Abstract
PGP9.5 is a controversial molecule from an oncologic point of view. We recently identified frequent methylation of PGP9.5 gene exclusively in primary head and neck squamous cell carcinoma (HNSCC), suggesting that it could be a tumor suppressor gene. On the other hand, PGP9.5 was reported to be overexpressed in a subset of human cancers presumably due to intrinsic oncogenic properties or as a result of transformation. To demonstrate that PGP9.5 possesses tumor suppressive activity, we examined forced expression by stable transfection of PGP9.5 in 4 HNSCC cell lines. Although all 4 cell lines demonstrated reduced log growth rates in culture after transfection, only 2 cell lines with wild type p53 (011, 022) demonstrated decreased growth in soft agar. In 2 cell lines with mutant p53 (013, 019), we observed no altered growth in soft agar and increased sensitivity to UV irradiation. We then tested for and found a high frequency of promoter methylation in a larger panel of primary tumors including HNSCC, esophageal SCC, gastric, lung, prostate and hepatocellular carcinoma. Our data support the notion that PGP9.5 is a tumor suppressor gene that is inactivated by promoter methylation or gene deletion in several types of human cancers.
Keywords: PTP9.5, cancer, methylation, tumor suppressor gene, tumorigenesis, apoptosis
DNA promoter hypermethylation is a common feature of human cancers.1 Moreover, genes showing cancer-specific methylation generally demonstrate tumor suppressive activity when unmethylated. Using our approach based on pharmacological unmasking and microarray hybridization, we identified several reactivated genes including PGP9.5.2,3 We confirmed cancer-specific PGP9.5 hypermethylation in 60% of primary head and neck squamous cell carcinomas (HNSCC). Pancreatic carcinoma also harbored frequent promoter hypermethylation of PGP9.5 in a cancer-specific manner.4 Moreover, PGP9.5 hypermethylation was demonstrated to be significantly associated with lymph node metastasis and patient prognosis in esophageal SCC (ESCC) by quantitative analysis.5 These observations suggest that PGP9.5 may be a tumor suppressor gene commonly inactivated in various cancers.
On the other hand, PGP9.5 has been reported to be overexpressed in various human cancers including SCC,6–8 and its expression seems to correlate with malignant behavior. For example, in non small cell lung carcinoma (NSCLC), PGP9.5 was identified as overexpressed by SAGE analysis.9,10 The promoter region of PGP9.5 was not methylated in the 3 NSCLC cell lines, from which the preliminary SAGE tags were generated.11 Discrepant data between epigenetic status and expression profiles in primary cancers led us to examine whether PGP9.5 possesses tumor suppressive properties. PGP9.5 is a neuron-specific protein with opposing biologic functions, functioning as both a ubiquitin carboxyl-terminal hydrolase and ligase,12,13 however little is known about the functional role of PGP9.5 in cancer. We found that PGP9.5 was hypermethylated in several types of cancers and demonstrated tumor suppressive activity in HNSCC cell lines.
Material and methods
Development of stable clones with PGP9.5 overexpression in HNSCC cell lines
A full-length cDNA of PGP9.5 was isolated from HNSCC cell line 028 using PCR with the primer sets 5′-AAAGCTTCCACCATGCAGCTCAAGCCGATGGAGATCA-3′ and 5′-CTCTAGAGGCTGCCTTGCAGAGAGCCACGGCA-3′. The restricted PCR products were ligated into p3xFLAG-CMV within the Hind III-Xba I sites (Sigma, St. Louis, MO). The sequence of the cloned PGP9.5 cDNA was verified by sequence analysis. Using this vector, we developed stable transfectants in 4 HNSCC cell lines (011,012,013,019) in which PGP9.5 mRNA was not constitutively expressed.3 We performed dot blot analysis for 20 clones resistant to G418 (600 µg/ml: Clontech, Palo Alto, CA) in each cell line using anti-mouse Flag antibody M2 and picked up clones with the most abundant expression of Flag-tagged PGP9.5 and finally confirmed by western blot analysis with M2 antibody (Fig. 1a). We used dot-blot-negative clones as negatively-transfected cells and confirmed that they did not express PGP9.5 (Fig. 1a). Cells were suspended in 200 µl of RIPA buffer (50 mM Tris-HCl, pH 7.5/150 mM NaCl/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS) containing 1 mM PMSF. The lysate was applied as described in the standard western blotting protocol.14
FIGURE 1.
