Abstract
Dense fibrosis, which is caused by desmoplastic reaction, is usually found in invasive ductal carcinoma and may represent the alteration of the tumor microenvironment preceding tumor invasion. Thus, the dense fibrotic zone around invasive ductal carcinoma can be considered to be the actual tissue site of tumor microenvironment, where the precedent alterations for tumor invasion occur. To characterize the dense fibrotic zone, we classified invasive ductal carcinoma tissue into a tumor zone, a normal zone, and the novel interface zone (IZ), which shows dense fibrosis. The postulated IZ is a 5-mm-wide belt that circles the tumor margin and overlaps with normal tissue. Of the extracellular matrix components, laminin-332 was specifically overexpressed in the IZ. Events that appear to be similar to the epithelial-mesenchymal transition, a novel source of myofibroblast formation from epithelial cells, were observed in the IZ, according to the following characteristics: overexpression of matrix metalloproteinase 3, membrane type 1−matrix metalloproteinase, snail, and zinc finger E-box-binding homeobox 1, and the gain of N-cadherin expression, as well as the down-regulation of miR200c. The myofibroblasts isolated from the IZ, which were designated interface zone-fibroblast, displayed laminin-332 and membrane type 1−matrix metalloproteinase overexpression, in contrast with both cancer-associated fibroblasts and normal breast fibroblasts. Taken together, our results suggest that the IZ, which shows dense fibrosis, may provide a specialized microenvironment for guiding tumor invasion: the fibrosis caused by laminin-332 overexpressing myofibroblast formation (interface zone-fibroblast) via epithelial-mesenchymal transition.
The tumor microenvironment (TME) is important for tumorigenesis and tumor progression.1,2 For breast cancer surgery and treatment, it is necessary to define the TME of a tissue, which is important in tumor progression. Nevertheless, no attempt has been made to define the TME of an actual tissue area. The dense fibrosis around the tumor burden, which is caused by desmoplastic reaction of the invading tumor, can be thought of as the alteration of TME for tumor invasion. Invasive ductal carcinoma (IDC), which is a representative type of invasive breast cancer, may be an ideal model for studying TME alterations during tumor invasion because of the accompanying desmoplasia. In our study, the dense fibrotic zone of IDC was first designated as “the interface zone” (IZ) and was then characterized. The initial concept of the IZ originated from a traditionally and widely used surgical margin showing low recurrence: a 5-mm-wide tissue zone surrounding the tumor burden and adjoining the normal tissue.3
Fibrosis is the formation of excess fibrous connective tissues caused by physiological stress, such as injury and inflammation. When a tumor becomes invasive or metastatic, dense fibrosis is detected around the tumor burden, especially in solid tumor tissue.4 In addition, basement membrane stiffening, one of the representative TME alterations for tumor progression, is caused by fibrosis.5 The transition of epithelia to myofibroblast via epithelial-mesenchymal transition (EMT), which can be induced by matrix metalloproteinase 3 (MMP3), has been reported to be a novel source of myofibroblast formation.6,7 Membrane type 1 (MT1)-MMP expression produces some of the same effects as MMP3: fibrosis in mammary glands8 and EMT in prostate cancer.9 Thus, the fibrotic zone in IDC could be formed by myofibroblasts that are transdifferentiated from nontumoral epithelial cells around the tumor burden during tumor invasion.10
Laminin-332, an extracellular matrix (ECM) component composed of LAMA3, LAMB3, and LAMC2 chains, is expressed by both tumor and stromal cells. Laminin-332 expression is increased in several types of tumors.11–13 However, some cancers, such as advanced breast and prostate cancer, reveal decreased expression of laminin-332.14,15 Similar to other laminins, laminin-332 primarily serves an adhesive function in mature normal tissue, but has a more complex role in tumor cell survival, invasion, and migration.12 During tumor progression, laminin-332 binds to integrin α6β4, specifically the β4 subunit, and signals through the PI3K and RAC1 pathways to promote the survival, invasion, and directional migration of tumor cells.16,17 Moreover, transforming growth factor-β (TGF-β) completes the EMT process in the presence of laminin-332.18
To understand the alterations of TME in IDC tissue during tumor invasion, we first introduced the novel concept of IZ in our study and focused on its external feature, dense fibrosis. Based on our results, the IZ may be considered the actual tissue site of TME, where tumor progression-inducing events actively occur. Therefore, the IZ may be a potent target for cancer treatment, such as neoadjuvant therapy, and should be further studied.
Materials and Methods
Tissue Acquisition
Breast cancer tissues were acquired from 11 patients (4 ductal carcinoma in situ (DCIS) and 7 IDC not otherwise specified) by macroscopic dissection with needle shredding, according to the spatial serial mapping: (1) real tumor burden, termed the tumor zone (TZ), around the epicenter of the main tumor; (2) directly adjacent normal-like tissue up to 5 mm from the peripheral margin of the tumor, termed the IZ; and (3) remote normal tissue located at least 10 mm from the tumor margin, termed the normal zone (NZ). For the conceptual construction, small IDCs less than 10 mm2 in gross dimension were selected as a prototype with reproducible spatial identification. Larger tumors more than 10 mm2 in dimension with a vague infiltrative boundary or cases other than ductal carcinomas were excluded for future evaluation. For validation of microscopic findings, other samples from corresponding areas were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned into 4-μm sections, and examined by H&E staining.
