Abstract
Cerebral palsy (CP) may lead to profound weakness in affected portions of the extremities and trunk. Knowing the mechanisms underlying muscle weakness will help to better design interventions for increasing force production in children with CP. This study quantified voluntary muscle activation, contractile properties, and fatigability of the quadriceps femoris and triceps surae in children with and without CP. Twelve children with CP (7–13 years) and 10 unaffected children (controls, 8–12 years) were assessed for (1) voluntary muscle activation during maximum voluntary isometric contractions (MVICs); (2) antagonist coactivation during agonist MVICs; (3) contractile properties, and (4) fatigability using electrically elicited tests. Children with CP were significantly weaker, had lower agonist voluntary muscle activation, and greater antagonist coactivation. In children with CP, the quadriceps normalized force–frequency relationship (FFR) was shifted upward at low frequencies and was less fatigable than controls. No differences were seen between groups in the normalized FFR and fatigability of the triceps surae. In addition, no differences were seen in the sum of the time to peak tension and half-relaxation times between groups for either muscle. Because children with CP demonstrated large deficits in voluntary muscle activation, using voluntary contractions for strength training may not produce forces sufficient to induce muscle hypertrophy. Techniques such as enhanced feedback and neuromuscular electrical stimulation may be helpful for strengthening muscles that cannot be sufficiently recruited with voluntary effort.
Keywords: antagonist coactivation, cerebral palsy, contractile properties, strength, voluntary muscle activation
Low force production has been documented in children with cerebral palsy (CP) compared to unaffected children, and attributed to either incomplete recruitment or decreased motor unit discharge rates during maximum voluntary contractions.7,8,12,13,31 We are unaware, however, of published studies that have investigated whether children with CP can obtain full muscle activation, and only a few studies have examined motor unit discharge rates at low force levels.19,28 In adults with poststroke hemiparesis, the involved side demonstrates lower initial and maximal motor unit discharge rates.14,15,23 Alternative techniques to single motor unit testing, however, are necessary to look at the overall activation of whole muscle. Superimposing electrical stimulation during maximum voluntary isometric contractions is one such technique,24,30 but it has not been applied to children with CP.
Increased antagonist coactivation could also contribute to the measured deficits in force production in CP. Increased antagonist coactivation occurs in children with CP during ambulation and standing balance.2 We are aware of only two studies, however, that have quantified antagonist coactivation during maximum voluntary isometric contractions in children with and without CP.11,21 At 60° of knee flexion, children with CP had 9.0% activation of their hamstrings during isometric knee extension compared to 4.3% in unaffected children.21 The contribution of coactivation of the hamstrings during isometric knee extension to the weakness of children with CP therefore appears to be modest.
Another source of CP-related weakness may lie within the morphology of single muscle fibers and whole muscle.3,22,29 The most common findings are an increased incidence of muscle-fiber atrophy,3,29 increased intramuscular fat and connective tissue in the most involved muscle groups,3,29 and an increase in the percentage of histochemically identified type I muscle fibers,22,29 all of which may contribute to weakness.
Noninvasive techniques that test the contractile and fatigue properties of muscle using transcutaneous electrical stimulation may be used to identify whole muscle contractile and fatigue properties. Harridge and colleagues18 examined the relationship between contractile/fatigue properties and fiber-type composition of three muscles (triceps surae, quadriceps femoris, and triceps brachii) each within 7 healthy adults and found that the twitch time to peak tension, the rate of force rise to a 50-Hz train, the ratio of 20-Hz:50-Hz forces, and the fatigue index were linearly related to the percentage of type II myosin heavy chain content when the data were collapsed across muscle groups. Noninvasive electrical stimulation techniques, therefore, may be able to provide some insight into whole muscle composition.
Clinically, evidence is accumulating that high-intensity strength training can improve force production, walking velocity, and gross motor function in children with CP.5,6 Knowledge of mechanisms underlying weakness is necessary to design more effective interventions for increasing force production in such children. The purpose of this study was to examine the muscle activation, contractile properties, and fatigability of the quadriceps femoris and triceps surae muscles in children with and without spastic diplegic CP. We hypothesized that the children with spastic diplegic CP would exhibit reduced voluntary muscle activation of the agonists (quadriceps femoris and triceps surae) and increased coactivation of the antagonists (hamstrings and dorsiflexors) during maximum voluntary isometric contractions (MVICs), slower contractile properties in response to electrical stimulation, and less fatigue during an electrically elicited fatigue test.