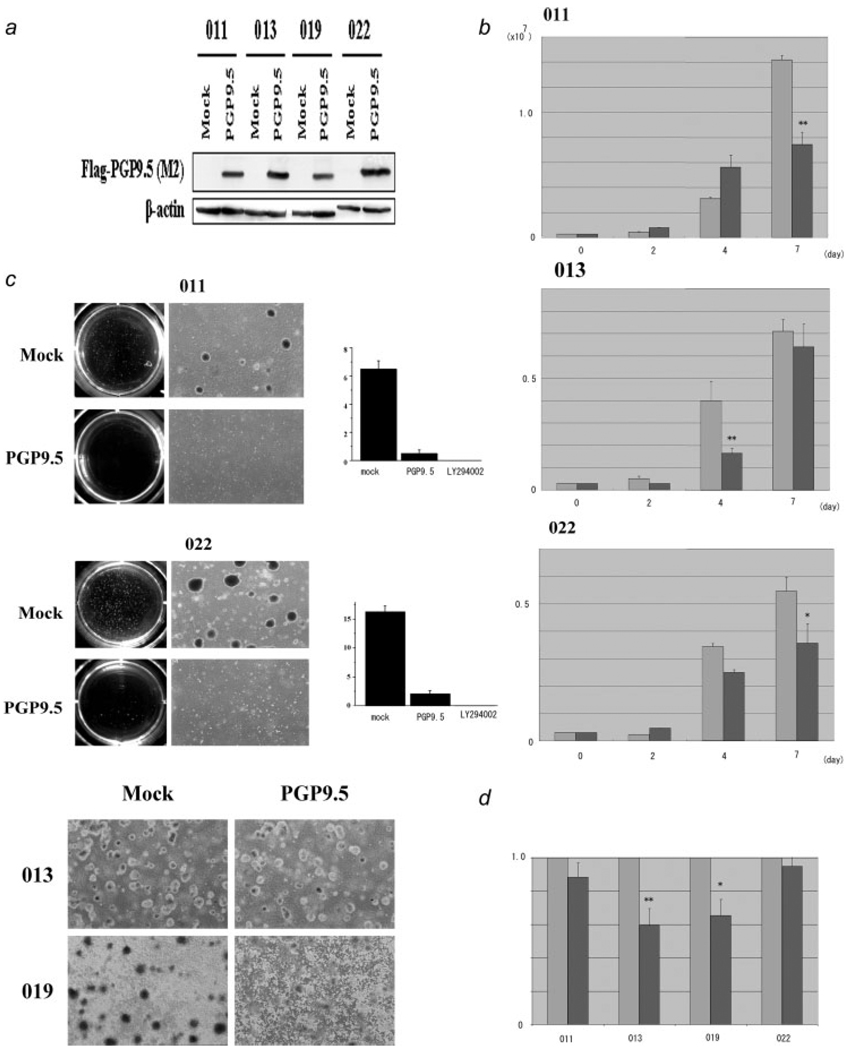
Tumor suppressor activity in PGP9.5 transfectants. (a) Western blot analysis in stable transfectants of PGP9.5. Mock cells were selected after transfection of control vector as described in Methods. Exogenous expression of PGP9.5 in 4 kinds of HNSCC cell lines (011, 013, 019 and 022) was detected by M2 antibody. β-actin was used as a loading control on the same membrane after removing M2 antibody with distilled water. (b) Proliferation in PGP9.5 transfectants and controls. Cell number was evaluated 2, 4, and 7 days after split. All transfected cell lines shown in this figure (011,013,022) showed a significant difference in proliferation rate in tissue culture. Mock-transfected cells (thin-colored box), and PGP9.5-transfected cells (thick-colored box) *p < 0.05, **p < 0.01. (c) Colony formation assay in soft agar for HNSCC cell lines with (PGP9.5) or without PGP9.5 (Mock). Both 011 and 022 showed a dramatic suppression for PGP9.5 stable clones in soft agar (Top and Middle panels). On the other hand, no suppression in soft agar was observed in the 013 and 019 PGP9.5 transfectants (Bottom panel). We also included the effects of PI3 kinase inhibitor (LY294002) in both 011 and 022 cell lines. (d) MTT analysis after UV irradiation. The amount of crystal violet dye determined by absorbance at 540 nm in Mock cells was defined as 1.0 to compare with PGP9.5 transfectants. Both 013 and 019 PGP9.5 clones showed approximately a 40% reduction of this value, suggesting increased sensitivity, while both 011 and 022 PGP9.5 transfectants remained resistant to UV irradiation. Mock-transfected cells (thin-colored box), and PGP9.5-transfected cells (thick-colored box) *p < 0.05, **p < 0.01.
Anchorage-independent colony formation analysis in soft agar
Anchorage-independent cell growth was analyzed by plating 0.36% top agarose (Bacto Agar: Becton, Dickinson, Sparks, MD) containing 1 × 105 cells mixed with corresponding medium (RPMI 1640) with 10% fetal bovine on a surface of 0.72% bottom agarose mixed with RPMI 1640 with 10% FBS in 6-well plates at 37°C. Cells were fed weekly by overlaying with fresh soft agar solution, and colonies were photographed and counted under a light microscope after 3 weeks of incubation. Each experiment was performed in triplicate wells and repeated 3 times.
MTT analysis after UV irradiation
Cell viability after UV irradiation was determined by the MTT assay. 011, 013, 019 and 022 HNSCC cell lines with or without PGP9.5 were grown at a density of 1 × 104 cells/well. Cells were grown in RPMI1640 containing 10% FBS on 24-well culture plates and incubated for 24 hr followed by UV irradiation (5 mJ/cm2) without medium using a UV STRATALINKER 1800 (Stratagene, La Jolla, CA). The medium was then replaced with 0.5 ml of RPMI1640 with 10% FBS medium. Twenty four hours after splitting, the medium was aspirated and the MTT assay was performed as previously described.15 The amount of uptake of crystal violet dye was determined by absorbance at 540 nm using plate reader AD340 (BECKMAN COULTER, Fullerton, CA). Each sample was assayed in triplicate, and each experiment was repeated twice.