ECM Array for Screening Interface-Specific Factors
Total RNA was extracted using a Protect Mini Kit (Invitrogen, Carlsbad, CA). After verifying the size and integrity of the RNA using Experion (Bio-Rad, Hercules, CA), total RNA was reverse transcribed using an RT2 First Strand Kit (SABiosciences, Frederick, MD). The human ECM and Adhesion Molecules RT2 Profiler PCR Array and RT2 Real-Time SYBR Green/Fluorescein PCR master Mix (SABiosciences) were used to analyze ECM gene transcripts. The PCR array was processed on an IQ5 iCycler (Bio-Rad), as described by the manufacturer, and the data were analyzed using the program provided on the manufacturer's website (http://www.sabiosciences.com/pcr/arrayanalysis.php). For data analysis, P < 0.05 values were considered statistically significant.
Quantitative Analysis of Gene Transcript Expression Using Real-Time PCR
Total RNA was transcribed using Superscript III (Invitrogen), and 25 ng of the resulting cDNA and 20 pM primers were added to a PCR mixture prepared with the QuantiTect SYBR green PCR kit (Qiagen, Valencia, CA). Primer sequences and annealing temperature are shown in Table 1. PCR reactions were performed in triplicate using the following cycling conditions: 10 minutes at 95°C, 35 cycles of 40 seconds at 94°C, 1 minute at a primer-specific annealing temperature, and 1 minute at 72°C, followed by 10 minutes at 72°C. The expression of gene transcripts was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression, and calculated according to the ΔΔCt method using Opticon Monitor 3 (ver. 3.1; Bio-Rad, Hercules, CA). Values of P < 0.05 were considered statistically significant.
Table 1.
Primer Sequence and Temperature for PCR
| Primer | Direction | Sequence | Annealing temperature (°C) |
|---|---|---|---|
| LAMA3 | Forward | 5-GCTCAGCTGTTTGTGGTTGA-3 | 55 |
| Reverse | 5-TGTCTGCATCTGCCAATAGC-3 | ||
| LAMC2 | Forward | 5-GATGGCATTCACTGCGAGAAG-3 | 56 |
| Reverse | 5-TCGAGCACTAAGAGAACCTTTGG-3 | ||
| ITGA6 | Forward | 5-GACTTGAAAGAAATGGTGAATGC-3 | 53 |
| Reverse | 5-TAGCACCTGTTTGCTGTTGC-3 | ||
| ITGB4 | Forward | 5-CCTGTACCCGTATTGCGACT-3 | 53 |
| Reverse | 5-AGGCCATAGCAGACCTCGTA-3 | ||
| Snail | Forward | 5-GAGGCGGTGGCAGACTAG-3 | 52 |
| Reverse | 5-GACACATCGGTCAGACCAG-3 | ||
| Twist | Forward | 5-CGGGAGTCCGCAGTCTTA-3 | 52 |
| Reverse | 5-TGAATCTTGCTCAGCTTGTC-3 | ||
| ZEB1 | Forward | 5-TGCACTGAGTGTGGAAAAGC-3 | 55 |
| Reverse | 5-TGGTGATGCTGAAAGAGACG-3 | ||
| GAPDH | Forward | 5-ACAACTTTGGTATCGTGGAA-3 | 55 |
| Reverse | 5-AAATTCGTTGTCATACCAGG-3 | ||
| URT | Forward | 5-AACGAGACGACGACAGACTTTTTTTTTTTTT-3 | 42 |
| Universal PCR | Forward | 5-AACGAGACGACGACAGACTTT-3 | 53 |
| miR200c | Forward | 5-ATACTGCCGGGTAATGATGG-3 | 53 |
URT, universal reverse transcription.
Differential Expression of Tumor Progression-Related Proteins
Tissues were lysed in 500 μl Protein Extraction Solution (Intron Biotech, Seongnan-si, Korea), homogenized with a 30-gauge needle, incubated for 30 minutes at 4°C, and then purified by centrifugation at 13,000 rpm. After quantifying proteins in extracts using the Bradford method, 20-μg protein was electrophoresed on 10% Tris/glycine gels (Invitrogen), transferred to polyvinylidene diflouride membranes (Millipore, Billerica, MA), and probed with primary antibodies against laminin α3, laminin γ2, integrin α6, integrin β4, SNAIL, TWIST, zinc finger E-box-binding homeobox 1 (ZEB1), N-cadherin, MMP3, MT1-MMP, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). Primary antibodies were detected by horseradish peroxidase-conjugated secondary antibodies (Invitrogen), and visualized using enhanced chemiluminescence reagents (Santa Cruz Biotechnology). Relative signal intensities were analyzed using TINA software (ver. 2.0; Raytest, Straubenhart, Germany); P values < 0.05 were considered statistically significant for all data.
MiR200c Expression as an EMT Phenotype
For amplification of microRNA, mature microRNA was first modified by the addition of a poly(A) tail, as described previously.19 Briefly, 1 μg total RNA in 15 μl DNase/RNase-free water was added to a mixture containing 2 μl 10 X E-PAP buffer and 1 μl 10 mmol/L ATP. One microliter Escherichia coli Poly(A) polymerase I (E-PAP; New England Biolabs, Ipswich, MA) was then added; the reaction mixture was incubated for 1 hour at 37°C. Poly(A)-tail-added microRNAs were transcribed using Superscript III (Invitrogen), and 25 ng of the resulting cDNA and 20 pM primers were added to a PCR mixture prepared with the QuantiTect SYBR green PCR kit (Qiagen). Primer sequences and annealing temperatures are shown in Table 1. PCR reactions were performed in triplicate using the following cycling conditions: 10 minutes at 95°C, 35 cycles of 40 seconds at 94°C, 1 minute at 53°C, and 1 minute at 72°C, followed by 10 minutes at 72°C. The expression of gene transcripts was normalized to endogenous GAPDH expression, and calculated according to the ΔΔCt method using Opticon Monitor 3 (ver. 3.1). Values of P < 0.05 were considered statistically significant.