METHODS
Subjects
Twelve children (7 boys) with spastic diplegic CP (mean age, 10.46 years; SD, 1.86 years; range, 7.46 –13.46 years) were recruited and screened by a physical therapist and orthopedic surgeon for eligibility. Inclusion criteria were as follows: (1) a diagnosis of spastic diplegic CP; (2) ability to ambulate with or without assistive devices but with limitations outdoors and in the community; (3) between the age of 7 and 12 years at time of recruitment; (4) cleared for risk of hip subluxation or dislocation; (5) less than 40° scoliosis; (6) well-controlled seizure activity or seizure free; (7) visuoperceptual skills and cognitive/communication skills sufficient to follow multiple-step commands and to attend to tasks associated with data collection; (8) more than 1 year postsurgery of either lower extremity; (9) passive range of motion of more than 20° of hip abduction, a popliteal angle of more than 45°, and at least ankle dorsiflexion to neutral (knee extended and foot in varus); and (10) less than 10° flexion contracture at the hips as measured by the Thomas test and less than 5° flexion contracture at the knee. Ten unaffected children (controls; 4 boys; mean age, 9.84 years; SD, 1.47 years; range, 8.11–12.90 years) were screened and had no known history of neurological, orthopedic, or cardiovascular disease. All children (CP and controls) and parents or guardians were informed of the purpose and experimental methods and gave written and verbal consent to be participants. The Human Subjects Review Boards of the University of Delaware and Temple University (for Shriners Hospitals for Children) approved the experimental procedures.
Experimental Procedures
Dynamometry
All testing was performed on a computer-controlled dynamometer (Kin-Com II, Chattecx Corp., Chattanooga, TN) on the same day for each subject. The right quadriceps femoris was tested in the morning, and the right triceps surae in the afternoon. For the quadriceps femoris, subjects were seated with their right leg, thigh, pelvis, and shoulders stabilized with Velcro straps and seat belts. Hips were flexed to approximately 85°, knees flexed to 60°, and subjects instructed to keep their arms folded across their chest or lap. The axis of the dynamometer was aligned with the lateral femoral condyle, and the force transducer pad was placed approximately 3.5 cm above the apex of the lateral malleolus on the anterior aspect of the leg. For the triceps surae, subjects were supine with their right foot placed in the dynamometer’s foot apparatus with the axis of the dynamometer aligned to bisect the lateral and medial malleoli. The heel was secured in the heel counter with athletic tape and the foot, heel, and lower leg were additionally stabilized with Velcro straps. The ankle was flexed to neutral for isometric testing. In addition, both set-ups allowed the children to watch videos during rest breaks and during tests when their attention was not required. These videos were used to fight boredom and to distract their attention from the stimulation.
MVIC and Voluntary Muscle Activation
Self-adhesive electrodes covered the width of the muscle being tested (quadriceps femoris or triceps surae muscles) with sizes ranging from 3.8 × 6.35 cm to 7.6 × 12.7 cm (Axelgaard, Fallbrook, CA; ConMed Corp., Utica, NY). Two hours before testing, the electrode size was selected, and a slightly larger skin area was marked with a permanent marker. An anesthetic cream (EMLA; 2.5% prilocaine, 2.5% lidocaine; Astra-Zeneca, Wilmington, DE) was applied to the area and covered with a self-adhesive occlusive dressing. This anesthetic cream was used to reduce discomfort during electrical stimulation and to reduce the possibility of reflex responses. After approximately 2 h, the cream was removed and the skin cleansed with alcohol prior to electrode placement. Electrodes were placed transversely across the width of the proximal and distal quadriceps femoris. For the triceps surae, electrodes were placed across the width of the proximal portion of the medial and lateral gastrocnemius muscles just below the knee joint line, and longitudinally over the distal portion of the soleus superior to the Achilles tendon. The quadriceps and triceps muscles were stimulated with a Grass Instruments S88 stimulator equipped with a Grass model SIU8T stimulus isolation unit (West Warwick, RI). Force data during MVIC testing were sampled at 2000 Hz and analyzed with custom-written software (LabView, 4.0.1, Austin, TX).
Once positioned on the dynamometer, subjects performed a submaximal knee extension or ankle plantar flexion isometric contraction, rested for approximately 2 min, and then performed a practice MVIC. After another rest for 2 min, two each of either knee flexion or ankle dorsiflexion MVICs were performed for normalization of the electromyogram (EMG) of the antagonist (see below). Next, the electrical stimulation intensity used for testing voluntary muscle activation was set using a 13-pulse, 100-Hz electrical train (each pulse was 600 µs in duration) by increasing the output in 5–10 V increments, until a force plateau was achieved. We termed this stimulus the maximum burst. On the dynamometer’s feedback monitor, visual force targets were set approximately at the force produced during the practice MVIC trial. After a 5-min rest, two to three attempts at knee extension or ankle plantar flexion MVICs lasting 3–5 s were performed to quantify peak voluntary force production, voluntary muscle activation, and antagonist coactivation (see below). Each attempt was separated by rest for 5 min, and peak voluntary forces were normalized to each participant’s body weight. Voluntary activation of the quadriceps femoris and triceps surae muscles was assessed by delivering the maximum burst during a MVIC and again immediately after the MVIC attempt when the force returned to baseline. A ratio that represents the degree of voluntary muscle activation was calculated by dividing the force augmentation from the stimulation during a MVIC by the force of the stimulation at baseline, and subtracting the resultant from 1.0. A ratio of 1.0 represents full voluntary muscle activation and anything less than 1.0 represents incomplete muscle activation.