Cell proliferation analysis
The cells were split at a density of 3 × 105 cells/well in 6-well plates. The medium was changed at 2, 4 and 7 day intervals after splitting. The cells were trypsinized to detach the cells and the cells were then collected and counted. Each experiment was performed in triplicate.
Cell lines and tissue samples
Six HNSCC cell lines, 011, 012, 013, 019, 022 and 028 were established in Johns Hopkins University, Department of Otolaryngology-Head and Neck Surgery (Baltimore, MD). Seven ESCC cell lines (KYSE30, KYSE70, KYSE140, KYSE150, KYSE200, KYSE410 and KYSE520) were kindly provided by Dr. Shimada of the Department of Surgery and Surgical Basic Science, Graduate School of Medicine, Kyoto University (Kyoto, Japan). Three ESCC cell lines (TE3, TE4 and TE5) and 6 gastric cancer cell lines (NUGC3, NUGC4, KATOIII, MKN1, MKN7, AZ521) were obtained from the Cell Response Center for Biomedical Research Institute of the Department of Aging and Cancer, Tohoku University (Sendai, Japan) and other cell lines were cultured according to ATCC recommendations. HNSCC, ESCC and gastric cancer cell lines were grown in RPMI 1640 media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), and other cell lines were grown in specific conditions according to ATCC recommendations.
All primary cancers and normal tissues (except primary ESCC and gastric cancers) were obtained from surgical specimens resected at the Johns Hopkins University Hospital. ESCC and gastric cancer and their paired normal mucosa specimens were obtained from patients who underwent surgery at the Medical Institute of Bioregulation Hospital, Kyushu University as previously described.2
Reverse transcription polymerase chain reaction analysis for cell lines after 5-aza-2′-deoxycytidine and trichostatin A treatment
Cells were split to low density (1 × 106 per T-75 flask) 12–24 hr before treatment. Cells were then treated for 4 days with 5 µM 5Aza-dC (Sigma, St. Louis, MO) dissolved in 50% acetic acid or were mock-treated with phosphate buffered saline (PBS) including the same amount of acetic acid. As indicated, for some cell lines trichostatin A was used to inhibit hypoacetylations as performed previously.2,3 Reverse transcription polymerase chain reaction (RT-PCR) conditions for PGP9.5 were previously described.3
Sequencing and real-time quantitative PCR for bisulfite treated DNA
DNA was prepared and the primers for sequencing were designed to recognize the DNA sequence alterations caused by bisulfite treatment as previously described.2,3 For TaqMan MSP, the fluorescent probe and primers were designed to hybridize to the amplified region of DNA.5 The β-actin primer sequences were used as an internal control and previously described.3 For all reactions, 3 µl of bisulfite-treated DNA was added to a final volume of 20 µl. Serial dilutions of human leukocyte DNA, methylated in vitro were used to construct a calibration curve and all reactions were performed in duplicate. The methylation ratio was defined as the quantity of fluorescence intensity derived from PGP9.5 promoter amplification divided by fluorescence intensity from β-actin amplification, multiplied by 100 (we designated this value as the TaqMan methylation value: TaqMeth V).
Results
Evaluation of tumor suppressor activity of PGP9.5 using stable clones with 3xFlag-tagged
We first compared growth ability in culture between PGP9.5 and mock clones. All 4 cell lines with PGP9.5 overexpression showed reduced log phase proliferation on plastic culture plates (Fig. 1b, not shown for 019). Expression levels of PGP9.5 in transfectants were similar to those in other HNSCC cell lines (028 or 012), which constitutively expressed PGP9.5 mRNA (data not shown), hence indicating that PGP9.5 transfectants made exogenous mRNA to a level similar to actual tumors. On the other hand, PGP9.5 expression in normal tissues varied (data not shown) to such an extent that we could not establish a physiological expression level of PGP9.5. We then performed anchorage-independent colony formation analysis in soft agar with 4 HNSCC cell lines with expressed PGP9.5 and controls. 011 and 022 HNSCC cells (with wild type p53) showed marked suppression of anchorage independent growth with expressed PGP9.5 (Fig. 1c), while 013 and 019 HNSCC cells (with mutant p53) were not suppressed (Fig. 1c). None of the cell lines with PGP9.5 overexpression showed augmented growth in soft agar.
Recently, PGP9.5 knockout mice (gad mice) were found to harbor cells resistant to UV irradiation associated with reduced ubiquitin accumulation.16 We therefore performed MTT analysis after UV irradiation to compare the apoptotic sensitivity of cancer cells with or without PGP9.5. All cells examined were treated with 5 µJ/cm2 UV irradiation; the number of attached cells after treatment was almost identical (data not shown). Interestingly, PGP9.5 stable transfectants in p53 mutant 013 and 019 cells, showed significantly increased apoptosis after UV irradiation in comparison to mock transfected cells. Conversely, 011 and 022 p53 wild type cells did not show any significant difference after UV irradiation between mock transfected cells and PGP9.5 (Fig. 1d).