Isolation of Primary Fibroblasts
Each spatial fraction from fresh tissues was used to isolate primary fibroblasts. Fresh tissues obtained from each different zone under sterile conditions were minced into smaller pieces, placed in a digestion solution of Enzyme Cocktail (ISU ABXIS Co., Ltd., Seoul, Korea) and incubated at 37°C in a humidified 5% CO2 incubator overnight. Digested tissue was filtered through a 70-μm cell strainer. The cells were suspended in medium: Ficoll (3:2) and separated by differential centrifugation at 90 × g for 2 minutes. The supernatant containing fibroblasts was centrifuged at 485 × g for 8 minutes, resuspended in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10% fetal bovine serum and 100 IU/ml penicillin-100 μg/ml streptomycin (Gibco BRL, Grand island, NY), and cultured at 37°C in a humidified 5% CO2 environment. The fibroblastic nature of the isolated cells was confirmed by microscopic determination of morphology portrait and immunofluorescence characterization using an antibody against vimentin and α-smooth muscle actin (Abcam, Cambridge, UK).
Direct Co-Culture Experiments
Primary fibroblasts were directly co-cultured with human less-invasive MCF7 and highly invasive MDA-MB-231 (Korean Cell Line Bank, Seoul, Korea) breast carcinoma cells, as described previously.20 Briefly, adherent fibroblasts or breast cancer cells were stained by incubation for 45 minutes at 37°C in serum-free DMEM/F12 containing 5 μmol/L CellTracker Green 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen), a green fluorescent dye. Subsequently, the dye solution was replaced with fresh, prewarmed medium, and the cells were incubated for an additional 2 hours at 37°C. The cells were then washed twice with PBS, and unstained cancer cells or fibroblasts were seeded onto plates containing CMFDA-stained fibroblasts or cancer cells, respectively. Finally, the co-cultures were incubated with minimum serum medium composed of DMEM/F12, 1% FBS, 100 IU/ml penicillin-100 μg/ml streptomycin for 1 week. CMFDA-stained cells were easily distinguished from unstained cells by fluorescent microscopy and flow cytometry, allowing separate measurements of each cell type to be made independently.
Results
Distinct Gene Expression in the IZ of IDC
The IZ was defined as a 5-mm-wide belt zone surrounding the TZ and adjoining the NZ (Figure 1A). The samples were predominantly IDCs less than 10 mm2 in dimension. The TZ was obtained from the epicenter of the tumor burden without necrosis or hemorrhage. The IZ was predominantly characterized by dense fibrosis with a few scattered lobuloductal units and without evidence of a tumor. The NZ was entirely fibrofatty tissue at least 10 mm away from the tumor margin with a few scattered normal lobuloductal units and a scant amount of fibrosis or no apparent preneoplastic lobules. The counterparts of DCIS were obtained from the same histological location as those of IDC to serve as controls.
Figure 1.
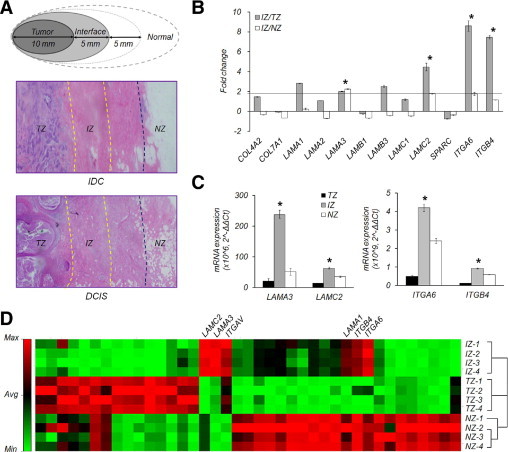
Distinct gene expression in the interface zone of invasive ductal carcinoma. A: Breast cancer tissues were acquired by macroscopic dissection based on geographical mapping as follows: (1) tumor burden at the epicenter of the tumor zone (TZ); (2) a 5-mm-wide interface tissue (IZ) located between the periphery of the tumor tissue and the adjacent normal tissue; and (3) normal zone (NZ) located at least 10 mm away from the tumor margin. Tissue sections (0.4-μm thick) were stained with H&E. B: Fold-differences in the expression of basement membrane and cell-matrix adhesion-related constituents of the ECM in the IZ of IDC relative to the TZ and/or NZ were determined using an ECM gene transcript array. LAMA3 transcripts were upregulated ∼2-fold in the IZ compared with the TZ and NZ. LAMC2, ITGA6, and ITGB4 transcripts were also highly expressed in the IZ compared to the TZ. C: Quantitative analysis of LAMA3, LAMC2, ITGA6, and ITGB4 using real-time PCR. LAMA3, LAMC2, ITGA6, and ITGB4 were all significantly upregulated in the IZ of IDC. D: Clustergram based on ECM transcript expression. ECM gene expression pattern of IZ was closer to that of the TZ than the NZ in IDC. Asterisk denotes more than twofold increased expression and the values of P < 0.05 in the IZ.
To determine whether the IZ of IDC shows distinct gene alterations compared to the TZ and NZ, ECM genes, major components of the TME, were analyzed using an mRNA array. LAMA3, LAMC2 (basement membrane constituents), ITGA6, and ITGB4 (cell-matrix adhesion-related molecules) were specifically upregulated in the IZ of IDC (Figure 1B). The expression of these genes was confirmed using real-time PCR (Figure 1C). In addition, the degree of similarity among the TZ, IZ, and NZ was analyzed by drawing a clustergram, and the IZ was relatively close to the TZ (Figure 1D).