Antagonist Coactivation
Surface EMG was recorded from the semitendinosus muscles during quadriceps testing and from the tibialis anterior during triceps surae testing. Electrodes were placed at one half the distance from the medial tibial condyle to the ischial tuberosity for the semitendinosus, and at one third the distance from the inferior pole of the patella to the apex of the lateral malleolus for the tibialis anterior. The skin was shaved, lightly abraded with a synthetic exfoliating pad, and cleaned with alcohol to reduce skin impedance. The EMG electrodes were secured to the skin with a double-sided, medical-grade adhesive tape. Signal conditioning electrodes (DE-2.3; DelSys Inc., Boston, MA) with a parallel bar arrangement (contact area 1 × 10 mm, 10-mm interelectrode distance) were used to detect the electrical activity of the muscle. The EMG signal was preamplified and filtered within the electrode housing (gain of 1000 and bandpass filtered between 20–450 Hz) and sampled at 2000 Hz.
The EMG signal was used to quantify the amount of antagonist muscle coactivation during isometric MVICs with electrical burst superimposition of the quadriceps femoris and triceps surae. The root mean square (RMS) of the EMG signal (1000-ms prior to electrical burst superimposition during activation tests) was calculated with custom-written software (LabView 5.1, Austin, TX). The RMS EMG from the semitendinosus and tibialis anterior during knee extension and plantar flexion MVICs were normalized by dividing these values by the RMS EMG from knee flexion and dorsiflexion MVICs, respectively.
Contractile and Fatigue Properties
After rest for 5 min from the MVIC–voluntary muscle activation tests, the force–frequency relationship (FFR) and fatigue tests were performed to compare the contractile and fatigue properties of muscles. First, the stimulator was set to deliver a 13-pulse, 40-Hz electrical train (each pulse 600 µs) and the muscle was potentiated with this train by delivering it once every 5 s for 15 repetitions. By the end of potentiation, the intensity was adjusted to elicit a force equivalent to 40% of the maximum burst force and was not adjusted for the remainder of the FFR and fatigue tests. For FFR and fatigue testing, the stimulator was driven by a personal computer using custom-written software (LabView 4.0.1, Austin, TX) to control the timing of each stimulation train. All force data was sampled at 200 Hz and analyzed with custom software. Within 5–10 s after potentiation, the FFR test began with a random sequence of 14 trains of the following frequencies: 1 (twitch), 5, 8, 10, 12.5, 14.3, 16.7, 25, 33.3, 40, 50, 66.7, 80, and 100 Hz. The random sequence of trains was then repeated in reverse order for a total of 28 trains. The stimulation trains were 1 s in duration or contained 50 pulses, depending on which limit was reached first. One train was delivered once every 10 s to prevent fatigue. The peak forces of the two trains from each frequency were averaged and normalized by dividing the averaged peak force produced at each frequency by the force response of the train frequency that produced the highest average peak force during that subject’s force–frequency test. The variability of the peak forces between each repeated train frequency was low, typically less than 5%. If the two trains had a greater than 10% difference or if the child moved or had a reflex response during testing, we reviewed the recorded force traces and eliminated the trace that appeared to have inconsistent recruitment or any motion artifact. Thus, in a few instances, a subject’s peak force for a given train frequency consisted of a single force response. Twitch to tetanus ratios were calculated for each subject by dividing the averaged peak force of the 1-pulse train (twitch) by the averaged peak force of the 100-Hz train. The frequencies needed to produce 25% and 50% of the maximum normalized force (f25% and f50%, respectively) that occurred during the FFR test were calculated by linear interpolation and used to compare the relative position of the FFR between children with and without CP.1 Time to peak tension (TPT) was calculated as the time it took the twitch force to reach its peak and half-relaxation time (½RT) was calculated as the time it took the twitch force to decline to 50% of its peak. The sum of the TPT and ½RT was used to make comparisons between CP and control groups.
Fatigue testing followed the FFR testing after rest for approximately 3–5 min. The muscle was repotentiated with 30 13-pulse, 40-Hz trains delivered once every 5 s and the fatigue test commenced within 5 s after the last potentiation train. The fatigue test consisted of a 13-pulse, 40-Hz train delivered once per second for a total of 180 contractions. The peak forces from the 180 fatiguing trains were normalized to the peak force produced by the first train in the fatigue sequence. The average of the normalized force over the last five contractions in the fatigue sequence was used to compare fatigability between CP and control groups.
Data Analysis
All comparisons between CP and control groups were made using independent t-tests and significance was accepted at the 0.05 level.
RESULTS
Most participants had little difficulty in tolerating the electrical stimulation. Several factors may have helped with stimulation tolerance: the use of a topical anesthetic cream, a gradual introduction to the stimulation, and distraction with videos during rest breaks and all tests except MVIC testing.