Evaluation of PGP9.5 methylation relevance in various types of cancers
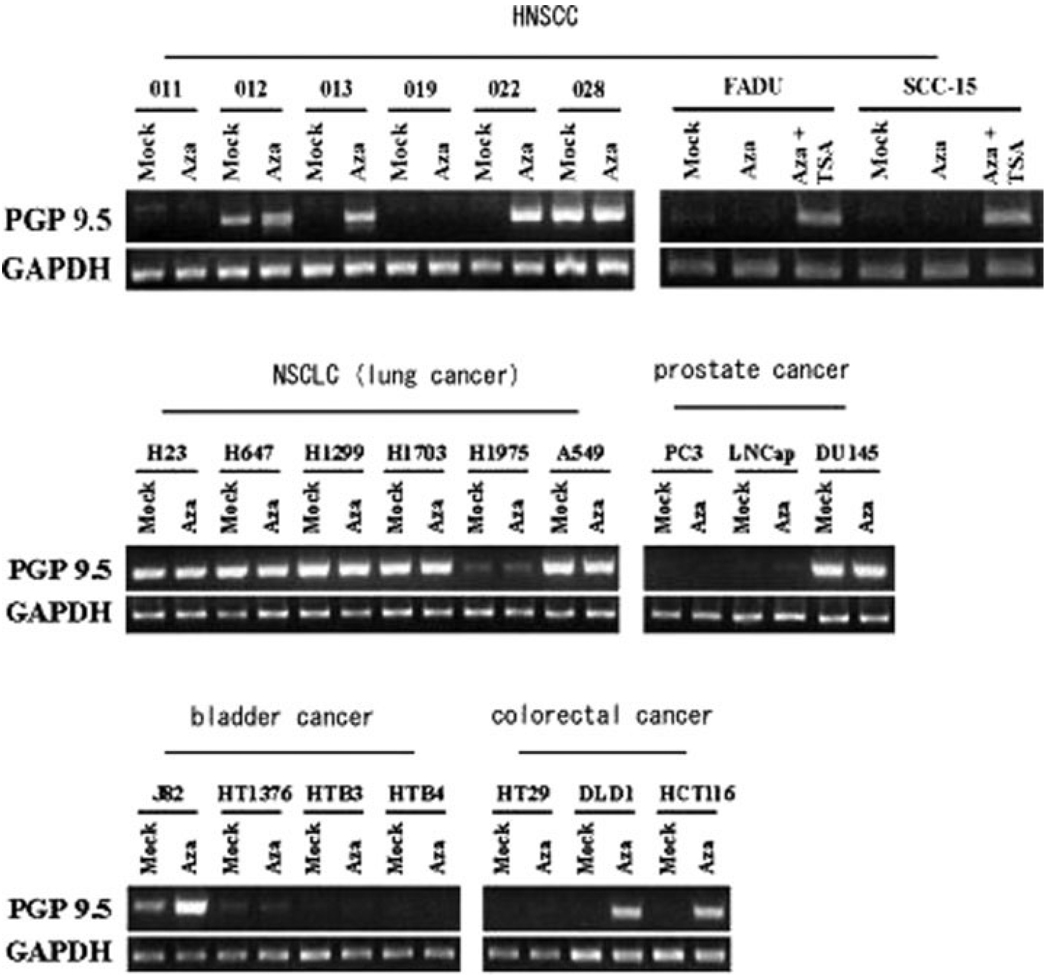
We then evaluated the methylation status of PGP9.5 in various cancers using cell lines and primary cancer tissue specimens. First, we checked the cytosine methylation status of the PGP9.5 promoter in various cancer cell lines by bisulfite sequencing and guanine bands representing CpG methylation were designated as “+” cases (Table I). Overall, 30 out of 46 (65%) cancer cell lines showed dense CpG promoter PGP9.5 methylation. Furthermore, RT-PCR revealed that PGP9.5 expression was completely silenced (21/24: 88%) and promptly reactivated after 5Aza-dC treatment (18/24: 75%) in methylated cell lines (Fig. 2 and Table I). On the other hand, PGP9.5 expression was abundantly found in unmethylated cell lines (14/14: 100%), and reactivation by 5-aza-2′-deoxycytidine (5 Aza-dC) was not recognized in almost such cells (13/14: 93%). Six cell lines with methylated PGP9.5 that did not change their methylated status after 5 Aza-dC treatment implies that mechanisms other than epigenetic, such as homozygous deletion, may be responsible for silencing of PGP9.5 expression. These findings confirmed that the expression of PGP9.5 was predominantly silenced by promoter hypermethylation.
TABLE I.