Overexpression of Tumor Progression-Related Factors, Laminin-332 and Integrin α6β4, in the IZ
To determine whether the 5-mm-wide IZ displays detectable zone-specific alterations, we compared laminin-γ2 and integrin β4 expression, which were identified in Figure 1, in serially dissected IZs at 1-mm intervals. The expression of laminin-γ2 and integrin β4 increased particularly from 3 to 4 mm in the IZ of IDC, but not in the counterparts of DCIS (Figure 2A). To confirm that the distinct expression of laminin-332 and integrin α6β4 in the IZ of IDC is reproducible, we compared their expression in other samples of breast cancer tissue. Western blot analyses showed the same pattern of expression as the mRNA array: laminin-α3, laminin-γ2, integrin α6, and integrin β4 were overexpressed in the IZ of IDC, but not in the counterparts of DCIS (Figure 2B).
Figure 2.
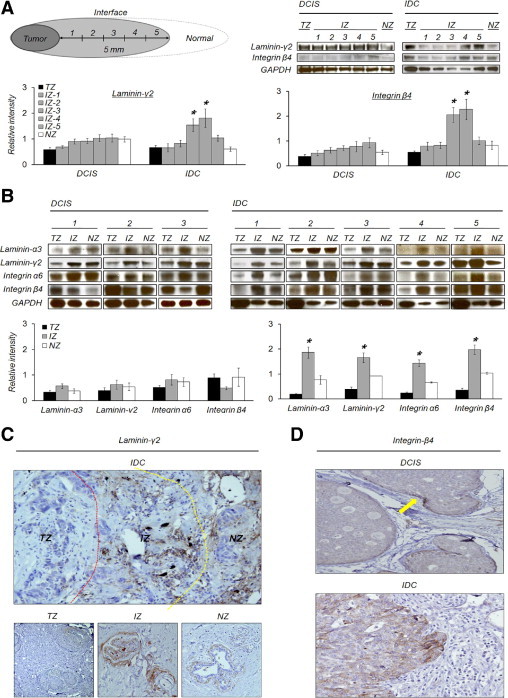
Overexpression of tumor progression-related factors in the interface zone. A: Zonal expression of laminin-γ2 and integrin β4. In the IZ of IDC, especially from 3 to 4 mm, laminin-γ2 and integrin β4 were significantly increased. B: Specific overexpression of laminin-332 and integrin α6β4 in the IZ of IDC. Both laminin-332 and integrin α6β4 were significantly increased in the IZ of IDC compared to the TZ and NZ. There was no significant difference between zones of DCIS. Asterisk denotes more than twofold increased expression, and P < 0.05 in the IZ. C: Laminin-γ2 expression in IDC tissue. Laminin-γ2 showed a strong immunoreactivity to ECM belonging to the IZ to compare to those of the TZ and NZ. At higher magnification, laminin-γ2 expression was restricted to the perilobular or ductal matrix in the IZ and also weakly positive in the NZ. D: Integrin β4 expression in DCIS and IDC tissue. DCIS showed focal accentuation of integrin β4 along the invading fronts of the tumor nest (arrow). IDC displayed diffusely strong membranous staining for integrin β4 with a tendency of accentuation in the periphery of the nest.
The spatial expression of laminin-γ2 in the IZ was relatively strong compared to those of the TZ and NZ in IDC tissue. In the IZ, laminin-γ2 was intensively and predominantly expressed in the matrix around lobules, including basement membrane, whereas it was undetectable in the TZ. The lobules in the NZ also expressed laminin-γ2, but not as much as in the IZ (Figure 2C). In DCIS samples, laminin-γ2 expression was weak or negative in both the TZ and IZ (data not shown).
Integrin β4 expression in IDC was expressed in a membranous pattern around vessels and within the tumor burden. However, it showed intratumoral heterogeneity, according to spatial area. Within the tumor nests, a peripheral-nested pattern was dominant, whether continuous or not. Integrin β4 was remarkably accentuated along the invading front of the tumor nests. DCIS was predominantly negative except in the front margin (arrow-marked) of the budding nest (Figure 2D).
Transition of Epithelial Cells to Myofibroblasts via EMT in the IZ
The dense fibrotic zone, a distinct characteristic of the IZ of IDC, allowed us to examine the expression of TGF-β, MMP3, MT1-MMP, and EMT-related factors. The TGF-β mRNA transcript was highly expressed in the TZ and moderately maintained in the IZ where it was higher than in the NZ (Figure 3A). MMP3 and MT1-MMP were specifically overexpressed in the IZ of IDC tissue (Figure 3B). Of EMT inducers, Twist, which is known as a tumor-specific marker and master regulator of EMT, was expressed merely in the TZ of both IDC and DCIS. On the other hand, Snail and ZEB1 were particularly overexpressed in the IZ of IDC (Figure 3B). The gain of N-cadherin expression was observed as an EMT phenotype (Figure 3B). The down-regulation of miR200c, which was coupled with an upregulation of ZEB1 in the level of mRNA, another EMT phenotype, was also detected in the IZ of IDC (Figure 3C).
Figure 3.