MVIC and Activation Testing
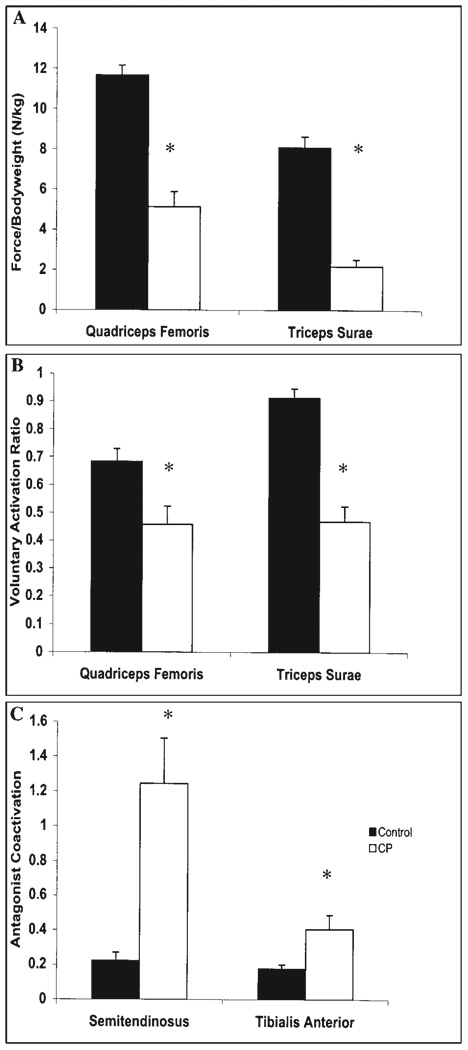
All children participated in MVIC testing of the quadriceps femoris. The knee extension forces (Fig. 1A) were 56% less in the CP than the control group (t = 6.81; P < 0.001). For the triceps surae (Fig. 1A), 12 children with CP and 8 unaffected children participated in MVIC testing, and the CP group produced 73% less force than the control group (t = 8.48; P < 0.001).
FIGURE 1.
(A) Maximum voluntary isometric contraction (MVIC) force normalized to body weight (kg) for the quadriceps femoris and triceps surae in CP (n = 12) and control (n = 10 for quadriceps, n = 8 for triceps surae) groups. (B) The degree of voluntary muscle activation in the quadriceps femoris and triceps surae in CP and control groups. (C) The degree of antagonist coactivation of the semitendinosus and tibialis anterior during MVICs of the quadriceps femoris and triceps surae, respectively, in CP and control groups. *Represents a difference between CP and control groups where P < 0.05.
Agonist voluntary muscle activation was lower in the CP than control group for both muscle groups (Fig. 1B). Children with CP had voluntary muscle activation ratios that were 33% lower in the quadriceps femoris (t = 2.70; P < 0.01) and 49% lower in the triceps surae (t = 6.58; P < 0.001).
Antagonist muscle coactivation during the agonist MVICs was greater in the CP than control group (Fig. 1C). Children with CP demonstrated increased semitendinosus activity during quadriceps femoris MVICs than unaffected children (t = −3.62; P = 0.002). Increased tibialis anterior activity in the CP group was also present during triceps surae contractions (t = −2.60; P = 0.012).
Contractile and Fatigue Properties
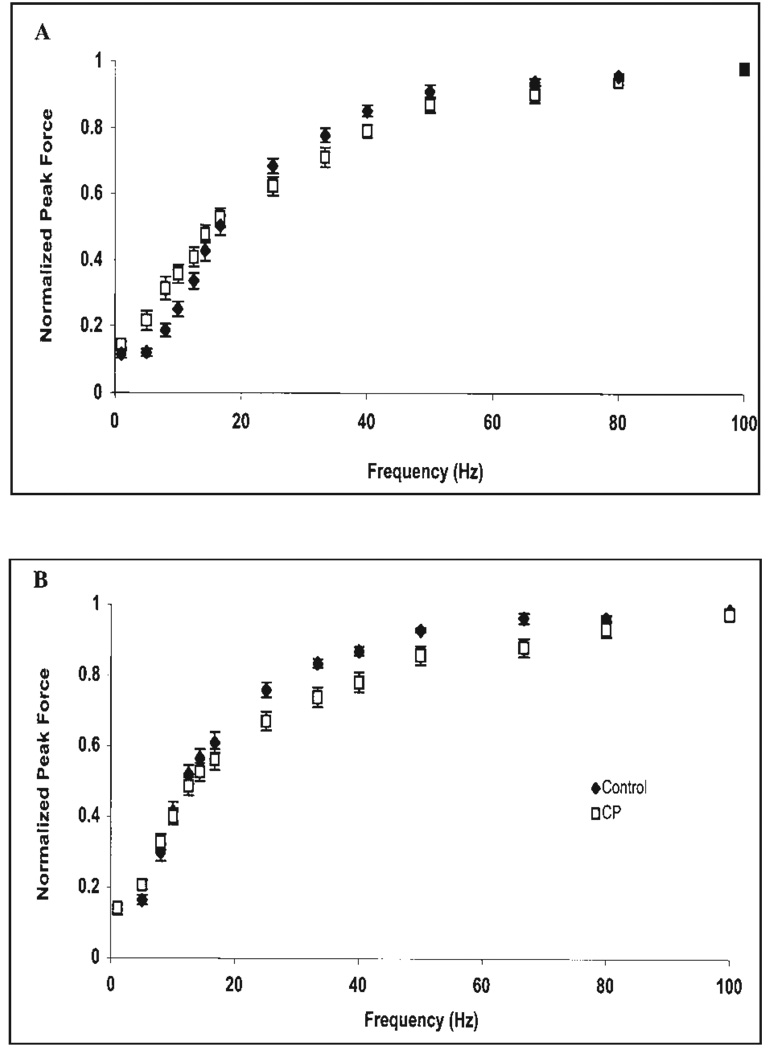
The quadriceps femoris normalized FFR in children with CP (n = 12) was shifted upward from the control group (n = 10) at frequencies below 16.67 Hz (Fig. 2A). In the triceps surae, the normalized FFR of the CP group (n = 12) was unchanged at low frequencies compared to the controls (n = 9; Fig. 2B). To quantify the shift in the FFR, we calculated the f25% and f50% values and found that the only difference between CP and control groups was for the f25% value of the quadriceps femoris (CP = 6.85 ± 2.97 Hz vs. controls = 10.14 ± 2.10 Hz; t = −2.89; P = 0.004). One possible explanation