, Durstine JL, Hand GA, Martin JC. Determinants of metabolic cost during submaximal cycling. J Appl Physiol. 2002;93:823–828. doi: 10.1152/japplphysiol.00982.2001. [DOI] [PubMed] [Google Scholar]
- 49.Takaishi T, Yasuda Y, Ono T, Moritani T. Optimal pedaling rate estimated from neuromuscular fatigue for cyclists. Med Sci Sports Exerc. 1996;28:1492–1497. doi: 10.1097/00005768-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Hansen EA, Jorgensen LV, Jensen K, et al. Crank inertial load affects freely chosen pedal rate during cycling. J Biomech. 2002;35:277–285. doi: 10.1016/s0021-9290(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 51.Gregor RJ, Rugg SG. Effects of saddle height and pedaling cadence on power output and efficiency. In: Burke ER, editor. Science of Cycling. Champaign, Ill: Human Kinetics Inc; 1986. pp. 69–90. [Google Scholar]
- 52.Baum BS, Li L. Lower extremity muscle activities during cycling are influenced by load and frequency. J Electromyogr Kinesiol. 2003;13:181–190. doi: 10.1016/s1050-6411(02)00110-4. [DOI] [PubMed] [Google Scholar]