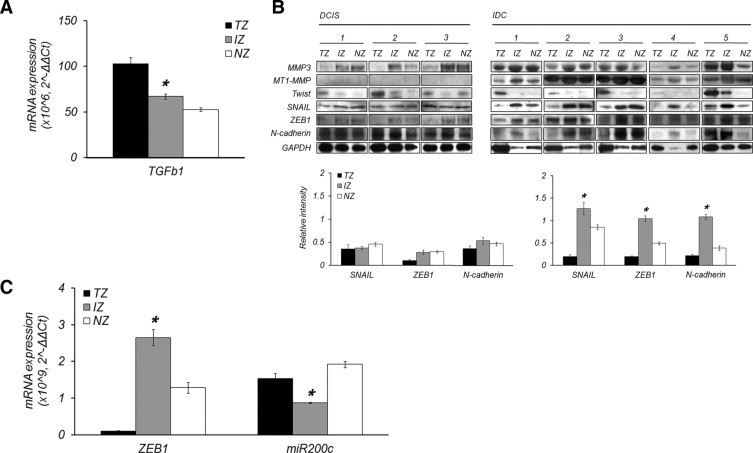
Epithelial-mesenchymal transition in the interface zone for myofibroblast formation. A: Expression of TGF-β transcript in IDC. TGF-β was highly upregulated in the TZ, and also moderately maintained in the IZ. B: Expression of MMP3, MT1-MMP, Twist, Snail, ZEB1, and N-cadherin in each tissue zone of both DCIS and IDC. In the IZ of IDC, MMP3 and MT1-MMP were significantly overexpressed compared with the TZ and NZ. There was no difference between tissue zones in DCIS, and even MT1-MMP was not expressed. Snail, ZEB1, and N-cadherin were overexpressed in the IZ of IDC relative to the TZ and NZ, whereas Twist was overexpressed only in the TZ of both DCIS and IDC. C: miR 200c down-regulation as an EMT phenotype. miR200c was down-regulated in the IZ coupled with the upregulation of ZEB1. Asterisk denotes more than twofold increased expression and P < 0.05 in the IZ.
Specialized Function of Fibroblasts Derived from the IZ in Tumor Invasion
To examine whether fibroblasts derived from the TZ, IZ, and NZ have distinct properties, we compared the molecules previously confirmed in tissues − laminin-γ2, integrin β4, MMP3, MT1-MMP, Snail, and ZEB1 − in all three fibroblast types. Laminin-γ2, Snail, and MT1-MMP were overexpressed only in interface zone-fibroblast (InF) cells, the myofibroblasts derived from the IZ of IDC. Integrin β4 was not expressed in any of the three fibroblast types (Figure 4A), and there was no significant difference in other factors among the tested fibroblasts (data not shown). To understand how the invasiveness of tumor cells affect the behavior of fibroblasts, we co-cultured each fibroblast type with less-invasive (MCF7) or high-invasive (MDA-MB-231) breast cancer cell lines, and then compared the same factors examined previously. MDA-MB-231, but not MCF7, induced laminin-γ2 and integrin β4 expression in all fibroblasts (Figure 4B).
Figure 4.
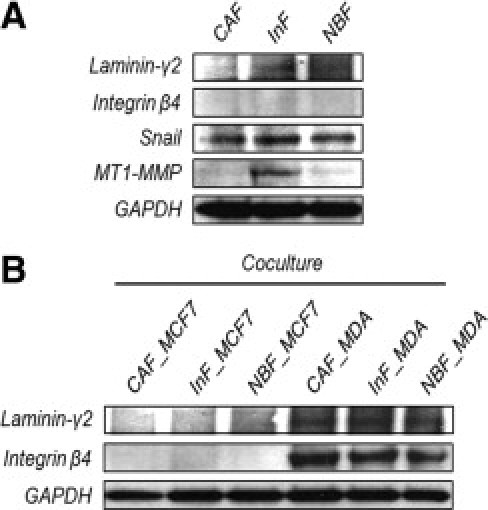
Tumor progression-specialized protein expression of the fibroblast derived from the interface zone. A: Comparison of fibroblasts derived from the TZ, IZ, and NZ of IDC. Laminin-γ2, Snail, and MT1-MMP were significantly overexpressed in fibroblasts from the IZ. B: Co-culture of fibroblasts with less (MCF-7) or high (MDA-MB-231) invasive breast cancer cell line. High invasive breast cancer could induce laminin-γ2 and integrin β4 in all fibroblasts, regardless of their origins.
Discussion
There have been many studies aimed at understanding tumorigenesis and various trials to develop cancer treatments to inhibit tumor formation. Unfortunately, because the causes of tumor formation are diverse and multiple, such studies have achieved limited success and have not been widely applied to cancer therapies. To overcome the limitations of previous studies, scientists have recently begun to turn their attention to the TME, as well as the tumor itself. Cross talk between tumor cells and the TME is now recognized as critical for tumor progression, which is closely associated with the malignancy of cancer.21 As mentioned previously, the wide variety of causes of tumorigenesis is hampering the advancement of cancer research and treatments. For this reason, the common characteristic shared by malignant tumors may be the best target for cancer treatment. Invasion is the early and primary phase of tumor progression in almost all malignant tumors. Thus, the relationship between the tumor invasiveness and alterations in TME should be studied. To understand this relationship, we first introduced the novel concept of IZ, the tissue area showing dense fibrosis, which we defined as a 5-mm-wide belt surrounding the tumor burden and adjoining normal tissue. A 5-mm-surgical margin is widely accepted for margin controlled surgery.3 In our study, the IZ was hypothesized to be the actual tissue site of TME where tumor invasion-promoting alterations actively occur, and we compared the IZ with the TZ and NZ of both DCIS and IDC tissues.