for the upward shift in the FFR is an elevated twitch:tetanus ratio. The twitch: tetanus ratio, however, only demonstrated a trend to be elevated in the CP group for the quadriceps (CP: 0.14 ± 0.04; controls: 0.12 ± 0.03; t = 1.55; P = 0.068) and no difference for the triceps surae (CP: 0.15 ± 0.03; controls: 0.14 ± 0.05; t = 0.248; P = 0.404). Another possible explanation for the upward shift could stem from longer twitch TPT and ½RT times in children with CP, thus producing greater force summation at unfused stimulation frequencies. The sum of the TPT and ½RT times in CP and control groups for the quadriceps femoris were 148.10 ± 14.08 ms and 141.35 ± 8.76 ms, respectively, which did not reach significance (t = 1.31; P = 0.103). Similarly the sum of the TPT and ½RT for the triceps surae was not significantly different (CP: 198.05 ± 23.01 ms; controls: 195.64 ±20.80 ms; t = 0.238; P = 0.407).
FIGURE 2.
The normalized force–frequency relationship for the (A) quadriceps femoris and (B) triceps surae in CP (n = 12) and control (n = 10 for quadriceps; n = 9 for triceps surae) groups plotted using forces normalized to the highest force produced during the test.
To gain further insight as to why the normalized FFR of children with CP was shifted upward at low frequencies for the quadriceps and not triceps surae, we looked for differences in absolute twitch forces between CP and control groups. The quadriceps twitch forces between groups were not significantly different (CP: 15.44 ± 7.25 N; controls: 20.56 ± 9.96 N; t = 1.326; P = 0.100); however, the triceps surae twitch forces of the control group were larger than the CP group (CP: 10.55 ± 4.05 N; controls: 20.70 ± 9.56 N; t = 3.144; P = 0.003).
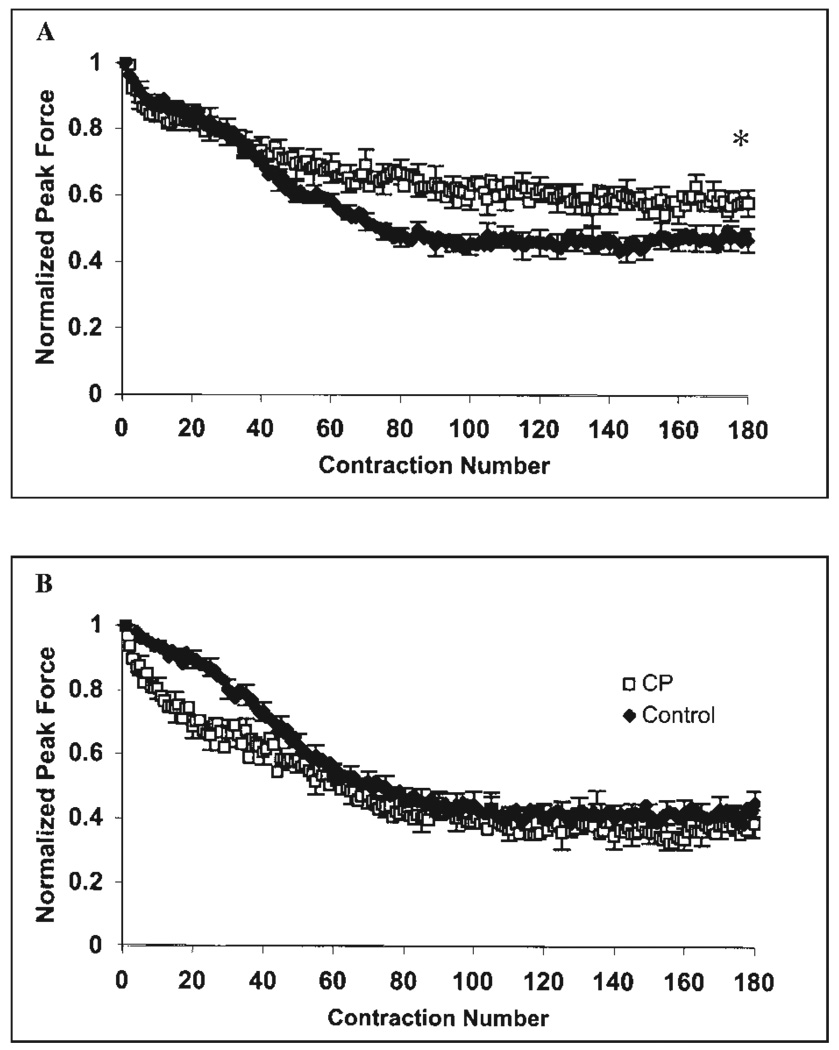
During fatigue testing, the quadriceps femoris of the CP (n = 11) and control (n = 10) groups had 42% and 52% declines in normalized peak force, respectively (Fig. 3A). The average normalized peak force over the last five quadriceps femoris contractions was greater for the CP group (t = −1.94; P = 0.034). In the triceps surae (Fig. 3B), the CP (n = 11) group had a more precipitous drop in force over approximately the first 40 contractions compared to controls (n = 9); however, both groups experienced approximately a 60% decline in normalized peak force by the end of the protocol (t = 0.692; P = 0.249).