Methylation anaysis of PGP9.5 in various panels of human cancers
| Cell line name | Methylation status in cell line1 | PGP9.5 expression2 | Methylation status in tissue specimens3 |
p value | |||
|---|---|---|---|---|---|---|---|
| without 5Aza | with 5Aza treatment | Primary ca | Normal | ||||
| Head and neck4 | 011 | M | (−) | (−) | 46/72 (64%) | 0/11 (0%) | <0.0001 |
| 012 | M | (+) | (++) | ||||
| 013 | M | (−) | (+) | ||||
| 019 | M | (−) | (−) | ||||
| 022 | M | (−) | (+) | ||||
| 028 | U | (+) | (+) | ||||
| Fadu | M | (−) | (+) | ||||
| SCC-15 | M | (−) | (+) | ||||
| Lung | H23 | U | (+) | (+) | 6/20 (30%) | ND | ND |
| H647 | U | (+) | (+) | ||||
| H1299 | U | (+) | (+) | ||||
| H1703 | U | (+) | (+) | ||||
| H1975 | U | (+) | (+) | ||||
| A549 | U | (+) | (+) | ||||
| Prostate | PC3 | M | (−) | (−) | 11/20 (55%) | 2/10 (20%) | NS |
| LNCap | M | (−) | (+) | ||||
| DU145 | U | (+) | (+) | ||||
| Bladder | J-82 | U | (+) | (++) | ND | ND | ND |
| HT1376 | U | (+) | (+) | ||||
| HTB3 | M | (−) | (−) | ||||
| HTB4 | M | (−) | (−) | ||||
| Colon | HCT116 | M | (−) | (+) | ND | ND | ND |
| HT29 | M | (−) | (−) | ||||
| DLD1 | M | (−) | (+) | ||||
| Breast | MCF7 | M | (−) | (+) | ND | ND | ND |
| BT20 | M | (−) | (+) | ||||
| 231 | M | (−) | (+) | ||||
| 436 | U | (+) | (+) | ||||
| Hepatocellular | Hep3B | U | (+) | (+) | 5/20 (25%) | 0/10 (0%) | NS |
| HepG2 | M | (+) | (++) | ||||
| Esophageal SCC* | KY30 | M | (−) | (+) | 9/20 (45%) | 5/20 (25%) | NS |
| KY70 | U | (+) | (+) | ||||
| KY140 | U | (+) | (+) | ||||
| KY150 | M | (−) | (+) | ||||
| KY200 | M | (−) | (+) | ||||
| KY410 | M | (−) | (+) | ||||
| KY520 | M | (−) | (+) | ||||
| TE3 | M | (+) | (++) | ||||
| TE4 | M | ND | ND | ||||
| TE5 | M | ND | ND | ||||
| Gastric | NUGC3 | M | ND | ND | 14/20 (70%) | 4/10 (40%) | NS |
| NUGC4 | M | ||||||
| KATO III | M | ||||||
| MKN1 | M | ||||||
| MKN7 | U | ||||||
| AZ521 | U | ||||||
Methylation status was evaluated by TaqMan MSP as described in Results.
ND, not determined in this study; NS, not significant.
Determined by bisulfite sequenclng.
RT-PCR7.
TaqMan MSP.
These results were partially published except primary cancer analysis in previous press by Tokumaru et al. and Mandelker et al.
FIGURE 2.
Expression status of PGP9.5 in various cancer cell lines after demethylation treatment. HNSCC, NSCLC (lung cancer), prostate cancer, bladder cancer, colorectal cancer, and breast cancer showed reexpression of PGP9.5 at the mRNA level by RT-PCR. Pharmacological treatments include 5 µM Aza-dC treatment and/or trichostatin A (TSA) (only in both FADU and SCC-15).