Although TME has been studied in various contexts, such as angiogenesis, and the roles of fibroblasts and adipocytes in tumor progression, no studies have defined the extent of TME in tissue that directly affects tumor behavior. This may be because TME is defined as the entire group of stromal cells activated by a tumor or tumor progression-attending stroma. Thus, the definition of TME seems rather abstract and difficult to attain. In our study, we considered the dense fibrotic zone, or the IZ, around the tumor burden of IDC as the actual tissue site of TME, because fibrosis is one of the common features of tumor progression and contributes to TME alterations such as basement membrane stiffening.5 Before studying the alterations in the IZ during tumor invasion, we had to confirm the distinctiveness of the IZ by comparing it with the TZ and NZ in the context of gene expression. The ECM is a major component of the TME, and ECM alterations accompany tumor progression.22 As expected, the IZ of IDC showed ECM gene expression patterns that were distinct from both the TZ and NZ, including the specific upregulation of LAMA3, LAMC2, ITGA6, and ITGB4, overexpression of the corresponding proteins, laminin-332 and integrin α6β4, intensive expression of laminin-γ2 in the matrix around lobules, and a strong membranous pattern of integrin β4 around vessels. The entire gene expression pattern of the IZ was also distinct from those of the TZ and NZ, but was relatively similar to that of the TZ. Overexpression of laminin-332 in the IZ of IDC was rather surprising because laminin-332 expression decreases as breast cancer progresses.14,15 The functions of laminin-332 in tumor progression are to lead tumor migration and to maintain tumor survival during migration by binding its receptors, integrin α6β4 or α3β1, on tumor cells.23–25 Therefore, increased expression of laminin-332 in the IZ may be the product of the proximate paracrine intercellular interaction between invasive tumor cells in the TZ and the myofibroblasts (InF) in the IZ to prepare a suitable microenvironment for tumor invasion. Laminin-332-rich tumor microenvironment in the IZ may be able to continuously stimulate integrin-related signaling pathways for migration and survival of the invading tumor cells. This may be supported by laminin-γ2 overexpression in InF myofibroblasts, and this overexpression may be a key player in the formation of laminin-332-rich tumor microenvironment in the IZ for tumor migration and survival. Both cancer-associated fibroblasts (CAF) and normal breast fibroblasts expressed significantly less laminin-γ2. Interestingly, both CAF and normal breast fibroblasts could express laminin-γ2 after interacting directly with the highly invasive tumor cells, MDA-MB-231. The induction of laminin-332 expression in fibroblasts may be closely associated with tumor invasiveness. Increased expression of integrin α6β4 in the IZ of IDC can also be evidence of TME alteration for tumor invasion. Integrin α6β4 expression may be helpful or necessary for stromal cell survival during ECM remodeling. Due to the loss of connections during ECM remodeling, stromal cells should die from anoikis. However, some stromal cells, such as fibroblasts and adipocytes, should be protected from anoikis to assist tumor progression. In this sense, integrin α6β4 expression might support TME alterations in the IZ for tumor progression. Integrin β4 expression was not observed in all fibroblast types. However, integrin β4 was expressed in all fibroblast types after direct interaction with invasive tumor cells. It may be that some alterations of TME, such as integrin β4, require direct contact with tumor cells.
Fibroblasts are known to play a positive role in tumor progression. Thus, fibrosis of the IZ of IDC could be the phenotype of tumor progression-promoting alterations in the actual site of TME. EMT, a novel source of fibroblast formation that causes fibrosis, has been observed during the formation of interstitial fibroblasts from organ epithelium, oncogenesis, and fibrotic tissue repair following injury − a process known as fibrogenesis.26–28 In the IZ of IDC, not a counterpart of DCIS, the following EMT-like events were observed: the upregulation of Snail and ZEB1, the gain of N-cadherin, and the down-regulation of miR200c coupled with the upregulation of ZEB1. Moreover, TGF-β was primarily expressed in the TZ, but also maintained at moderate levels in the IZ. TGF-β can induce the expression of MMP3 in stromal cells, leading to Snail upregulation through reactive oxygen species caused by Rac1b in nontumoral epithelial cells, and then finally the transition of epithelial cells to myofibroblasts.6,7 TGF-β can also induce MT1-MMP,29 which leads to similar results as MMP3, such as fibrosis in mammary glands8 and EMT in prostate cancer.9 Therefore, we suggest that TGF-β produced in the tumor cells paracrinically expended to the IZ and triggered the transition of epithelial cells into myofibroblasts via EMT process induced by MMP3 and MT1-MMP. This may be supported by both MT1-MMP overexpression and Snail upregulation in InF. Although MMP3 expression was not observed in InF, it may play a more important function for myofibroblast formation in the IZ than MT1-MMP. In the initial stage of myofibroblast formation, MMP3 may first trigger the EMT of epithelial cells in the IZ, and then transdifferentiated myofibroblasts could express MT1-MMP, which may enhance the transition of epithelial cells into myofibroblasts by helping MMP3. The co-presence of laminin-332 with TGF-β in the IZ may also support complete EMT, leading to myofibroblast formation.18 In previous studies, the transition of tubular epithelial cells to myofibroblasts27,30 and the susceptibility of tubular epithelial cells to EMT31 were reported. Therefore, the origin of InF may be the epithelial cells from ductolobular units in the IZ based on both intensive laminin-γ2 expression in the matrix around lobules and laminin-γ2 overexpression of InF. Taken together, we propose that InF may be responsible for tumor invasion based on laminin-332 and MT1-MMP overexpression. Laminin-332 is now widely recognized to induce the motility of cancer cells with integrins,25 and is associated with breast cancer metastasis.32 In addition, MT1-MMP derived from fibroblasts promotes tumor progression.33
Based on our results, we summarize the events in the IZ of IDC leading to the formation of dense fibrosis during tumor invasion as the following: (1) tumor cells in the TZ initially interact with CAF and some in the TZ undergo EMT; (2) at the same time, TGF-β produced by tumor cells in the TZ begins to spread out to adjacent tissue areas, the IZ; (4) TGF-β leads to MMP3 production in some stromal cells in the IZ; (5) secreted MMP3 induces Snail and ZEB1 upregulation in epithelial cells in the IZ through Rac1b; (6) the epithelial cells begin to transdifferentiate via EMT and become myofibroblasts or InF; (7) InF myofibroblasts continue to increase in number and finally form dense fibrosis in the IZ; (8) InF can enhance myofibroblast formation by expressing MT1-MMP, which can enhance the EMT of epithelial cells together with MMP3, secrete laminin-332 to guide tumor progression, and activate other soluble MMPs for ECM remodeling by MT1-MMP (Figure 5).