FIGURE 3.
Electrically elicited fatigue tests in CP (n = 11) and control groups for (A) quadriceps femoris (control, n = 10) and (B) triceps surae (control, n = 9). *Represents a difference between CP and control groups where P < 0.05
DISCUSSION
The major findings of this study are that the weakness seen in children with spastic diplegic CP is, in part, attributable to deficits in voluntary muscle activation and to increases in antagonist coactivation. Secondary findings demonstrate that the quadriceps femoris of children with CP have greater force summation at lower frequencies of stimulation and are less fatigable than unaffected children.
MVIC and Activation Testing
Voluntary muscle activation of the quadriceps femoris and triceps surae in the CP group was reduced by approximately 33% and 49%, respectively. These deficits in voluntary muscle activation in children with CP are similar in magnitude to deficits reported for the quadriceps femoris of adults after a stroke (25–40%) or with multiple sclerosis (25%).9,25 Voluntary muscle activation of the quadriceps femoris in our control group is lower than that attained by adults (~ 20–35 years) using the same technique (0.98; Stackhouse & Lee, unpublished data). Ramsay and colleagues26 also demonstrated incomplete activation of the quadriceps in unaffected prepubescent children using a twitch interpolation technique; therefore, this incomplete activation may represent maturational differences in recruitment between children and adults.
Our results support the hypothesis that increased antagonist coactivation explains part of the observed lower peak forces produced in children with CP. Greater EMG activity of the semitendinosus was produced during quadriceps MVICs than during hamstring MVICs (coactivation ratio = 1.24). This high semitendinosus coactivation was mainly the result of 3 subjects with coactivation during quadriceps MVICs that were two to three times greater than during semitendinosus MVICs. If these 3 subjects are excluded, the semitendinosus coactivation for the CP group is still greater than controls (0.74 vs. 0.22). Ikeda and colleagues21 demonstrated hamstring co-activation ratios of approximately 0.09 and 0.04 for CP and control groups, respectively. Our study differs in mainly one aspect: their children were positioned supine, which may put the hamstring muscles in a position that inhibits abnormal activation. This different posture may explain why our study had greater antagonist coactivation in both CP and control groups. In addition, our values may be overestimating the degree of antagonist coactivation for those children unable to achieve full activation of their semitendonosis during knee flexion MVIC. For these children, a smaller denominator in the calculation of antagonist coactivation would inflate the relative amount of antagonist activity during knee extension. This concern emphasizes the need to evaluate further the validity of this measure in people with CNS dysfunction.
Contractile and Fatigue Properties
The quadriceps femoris of our CP group had greater normalized force summation at unfused frequencies and less fatigability during an electrically elicited fatigue test than unaffected children. The triceps surae, however, were similar between the two groups. In an attempt to explain differences in the normalized quadriceps FFR between the groups, we examined the sum of the TPT and ½RT and the twitch:tetanus force relationship. We found a slightly longer TPT + ½RT of the quadriceps femoris in children with CP than in unaffected children, but this difference was not significant and is unlikely to have increased the relative force summation during the FFR test as dramatically as depicted in Figure 2B. Differences in the proportions of myosin heavy chain content toward type I in CP, therefore, may not account for the observed shift in the normalized quadriceps FFR.
The twitch:tetanus ratio showed a trend towards elevation in the quadriceps femoris of children with CP (P = 0.068). An elevated twitch:tetanus ratio has been observed previously in denervated muscle,20 in multiple sclerosis,27 and after spinal cord injury.16 When absolute twitch forces were compared between controls and subjects with multiple sclerosis,27 spinal cord injury,16 and CP (this study), no differences in force were observed. Preservation of the quadriceps twitch force and attenuation of force at high frequencies (> 20 Hz) of stimulation in the CP could explain why the normalized FFR was shifted upward at low frequencies. The preservation of the twitch force especially seems at odds with the degree of muscle atrophy that occurs in muscles after spinal cord injury4 and denervation.10
Gerrits and colleagues17 recently suggested that the increase in twitch forces after spinal cord injury could be the result of an increased level of activation (crossbridge formation) per stimulation impulse. Increased activation per impulse could occur from increased calcium release by the sarcoplasmic reticulum, a greater affinity of troponin for free calcium, or increased light-chain phosphorylation as seen with posttetanic potentiation.17 If increased activation does occur, it may explain why the absolute twitch forces were not different in the quadriceps femoris between CP and control groups.