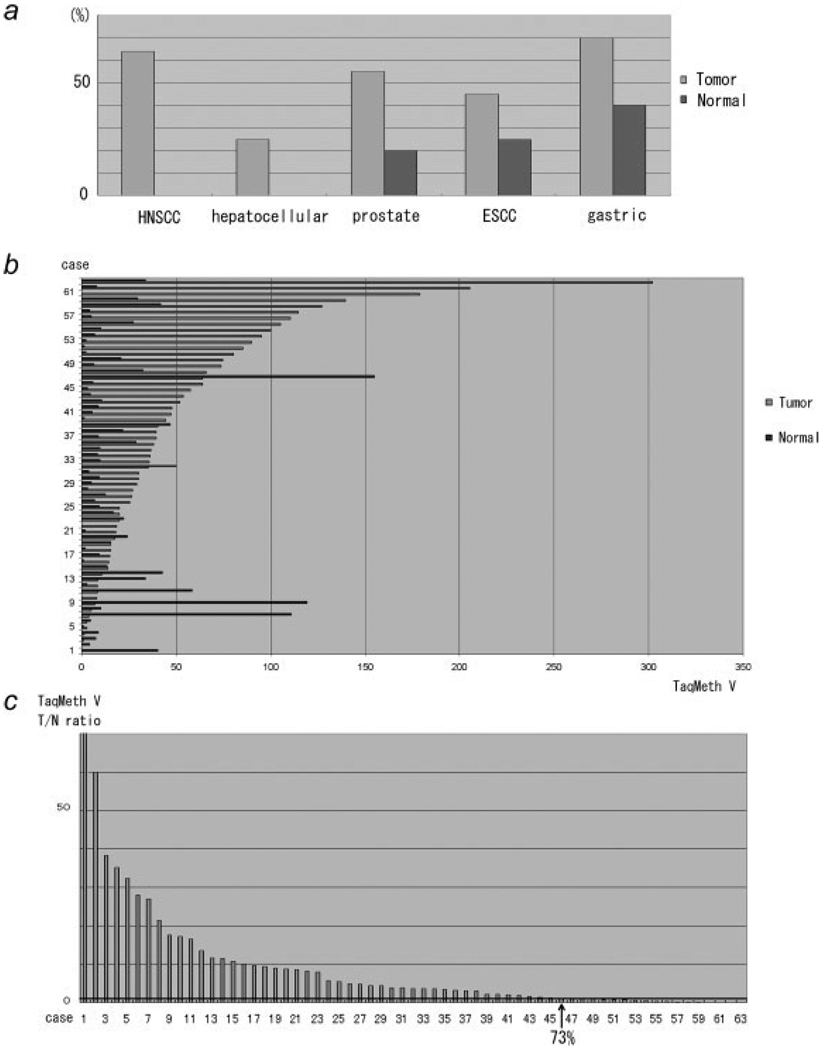
Next, we examined PGP9.5 methylation status in primary cancer tissue specimens. Using optimized real time MSP,5 PGP9.5 was methylated in 64% of head and neck cancers, 30% of lung cancers, 55% of prostate cancers, 25% of hepatocellular cancers, 45% of esophageal cancers and 70% of gastric cancer samples (Table I and Fig. 3a). We previously examined primary HNSCC and NSCLC tissues, and found no expression of PGP9.5 in a subset of cancer tissues.3,10 In addition, normal samples revealed no methylation (HNSCC and hepatocellular cancer) or quantitatively low level of methylation compared to cancer samples (prostate, esophageal and gastric cancers). There was no case with homozygous deletion because bisulfite DNA was all amplified in the tested cases. As small in number in each organ, it could not reach the significant difference of methylation level between cancer and normal tissues as in Table I. By expanding the numbers examined, we did actually and clearly showed caner-prone methylation using 50 gastric cancer samples (p < 0.001: Fig. 3b), and 73% of gastric cancer was hypermethylation in the tumor tissues (Fig. 3c).
FIGURE 3.
PGP9.5 methylation in primary cancer tissues and the corresponding normal tissues. (a) Methylation frequency of each cancer evaluated by Q-MSP as previously described.5 Methylation was defined as TaqMeth V of 20. (b) Raw data of Q-MSP results of the 50 gastric cancer cases. (c) T/N ratio of TaqMeth V was shown. Note that 73% cases showed hypermethylation in primary gastric cancer.
Discussion
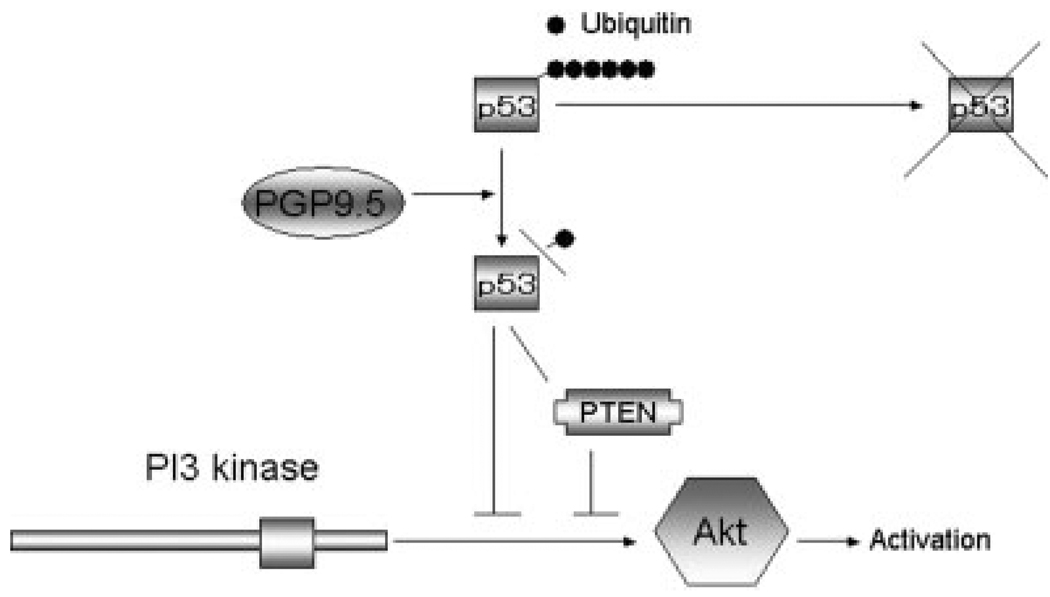
In this study, we sought to address the issue of whether PGP9.5 actually has tumor suppressor activity in human cancers. In 2 HNSCC cell lines with wild type p53, 011 and 022, PGP9.5 significantly suppressed anchorage independent growth in soft agar. Anchorage independent growth has been linked to the PI3 kinase pathway and is associated with metastatic ability of cancer cells.17 Our experiments also confirmed that all 4 HNSCC cell lines tested were inhibited by the PI3 kinase inhibitor, LY294002, completely (data not shown). Although the effect of PGP9.5 on this key pathway remains to be explored as to anchorage-independent growth ability, it is likely that p53 is a critical upstream regulator of PI3 kinase pathway through PTEN18 or other mechanism19 on the downstream of PGP9.5 in HNSCC (Fig. 4). That may be why (in p53 wild type cell lines) tumorigenesis was suppressed by PGP9.5 whereas p53 mutant cell lines activated the Akt/PI3 kinase pathway irrespective of PTEN or other mechanisms.