Figure 5.
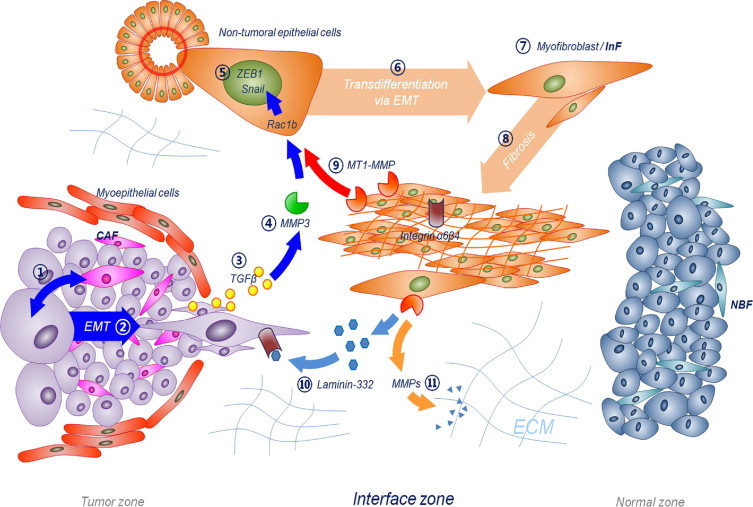
Interface zone alterations that may occur in the early stages of tumor invasion. Peritumoral dense fibrotic zone of IDC may be formed by following processes: (1) tumor cells in the TZ initially interact with CAF, and (2) some of them can undergo EMT and become invasive. Simultaneously, (3) TGF-β produced by tumor cells in the TZ begins to spread out to the IZ. (4) TGF-β leads to MMP3 production in some stromal cells in the IZ. (5) Secreted MMP3 induces Snail and ZEB1 upregulation in epithelial cells in the IZ through Rac1b. (6) The epithelial cells begin to transdifferentiate via EMT, (7) they become myofibroblasts or specifically InF. (8) InF myofibroblasts continue increasing in number and finally form dense fibrosis in the IZ. (9) InF can enhance myofibroblast formation by expressing MT1-MMP, which can induce the EMT of epithelial cells together with MMP3. In addition, (10) InF can secrete laminin-332 to guide tumor progression, and (11) activates other soluble MMPs for ECM remodeling by MT1-MMP expression.
The findings in our study raise some questions as follows:
i) Is the InF cell population truly distinct from CAF?
ii) If so, how valuable is the InF population as a candidate for cancer therapy?
iii) What is the major source of InF cells in breast cancer?
iv) Are there other factors required for InF formation besides TGF-β, MMP3, and MT1-MMP?
v) Are InF cells specialized for tumor invasion, as we expect?
We will try to answer these questions in our future studies and hope to better understand the relationship between tumor invasiveness and TME alterations.
In conclusion, the IZ of IDC may be a distinct tissue area based on its global gene expression pattern, specific molecular expression, and the dense fibrosis that may be formed by the transition of epithelial cells to myofibroblasts via EMT. In addition, a laminin-332 overexpressing myofibroblast type (InF) in the IZ of IDC may be critical for tumor invasion and survival. Therefore, the IZ of IDC may be considered to be the actual tissue site of TME where precedent alterations to enhance tumor invasion actively occur. The IZ may prove to be a potent target for cancer treatment, such as neoadjuvant therapy, and a tissue area worthy of further study.
Footnotes
Supported by the Korea Health Care Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea A084550 (N.C.H.); and by Mid-career Researcher Program through NRF grant funded by the MEST No. 2010-0000357 (N.C.H.).