The quadriceps femoris of children with CP fatigued to a lesser extent than unaffected children, but no differences were detected for triceps surae. An attractive explanation for this observation is that the quadriceps femoris in children with CP has a greater proportion of fatigue-resistant muscles fibers (slow oxidative and fast oxidative glycolytic). Studies by others22,29 have demonstrated an increase in type I muscle fibers in some lower-extremity muscles in children with CP using myosin ATPase staining. Unfortunately, the quadriceps femoris muscle was not sampled in these studies,22,29 and other morphological and metabolic properties were not analyzed that might have clarified our observations. It is possible that our children with spastic diplegic CP had more quadriceps femoris muscle fibers with metabolic characteristics of slow oxidative and fast oxidative glycolytic fibers, which could have reduced relative fatigue by the observed 10%. One hypothesis is that ambulatory children with spastic diplegia may use the same motor units more repetitively and for longer durations during standing and gait as a result of limitations with muscle recruitment and increased levels of muscle coactivation, leading to metabolic adaptations to meet the demand.
In the triceps surae, we did not observe the same difference in fatigability as in the quadriceps. Although not formally evaluated, we saw a greater decline in force in the CP group over the first 40 contractions, but by contraction 60 both groups reached the same level of fatigue that then remained stable. The relative force fatigue measured in this study should not be confused with clinically reported fatigue in children with CP. During functional tasks such as ambulation over long distances, the lower force-generating capacity in children with CP creates a lower force reserve to sustain activity, so that children with CP are functionally more fatigable. Future studies should further investigate the rate of fatigue development and its relationship to the metabolic and morphologic properties of the muscle.
CLINICAL IMPLICATIONS
Because children with CP in this study have demonstrated large deficits in voluntary muscle activation (~20–50%), using voluntary contractions for strength training may not produce forces sufficient to induce muscle hypertrophy. Although studies have demonstrated that force production and some aspects of function can be improved by high-intensity volitional strength training in CP,5,6 programs that combine enhanced feedback techniques with high-intensity volitional strength training may have the potential to further augment gains in force by facilitating agonist muscle activation and inhibiting antagonist coactivation. Additionally, neuromuscular electrical stimulation may be useful for strengthening muscles that cannot be sufficiently recruited with voluntary effort.
Acknowledgments
Scott K. Stackhouse submitted this research as partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Delaware. This research was supported in part by the Foundation for Physical Therapy (PODS 1), the University of Delaware Alumni Association, and the Section on Pediatrics of the American Physical Therapy Association to S. K. Stackhouse; by Shriners Hospitals for Children Research Grant 8530; and NIH grant (HD043859) to S. C. K. Lee. In addition, the authors thank Tracy Moretta, Shannon Stuckey, Katie Parker, and Chris Gorrell for their assistance in data processing.
Abbreviations
- CP
cerebral palsy
- EMG
electromyography
- FFR
force–frequency relationship
- MVIC
maximum voluntary isometric contraction
- RMS
root mean square
- TPT
time to peak tension
- ½RT
half-relaxation time
REFERENCES
- 1.Binder-Macleod SA, Halden EE, Jungles KA. Effects of stimulation intensity on the physiological responses of human motor units. Med Sci Sports Exerc. 1995;27:556–565. [PubMed] [Google Scholar]
- 2.Burtner PA, Qualls C, Woollacott MH. Muscle activation characteristics of stance balance control in children with spastic cerebral palsy. Gait Posture. 1998;8:163–174. doi: 10.1016/s0966-6362(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 3.Castle ME, Reyman TA, Schneider M. Pathology of spastic muscle in cerebral palsy. Clin Orthop. 1979;142:223–233. [PubMed] [Google Scholar]
- 4.Castro MJ, Apple DF, Staron RS, Campos GER, Dudley GA. Influence of complete spinal cord injury on skeletal muscle with 6 mo of injury. J Appl Physiol. 1999;86:350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 5.Damiano DL, Abel MF. Functional outcomes of strength training in spastic cerebral palsy. Arch Phys Med Rehabil. 1998;79:119–125. doi: 10.1016/s0003-9993(98)90287-8. [DOI] [PubMed] [Google Scholar]
- 6.Damiano DL, Kelly LE, Vaughan CL. Effects of quadriceps femoris muscle strengthening on crouch gait in children with spastic diplegia. Phys Ther. 1995;75:658–671. doi: 10.1093/ptj/75.8.658. [DOI] [PubMed] [Google Scholar]