FIGURE 4.
Activation of p53 on Akt/PI3 kinase may be critical downstream of PGP9.5, which affects tumorigenesis. Our hypothesis is based on the notion that p53 might be a direct or indirect substrate of PGP9.5, and it is consistent with the previous report that p53 protein level was associated with PGP9.5 protein level in a stress-induced states of p53 wild type myeloma cells KMS27. PI3 kinase/Akt could be regulated by PTEN-dependent or PTEN-independent inhibition by p53,18,19 which may in turn be regulated by PGP9.5. That is why PGP9.5 induced growth suppression of p53 wild type cells (011 and 022), and the PI3 kinase pathway is constantly activated irrespective of PTEN or other mechanism in p53 mutant cells (013 and 019).
We also confirmed initial observations in mice suggesting that PGP9.5 is involved in the sensitivity to UV irradiation.16 HNSCC cell lines with mutant p53 and PGP9.5 overexpression became more sensitive to UV irradiation. On the other hand, HNSCC cell lines with wild type p53 and PGP9.5 overexpression showed the same level of sensitivity to apoptosis as compared to mock cells. A recent article on myeloma cells reported that p53 wild type KMS-27 cells were less effective in expressing PGP9.5 following stressful stimuli, compared to p53 mutant KMS-11 cells.20 These findings suggested that PGP9.5-induced apoptosis is independent of p53 differently from the issue of tumorigenesis and the pathways are more relevant in the absence of functional p53 protein. In support of this observation, PGP9.5 methylation status was independent of p53 mutational status in primary HNSCC tumors.3 In PGP9.5 knockout mice, retinal cells were resistant to apoptosis following stress, and the effect was accompanied by suppressed ubiquitin induction and subsequent accumulation of oncogenic molecules such as bcl-2 and XIAP.16 Our findings suggest that increased PGP9.5 levels are likely to suppress these oncogenes after stress, leading to the observed growth suppression and sensitivity to apoptosis. Since our results were obtained by using only a relatively few cell lines composed of 2 p53 WT cells and 2 p53 mutant cells, we could not generalize on this supposition based on the results from our study.
Initially, PGP9.5 was identified as overexpressed in NSCLC tissues by the SAGE library. Preliminary data, examining 3 NSCLC cell lines, revealed that PGP9.5 was not methylated in NSCLC11. We examined log phase growth, anchorage independent growth and apoptosis in HNSCC cell lines and found diminished growth or increased apoptosis after forced expression of PGP9.5. In all 6 NSCLC cell lines, we tested, PGP9.5 promoter methylation was absent and expression retained (Table I and Fig. 2). However, PGP9.5 showed dense methylation in 52% (91/172) of all primary cancers including NSCLC (range 25–70%). Recently, PGP9.5 methylation was found to correlate with poor patient prognosis independent of other clinicopathological factors in esophageal squamous cell carcinoma.5 Moreover, pancreatic cancers, with abysmal 5-year survival, were found to harbor methylation of PGP9.5 in 100% of tested cases.4 These findings suggested that PGP9.5 methylation may predict invasion and the presence of a more malignant clone with poor prognosis in several cancers. Hence, our work supports the notion that PGP9.5 is a tumor suppressor gene inactivated by promoter methylation in several types of human cancers.
In this study, we could not fully elucidate the clinical significance of PGP9.5 by phenotypic analysis, because PGP9.5 methylation and concomitant overexpression were recognized in primary cancers. This issue is general contradiction for methylation genes, not specific to PGP9.5. For example, p16 has been reported to be hypermethylated in human cancers, while p16 is sometimes overexpressed in a subset of cancer tissues as compared to the corresponding normal tissues (data not shown). To resolve these issues, we have to examine both methylation and expression status of PGP9.5 simultaneously in tumors through a more detailed analysis involving microdissection. It is speculated that PGP9.5 may be overexpressed by oncogenic signals in a feedback manner, and therefore, the remaining unmethylated alleles still represent PGP9.5 overexpression. Even in such a case, the relative reduction of PGP9.5 expression by incomplete methylation may play a significant role in tumor progression by tumor suppressive activity. The most interesting finding in the current study is that a gene of cancer-prone methylation again represents possession of tumor suppressor activity in human cancer, and cancer-prone methylation could be excellent landmarks of TSG.