References
- 1.Liotta L.A., Kohn E.C. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 2.Montel V., Mose E.S., Tarin D. Tumor-stromal interactions reciprocally modulate gene expression patterns during carcinogenesis and metastasis. Int J Cancer. 2006;119:251–263. doi: 10.1002/ijc.21757. [DOI] [PubMed] [Google Scholar]
- 3.Taghian A., Mohiuddin M., Jagsi R., Goldberg S., Ceilley E., Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005;241:629–639. doi: 10.1097/01.sla.0000157272.04803.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radisky D.C., Kenny P.A., Bissell M.J. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT. J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 6.Radisky D.C., Levy D.D., Littlepage L.E., Liu H., Nelson C.M., Fata J.E., Leake D., Godden E.L., Albertson D.G., Nieto M.A., Werb Z., Bissell M.J. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radisky D.C., Przybylo J.A. Matrix metalloproteinase-induced fibrosis and malignancy in breast and lung. Proc Am Thorac Soc. 2008;5:316–322. doi: 10.1513/pats.200711-166DR. [DOI] [PubMed] [Google Scholar]
- 8.Ha H.Y., Moon H.B., Nam M.S., Lee J.W., Ryoo Z.Y., Lee T.H., Lee K.K., So B.J., Sato H., Seiki M., Yu D.Y. Overexpression of membrane-type matrix metalloproteinase-1 gene induces mammary gland abnormalities and adenocarcinoma in transgenic mice. Cancer Res. 2001;61:984–990. [PubMed] [Google Scholar]
- 9.Cao J., Chiarelli C., Richman O., Zarrabi K., Kozarekar P., Zucker S. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J Biol Chem. 2008;283:6232–6240. doi: 10.1074/jbc.M705759200. [DOI] [PubMed] [Google Scholar]
- 10.Xouri G, Christian S: Origin and function of tumor stroma fibroblasts, Semin Cell Dev Biol 21:40–46 [DOI] [PubMed]
- 11.Mizushima H., Miyagi Y., Kikkawa Y., Yamanaka N., Yasumitsu H., Misugi K., Miyazaki K. Differential expression of laminin-5/ladsin subunits in human tissues and cancer cell lines and their induction by tumor promoter and growth factors. J Biochem. 1996;120:1196–1202. doi: 10.1093/oxfordjournals.jbchem.a021541. [DOI] [PubMed] [Google Scholar]
- 12.Berndt A., Hyckel P., Konneker A., Katenkamp D., Kosmehl H. Oral squamous cell carcinoma invasion is associated with a laminin-5 matrix re-organization but independent of basement membrane and hemidesmosome formation: Clues from an in vitro invasion model. Invasion Metastasis. 1997;17:251–258. [PubMed] [Google Scholar]
- 13.Giannelli G., Fransvea E., Bergamini C., Marinosci F., Antonaci S. Laminin-5 chains are expressed differentially in metastatic and nonmetastatic hepatocellular carcinoma. Clin Cancer Res. 2003;9:3684–3691. [PubMed] [Google Scholar]
- 14.Martin K.J., Kwan C.P., Nagasaki K., Zhang X., O'Hare M.J., Kaelin C.M., Burgeson R.E., Pardee A.B., Sager R. Down-regulation of laminin-5 in breast carcinoma cells. Mol Med. 1998;4:602–613. [PMC free article] [PubMed] [Google Scholar]
- 15.Hao J., Jackson L., Calaluce R., McDaniel K., Dalkin B.L., Nagle R.B. Investigation into the mechanism of the loss of laminin 5 (alpha3beta3gamma2) expression in prostate cancer. Am J Pathol. 2001;158:1129–1135. doi: 10.1016/s0002-9440(10)64060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pullar C.E., Baier B.S., Kariya Y., Russell A.J., Horst B.A., Marinkovich M.P., Isseroff R.R. beta4 Integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell. 2006;17:4925–4935. doi: 10.1091/mbc.E06-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W., Giancotti F.G. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 18.Giannelli G., Bergamini C., Fransvea E., Sgarra C., Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129:1375–1383. doi: 10.1053/j.gastro.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 19.Hurteau G.J., Spivack S.D., Brock G.J. Potential mRNA degradation targets of hsa-miR-200c, identified using informatics and qRT-PCR. Cell Cycle. 2006;5:1951–1956. doi: 10.4161/cc.5.17.3133. [DOI] [PubMed] [Google Scholar]
- 20.Olumi A.F., Grossfeld G.D., Hayward S.W., Carroll P.R., Tlsty T.D., Cunha G.R. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissell M.J., Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver V.M., Fischer A.H., Peterson O.W., Bissell M.J. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinkovich M.P. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki K. Laminin-5 (laminin-332): unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006;97:91–98. doi: 10.1111/j.1349-7006.2006.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter P.M., Dao A.V., Arain Z.S., Chang M.K., Nguyen H.P., Arain S., Wang-Rodriguez J., Kwon S.Y., Wilczynski S.P. Motility induction in breast carcinoma by mammary epithelial laminin 332 (laminin 5) Mol Cancer Res. 2009;7:462–475. doi: 10.1158/1541-7786.MCR-08-0148. [DOI] [PubMed] [Google Scholar]
- 26.Flier S.N., Tanjore H., Kokkotou E.G., Sugimoto H., Zeisberg M., Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng Y.Y., Huang T.P., Yang W.C., Chen Z.P., Yang A.H., Mu W., Nikolic-Paterson D.J., Atkins R.C., Lan H.Y. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998;54:864–876. doi: 10.1046/j.1523-1755.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- 28.Strutz F., Okada H., Lo C.W., Danoff T., Carone R.L., Tomaszewski J.E., Neilson E.G. Identification and characterization of a fibroblast marker: fSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munshi H.G., Wu Y.I., Mukhopadhyay S., Ottaviano A.J., Sassano A., Koblinski J.E., Platanias L.C., Stack M.S. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-beta1-induced pericellular collagenolysis. J Biol Chem. 2004;279:39042–39050. doi: 10.1074/jbc.M404958200. [DOI] [PubMed] [Google Scholar]
- 30.Rhyu D.Y., Yang Y., Ha H., Lee G.T., Song J.S., Uh S.T., Lee H.B. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 31.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter P.M., Wang-Rodriguez J., Chan O.T., Wilczynski S.P. Laminin 5 expression in metaplastic breast carcinomas. Am J Surg Pathol. 2008;32:345–353. doi: 10.1097/PAS.0b013e3181592201. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W., Matrisian L.M., Holmbeck K., Vick C.C., Rosenthal E.L. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]