- 7.Damiano DL, Martellotta TL, Quinlivan JM, Able MF. Deficits in eccentric versus concentric torque in children with spastic cerebral palsy. Med Sci Sports Exerc. 2001;33:117–122. doi: 10.1097/00005768-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Damiano DL, Vaughan CL, Able MF. Muscle response to heavy resistance exercise in children with spastic cerebral palsy. Dev Med Child Neurol. 1995;37:731–739. doi: 10.1111/j.1469-8749.1995.tb15019.x. [DOI] [PubMed] [Google Scholar]
- 9.de Haan A, de Ruiter CJ, van der Woude LHV, Jongen PJH. Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle Nerve. 2000;23:1534–1541. doi: 10.1002/1097-4598(200010)23:10<1534::aid-mus9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Eberstein A, Eberstein S. Electrical stimulation of denervated muscle: is it worthwhile? Med Sci Sports Exerc. 1996;28:1463–1469. doi: 10.1097/00005768-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, Leahey L. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–550. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- 12.Engsberg JR, Olree KS, Ross SA, Park TS. Spasticity and strength changes as a function of selective dorsal rhizotomy. J Neurosurg. 1998;88:1020–1026. doi: 10.3171/jns.1998.88.6.1020. [DOI] [PubMed] [Google Scholar]
- 13.Engsberg JR, Ross SA, Park TS. Changes in ankle spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. J Neurosurg. 1999;91:727–732. doi: 10.3171/jns.1999.91.5.0727. [DOI] [PubMed] [Google Scholar]
- 14.Frascarelli M, Mastrogregori L, Conforti L. Initial motor unit recruitment in patients with spastic hemiplegia. Electromyogr Clin Neurophysiol. 1998;38:267–271. [PubMed] [Google Scholar]
- 15.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 16.Gerrits HL, de Haan A, Hopman MTE, van der Woude LHV, Jones DA, Sargeant AJ. Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve. 1999;22:1249–1256. doi: 10.1002/(sici)1097-4598(199909)22:9<1249::aid-mus13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Gerrits HL, Hopman MTE, Sargeant AJ, Jones DA, de Haan A. Effects of training on contractile properties of paralyzed quadriceps muscle. Muscle Nerve. 2002;25:559–567. doi: 10.1002/mus.10071. [DOI] [PubMed] [Google Scholar]
- 18.Harridge SDR, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjörnsson M, Saltin B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch. 1996;432:913–920. doi: 10.1007/s004240050215. [DOI] [PubMed] [Google Scholar]
- 19.Harrison A, Connolly K. The conscious control of fine levels of neuromuscular firing in spastic and normal subjects. Dev Med Child Neurol. 1971;13:762–771. doi: 10.1111/j.1469-8749.1971.tb08349.x. [DOI] [PubMed] [Google Scholar]
- 20.Herbison GJ, Jaweed MM, Ditunno JF. Contractile properties of reinnervating skeletal muscle in the rat. Arch Phys Med Rehabil. 1981;62:35–39. [PubMed] [Google Scholar]
- 21.Ikeda AJ, Abel MF, Granata KP, Damiano DL. Quantification of cocontraction in spastic cerebral palsy. Electromyogr Clin Neurophysiol. 1998;38:497–504. [PubMed] [Google Scholar]
- 22.Ito J, Araki A, Tanaka H, Tasaki T, Cho K, Yamazaki R. Muscle histopathology in spastic cerebral palsy. Brain Dev. 1996;18:299–303. doi: 10.1016/0387-7604(96)00006-x. [DOI] [PubMed] [Google Scholar]
- 23.Jakobsson F, Grimby L, Edstrom L. Motoneuron activity and muscle fibre type composition in hemiparesis. Scand J Rehab Med. 1992;24:115–119. [PubMed] [Google Scholar]
- 24.Merton PA. Voluntary strength and fatigue. J Physiol (Lond) 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newham DJ, Hsiao SF. Knee muscle isometric strength, voluntary activation and antagonist co-contraction in the first six months after stroke. Disabil Rehabil. 2001;23:379–386. doi: 10.1080/0963828001006656. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay JA, Blimkie CJR, Smith K, Garner S, MacDougall JD, Sale DG. Strength training effects in prepubescent boys. Med Sci Sports Exerc. 1990;22:605–614. doi: 10.1249/00005768-199010000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Rice CL, Vollmer TL, Bigland-Ritchie B. Neuromuscular responses of patients with multiple sclerosis. Muscle Nerve. 1992;15:1123–1132. doi: 10.1002/mus.880151011. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D, Lee WA, Jacobs M. Single motor-unit control by normal and cerebral-palsied males. Dev Med Child Neurol. 1984;26:323–327. doi: 10.1111/j.1469-8749.1984.tb04448.x. [DOI] [PubMed] [Google Scholar]
- 29.Rose J, Haskell WL, Gamble JG, Hamilton RL, Brown DA, Rinsky L. Muscle pathology and clinical measures of disability in children with cerebral palsy. J Orthop Res. 1994;12:758–768. doi: 10.1002/jor.1100120603. [DOI] [PubMed] [Google Scholar]
- 30.Stackhouse SK, Dean JC, Lee SC, Binder-Macleod SA. Measurement of central activation failure of the quadriceps femoris in healthy adults. Muscle Nerve. 2000;23:1706–1712. doi: 10.1002/1097-4598(200011)23:11<1706::aid-mus6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40:100–107. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]