References
- 1.Herman J, Baylin S. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita K, Upadhyay S, Osada M, Hoque MO, Xia Y, Mori M, Sato F, Meltzer SJ, Sidransky D. Pharmacological unmasking of epigenetically silenced tumor suppressor genes in esophageal sqaumous cell carcinoma. Cancer Cell. 2002;2:485–495. doi: 10.1016/s1535-6108(02)00215-5. [DOI] [PubMed] [Google Scholar]
- 3.Tokumaru Y, Yamashita K, Osada M, Nomoto S, Sun DI, Xiao Y, Hoque MO, Westra WH, Califano JA, Sidransky D. Inverse correlation between cyclin A1 hypermethylation and p53 mutation in head and neck cancer identified by reversal of epigenetic silencing. Cancer Res. 2004;64:5982–5987. doi: 10.1158/0008-5472.CAN-04-0993. [DOI] [PubMed] [Google Scholar]
- 4.Sato N, Fukushima N, Maitra A, Matsubayashi H, Yeo CJ, Cameron JL, Hruban RH, Goggins M. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–3742. [PubMed] [Google Scholar]
- 5.Mandelker D, Yamashita K, Tokumaru Y, Mimori K, Howard DL, Tanaka Y, Carvalho AL, Jiang W, Park HL, Kim MS, Osada M, Mori M, et al. PGP9.5 promoter methylation is an independent prognostic factor for esophageal squamous cell carcinoma. Cancer Res. 2005;65:4963–4968. doi: 10.1158/0008-5472.CAN-04-3923. [DOI] [PubMed] [Google Scholar]
- 6.Tezel E, Hibi K, Nagasaka T, Nakao A. PGP9.5 as a prognostic factor in pancreatic cancer. Clin Cancer Res. 2000;6:4764–4767. [PubMed] [Google Scholar]
- 7.Yamazaki T, Hibi K, Takase T, Tezel E, Nakayama H, Kasai Y, Akiyama S, Nagasaka T, Nakao A. PGP9.5 as a marker for invasive colorectal cancer. Clin Cancer Res. 2002;8:192–195. [PubMed] [Google Scholar]
- 8.Takase T, Hibi K, Yamazaki T, Nakayama H, Taguchi M, Kasai Y, Akiyama S, Nagasaka T, Nakao A. PGP9.5 overexpression in esophageal squamous cell carcinoma. Hepatogastroenterology. 2003;50:1278–1280. [PubMed] [Google Scholar]
- 9.Hibi K, Liu Q, Beaudry G, Madden SL, Westra WH, Wehage SL, Heitmiller RF, Bertelsen AH, Sidransky D, Jen J. Serial analysis of gene expression in non-small cell lung cancer. Cancer Res. 1998;58:5690–5694. [PubMed] [Google Scholar]
- 10.Hibi K, Westra W, Borges M, Goodman S, Sidransky D, Jen J. PGP9.5 as a candidate tumor marker for non-small cell lung cancer. Am J Pathol. 1999;155:711–715. doi: 10.1016/S0002-9440(10)65169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bittencourt Rosas S, Caballero O, Dong S, da Costa Carvalho G, Sidransky D, Jen J. Methylation status in the promoter region of human PGP9.5 gene in cancer and normal tissues. Cancer Lett. 2001;170:73–79. doi: 10.1016/s0304-3835(01)00449-9. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson K, Lee K, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP9.5 is a ubiquitin carboxy-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Fallon L, Lashuel H, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect α-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 14.Hibi K, Trink B, Patturajan M, Westra WH, Ceballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000;97:5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M, Lee Y, Moon E, Kim SE, Lee JJ, Kim KW. Anti-angiogenic activity of torilin, a sesquiterperne compound isolated from Torilis japonica. Int J Cancer. 2000;87:269–275. [PubMed] [Google Scholar]
- 16.Harada T, Harada C, Wang Y, Osaka H, Amanai K, Tanaka K, Takizawa S, Setsuie R, Sakurai M, Sato Y, Noda M, Sada K. Role of ubiquitin carboxy terminal hydrolase-L1 in neural cell apoptosis induced by ischemic retinal injury in vivo. Am J Pathol. 2004;164:59–64. doi: 10.1016/S0002-9440(10)63096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi K, Sakamoto M, Yasuda J, Takamura M, Fujita N, Tsuruo T, Todo S, Hirohashi S. Critical involvement of the phosphatidylinositol 3-kinase/Akt pathway in anchorage-independent growth and hematogeneous intrahepatic metastasis of liver cancer. Cancer Res. 2002;62:2971–2975. [PubMed] [Google Scholar]
- 18.Mayo L, Rok Seo Y, Jachson M, Smith ML, Rivera Guzman JR, Korgaonkar CK, Donner DB. Phosphorylation of human p53 at serine 46 determines promoter selection and whether apoptosis is attenuated or amplified. J Biol Chem. 2005;280:25953–25959. doi: 10.1074/jbc.M503026200. [DOI] [PubMed] [Google Scholar]
- 19.Singh B, Reddy P, Goberdhan A, Walsh C, Dao S, Ngai I, Chou TC, O-Charoenrat P, Levine AJ, Rao PH, Stoffel A. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuki T, Yata K, Takata-Tomokuni A, Hyoudoh F, Miura Y, Sakaguchi H, Hatayama T, Hatada S, Tsujioka T, Sato Y, Murakami H, Sadahira Y, et al. Expression of protein gene product 9.5 (PGP9.5)/ubiquitin-C-terminal hydrolase (UCHL-1) in human myeloma cells. Br J Hemat. 2004;127:292–298. doi: 10.1111/j.1365-2141.2004.05205.x. [DOI] [PubMed] [Google Scholar]